Abstract
The primary objective of this study was to determine whether or not Spiroplasma mirum would be capable of producing lesions of transmissible spongiform encephalopathy (TSE) when inoculated in raccoons (Procyon lotor) and, if that was possible, to compare the clinicopathological findings with those of transmissible mink encephalopathy (TME) in the same experimental model. For this purpose, 5 groups (n = 5) of raccoon kits were inoculated intracerebrally with either S. mirum and/or TME. Two other groups (n = 5) of raccoon kits served as sham-inoculated controls. All animals inoculated with TME, either alone or in combination, showed clinical signs of neurologic disorder and were euthanized within 6 mo post-inoculation (MPI). None of the carcasses revealed gross lesions. Spongiform encephalopathy was observed by light microscopy and the presence of abnormal disease-causing prion protein (PrPd) was detected by immunohistochemistry (IHC) and Western blot (WB) techniques in only the raccoons administered TME. Raccoons inoculated with Spiroplasma, but not administered TME agent, were euthanized at 30 MPI. They did not show clinical neurologic signs, their brains did not have lesions of spongiform encephalopathy, and their tissues were negative for S. mirum by polymerase chain reaction (PCR) and for PrPd by IHC and WB techniques. The results of this study indicate that Spiroplasma mirum does not induce TSE-like disease in raccoons.
Résumé
L’objectif principal de cette étude était de déterminer si Spiroplasma mirum serait en mesure de causer des lésions d’encéphalopathie spongiforme transmissible (TSE) lorsqu’inoculé à des ratons-laveurs (Procyon lotor) et, si cela était possible, de comparer les trouvailles clinico-pathologiques avec celles de l’encéphalopathie transmissible du vison (TME) dans le même modèle expérimental. À cette fin, 5 groupes (n = 5) de jeunes ratons-laveurs ont été inoculés par voie intracérébrale avec S. mirum et/ou TME. Deux autres groupes (n = 5) de jeunes ratons-laveurs ont servi de témoins inoculés de manière simulée. Tous les animaux inoculés avec TME, soit seul ou en combinaison, ont montré des signes cliniques de désordre neurologique et ont été euthanasiés dans les 6 mois post-inoculation (MPI). Aucune des carcasses n’a montré de lésions macroscopiques. L’encéphalopathie spongiforme a été observée en microscopie photonique et la présence de protéine prion anormale causant la maladie (PrPd) a été détectée par immuno-histochimie (IHC) et par immuno-buvardage (WB) seulement chez les ratons-laveurs ayant reçu TME. Les ratons-laveurs inoculés avec Spiroplasma mais auxquels on ne donna pas l’agent du TME, ont été euthanasiés 30 MPI. Ils n’ont démontré aucun signe clinique neurologique, leurs cerveaux n’avaient pas de lésions d’encéphalopathie spongiforme, et leurs tissus étaient négatifs pour la présence de S. mirum par réaction d’amplification en chaîne par la polymérase (PCR) et pour PrPd par IHC et WB. Les résultats de la présente étude indiquent que Spiroplasma mirum ne cause pas des lésions similaires au TSE chez les ratons-laveurs.
(Traduit par Docteur Serge Messier)
Introduction
Transmissible spongiform encephalopathies (TSEs) are fatal neurologic diseases. Three animal TSEs, namely scrapie, transmissible mink encephalopathy (TME), and chronic wasting disease (CWD), have been present in the United States for some time and 3 cases of bovine spongiform encephalopathy (BSE) were recently diagnosed. While the consensus is that abnormal prion protein (PrPd) is the causative agent of TSEs, some researchers believe that a bacterial agent, Spiroplasma mirum, can cause TSE-like disease (1,2). When examined, however, the brains of animals with experimental TSE disease were found to be negative for S. mirum nucleic acid by polymerase chain reaction (PCR) (3).
It has recently been demonstrated that TME and sheep scrapie can be transmitted to raccoons (Procyon lotor) within 6 mo and 2 y, respectively (4,5). Based on these findings, raccoons have been used as an animal model for identification of indigenous TSEs in the United States (4,5). The advantages of raccoons over a mouse model are that the raccoon is susceptible to TME (whereas conventional mice are not) and the tissue distribution of abnormal prion protein in the raccoon is similar to the cow in that it is restricted to tissues in the central nervous system. The raccoon has the additional benefit of being a more rapid model than other non-murine species.
This study was therefore undertaken to determine whether or not S. mirum would be capable of producing lesions of transmissible spongiform encephalopathy (TSE) when inoculated into raccoons and, if that was possible, to compare the clinicopathological findings with that of transmissible mink encephalopathy (TME) in the same experimental model.
