Abstract
The Kitasato Symposium 2009: New Prospects for Cytokine Inhibition was held in Berlin, Germany from 7 to 9 May 2009. The key aims of this meeting were to bring together a group of front-line researchers and rheumatologists to evaluate the use of cytokine blockade and to examine the role of certain cytokines in the pathogenesis of rheumatoid arthritis and other autoimmune diseases. A keynote lecture delivered by Professor Jean-Michel Dayer provided an up-to-date overview of the interactions occurring between the immune system and acute phase proteins. Other speakers discussed the role of cytokines in rheumatoid arthritis, including their role in joint destruction, as well as their regulatory role upon T cells and B cells. The involvement of cytokines in other autoimmune diseases was also addressed.
Introduction
During May 2009 the first Kitasato meeting was held at the Palais am Festungsgraben, Berlin in memory of Professor Shibasaburo Kitasato (1853-1931), who worked in Berlin between 1885 and 1892 together with Robert Koch, Emil von Behring and other scientists. This 2-day meeting gathered together a group of front-line researchers and rheumatologists to discuss the protective and pathogenic role of cytokines in rheumatoid arthritis (RA) and other autoimmune diseases. Cytokine-related therapeutic approaches for these conditions and their underlying mechanisms were also considered.
A key aim of this meeting was to bring together not only those individuals with an interest in the clinical aspects of rheumatology and biological therapies, but also those involved in basic immunological research. Approximately 50 individuals from around the world attended this meeting, which it is hoped may be the first of many, thus furthering the development of biological therapies and improving outcomes for individuals with RA or other autoimmune diseases.
Keynote lecture
The meeting began with a keynote lecture delivered by Professor Jean-Michel Dayer (Geneva, Switzerland). Professor Dayer proposed that acute phase proteins, which were first identified in patients with pneumococcal infections during the 1930s [1] and 1940s [2,3], may be the first cytokines to have been identified.
Acute phase proteins can be positive (that is, their concentrations increase in response to inflammation - for example, C-reactive protein, serum amyloid A) or negative (that is, their concentrations decrease in response to inflammation - for example, apolipoproteins that protect from inflammation by inhibiting the contact between activated lymphocytes and monocytes for the production of IL-1 and TNF) (Figure 1) [4]. Describing autoimmune diseases as a hyperactivity of the immune system, and using IL-1 receptor agonist (IL-1Ra) and apolipoprotein A1 as examples, Professor Dayer went on to consider how cytokines interact with one another in the body and the role that they might play in the development of autoimmune diseases. Discussing the delicate balance that exists between IL-1 and IL-1Ra, Professor Dayer noted that, during inflammation, leptin produced by adipocytes can stimulate the production of IL-1 by the hypothalamus (Figure 2) [5]. Acting at this level, IL-1 becomes a cachexin resulting in the loss of both adipose tissue and lean body mass. The adipocytes also produce IL-1Ra, however, which is able to block the cachectic action of IL-1 and increase appetite [6].
Figure 1.
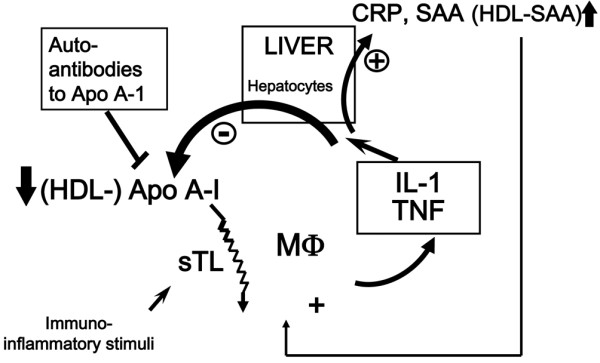
Apolipoproteins protect from inflammation. Apolipoprotein (Apo) A-I inhibits T-cell/monocyte interactions, thus blocking the production of IL-1 and TNF and reducing inflammation. CRP, C-reactive protein; SAA, serum amyloid A; HDL, high-density lipoprotein; sTL, stimulated T lymphocyte; MΦ, macrophages. Figure kindly provided by Prof. Jean-Michel Dayer (Geneva, Switzerland).
Figure 2.
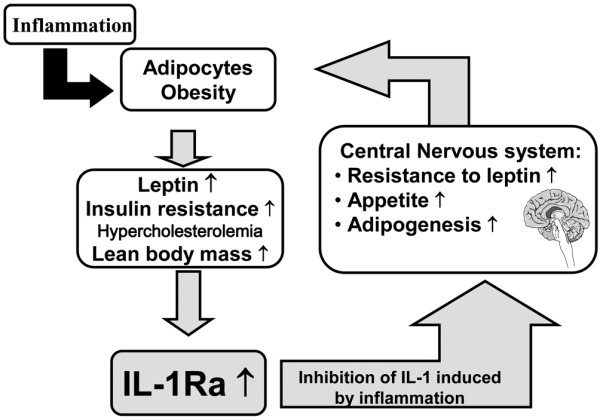
Leptin produced by adipocytes can stimulate production of IL-1 by the hypothalamus. Relationship between inflammation, adipocytes, IL-1 receptor antagonist (IL-1Ra), leptin, and obesity. Figure kindly provided by Prof. Jean-Michel Dayer (Geneva, Switzerland).
