Abstract
Strong correlations between clinical signs on farms and the presence of lesions at slaughter have been reported. The objective of this study was to determine if changes in condemnation rates provide a data source for surveillance of disease outbreaks in pigs. The data were obtained from 1 abattoir in Ontario (2005–2007). The epidemiological relevance of the results was based on an outbreak of porcine circovirus associated disease (PCVAD) in Ontario in 2005. The total condemnations and condemnations due to arthritis and pneumonia patterns reflected the field infection of PCVAD in 2005 followed by the widespread use of porcine circovirus type 2 (PCV-2) vaccine in 2007. In contrast, increased rates of nephritis and enteritis suggested areas for enhanced surveillance for unexplained changes in disease patterns not identified through traditional passive surveillance. Further studies looking at the benefits of using abattoir data should compare condemnation patterns with multiple sources of swine health data.
Résumé
Schémas des taux de réforme chez les porcs dans un abattoir inspecté par le gouvernement fédéral en rapport avec l’information sur l’éclosion de maladies en Ontario (de 2005 à 2007). De fortes corrélations entre les signes cliniques à la ferme et la présence de lésions lors de l’abattage ont été signalées. L’objectif de la présente étude consistait à déterminer si les changements des taux de réforme fournissent une source de données pour la surveillance des éclosions de maladie chez les porcs. Les données ont été obtenues dans 1 abattoir en Ontario (de 2005 à 2007). La pertinence épidémiologique des résultats s’est fondée sur une éclosion de maladie associée au circovirus porcin (MACVP) en Ontario en 2005. Le total des réformes et les réformes attribuables aux patrons d’arthrite et de pneumonie ont reflété l’infection sur le terrain par le MACVP en 2005, suivie de l’usage généralisé du vaccin contre le circovirus porcin de type 2 (PCV-2) en 2007. Par contraste, les taux accrus de néphrite et d’entérite ont suggéré des domaines de surveillance accrue pour des changements inexpliqués au niveau des patrons de maladies non identifiés lors de la surveillance passive traditionnelle. De nouvelles études examinant les avantages de l’utilisation des données de l’abattoir devraient comparer les patrons de réforme avec des sources multiples de données sur la santé porcine.
(Traduit par Isabelle Vallières)
Introduction
Reliable surveillance systems may provide early warning to identify outbreaks of disease in animal populations (1,2). Syndromic surveillance focuses on clinical signs or lesions rather than specific diagnoses and has become increasingly important as it may identify natural disease outbreaks more quickly than surveillance systems based on laboratory diagnoses (3–5). Syndromic information can be obtained from farms, veterinary clinics, diagnostic laboratories, and abattoirs. The validity of the classification of lesions at abattoirs has been questioned because of the difficult conditions under which diagnostic work is carried out during meat inspection (6) and the variability of classification of lesions in different abattoirs (7). Detection of changes in condemnation rates has been used in some epidemiological studies to evaluate changes in the health status of pig populations so control strategies of common infectious diseases could be evaluated (1,6–10). Correlations between clinical signs at the farm level and lesions at slaughter have been reported for pneumonia and diarrhea and other complex diseases (6,11). Willeberg et al (6) reported highly significant associations between chronic respiratory lesions at slaughter and pigs with clinical signs of respiratory disease on-farm (RR = 3.5, P < 0.001). In addition, a strong correlation was observed between specific lung infections prior to slaughter and postmortem diagnoses of particular diseases affecting the animal (6). However, the usefulness of slaughter data as a source of surveillance data to detect specific outbreaks of disease has not been evaluated.