Materials and methods
Animals
Thirty-five 5.5-month-old raccoon kits, vaccinated against canine distemper, were purchased from a commercial breeder who had no prior history of TSE-like disease in his breeding animals. The raccoons were randomly divided into 7 groups (n = 5 each). The kits were group-housed (Table I) in a level-2 biosafety containment facility at the National Animal Disease Center in Ames, Iowa. The raccoons were fed dry dog food (Premium formula; PMI Nutrition, Brentwood, Missouri, USA) twice a day and clean water was available at all times. Personnel wore protective clothing while in the isolation facility and showered before leaving the facility.
Table I.
Findings in raccoons intracerebrally inoculated with TME and/or Spiroplasma mirum and various control treatments
| Group | Raccoon no. | Sex | Material inoculated | CNS signs | Survival time (mean)* | Brain sections with SE/PrPd (IHC) | WB (PrPd) Brainstem |
Spiroplasma PCR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebrum | Hippocampus | Colliculus | Cerebellum | Brainstem | |||||||||
| 1 | G-1 | M | Cult. media | − | 916 | −/− | −/− | −/− | −/− | −/− | − | −** | − |
| G-2 | M | Cult. media | − | 916 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| G-3 | M | Cult. media | − | 912 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| G-4 | M | Cult. media | − | 917 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| G-5 | M | Cult. media | − | 916 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| Mean | 915 | ||||||||||||
| 2 | H-1 | M | Spiro. (HD) | − | 912 | −/− | −/− | −/− | −/− | −/− | − | − | − |
| H-2 | M | Spiro. (HD) | − | 910 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| H-3 | M | Spiro. (HD) | − | 910 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| H-4 | M | Spiro. (HD) | − | 912 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| H-5 | M | Spiro. (HD) | − | 910 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| Mean | 911 | ||||||||||||
| 3 | I-1 | M | Spiro. (LD) | − | 909 | −/− | −/− | −/− | −/− | −/− | − | NE | − |
| I-2 | M | Spiro. (LD) | − | 909 | −/− | −/− | −/− | −/− | −/− | − | − | + | |
| I-3 | M | Spiro. (LD) | − | 909 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| I-4 | M | Spiro. (LD) | − | 909 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| I-5 | NR | Spiro. (LD) | NA | < 1 | −/− | −/− | −/− | −/− | −/− | NE | NE | NE | |
| Mean | 909 | ||||||||||||
| 4 | J-1 | F | TME | + | 173 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − |
| J-2 | F | TME | + | 200 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| J-3 | F | TME | + | 169 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| J-4 | M | TME | + | 154 | +/+ | +/+ | +/+ | −/+ | +/+ | + | + | − | |
| J-5 | M | TME | + | 200 | +/+ | +/+ | +/+ | +/+ | +/+ | + | NE | − | |
| Mean | 179 | ||||||||||||
| 5 | K-1 | F | TME + Spiro. | + | 162 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − |
| K-2 | NR | TME + Spiro. | + | 162 | +/+ | +/+ | +/+ | −/+ | +/+ | − | + | − | |
| K-3 | F | TME + Spiro. | + | 162 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| K-4 | M | TME + Spiro. | + | 162 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| K-5 | F | TME + Spiro. | + | 162 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| Mean | 162 | ||||||||||||
| 6 | L-1 | F | TME + media | + | 166 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − |
| L-2 | F | TME + media | + | 166 | +/+ | +/+ | +/+ | −/+ | +/+ | − | + | − | |
| L-3 | F | TME + media | + | 166 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| L-4 | F | TME + media | + | 186 | +/+ | +/+ | +/+ | −/+ | +/+ | − | + | − | |
| L-5 | F | TME + media | + | 166 | +/+ | +/+ | +/+ | −/+ | +/+ | + | NE | − | |
| Mean | 170 | ||||||||||||
| 7 | M-1 | F | Normal brain | − | 910 | −/− | −/− | −/− | −/− | −/− | − | NE | − |
| M-2 | F | Normal brain | − | 910 | −/− | −/− | −/− | −/− | −/− | − | − | − | |
| M-3 | F | Normal brain | − | 910 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| M-4 | F | Normal brain | − | 909 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| M-5 | F | Normal brain | − | 909 | −/− | −/− | −/− | −/− | −/− | − | NE | − | |
| Mean | 910 | ||||||||||||
IHC — immunohistochemistry; WB — Western blot; PCR — polymerase chain reaction; NR — not recorded; Spiro. (HD) — high dose of Spiroplasma; Spiro. (LD) — low dose of Spiroplasma; NA — not applicable; NE — not examined; M — male; F — female; NR — not recorded; + — clinical signs, lesions, or antigen present; — clinical signs, lesions, or antigen absent;
days post-inoculation;
Western blot following enrichment.