If used therapeutically, cytokines may offer a less toxic treatment option for individuals with autoimmune disease owing to their abilities to modulate inflammation. Moreover, recombinant cytokines may offer novel treatment approaches for individuals found to demonstrate mutations in genes responsible for the production of cytokines.
Cytokines and arthritis
The involvement and therapeutic potential of lymphotactin (activation-induced, T-cell-derived and chemokine-related cytokine (ATAC)) in Th-cell autoimmune reactions was the subject of a presentation by Professor Richard Kroczek (Berlin, Germany).
ATAC was first cloned in both mice [7] and humans [8] more than 15 years ago, and was initially believed to act as a chemoattractant for lymphocytes [7]. Subsequent studies in humans indicated that ATAC/lymphotactin was primarily produced in the synovium of RA patients and so, given its role as a chemoattractant, might be a key modulator for T-cell trafficking in the pathogenesis of RA [9]. Studies using murine models suggest that the receptor for ATAC/lymphotactin is only present on CD8+ dendritic cells, such as those found in the spleen, which, given the role of CD8 cells in the development of self-tolerance by the immune system, may be implicated in the development of autoimmunity.
Cartilage destruction and bone erosion are major problems in RA, and studies have shown that these processes may be mediated by cytokines. Murine arthritis models have demonstrated the therapeutic potential of anti-TNFα and anti-IL-1 antibodies [10]. During this session on cytokines and arthritis, Professor Wim van den Berg (Nijmegen, The Netherlands) postulated that different cytokines may dominate at different stages of the inflammatory process. For example, in early-stage collagen-induced arthritis both anti-TNFα and anti-IL-1 treatments have been shown to be effective [11]. Moreover, IL-17 - a T-cell cytokine expressed in the synovium and synovial fluid of patients with RA - has been shown to be a potent inducer of TNFα and IL-1, and is involved in both the initiation and progression of murine arthritis models [12]. IL-17 not only synergizes with TNFα, but also enhances inflammation and destruction independent of IL-1 and TNFα, making it an additional potential target for the treatment of RA. As such, tailor-made treatment is required for the different patient groups.
Professor Stefan Rose-John (Kiel, Germany) discussed the inflammatory properties of IL-6 and the complexity of IL-6 signalling, together with the consequences of and various techniques employed in IL-6 blockade. During his presentation, Professor Rose-John noted that all IL-6 signalling is mediated via binding of the IL-6 receptor to the ubiquitously expressed glycoprotein 130. The IL-6 receptor is normally membrane bound and expressed only on hepatocytes and some leukocytes; however, this receptor can be cleaved, or shed, from the cell via the actions of a metalloprotease (ADAM17), producing a soluble IL-6 receptor that can then bind to cells which do not normally express this receptor. This phenomenon, so-called trans-signalling, enables IL-6 to exert its effects upon a much wider range of cell types, including smooth muscle cells, endothelial cells and neural cells [13]. Binding of IL-6 to soluble IL-6 receptor has been shown to be proinflammatory, and has thus been implicated in the pathogenesis of RA - making this pathway a target for therapeutic interventions [13]. Recent studies have revealed that selective blockade of this alternative IL-6 signalling pathway using an engineered variant of soluble glycoprotein 130 (sgp130Fc) led to substantial clinical improvement in a preclinical arthritis model [14].
Although TNFα has been implicated in the pathogenesis of RA, it also plays an important role in host defence, with complete TNFα blockade associated with an increased risk of mycobacterial infection [15]. Professor Sergei Nedospasov (Berlin, Germany and Moscow, Russia) described in detail the development of a novel humanized murine model for the study of TNFα in collagen-induced arthritis [16]. In this heterozygous model, both alleles are active - producing human and murine TNFα, and thus allowing detailed comparison of human versus mouse regulation of TNFα expression. In the homozygous model, approximately 40 to 50% of humanized mice will develop arthritis (mediated by human TNFα), which can be treated clinically using TNF blockers. Using this, and several other murine models that they have developed, Professor Nedospasov and his team aim to determine the source of TNFα that protects against infection and to develop TNFα inhibitors that target specific cell types or compartments in the body where TNFα is overproduced.