In Ontario, swine disease outbreaks in finisher herds associated with swine influenza virus (SIV), porcine reproductive and respiratory syndrome virus (PRRSv), and porcine circovirus type 2 (PCV-2) occurred in early 2005 (12–14). In particular, the expression of PCVAD was different than previously experienced with older animals affected and higher reported mortality rates (15). Affected pigs had rasping coughs and watery diarrhea. Postmortem findings included various degrees of broncho-interstitial pneumonia and thickening of the jejunum, ileum, and colon. Affected animals also had lesions associated with interstitial nephritis (15,16). This change in the expression of PCVAD coincided with a change in the predominant viral restricted fragment length polymorphism (RFLP) pattern from 422 to 321 (16). The number of cases typed as RFLP type 321 increased from 1 in 2004 to 135 out of 175 cases typed in 2005 by the Animal Health Laboratory (AHL) at the University of Guelph, Guelph, Ontario. In 2006, 130 out of 159, and in 2007 53 out of 58 typed cases were associated with RFLP 321 (17). A total of 350 PCVAD cases were presented to the AHL in 2005 and 408 in 2006; however, only 222 new PCVAD cases were reported in 2007 (17). Between 2005 and 2006, the percentage of total submissions diagnosed with PCV-2 increased from 8.9% to 10.1% (18–20); however, the percent of PCVAD cases compared to the total swine submissions decreased to 6.3% in 2007 (16), when sufficient quantities of PCV-2 vaccine became available to producers (21).
A wide spectrum of infectious agents was observed concomitant with PCV-2 on affected farms during the outbreak. These include Mycoplasma hyopneumiae, Mycoplasma hyorhinis, Haemophilus parasuis, Streptococcus suis, Staphylococcus aureus, and Pasteurella multocida (16,18–20,22). The association of S. aureus and M. hyorhinis with pathological lesions related to arthritis has been reported (23–25).
The objective of this study was to determine if information from abattoirs can provide an additional and reliable data source for surveillance of disease outbreaks in finisher pigs in Ontario. The validity of these data for disease surveillance was evaluated by comparing the changes in patterns in lesions among condemned carcasses with the expected changes based on the documented outbreak of PCVAD that occurred in 2005 and ended in 2007 with the introduction and widespread use of a PVC-2 vaccine. The potential changes were examined using a statistical model and a temporal cluster detection technique commonly used for disease surveillance; these 2 methods were performed to evaluate if peaks in rates observed in the scan statistics could be compared with the trends observed in negative binomial models. The temporal scan statistic is a standard method for the detection of disease clusters, and it was performed to determine its potential role in a disease surveillance system.
Materials and methods
Data source
The data, including lesion codes from January 2005 to December 2007, were obtained from the database of 1 federally inspected abattoir in Ontario. The data included total condemnations due to at least 1 of the following: abscess, arthritis, bruises, emaciation, icterus, imperfect bleeding, necrosis, nephritis, odor, over-scald, peritonitis, pleuritis, pneumonia, septicemia, toxemia, erysipelas, ascites, neoplasm, pericarditis, anemia, uremia, and enteritis. Pathological lesions that were considered to be easily identifiable, and associated with problems related to PCVAD infection (arthritis, nephritis, pneumonia, enteritis) were analyzed. Additional data recorded included: date of condemnation, total animals slaughtered, total number of condemned and total number of animals approved. The recording of lesions was performed on a daily basis by 5 to 6 veterinary meat inspectors.
Description of pathological lesions
The meat inspectors have standard criteria for classifying pathological lesions. Arthritis was associated with inflammation and presence of excessive synovial fluid in joints as well as enlarged iliac and inguinal lymph nodes. Nephritis was associated with enlargement, change in color and/or firmness of the kidneys. For arthritis and nephritis, the general appearance of the carcass was also evaluated before total condemnation. Pneumonia was associated with firm discolored lungs that could have fluid, abscesses or consolidated areas. Enteritis was associated with thickening of the intestinal walls and mega colon.
Statistical analyses
Descriptive statistics
The total number of condemned carcasses, the number condemned over the study period and by year, and rates of condemned per 10 000 animals were reported for each of the condemnation codes included in the analysis. The total and yearly condemnation rates were calculated by dividing the number of animals condemned for each specific lesion by the total number of animals entering the abattoir in specific years. This value was then multiplied by 10 000 to represent the condemnation rate per 10 000 animals.