TME inoculum
The TME inoculum was obtained from a raccoon that had developed clinical signs of TSE during a previous study (5). The brain tissue of that raccoon had microscopic lesions of spongiform encephalopathy and tested positive for PrPd by immunohistochemistry and Western blot techniques. Material for the inoculum was ground in a hand-held tissue grinder and the final concentration of 10% (wt/vol) was made with phosphate-buffered saline (PBS). The inoculum dose was 0.1 mL of the 10% solution.
Spiroplasma inoculum — strain and culture
Spiroplasma mirum strains SMCAT and GT 48 were obtained from the ATCC (American Type Culture Collection; Manassas, Virginia, USA) as lyophilized stock cultures, designated as ATCC 29335 and ATCC 29334, respectively. These strains were initially isolated from rabbit ticks (1,2). Strains SMCAT (suckling mouse cataract agent) and GT 48 produce persistent brain infections in experimentally infected, 1- to 2-day-old suckling rats or mice (1–3,6).
Spiroplasma strains were cultured in ATCC No. 988 broth, also known as Spiroplasma medium SP-4. These strains were used in a previous study to develop a PCR assay for detecting Spiroplasma SMCAT 16S rRNA gene in prion-positive brains of various animals (Hamir et al, unpublished). Medium supplements were added as recommended by the ATCC. They included heat-inactivated fetal bovine serum, mycoplasma growth supplement ATCC 20-2207, and fresh yeast extract. The yeast extract was prepared by suspending 250 g of Baker’s yeast in 1 L of distilled water, autoclaving the suspension for 90 min, adjusting the pH to 6.6 to 6.8 with sterile 30% NaOH (1.4 mL per L), and aseptically removing the cell debris by centrifugation (20 000 × g, 30 min). Agar plates were made by including 1.2% (w/v, final concn) of Noble agar in the basal broth before autoclaving.
The spiroplasmas were cultured aerobically at 31°C in 15-mm capped tubes containing 4.5 mL of 988 broth. Inoculum volumes were 10%. After 4 to 5 d of incubation, cells reached a population density of ~1.5 to 2.5 × 109 cells/mL (direct cell counts, Petroff-Hausser counting chamber).
Cultures were monitored daily for acid accumulation based on color changes (from red to orange to yellow) of the phenol red indicator included in the broth medium. Cultures were also monitored by phase-contrast microscopy for cell population densities, purity, and viability (vigorous cell motility). Spiroplasma cells used in experiments were actively growing and had been minimally passaged, from 3 to 5 times, since receipt of lyophilized stocks. Cultures were checked for contamination by spotting 0.1 mL on medium #988 agar plates, which were then incubated aerobically at 37°C for 2 wk.
High-and low-dose Spiroplasma inocula
Actively growing cultures of S. mirum GT48 were harvested by centrifugation (17 000 × g, 30 min). The pelleted cells were resuspended in an equal volume of fresh culture broth, harvested by centrifugation, and resuspended in a 0.1 × volume of fresh broth. This concentrated cell suspension was used as the high-dose inoculum and contained an estimated 2.5 × 109 Spiroplasma cells/mL based on direct microscopy cell counts. The suspension was diluted 1/100 in fresh medium to create a low-dose inoculum.
Electron microscopic examination of Spiroplasma
An aliquot of the low-dose inoculum was fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and post-fixed in 1% osmium tetroxide. It was then pelleted and placed into 2% agar before being processed for transmission electron microscopy (TEM).
Inoculation procedure
All groups of experimental raccoons were inoculated intracerebrally with either sterile culture media (Group 1), high-dose Spiroplasma (Group 2), low-dose Spiroplasma (Group 3), TME (Group 4), TME plus Spiroplasma (Group 5), TME plus cell culture media (Group 6), or with normal raccoon brain (Group 7) (Table I). The procedure for inoculation has been described previously (4–5,7). Briefly, the raccoon kits were sedated with a mixture of ketamine and xylazine, a midline incision was made in the skin at the junction of the parietal and frontal bones, and a hole 2 mm in diameter was drilled through the calvarium. The inoculum (total volume = 0.1 mL per animal) was injected into the midbrain via a disposable 22-gauge, 1.5-inch long needle, while withdrawing the needle from the brain. The skin incision was closed with a single suture. The surgical instruments, including the drill bit, were replaced after each experimental group was inoculated. Two kits (controls) were not inoculated (Table I).