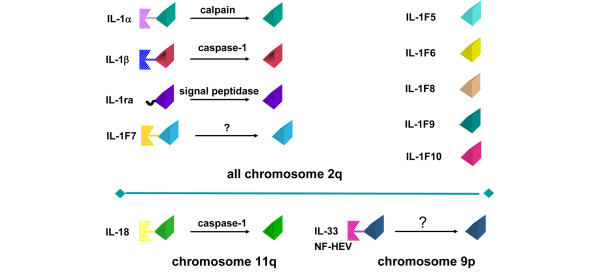
Session 2 continued the theme of cytokines in arthritis and began with an overview of the biological role of IL-1 and its novel homologue IL-33 in inflammatory responses. The original members of the IL-1 superfamily were IL-1α, IL-1β and IL-1Ra; however, several additional molecules with structural homology have been added to this family in recent years, namely IL-1F5, IL-1F6, IL-1F7, IL-1F8, IL-1F9, IL-1F10, IL-18 and IL-33 (Figure 3). Professor Cem Gabay (Geneva, Switzerland) discussed how the balance between IL-1and IL-1Ra influences the development and severity of arthritis. Using conditional knockout mice in which the expression of IL-1Ra has been selectively targeted in myeloid cells, Professor Gabay's group showed that these mice had a more rapid onset and severe form of collagen-induced arthritis with lower levels of IL-1Ra in lymph nodes and increased Th1 and Th17 responses [17]. IL-33 - which binds to a member of the IL-1 receptor family, inducing similar intracellular signals to IL-1 - is also expressed in human synovial fibroblasts with increased expression in arthritic joints of murine models, suggesting a potential role in the pathogenesis of arthritis [18].
Figure 3.
The IL-1 superfamily. The original members of the IL-1 superfamily were IL-1α, IL-1β and IL-1 receptor antagonist (IL-1Ra). IL-1F5, IL-1F6, IL-1F7, IL-1F8, IL-1F9, IL-1F10, IL-18 and IL-33 have been added to this family in recent years owing to their structural homology. NF-HEV, nuclear factor from high endothelial venules. Figure kindly provided by Dr John Sims (Amgen Inc., Thousand Oaks, CA, USA).
Interferons are a family of naturally secreted proteins with immunomodulatory functions. IFNβ has anti-inflammatory properties and plays a role in bone homeostasis. IFNβ treatment has been shown to reduce the severity of collagen-induced arthritis in mice [19,20] and rhesus monkeys [21]. Conversely, IFNβ deficiency resulted in the development of severe collagen-induced arthritis in mice as a result of increased activation of stromal cells and osteoclasts [22]. After reviewing the lack of efficacy of subcutaneous injections of IFNβ protein three times weekly for the treatment of RA [23], Dr Margriet Vervoordeldonk (Amsterdam, The Nether-lands) went on to discuss the potential of intra-articular IFNβ gene therapy for the treatment of RA. In a set of proof-of-principle studies using an adenoviral vector and recombinant adeno-associated virus type 5 for local delivery of the rat IFNβ gene [24,25], a beneficial effect has been shown on arthritis development in two different rat models of arthritis.
Local delivery of adenoviral vector or recombinant adeno-associated virus type 5 vectors expressing rat IFNβ after the onset of disease reduced paw swelling impressively in both injected and uninjected joints. Strikingly, IFNβ treatment protected against bone and cartilage erosions. Together, the results provide a rationale for IFNβ as a therapeutic target for intra-articular gene therapy for arthritis.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to be produced locally in the synovium of individuals with RA, but not in those with osteoarthritis [26,27]. Dr Christine Plater-Zyberk (Munich, Germany) described the validation of an anti-GM-CSF monoclonal antibody (22E9) for the treatment of RA, and noted how administration of this antibody decreased arthritis severity in several experimental models [28-30]. Dr Plater-Zyberk went on to discuss the development of MT203, a human anti-GM-CSF antibody with subnanomolar affinity and a very slow off-rate, which has been derived from phage display-guided selection. MT203 has been shown to be stable in human serum and to inhibit the production of IL-8 at subnanomolar concentrations. Preclinical studies suggest that MT203 may be useful for the treatment of RA and other indications, including psoriasis and multiple sclerosis. The first clinical trial application for MT203 was submitted in Europe early in 2009 and the trial has now started.