Statistical models
Negative binomial models were used to model the effect of year and season on the rate of all condemnations consistent with PCVAD, and specific lesions associated with PCVAD. Seasons were defined as follows: winter = January, February, March; spring = April, May, June; summer = July, August, September; and fall = October, November, December. Season and year were modeled as dummy variables. Winter and 2005 were the referent variables for each model. Interactions between year and season were also examined. The model building process included the evaluation of the contribution of subsets of predictors (season, year, and interactions among these variables) in the model by using the likelihood ratio test. Nested models with and without interaction terms were compared. If the likelihood ratio test between a simpler and the full model was significant (P < 0.05) the interaction term was included in the model (26). In addition, models with and without the interaction terms were compared using the AIC (Akaike information criterion) and BIC (Bayesian information criterion) values. The appropriateness of using a negative binomial model, as opposed to a Poisson model, was based on the statistical significance of the over-dispersion term, alpha (26). The exposure for the negative binomial models was the total number of animals entering the abattoir each day and the outcome was the number of animals condemned for each of the condemnation codes included in this study. Anscombe and deviance residuals were calculated for each of the negative binomial models to evaluate the presence of unusual observations that would require further investigation. In addition, the normality of the Anscombe residuals was assessed to evaluate if the data fitted the model (26). In order to evaluate whether or not significant differences existed among seasons between years, contrasts were performed using the lincom command in STATA (Stata Corp., College Station, Texas, USA). This approach allows for the comparison of 2 groups based on the differences in linear combinations of the coefficients for the groups being contrasted (26). Using this approach, incidence rate ratios, standard errors, Z-scores, confidence intervals (CI), and P-values can be estimated for contrasts between 2 groups based on the model derived coefficients. The analyses of data were performed in STATA 9 (Stata Corp.). All tests performed were 2-tailed with a statistical significance level of 5%.
Temporal scan statistics
Retrospective temporal scan statistics were performed to identify the most likely temporal clusters for each condemnation code occurring from 2005 to 2007 using Bernoulli models in SaTScan Version 7.0 (27). In the temporal scan statistic, the scanning begins as a point at the smallest scale defined in the study at each point in time. Each time an additional time is reached, a likelihood ratio and the relative risk are calculated to determine if the rate of disease within each window is different from outside the window based on a Bernoulli model for these data. Monte Carlo simulations generating random replications of the dataset under the appropriate null hypothesis are used to determine the significance of the results (27).
Although we had information on the total population, the Poisson model was not used due to potential biases in estimating clusters associated with periods, such as holidays and weekends, when the abattoir was not open for slaughter, but the model would include these time periods in estimating background population levels. The Bernoulli model uses case and control data. Cases included the number of animals condemned for a specific lesion. Controls included all carcasses not condemned for the specific lesion being analyzed. All condemnation codes were analyzed separately. The maximum scanning window was restricted to 50% of the study period (2005–2007), and 9999 Monte Carlo replications were performed to estimate the statistical significance of the clusters. All scans were restricted to identify clusters with high rates of condemnations, and only the most likely significant (P < 0.05) cluster was reported for each condemnation code.
Validation of temporal trends
The temporal patterns observed in the negative binomial models and the significant clusters of pathological lesions found in the retrospective temporal scan statistics were qualitatively compared to PCVAD diagnostic reports from the AHL at the University of Guelph from 2005 to 2008 (Available from http://www.labservices.uoguelph.ca/labserv/units/ahl/news_notes.cfm Last accessed November 8, 2010). The AHL offers a wide range of veterinary diagnostic tests and laboratory services in various specialty areas, such as pathology, bacteriology, and virology, and it is the primary location for swine practitioners in Ontario to submit case material for diagnostic and routine monitoring services.
Results
Descriptive statistics
A total of 6 204 702 animals originating from 1132 herds were slaughtered in this abattoir from 2005–2007. A total of 22 980 pigs (37 condemnations/10 000 animals) were condemned during this period. Although the number of animals slaughtered increased from 2005 to 2007, the number and rates of total condemnations decreased. The rates of the lesions included in this study ranged between 1 to 4 condemnations/ 10 000 animals/y, and out of the total condemned, 5419 (23.6%) animals were condemned due to the pathological lesions commonly associated with PCVAD (Table 1). A decrease in the rates of arthritis and pneumonia was observed from 2005 to 2007. In contrast, an increase in the rates of nephritis and enteritis were observed from 2005 to 2007 (Table 1).