Necropsy and samples
Raccoons were euthanized with a pentobarbital overdose and a complete necropsy was conducted on each of the carcasses. Half of the brain (cut longitudinally), together with representative samples of striated muscles (heart, diaphragm, tongue, masseter, psoas major, triceps, and biceps femoris), lung, liver, kidney, skin, spleen, eye, mesenteric lymph node, palatine tonsil, salivary glands, rectal mucosa, and urinary bladder, were immersion-fixed in 10% neutral buffered formalin for histopathology. The fixed brain was cut in serial coronal sections 2 to 4 mm wide and sections of the medulla, cerebellum, superior colliculus, and rostral cerebrum were processed for routine histopathology and stained with hematoxylin and eosin. Selected brain sections of one raccoon (No. I-5) were stained with ZN, Gram, GMS, Geimsa, Stiner’s silver, and toluidine blue to identify Spiroplasma. All sections of tissue were also stained using an immunohistochemical (IHC) method for detection of PrPd. The primary antibody was a cocktail of 2 monoclonal antibodies, F89/160.1.5 (8) and F99/97.6.1 (9), each used at a concentration of 5 μg/mL and incubated at 37°C for 32 min. These antibodies recognize PrP sequences conserved in most mammalian species in which natural TSEs have been reported and have previously been shown to detect PrPd in raccoons with TME.
Selected areas of formalin-fixed, paraffin-embedded brain of one raccoon (No. I-5) were placed in 2.5% glutaraldehyde in 0.1M cacodylate buffer. They were rinsed in the buffer, post-fixed in 1% osmium tetroxide, and embedded in Eponate 12 resin. Thick sections (1 μm) were stained with toluidine blue and selected areas were thin-sectioned and stained with 5% uranyl acetate in methanol and Reynolds lead citrate. They were examined on a Tecnai 12 transmission electron microscope.
The other half of the brain was frozen for Western blot analysis (Prionics-Check; Zurich, Switzerland) for PrPd as described previously (4,5) and for PCR detection of S. mirum (unpublished data).
Results
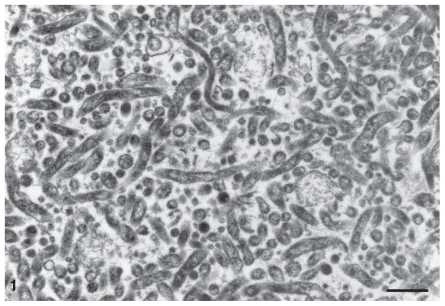
The Spiroplasma bacteria used to inoculate raccoons in these studies were tested for viability by serially diluting the high-dose inoculum (2.5 × 109 bacteria/mL, direct microscopy cell counts) 10-fold to a final dilution of 10−8 in tubes of fresh SP-4 broth. After 3 to 4 wk of incubation at 31°C, all cultures representing dilutions of the high-dose inoculum had turned orange to yellow in color (acidic pH due to metabolic end products of actively growing Spiroplasma cells) and contained actively motile Spiroplasma cells visible by phase-contrast microscopy. Both high-dose and low-dose inocula were tested and found to be free of contamination from extraneous bacteria. Electron microscopic examination of the aliquot of the low-dose inoculum revealed numerous slender organisms consistent with S. mirum. Most had a cross-section of 150 nm and were up to 1500 nm in length (Figure 1).
Figure 1.
Electron micrograph of inoculum of Spiroplasma mirum showing sections of numerous slender organisms. Uranyl acetate and lead citrate. Bar = 500 nm.
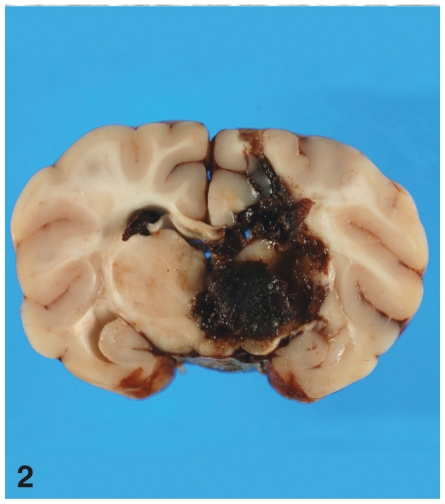
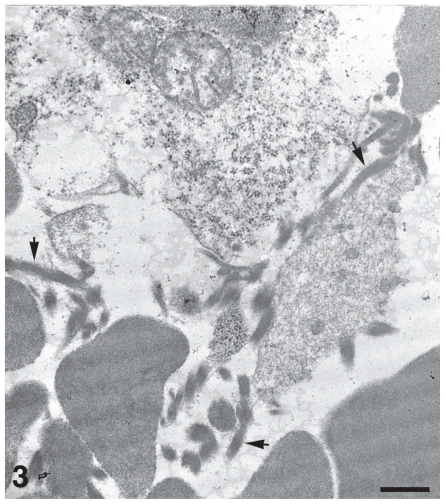
As a result of the inoculation procedure, one of the raccoons inoculated with a low-dose of S. mirum (No. I-5, Group 3) died from an iatrogenic acute cerebral hemorrhage. Examination of the brain of this raccoon revealed extensive acute hemorrhage in the brain parenchyma (Figure 2). None of the special stains (ZN, Gram, GMS, Geimsa, silver, and toluidine blue) was able to unequivocally confirm the presence of Spiroplasma in tissue sections of this raccoon. However, electron microscopic examination of the brain and an adjacent hemorrhagic region revealed moderate numbers of partially intact S. mirum organisms (Figure 3).