Cytokines and T-cell regulation
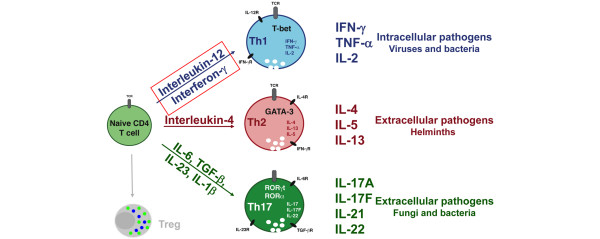
The first presentation in the next session of the meeting was given by Professor Max Löhning (Berlin, Germany) and focused on memory imprinting of T cells by cytokines. Immunological memory, which can be defined as a more rapid response in antigen-primed individuals, is a key characteristic of the adaptive immune system. Increasing the availability of T cells reactive to specific antigens is the aim of the majority of vaccinations and immune cell therapies. Professor Löhning described a series of experiments to identify how IFNγ and IL-12, as opposed to IL-4 or IL-6 and transforming growth factor beta, induce the differentiation of relatively short-lived naïve T cells into long-lived memory T cells with distinct functional properties (Figure 4) [31,32].
Figure 4.
Role of cytokines in T-cell differentiation. Effector cytokine induction is part of Th-cell differentiation programmes - Th1 inducers? ROR, retinoic acid-related orphan receptor; T-bet, T-box expressed in T-cells; TCR, T-cell receptor; TGF, transforming growth factor; Treg, regulatory T cell. Figure kindly provided by Prof. Max Löhning (Berlin, Germany).
Techniques that may be employed to manipulate the cytokine environment using tolerogenic dendritic cells were presented by Professor John Isaacs (Newcastle, UK). Dendritic cells are antigen-presenting cells that initiate and orchestrate immune responses. By generating tolerogenic dendritic cells, it is possible to downregulate immune responses. Tolerogenic dendritic cells have been generated by culturing monocytes in the presence of IL-4 and GM-CSF and then exposing them to immunosuppressive agents such as dexamethasone [33]. Tolerogenic dendritic cells have been shown to be highly stable and refractory to further stimulation by either lipopolysaccharide or peptidoglycan. They also maintain their tolerogenic phenotype in a proinflammatory cytokine environment. Professor Isaacs described preclinical studies where intravenous administration of tolerogenic dendritic cells has been shown to decrease the severity of inflammatory arthritis and to inhibit disease progression. Studies in humans are planned in the near future, and it is hoped that this approach may provide a route for immune reprogramming in autoimmunity.
An update on IL-17 and the growing IL-17 family of cytokines was provided by Professor Pierre Miossec (Lyon, France). Studies have shown production of functional IL-17 by RA synovium explants and have shown that overexpression of IL-17 in the knee joint of mice induces bone destruction and cartilage damage [34]. A whole family of IL-17 molecules, now known as IL-17A to IL-17F, has been identified together with a range of IL-17 receptors. The highest degree of homology (50%) is observed between IL-17A and IL-17F, both of which are associated with the pathogenesis of RA [35]. In contrast, IL-17E displays the lowest level of homology with IL-17A (17%) and has not been shown to play any role in RA [36-38]. IL-17 has been shown to induce the expression of IL-1, IL-6, IL-8, GM-CSF and TNFα, all of which may contribute to the pathogenesis of RA. In concluding, Professor Miossec noted that the biology of IL-17A indicates a role in the development of inflammation and joint destruction, and that the contribution of other IL-17 family members to this process must be considered. Different novel therapeutic approaches targeted at these pathways are now under investigation with interesting preliminary results.
The role of IL-17 in autoimmune disease was discussed during a presentation given by Professor Thomas Kamradt (Jena, Germany). The pathogenesis of autoimmune diseases was previously thought to be largely driven by IL-12 acting via Th1 cells. Knockout studies, however, have revealed an increased susceptibility to autoimmunity when the p35 chain of the IL-12 receptor is removed. These findings resulted in the hypothesis that Th17 cells underlie the majority of autoimmune diseases and are therefore a good target for the development of novel therapies [39]. More recent research has demonstrated that T cells isolated from inflammatory lesions often co-express IL-17 together with IFNγ (the prototypical Th1 cytokine) and TNFα. A major current topic of investigation into Th17 cells is the question of whether Th cells form a stable memory for IL-17 expression [40].
Cytokines related to B-cell functions
The role of two cytokines that belong to the TNFα family-B-cell activating factor (BAFF), which plays a role in the development and survival of autoreactive B-cells, and a proliferation-inducing ligand (APRIL), which promotes B-cell activation and survival together with the humoral immune response - were considered during a presentation by Professor Thomas Dörner (Berlin, Germany). Both BAFF and APRIL have been found to be elevated in autoimmune diseases such as RA, systemic lupus erythematosus, Sjögren's syndrome, autoimmune thrombocytopenia and autoimmune polyglandular syndrome type 1, and so have become potential therapeutic targets. Professor Dörner outlined a number of studies using the monoclonal antibody belimumab to inhibit BAFF [41-46]. Although the clinical efficacy of this approach, particularly in RA, has not so far been convincing, a recent press release (GSK/HGS) reported positive results of the use of belimumab (anti-BAFF/BLyS) in a 1-year trial of systemic lupus erythematosus (BLISS-52). Finally, Professor Dörner speculated that targeting the events that lead to BAFF production may be more successful.