Table 1.
Numbers and rates per 10 000 hogs slaughtered over the study period and per year of total condemned and condemned due to arthritis, enteritis, nephritis, and pneumonia from a federally inspected abattoir in southern Ontario from 2005–2007
| Total |
Condemned |
Arthritis |
Enteritis |
Nephritis |
Pneumonia |
|
|---|---|---|---|---|---|---|
| Numbera slaughtered | Number rateb | Number rate | Number rate | Number rate | Number rate | |
| 2005–2007 | 6 204 702 | 22 980 | 1911 | 658 | 1595 | 1255 |
| 37 | 3.1 | 1.0 | 2.6 | 2.0 | ||
| 2005 | 1 991 726 | 9707 | 783 | 86 | 346 | 589 |
| 49 | 3.9 | 0.4 | 1.7 | 2.9 | ||
| 2006 | 2 047 321 | 7000 | 610 | 230 | 516 | 383 |
| 34 | 2.9 | 1.1 | 2.5 | 1.9 | ||
| 2007 | 2 165 655 | 6276 | 518 | 342 | 733 | 283 |
| 29 | 2.4 | 1.6 | 3.4 | 1.3 |
Number of animals.
Rates per 10 000 animals slaughtered per year.
Statistical models
The negative binomial models showed that season, year and an interaction term for season and year were statistically significant for each model (Table 2). The likelihood ratio tests for alpha were highly significant for all the negative binomial models of each condemnation code (P < 0.001) indicating that over-dispersion was present and negative binomial models would fit the data better than Poisson models. The Anscombe residuals showed that the data fitted the model and there were no unusual observations when plotting the deviance residuals. Contrasts were constructed from the fitted models to determine when there were significant changes in condemnation rates for the condemnation lesions included in this study (Table 3).
Table 2.
Negative binomial models that include the effect of season, year, and the interaction between season and year on the rate of different condemnation codes from a federally inspected abattoir in southern Ontario from 2005–2007
| Total condemned |
Arthritis |
Pneumonia |
Enteritis |
Nephritis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRRa | 95% CI b | P-value | IRR | 95% CI | P-value | IRR | 95% CI | P-value | IRRa | 95% CI b | P-value | IRR | 95% CI | P-value | |
| Seasonc | |||||||||||||||
| Spring | 0.93 | 0.83–1.04 | 0.22 | 1.39 | 1.06–1.82 | 0.02 | 0.84 | 0.58–1.22 | 0.35 | 1.01 | 0.53–1.93 | 0.97 | 2.23 | 1.50–3.33 | < 0.001 |
| Summer | 1.01 | 0.90–1.13 | 0.83 | 1.33 | 1.02–1.73 | 0.03 | 1.03 | 0.72–1.46 | 0.85 | 1.10 | 0.60–2.04 | 0.74 | 1.73 | 1.16–2.60 | 0.007 |
| Fall | 1.05 | 0.94–1.17 | 0.37 | 1.13 | 0.86–1.49 | 0.34 | 0.68 | 0.47–0.98 | 0.04 | 0.71 | 0.36–1.41 | 0.33 | 2.88 | 1.97–4.21 | < 0.001 |