Figure 2.
Brain of raccoon No. I-5 that died soon after inoculation with low-dose Spiroplasma mirum. There is locally extensive acute hemorrhage in almost half of the brain parenchyma on one side and extending to the opposite side.
Figure 3.
Electron micrograph of brain of raccoon No. I-5 (shown in Figure 2). Moderate numbers of Spiroplasma mirum, indicated by arrows, are seen among erythrocytes and necrotic cellular debris. Bar = 500 nm. Uranyl acetate and lead citrate.
All raccoons inoculated with the TME agent (Groups 4, 5, and 6) developed clinical neurological signs and were euthanized 154 to 200 days after the intracerebral inoculations (Table I). All were found to be lethargic, did not respond to external stimuli as normal raccoons would, could not climb up to their sleeping quarters, and were generally unaware of their surroundings. The remainder of the raccoons, including the sham-inoculated controls, remained healthy and were euthanized 900 d after the experiment was initiated (Table I).
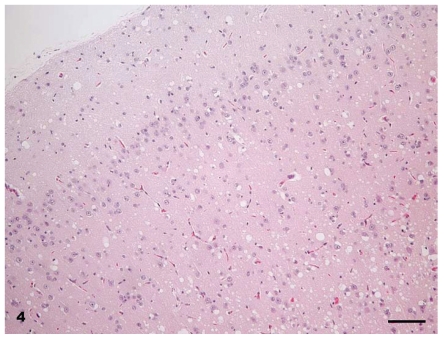
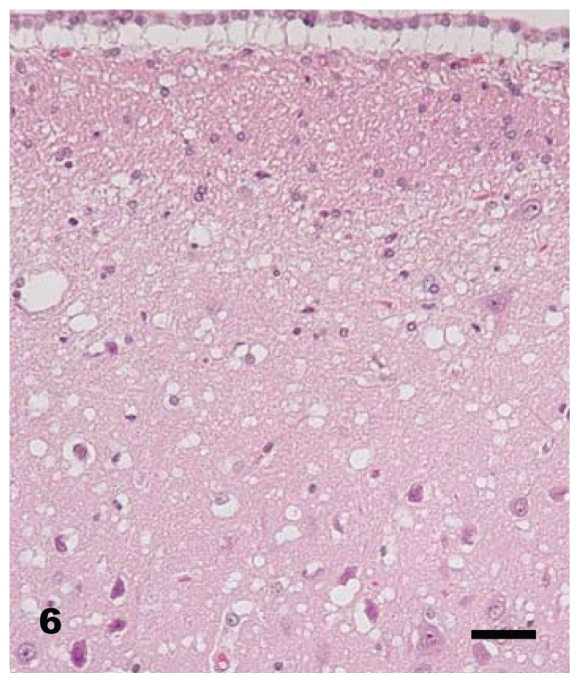
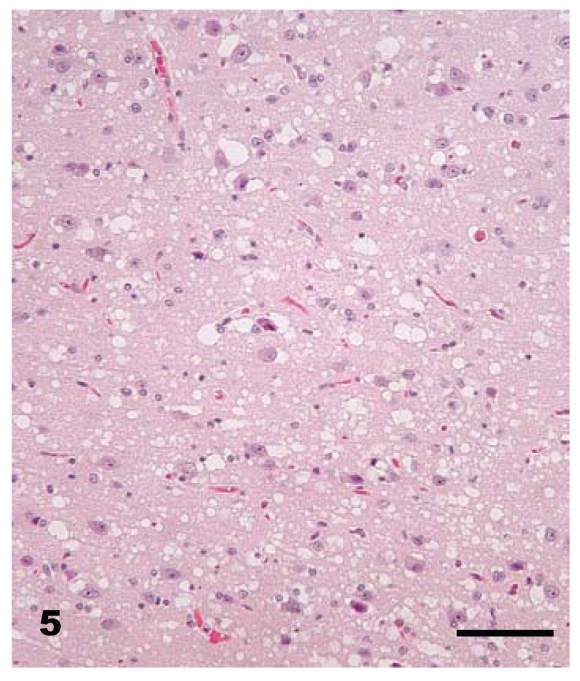
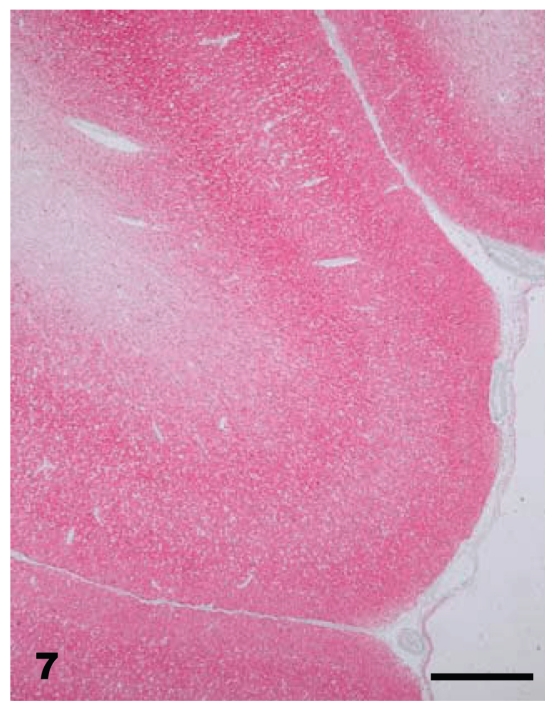
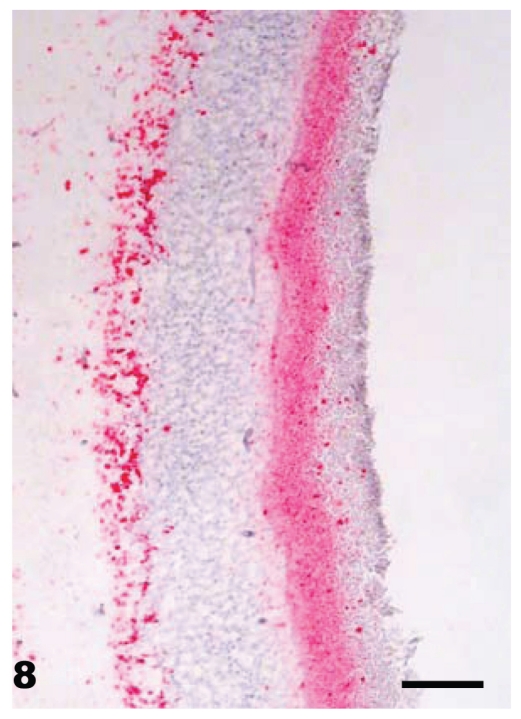
At necropsy, all raccoons were in good nutritional condition. Four of 16 females had mild to moderate distension of the uterus which from past experience indicated that there was mild to moderate hydrometra. No other gross lesions were seen in any of the carcasses. Microscopic lesions of spongiform encephalopathy were present in the gray matter of the brains of all clinically affected raccoons. The severity of lesions varied among individual animals and within the various neuroanatomic sites. The 5 hemisections of the brain (rostral cerebrum, hippocampus, rostral colliculus, cerebellum, and brainstem) that were examined revealed severe and extensive spongiform encephalopathy (Figures 4 to 6) in all areas except the cerebellum where it was seen in only one animal (No. J-5; Table I). Spongiform change was most prominent in the cerebral cortex and consisted of severe extensive vacuolation