The role of B cells in autoimmune diseases was further discussed during a presentation by Dr Simon Fillatreau (Berlin, Germany). Murine models of multiple sclerosis have been employed in an attempt to elucidate the interactions that may occur between cytokines, B cells and T cells. Experimental autoimmune encephalomyelitis, induced by immunizing mice with myelin proteins, is mediated by Th1 and Th17 CD4+ T cells and is clinically manifested by paralysis. Experiments have shown that recovery from experimental autoimmune encephalomyelitis requires the production of IL-10 by B cells [47]. Myeloid differentiation primary response gene 88 is a universal adapter protein that signals via Toll-like receptors to activate the transcription factor NF-κB and trigger the regulatory function of B cells, and hence control the inflammatory T-cell response. In addition, B-cell activation via the B-cell receptor and CD40 has also been implicated in recovery from experimental autoimmune encephalomyelitis. A two-step process whereby B cells limit inflammation and aid recovery from experimental autoimmune encephalomyelitis thus operates within this murine model. Firstly, myeloid differentiation primary response gene 88 signalling in B cells induces potent anti-inflammatory cascades capable of suppressing chronic immune responses. Secondly, the B-cell receptor and CD40 then act to amplify these B-cell-mediated cascades. These findings may have implications for the study of multiple sclerosis in humans.
In addition to its involvement in metabolism and energy balance, adipose tissue has been suggested to play a role in the immune system via the secretion of cytokines known as adipokines. Adipokines such as adiponectin, visfatin and resistin have all been implicated in the pathogenesis of RA. Dr Elena Neumann (Giessen, Germany) described a number of studies that suggest adipokines may play a role in RA by modulating inflammatory and/or destructive mechanisms [48,49]. For example, both adiponectin and visfatin have been shown to have several destructive effects in RA, inducing proinflammatory factors, chemokines and matrix-degrading enzymes [50-53]. Studies in murine models, however, suggest that adiponectin may mitigate the severity of arthritis [54,55] - suggesting either that different isoforms of adiponectin exist or that different signalling cascades may be activated in different tissues. The role of resistin in inflammation, and thus RA, is less clear and requires further investigation.
Genetic aspects of cytokine regulation
A comprehensive review of the genomic aspects of autoimmunity was provided by Professor Gerd Burmester (Berlin, Germany). Professor Burmester discussed the various techniques available for gene expression profiling, together with the importance of optimal sample selection. Gene expression profiling of synovial tissue taken from individuals suffering from RA has revealed a diagnostic pattern with a dominance of cell infiltration-related changes. Interestingly, these techniques have also allowed analysis of gene expression in patients who did not respond to therapy, with nonresponding patients treated with anti-TNF showing similar genetic profiles to untreated patients.
Further insight into the genetics underlying cytokine regulation was provided in a presentation by Dr Leonid Padyukov (Stockholm, Sweden). Genetic polymorphisms can have functional consequences, mainly due to variation in regulatory sequences. Studying genetic predisposition is therefore important in the most homogeneous subgroup of the disease available. For example, autoantibodies to citrullinated proteins have been observed in 60 to 70% of patients with RA, and their early appearance suggests a possible role in the pathogenesis of this disease [56]. It has been suggested that the majority of genetic polymorphisms associated with RA are restricted to those individuals with autoantibody-positive disease and are found in only a few individuals with autoantibody negative RA [57].
Key cytokines related to autoimmunity
The final session of the meeting focused on the key cytokines that have been shown to play a role in autoimmunity: type I interferon, IFNα, IL-12, IL-23 and IL-21.
Professor Peggy Crow (New York City, USA) discussed how microarray analyses have enabled the identification of genetic signatures in the peripheral blood of individuals with autoimmune diseases. In addition, increased IFNα pathway activation has been associated with increased autoimmunity together with increased inflammation and tissue damage.
Continuing on the same theme, Professor Lars Rönnblom (Uppsala, Sweden) concurred that administration of IFNα can cause autoimmune disease and that many patients with autoimmune conditions have an ongoing production of IFNα. Moreover, anti-IFNα antibodies applied in preclinical and early clinical trials have been shown to attenuate autoimmunity.
Professor Joachim Sieper (Berlin, Germany) provided an overview of clinical data that clearly demonstrated the benefits of IL-12 and IL-23 blockade in individuals with psoriasis [58,59]. This approach has proved less beneficial in individuals with either Crohn's disease or psoriatic arthritis. Moreover, further investigations are required in order to establish whether joint IL-12 and IL-23 blockade is required, or whether blockade of IL-23 alone would be sufficient for the treatment of autoimmunity.