| Yeard | |||||||||||||||
| 2006 | 1.01 | 0.90–1.12 | 0.88 | 1.30 | 1.00–1.69 | 0.05 | 1.04 | 0.73–0.47 | 0.81 | 1.02 | 0.55–1.91 | 0.93 | 2.65 | 1.81–3.88 | < 0.001 |
| 2007 | 0.89 | 0.80–0.99 | 0.05 | 1.09 | 0.84–1.43 | 0.49 | 0.61 | 0.42–0.89 | 0.01 | 7.29 | 4.41–12.06 | < 0.001 | 5.48 | 3.82–7.84 | < 0.001 |
| Interactions | |||||||||||||||
| Spring 2006 | 0.62 | 0.53–0.73 | < 0.001 | 0.50 | 0.34–0.74 | < 0.001 | 0.44 | 0.25–0.76 | 0.003 | 1.99 | 0.86–4.63 | 0.10 | 0.38 | 0.23–0.64 | < 0.001 |
| Spring 2007 | 0.86 | 0.73–1.01 | 0.06 | 0.70 | 0.48–1.01 | 0.06 | 1.27 | 0.75–2.16 | 0.36 | 0.51 | 0.25–1.07 | 0.07 | 0.45 | 0.29–0.72 | 0.001 |
| Summer 2006 | 0.46 | 0.39–0.55 | < 0.001 | 0.32 | 0.22–0.48 | < 0.001 | 0.26 | 0.15–0.46 | < 0.001 | 2.59 | 1.17–5.74 | 0.02 | 0.62 | 0.38–1.02 | 0.06 |
| Summer 2007 | 0.39 | 0.39–0.46 | < 0.001 | 0.36 | 0.24–0.54 | < 0.001 | 0.29 | 0.16–0.53 | < 0.001 | 0.28 | 0.14–0.58 | 0.001 | 0.26 | 0.16–0.43 | < 0.001 |
| Fall 2006 | 0.67 | 0.57–0.78 | < 0.001 | 0.55 | 0.37–0.79 | 0.002 | 0.65 | 0.39–1.11 | 0.11 | 5.21 | 2.26–12.02 | < 0.001 | 0.46 | 0.29–0.74 | 0.001 |
| Fall 2007 | 0.46 | 0.39–0.53 | < 0.001 | 0.21 | 0.13–0.32 | < 0.001 | 0.29 | 0.15–0.55 | < 0.001 | 0.14 | 0.67–0.34 | < 0.001 | 0.13 | 0.87–0.20 | < 0.001 |
Incidence rate ratio.
Confidence intervals.
Referent winter.
Referent 2005.
Table 3.
Contrasts derived from the negative binomial models* for different condemnation codes to determine the differences among years and seasons
| Total condemned |
Pneumonia |
Arthritis |
Nephritis |
Enteritis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRRa | 95% CI b | P-value | IRR | 95% CI | P-value | IRR | 95% CI | P-value | IRRa | 95% CI b | P-value | IRR | 95% CI | P-value | |
| 2005 vs 2006 | |||||||||||||||
| Winter | 0.99 | 0.88–1.10 | 0.88 | 0.95 | 0.67–1.36 | 0.8 | 0.77 | 0.59–0.99 | 0.05 | 0.37 | 0.25–0.55 | < 0.001 | 0.97 | 0.52–1.81 | 0.94 |
| Spring | 1.59 | 1.41–1.79 | < 0.001 | 2.16 | 1.42–3.29 | < 0.001 | 1.51 | 1.15–1.99 | 0.003 | 0.97 | 0.70–1.33 | 0.84 | 0.48 | 0.27–0.86 | 0.01 |
| Summer | 2.12 | 1.89–2.37 | < 0.001 | 3.58 | 2.35–5.46 | < 0.001 | 2.35 | 1.75–3.15 | < 0.001 | 0.60 | 0.44–0.82 | 0.002 | 0.37 | 0.22–0.62 | < 0.001 |
| Fall | 1.47 | 1.32–1.64 | < 0.001 | 1.45 | 0.98–2.5 | 0.06 | 1.40 | 1.07–1.83 | 0.01 | 0.81 | 0.62–1.05 | 0.12 | 0.18 | 0.10–0.32 | < 0.001 |
| 2005 vs 2007 | |||||||||||||||