in the neuropil (Figures 4 and 5). Vacuoles were round to oval in shape and varied in size (up to 80 mm in diameter). There was mild to moderate gliosis in regions affected by spongiform change. Similar but less severe lesions were present in most nuclei of the brainstem. Vacuolation of neuronal perikarya, although present in isolated neurons, was not frequently seen in the cerebral cortex but was more common in the brainstem. Neuronal degeneration was not a prominent feature in areas with spongiform change. The choroid plexus of 4 raccoons revealed a few isolated blood vessels with concentric areas of mineralization (psammoma bodies) and the caudal medulla of 14 animals showed one or more foci of neuroaxonal degeneration (NAD). There were no spongiform lesions in the tissues of the central nervous system (CNS) in any raccoons in the control group (Groups 1, 2, 3, and 7) or in those inoculated only with S. mirum. On PrPd IHC staining, no positive tissues were identified from the following groups: culture media only (Group 1), Spiroplasma (high dose; Group 2), Spiroplasma (low dose; Group 3), and normal brain (Group 7). In general, the PrPd staining of the TME (Group 4), TME plus Spiroplasma (Group 5), and TME plus culture media (Group 6) appeared to be similar. The sections with positive immunoreactivity were from the rostral cerebrum (Figure 7), brainstem, cerebellum, hippocampus, colliculus, and retina (Figure 8). In a few cases, the spinal cord tissue was also positive. There were no non-CNS tissues with immunoreactivity different from background levels present in the control groups.
Figure 4.
Brain — cerebral cortex of raccoon No. K-4 inoculated with brain homogenate of transmissible mink encephalopathy. There is extensive vacuolation in superficial and deep cortex. Hematoxylin & eosin (H & E). Bar = 50 μm.
Figure 6.
Brain — hippocampal gyrus and hippocampus of raccoon No. K-4 inoculated with brain homogenate of transmissible mink encephalopathy. Although there is diffuse vacuolation in the neuropil, a few isolated neurons have cytoplasmic vacoules. H & E. Bar = 40 μm.