A comprehensive review of the properties of IL-21 and the IL-21 receptor was provided by Professor Peter Lipsky (Bethesda, MD, USA). Professor Lipsky outlined the role played by IL-21 in B-cell differentiation and T cell-B cell interactions, and commented that IL-21 is a new target for the downregulation of B-cell responsiveness (Figure 5). After noting that IL-21 is elevated in individuals with systemic lupus [60], Professor Lipsky speculated on the possible benefit to these individuals of effective IL-21 blockade.
Figure 5.
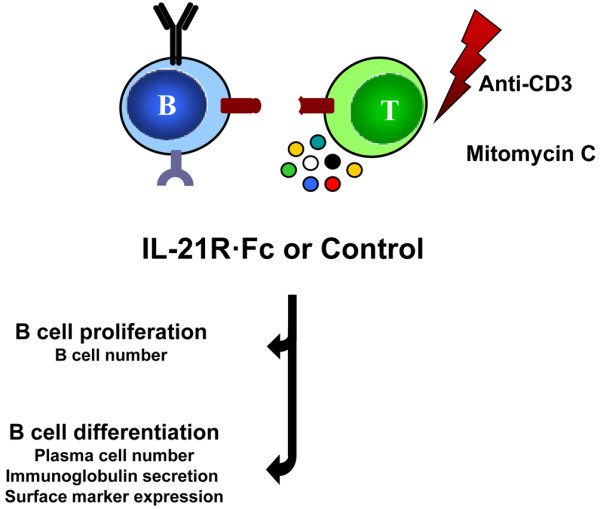
Properties of IL-21 and the IL-21 receptor. IL-21 plays a role in B-cell differentiation and T cell-B cell interactions, and is elevated in individuals with systemic lupus erythematosus.
Conclusions
In bringing this meeting to a close, Professor Burmester thanked all of the speakers for their thought-provoking presentations at a most enjoyable and interesting meeting. He commented on how pleased Professor Kitasato would have been with the advances that have been made within the field of cytokine research. Significant advances have been made in identifying the proinflammatory pathways that underlie the pathogenesis of RA and other autoimmune diseases. By building upon the research that was presented at this first Kitasato Symposium, it may be possible to develop new, more targeted therapies with the potential to significantly improve the prognosis for those individuals affected by autoimmunity.
Abbreviations
APRIL: a proliferation-inducing ligand; ATAC: activation-induced, T-cell-derived and chemokine-related cytokine; BAFF: B-cell activating factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN: interferon; IL: interleukin; IL-1Ra: IL-1 receptor antagonist; NF: nuclear factor; RA: rheumatoid arthritis; Th: T helper; TNF: tumour necrosis factor.
Competing interests
TD declares the following competing interests: support for clinical studies and consultancies from Roche, Chugai, UCB, Immunomedics and Genentech. GRB and PEL declare that they have no competing interests.
Note
*Kitasato Meeting Faculty: JR Kalden (Erlangen), JM Dayer (Geneva), R Kroczek (Berlin), W van den Berg (Nijmegen), S Rose-John (Kiel), S Nedospasov (Berlin, Moscow), C Gabay (Geneva), M Vervoordeldonk (AMC/University of Amsterdam, Amsterdam), Ch Plater-Zyberk (Munich), M Löhning (Berlin), J Isaacs (Newcastle, UK), P Miossec (Lyon), T Kamradt (Jena), S Fillatreau (Berlin), E Neumann (Giessen/Bad Nauheim), L Padyukov (Stockholm), P Crow (New York City), L Rönnblom (Uppsala), J Sieper (Berlin).
Contributor Information
Gerd R Burmester, Email: gerd.burmester@charite.de.
Peter E Lipsky, Email: peterlipsky@comcast.net.
Thomas Dörner, Email: thomas.doerner@charite.de.
Acknowledgements
The authors thank Sarah Birch who provided medical writing services supported by an unrestricted educational grant from Roche Pharma AG/Chugai. The Kitasato Symposium was supported by an unrestricted educational grant from Roche Pharma AG/Chugai.