| Winter | 1.11 | 1.00–1.24 | 0.05 | 1.62 | 1.12–2.34 | 0.01 | 0.91 | 0.69–1.19 | 0.5 | 0.18 | 0.13–0.26 | < 0.001 | 0.14 | 0.08–0.22 | < 0.001 |
| Spring | 1.29 | 1.16–1.46 | < 0.001 | 1.27 | 0.87–1.86 | 0.21 | 1.30 | 1.00–1.69 | 0.04 | 0.39 | 0.30–0.52 | < 0.001 | 0.26 | 0.15–0.45 | < 0.001 |
| Summer | 2.81 | 2.49–3.16 | < 0.001 | 5.47 | 3.46–864 | < 0.001 | 2.50 | 1.86–3.33 | < 0.001 | 0.68 | 0.50–0.94 | 0.02 | 0.48 | 0.28–0.79 | 0.005 |
| Fall | 2.43 | 2.17–2.73 | < 0.001 | 5.50 | 3.28–9.2 | < 0.001 | 4.31 | 3.04–6.12 | < 0.001 | 1.41 | 1.06–1.89 | 0.02 | 0.93 | 0.47–1.84 | 0.84 |
| 2006 vs 2007 | |||||||||||||||
| Winter | 1.13 | 1.01–1.25 | 0.03 | 1.69 | 1.18–2.4 | 0.004 | 1.18 | 0.92–1.52 | 0.18 | 0.48 | 0.37–0.62 | < 0.001 | 0.14 | 0.08–0.20 | < 0.001 |
| Spring | 0.81 | 0.72–0.92 | < 0.001 | 0.58 | 0.39–0.89 | 0.01 | 0.86 | 0.65–1.13 | 0.28 | 0.41 | 0.31–0.54 | < 0.001 | 0.54 | 0.35–0.83 | 0.005 |
| Summer | 1.32 | 1.17–1.50 | < 0.001 | 1.52 | 0.90–2.57 | 0.11 | 1.06 | 0.76–1.47 | 0.73 | 1.13 | 0.84–1.51 | 0.39 | 1.27 | 0.84–1.93 | 0.25 |
| Fall | 1.65 | 1.47–1.85 | < 0.001 | 3.77 | 2.22–6.39 | < 0.001 | 3.08 | 2.15–4.41 | < 0.001 | 1.74 | 1.32–2.31 | < 0.001 | 4.99 | 2.95–8.45 | < 0.001 |
Total condemned
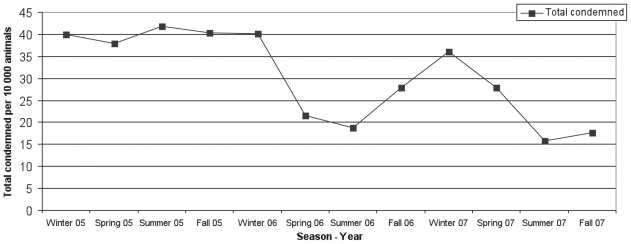
Significantly higher rates of total condemnations were observed in the winter of 2005 and 2006 compared to the winter of 2007 (Table 3; Figure 1). Significantly higher rates of total condemnations were observed in spring, summer, and fall of 2005 compared to the same seasons in 2006 and 2007 (Table 3; Figure 2).
Figure 1.
Total condemnation rates per 10 000 hogs slaughtered per season from a federally inspected abattoir in southern Ontario from 2005—2007.
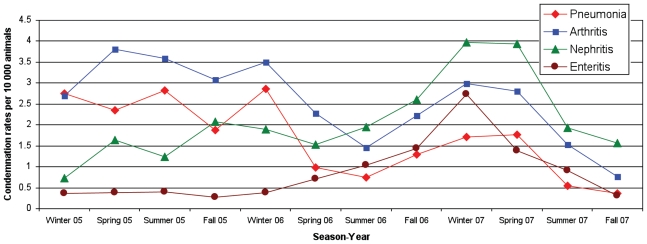
Figure 2.
Condemnation rates per 10 000 hogs slaughtered per season of condemnations due to pneumonia, arthritis, nephritis, and enteritis from a federally inspected abattoir in southern Ontario from 2005–2007.
Pneumonia
Significantly higher rates of pneumonia were observed in the spring and summer of 2005 compared to the same seasons in 2006 (Table 3; Figure 2). Significantly higher rates of pneumonia were also observed for winter, summer, and fall of 2005 compared to the same seasons in 2007 (Table 3; Figure 2). Higher rates of pneumonia were observed in 2006 compared to 2007, except for spring when a significantly lower rate of pneumonia was observed in 2006 compared to 2007, and summer when there was no significant difference between the 2 periods (Table 3; Figure 2).