Figure 5.
Brain — cerebral cortex of raccoon No. K-4 inoculated with brain homogenate of transmissible mink encephalopathy. This is a higher magnification of Figure 4. A majority of the vacuoles are located in the neuropil. H & E. Bar = 80 μm.
Figure 7.
Brain — cerebral cortex of raccoon No. K-4 inoculated with brain homogenate of transmissible mink encephalopathy. There is diffuse PrPd staining (red) present within all layers of the cortex. Stained for PrPd by immunohistochemistry (IHC). Bar = 256 μm.
Figure 8.
Eye — retina of raccoon No. K-4 inoculated with brain homogenate of transmissible mink encephalopathy. Diffuse PrPd staining (red) is present in both plexiform layers. Stained for PrPd by IHC. Bar = 50 μm.
Immunoreactivity in the brain was generally in a granular and globular pattern throughout the neuropil. Immunoreactivity in the retina was largely confined to the plexiform layers. White matter areas appeared to be spared as were neurons in brainstem nuclei, cerebrum, and hippocampus. Staining was more intense in the granular layer of the cerebellum than in the molecular layer.
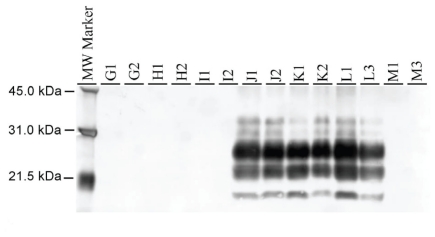
The Western blot (WB) analysis was performed with monoclonal antibody P4 and brainstem samples from all raccoons in this study were analyzed. As shown in Figure 9, only the raccoons inoculated with TME were positive for PrPd, showing the typical profile of 3 bands of proteinase-K-resistant isoforms of PrPd, representing the diglycosylated, monoglycosylated, and unglycosylated isoforms. No difference in molecular weight and glycoform profile was noted among the individual TME-inoculated raccoons. In contrast, none of the brainstem samples from the raccoons inoculated with Spiroplasma was positive for PrPd (Figure 9).
Figure 9.
Western blot of brainstems of raccoons inoculated with TME and those not inoculated (representing 14 animals). In the positive cases, a distinct profile of PrPd is observed in the brainstem of all inoculated animals. As expected, the negative control animal has no detectable PrPd. The molecular marker is on the left.
Discussion
Previous reports have found that spongiform encephalopathy is caused by S. mirum (6,1). Here we present findings on intracerebral inoculations of S. mirum into raccoons that have been used as a model of TSE disease. Previous studies have shown TME inoculation of raccoons to be a very reproducible TSE disease with a tissue distribution in raccoons that closely mimics that of bovine spongiform encephalopathy (BSE) in cattle.
As one of a group of fatal neurodegenerative diseases, TME is a rare food-borne disease of ranch mink that was first documented in Wisconsin, USA in 1947 (10). While the origin of TME is unknown, it is speculated that it came from sheep scrapie or from an unknown TSE in cattle (10,11). It has been suggested, however, that S. mirum can cause TSE-like disease experimentally, which calls into question the validity of prions as a sole causal agent of TSEs (2). We recently reported the results of examinations of a large number of brains of animals with TSEs for the presence of the Spiroplasma SMCAT 16S rRNA gene by a polymerase chain reaction (PCR) method, in which all findings were negative (unpublished data). Similarly, the present study did not show any raccoon brains to be positive for the SMCAT 16S rRNA gene of this bacterium and intracerebral inoculation of S. mirum did not result in the development of any spongiform lesions.
In this study, we used TME as a positive control inoculation that would result in TSE lesions and, as expected, the microscopic spongiform lesions in tissues of the central nervous system (CNS) were severe and diffuse in all TME-inoculated raccoons (Table I). The changes were most severe in the cerebral cortex and were characterized by diffuse vacuolation of neuropil and moderate gliosis without appreciable neuronal vacuolation or degeneration. These observations are similar to those described previously in raccoons experimentally infected with TME (4,5). By immunohistochemistry (IHC), only the CNS tissues of TME-inoculated raccoons were positive for PrPd; the brainstems of these raccoons were also found to be positive for PrPd using the Western blot (WB) technique.
Spiroplasma mirum is a bacterial organism that lacks cell walls (12). Therefore, none of the standard histochemical stains for bacterial cell walls was able to demonstrate these in histologic sections. In the present study, the raccoons inoculated intracerebrally with this bacteria did not exhibit lesions resembling spongiform encephalopathy and their CNS tissues were not positive for PrPd by IHC or WB techniques. Moreover, S. mirum did not cause any microscopic changes in the brains of raccoons inoculated intracerebrally and it did not appear to cause enhanced clinical signs or lesions when the raccoons were inoculated with TME. Also, the PCR test for S. mirum was negative in all the co-inoculated raccoons, which indicates that the animals were able to clear this organism from their system. The negative PCR test for S. mirum is in agreement with our previous study that failed to identify S. mirum DNA in brain tissues from TSE-infected animals (unpublished data). Recently, similar conclusions were derived when hamsters were infected with the scrapie agent (3).