References
- Tillett WS, Francis T Jr. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernethy TJ, Avery OT. The occurrence during acute infections of a protein not normally present in the blood: I. distribution of the reactive protein in patients' sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J Exp Med. 1941;73:173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM, Avery OT. The occurrence during acute infections of a protein not normally present in the blood: III. immunological properties of the C-reactive protein and its differentiation from normal blood proteins. J Exp Med. 1941;73:191–200. doi: 10.1084/jem.73.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmun Rev. 2002;1:111–117. doi: 10.1016/S1568-9972(01)00018-0. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Chicheportiche R, Juge-Aubry C, Meier C. Adipose tissue has anti-inflammatory properties: focus on IL-1 receptor antagonist (IL-1Ra) Ann N Y Acad Sci. 2006;1069:444–453. doi: 10.1196/annals.1351.043. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- Müller S, Dorner B, Korthaüer U, Mages HW, D'Apuzzo M, Senger G, Kroczek RA. Cloning of ATAC, an activation-induced, chemokine-related molecule exclusively expressed in CD8+ T lymphocytes. Eur J Immunol. 1995;25:1744–1748. doi: 10.1002/eji.1830250638. [DOI] [PubMed] [Google Scholar]
- Blaschke S, Middel P, Dorner BG, Blaschke V, Hummel KM, Kroczek RA, Reich K, Benoehr P, Koziolek M, Müller GA. Expression of activation-induced, T cell-derived, and chemokine-related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum. 2003;48:1858–1872. doi: 10.1002/art.11171. [DOI] [PubMed] [Google Scholar]
- Berg WB van den. Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res. 2001;3:18–26. doi: 10.1186/ar136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Helsen MM, Loo FA van de, Berg WB van den. Anti-cytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparative study using anti-TNFα, anti-IL-1α/β and IL-1Ra. Arthritis Rheum. 2008;58:S110–S122. doi: 10.1002/art.23152. [DOI] [PubMed] [Google Scholar]
- Koenders MI, Joosten LA, Berg WB van den. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65:iii29–iii33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Waetzig GH, Scheller J, Grötzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- Nowell MA, Williams AS, Carty SA, Scheller J, Hayes AJ, Jones GW, Richards PJ, Slinn S, Ernst M, Jenkins BJ, Topley N, Rose-John S, Jones SA. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J Immunol. 2009;182:613–622. doi: 10.4049/jimmunol.182.1.613. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Anti-cytokine therapeutics and infections. Vaccine. 2003;21(Suppl 2):S24–S34. doi: 10.1016/S0264-410X(03)00196-8. [DOI] [PubMed] [Google Scholar]
- Kruglov AA, Kuchmiy A, Grivennikov SI, Tumanov AV, Kuprash DV, Nedospasov SA. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev. 2008;19:231–244. doi: 10.1016/j.cytogfr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Lamacchia C, Palmer G, Seemayer CA. Myeloid-cell-specific interleukin-1 receptor antagonist deficiency enhances Th1 and Th17 responses and severity of arthritis. Arthritis Rheumatol. 2009. in press . [DOI] [PubMed]
- Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- van Holten J, Reedquist K, Sattonet-Roche P, Smeets TJ, Plater-Zyberk C, Vervoordeldonk MJ, Tak PP. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2004;6:R239–R249. doi: 10.1186/ar1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphyllopoulos KA, Williams RO, Tailor H, Chernajovsky Y. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum. 1999;42:90–99. doi: 10.1002/1529-0131(199901)42:1<90::AID-ANR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tak PP, Hart BA, Kraan MC, Jonker M, Smeets TJ, Breedveld FC. The effects of interferon beta treatment on arthritis. Rheumatology (Oxford) 1999;38:362–369. doi: 10.1093/rheumatology/38.4.362. [DOI] [PubMed] [Google Scholar]
- Treschow AP, Teige I, Nandakumar KS, Holmdahl R, Issazadeh-Navikas S. Stromal cells and osteoclasts are responsible for exacerbated collagen-induced arthritis in interferon-beta-deficient mice. Arthritis Rheum. 2005;52:3739–3748. doi: 10.1002/art.21496. [DOI] [PubMed] [Google Scholar]
- van Holten J, Pavelka K, Vencovsky J, Stahl H, Rozman B, Gen-ovese M, Kivitz AJ, Alvaro J, Nuki G, Furst DE, Herrero-Beaumont G, McInnes IB, Musikic P, Tak PP. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:64–69. doi: 10.1136/ard.2003.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaansen J, Tas SW, Klarenbeek PL, Bakker AC, Apparailly F, Firestein GS, Jorgensen C, Vervoordeldonk MJ, Tak PP. Enhanced gene transfer to arthritic joints using adeno-associated virus type 5: implications for intra-articular gene therapy. Ann Rheum Dis. 2005;64:1677–1684. doi: 10.1136/ard.2004.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaansen J, Kuhlman RR, van Holten J, Kaynor C, Vervoordel-donk MJ, Tak PP. Intraarticular interferon-beta gene therapy ameliorates adjuvant arthritis in rats. Hum Gene Ther. 2006;17:985–996. doi: 10.1089/hum.2006.17.985. [DOI] [PubMed] [Google Scholar]