Arthritis
Significantly higher arthritis rates were observed in spring, summer, and fall of 2005 compared to the same seasons in 2006 and 2007 (Table 3; Figure 2). A significantly higher rate of arthritis was observed in the fall of 2006 compared to the fall of 2007 (Table 3; Figure 2).
Nephritis
Significantly lower rates of nephritis were observed in winter and summer of 2005 compared to 2006 (Table 3; Figure 2). Significantly lower rates were observed in winter, spring and summer of 2005 compared to the same seasons in 2007 (Table 3; Figure 2). However, a significantly higher rate of nephritis was observed in the fall of 2005 compared to the fall of 2007 (Table 3; Figure 2). Highly significant lower rates of nephritis were observed in the winter and spring of 2006 compared to the same seasons in 2007 (Table 3; Figure 2); however, higher rates of condemnations were observed in the fall of 2006 compared to the fall of 2007 (Table 3; Figure 2).
Enteritis
Significantly lower rates of enteritis were observed in spring, summer, and fall of 2005 compared to 2006 (Table 3; Figure 2). Significantly lower rates were observed in winter, spring, and summer of 2005 compared to the same seasons in 2007, and in the winter and spring of 2006 compared to the same seasons in 2007 (Table 3; Figure 2). However, a significantly higher rate of enteritis was observed in the fall of 2006 compared to the fall of 2007 (Table 3; Figure 2).
Temporal scan statistics
Scan statistics indicated that the most likely statistically significant temporal clusters during the entire study period for total condemned, arthritis, and pneumonia occurred at similar time frames, from the January 2005 to spring 2006 (Table 4). In contrast, the most likely statistically significant temporal clusters for the entire period for nephritis and enteritis occurred near the end of the study (Table 4).
Table 4.
Significant most likely temporal clusters of different condemnation codes, using Bernoulli models in SaTScan from a federally inspected abattoir in Southern Ontario from 2005–2007
| Most likely cluster | Total condemned | Arthritis | Pneumonia | Nephritis | Enteritis |
|---|---|---|---|---|---|
| Total population in study period | 6 204 702 | 6 204 702 | 6 204 702 | 6 204 702 | 6 204 702 |
| Total number of cases in study period | 22 983 | 1911 | 1255 | 1595 | 658 |
| Total population in the cluster | 2 514 331 | 2 661 366 | 2 496 581 | 1 796 545 | 2 483 727 |
| Time frame of cluster | 2005/January/3 2006/March/29 |
2005/January/31 2006/June/1 |
2005/Janury/4 2006/March/28 |
2006/September/18 2007/July/20 |
2006/May/23 2007/July/31 |
| Number of cases in the cluster | 12 301 | 1089 | 777 | 763 | 505 |
| Expected cases in the cluster | 9 313.4 | 819.7 | 504.9 | 461.8 | 263.4 |
| Observed/expected | 1.32 | 1.32 | 1.54 | 1.65 | 1.92 |
| Relative Risk | 1.69 | 1.76 | 2.4 | 2.25 | 4.94 |
| Log likelihood ratio | 791.13 | 76.45 | 119.52 | 126.1 | 183.7 |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Validation of temporal trends
The following changes in PCVAD were noted from the review of the AHL reports between 2005 and 2007:
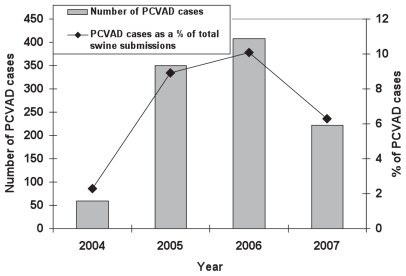
An increase in the number and proportion of PCVAD cases was observed in 2005 and 2006 compared to 2004 reflecting the outbreak of PCVAD in Ontario (Figure 3).
A decrease in PCVAD number and proportion of cases was reported in 2007 reflecting the wide use of PCV-2 vaccine in 2007 (17) (Figure 3).
In addition, the AHL reported a marked decline of PCV-2 diagnoses from 10.2% of the total swine submissions in the first trimester of 2006 to 8.4% by the fall of 2006 reflecting the initiation of the PCV-2 vaccine in the spring of 2006 (28).