The findings of hydrometra neuroaxonal degeneration (NAD) in the caudal medulla, and psammoma bodies in the choroid plexus of some of the raccoons have been reported previously, and all 3 conditions are considered incidental findings and appear to be more prevalent in older laboratory-confined raccoons (7).
In conclusion, in the present study, we found that S. mirum GT48 was not able to cause any pathology resembling a TSE-like disease in raccoons when inoculated intracerebrally. In addition, we were unable to detect the presence of S. mirum 16S rRNA in the brains of several hundred animals with experimental or naturally occurring TSE (unpublished data). Our findings, combined with the reported absence of Spiroplasma or other bacterial 16S rRNA genes in the brains of hamsters with scrapie (3) indicates that there have been multiple failures to support the Spiroplasma hypothesis for causation of TSE.
Acknowledgments
We thank the late Drs. Lauren Nusz and Jean Laufer for clinical assistance and James Fosse for the photomicrographs. Expert technical assistance was provided by Martha Church, Ginny Montgomery, Joseph Lesan, Trudy Tatum, Deb Clouser, Sam Humpherey, Kevin Hassall, and the TSE animal caretakers.
Footnotes
This study was carried out under the guidelines of the Institutional Animal Care and Use Committee at the National Animal Disease Center. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- 1.Bastian FO, Purnell DM, Tully JG. Neuropathology of spiroplasma infection in the rat brain. Am J Pathol. 1984;114:496–514. [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian FO, Sanders DE, Forbes WA, et al. Spiroplasma spp. from transmissible spongiform encephalopathy brains or ticks induce spongiform encephalopathy in ruminants. J Med Microbiol. 2007;56:1235–1242. doi: 10.1099/jmm.0.47159-0. [DOI] [PubMed] [Google Scholar]
- 3.Alexeeva I, Elliott EJ, Rollins S, Gasparich GE, Lazar J, Rohwer RG. Absence of Spiroplasma or other bacterial 16S rRNA genes in brain of hamsters with scrapie. J Clin Microbiol. 2006;44:91–97. doi: 10.1128/JCM.44.1.91-97.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamir AN, Miller JM, O’Rourke KI, Bartz JC, Stack MJ, Chaplin MJ. Transmission of transmissible mink encephalopathy (TME) to raccoons (Procyon lotor) by intracerebral inoculation. J Vet Diagn Investi. 2004;16:57–63. doi: 10.1177/104063870401600110. [DOI] [PubMed] [Google Scholar]
- 5.Hamir AN, Kunkle RA, Miller JM, Richt J. Experimental second raccoon passage of sheep scrapie and transmissible mink encephalopathy (TME) agents. Vet Pathol. 2005;42:844–851. doi: 10.1354/vp.42-6-844. [DOI] [PubMed] [Google Scholar]
- 6.Bastian FO, Jennings RA, Gardner WA. Antiserum to scrapie-associated fibril protein cross-reacts with Spiroplasma mirum fibril proteins. J Clin Microbiol. 1987;25:2430–2431. doi: 10.1128/jcm.25.12.2430-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamir AN, Kunkle RA, Miller MJ, et al. Age-related lesions in laboratory-confined raccoons (Procyon lotor) inoculated with the agent of chronic wasting disease of mule deer. J Vet Diagn Invest. 2007;19:680–686. doi: 10.1177/104063870701900610. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke KI, Baszler TV, Besser TE, et al. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol. 2000;38:3254–3259. doi: 10.1128/jcm.38.9.3254-3259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler-Riggleman I, Knowles DP. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol. 1998;36:1750–1755. doi: 10.1128/jcm.36.6.1750-1755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh RF, Bessen RA, Lehmann S, Hartsough GR. Epidemiological and experimental studies on a new incident of transmissible mink encephalopathy agent. J Gen Virol. 1991;72:589–594. doi: 10.1099/0022-1317-72-3-589. [DOI] [PubMed] [Google Scholar]
- 11.Marsh RF, Burger D, Eckroade R, Zu Rheim GM, Hanson RP. A preliminary report on the experimental host range of the transmissible mink encephalopathy agent. J Infect Dis. 1969;120:713–719. doi: 10.1093/infdis/120.6.713. [DOI] [PubMed] [Google Scholar]
- 12.Whitcomb RF. The genus Spiroplasma. Annu Rev Microbiol. 1980;34:677–709. doi: 10.1146/annurev.mi.34.100180.003333. [DOI] [PubMed] [Google Scholar]