- Ulfgren AK, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52:870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3:293–298. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C, Joosten LA, Helsen MM, Hepp J, Baeuerle PA, Berg WB van den. GM-CSF neutralisation suppresses inflammation and protects cartilage in acute streptococcal cell wall arthritis of mice. Ann Rheum Dis. 2007;66:452–457. doi: 10.1136/ard.2006.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C, Joosten LA, Helsen MM, Koenders MI, Baeuerle PA, Berg WB van den. Combined blockade of granulocyte-macrophage colony stimulating factor and interleukin 17 pathways potently suppresses chronic destructive arthritis in a tumour necrosis factor alpha-independent mouse model. Ann Rheum Dis. 2009;68:721–728. doi: 10.1136/ard.2007.085431. [DOI] [PubMed] [Google Scholar]
- Löhning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Höfer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grün JR, Löhning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Anderson AE, Swan DJ, Sayers BL, Harry RA, Patterson AM, von Delwig A, Robinson JH, Isaacs JD, Hilkens CM. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol. 2009;85:243–250. doi: 10.1189/jlb.0608374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- Zrioual S, Ecochard R, Tournadre A, Lenief V, Cazalis MA, Miossec P. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112–3120. doi: 10.4049/jimmunol.0801967. [DOI] [PubMed] [Google Scholar]
- Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, Olsen H, Ruben SM, Knyazev I, Cho YH, Kao V, Wilkinson KA, Carrell JA, Ebner R. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- Alber G, Kamradt T. Regulation of protective and pathogenic Th17 responses. Curr Immunol Rev. 2007;3:3–16. doi: 10.2174/157339507779802223. [DOI] [Google Scholar]
- Lexberg MH, Taubner A, Förster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Petri M. Novel combined response endpoint and systemic lupus erythematosus (SLE) flare index (SFI) demonstrate belimumab (fully human monoclonal antibody to BLyS) improves or stabilizes SLE disease activity and reduces flare rate over 2.5 years of therapy [abstract 1316] Arthritis Rheum. 2007;56:S527. [Google Scholar]
- Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ, Fernandez V, Chevrier MR, Zhong ZJ, Freimuth WW. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, Chatham WW, Strand V, Weinstein A, Chevrier MR, Zhong ZJ, Freimuth WW. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 2009;61:1143–1151. doi: 10.1002/art.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzler E. Novel combined response endpoint shows that belimumab (fully human monoclonal antibody to B-lymphocyte stimulator [BLyS]) improves or stabilizes SLE disease activity in a phase 2 trial [abstract OP0018] Ann Rheum Dis. 2007;66:56. [Google Scholar]
- Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, Merrill JT, Weinstein A, McCune WJ, Zhong J, Cai W, Freimuth W. Belimumab Study Group. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10:R109. doi: 10.1186/ar2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, Kalunian K, Dhar P, Vincent E, Pena-Rossi C, Wofsy D. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus ery-thematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- Mekala DJ, Alli RS, Geiger TL. IL-10-dependent suppression of experimental allergic encephalomyelitis by Th2-differentiated, anti-TCR redirected T lymphocytes. J Immunol. 2005;174:3789–3797. doi: 10.4049/jimmunol.174.6.3789. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Okubo-Fornbacher H, Aoki Y, Kamata Y, Kimura H, Kamimura T, Nara H, Iwamoto M, Yoshio T, Okazaki H, Minota S. Increase in plasma levels of adiponectin after administration of anti-tumor necrosis factor agents in patients with rheumatoid arthritis. J Rheumatol. 2008;35:936–938. [PubMed] [Google Scholar]
- Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:385–390. doi: 10.1007/s10165-007-0605-8. [DOI] [PubMed] [Google Scholar]
- Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Schölmerich J, Neumann E, Müller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-κB pathway. J Immunol. 2007;179:5483–5492. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, Gay S, Kyburz D. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007;56:2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, Leo O, So A, De Smedt T. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3:e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Kim JH, Park MC, Park YB, Lee SK. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand J Rheumatol. 2008;37:260–268. doi: 10.1080/03009740801910346. [DOI] [PubMed] [Google Scholar]
- Ebina K, Oshima K, Matsuda M, Fukuhara A, Maeda K, Kihara S, Hashimoto J, Ochi T, Banda NK, Yoshikawa H, Shimomura I. Adenovirus-mediated gene transfer of adiponectin reduces the severity of collagen-induced arthritis in mice. Biochem Biophys Res Commun. 2009;378:186–191. doi: 10.1016/j.bbrc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schellekens GA, de Jong BA, Hoogen FH van den, Putte LB van de, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Padyukov L, Lundström E, Seielstad M, Plenge RM, Oksenberg JR, Gregersen PK, Alfredsson L, Klareskog L. Different patterns of associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in the extended major histocompati-bility complex region. Arthritis Rheum. 2009;60:30–38. doi: 10.1002/art.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J. ABT-874 Psoriasis Study Investigators. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144:200–207. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, Dooley LT, Reich K. PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]