Figure 3.
Total number of PCVADa cases and total PCVAD cases as a percent of total swine submissions to the AHLb from 2004 to 2007.
a PCVAD — porcine circovirus-associated disease.
b AHL — Animal Health Laboratory, University of Guelph.
Discussion
The higher rates of condemnation observed in 2005 in the negative binomial models and the most likely temporal clusters identified in this study for total condemned, pneumonia, and arthritis are consistent with the presence of new strains of porcine circovirus Type 2 (16,18–19). Pneumonia is one of the most frequent clinical signs observed in PCVAD (15–16). However, a wide spectrum of infectious agents such as M. hyopneumoniae, M. hyorhinis, H. parasuis, S. suis, S. aureus, and P. multocida (16,18–20,21) were found concomitant with PCV-2 on affected farms during the outbreak in Ontario. The association of these specific pathogens such as M. hyorhinis and S. aureus with pathological lesions related to arthritis (23–25) may explain the similar patterns of condemnation rates observed for these lesions as with pneumonia. Associations between arthritis and pneumonia have been described in other studies (23); these conditions are often associated with lowered immunity which might be caused by infectious agents such as porcine circovirus, together with management and environmental stressors.
A decrease in the rate of pneumonia in the spring of 2006 and the dates when the temporal clusters identified for these condemnation codes ended coincides with a marked decline in the diagnoses of PCVAD at the AHL from 10.2% of the total swine submissions in the first months of 2006 to 8.4% by the fall of 2006 (28) likely reflecting the initiation of the use of PCV-2 vaccines in the April of 2006 in Ontario (21). In addition, a decline in the percent of positive PRRSV-PCR test results from 45% in 2005 to 33% in 2006 was also reported from the AHL that is consistent with the decline in pneumonia cases in this abattoir in 2006 (29).
The PCV-2 outbreak cases in Ontario were also associated with an increase in enteritis and nephritis (15,16). However, in contrast to what was expected, the rates of nephritis and enteritis were often higher in 2007 compared to 2005. The most common pathogens associated with a thickening of intestinal walls in slaughter pigs are Salmonella Typhimurium and Lawsonia intracellularis. These 2 pathogens are commonly found in pig populations in Ontario (30,31), but there are no records from the AHL to support an increase in the incidence of infections with these pathogens during this period. Similarly, nephritis in finisher pigs can also be caused by various conditions, and condemnations due to interstitial nephritis are still common in Canada (10,32). Recent studies report that porcine parvovirus (PPV), PCV-2, Leptospira, and PRRSv are common causes of nephritis in slaughter pigs (10,32–36). However, our results are inconsistent with changes in PCVAD clinical disease prevalence, and no diagnostic data exist to suggest that the other potential pathogens appeared at increasing rates during this period.
Due to the retrospective nature of our study, we were not able to support our results with serological or other sources of field data from the abattoir or the source farms, but our findings on the patterns of pneumonia and arthritis were consistent with the PCVAD outbreak that occurred in 2005 and decreased with the introduction of the PCV-2 vaccine in 2006. It also seems apparent that abattoir findings, in the case of nephritis and enteritis, may suggest areas for enhanced surveillance for unexplained changes in disease patterns that were not identified through traditional passive surveillance involving diagnostic laboratories. Identifying the causal agents behind these changing patterns could have considerable economic, public health, and animal health benefits. Systems such as the Pig Health Monitoring Scheme in Australia, the Health Production Surveillance System (HEPS), and PigMON in the USA were developed to inform producers and veterinarians of the presence and impact of disease by using slaughter inspection findings (1,9,37). The feasibility of using feedback from abattoirs has also been investigated in Canada (38) and various projects have attempted to implement this type of program so that slaughterhouse lesions might be used in herd health programs (39). However, these programs have not been well-utilized by producers. This information would be of much more value if slaughter data were incorporated into a surveillance program and validated with additional health data sources to monitor the whole industry rather than relying on individual producers utilizing the information for their own herd health programs.
We were only able to receive data from 1 of 5 federally inspected plants in Ontario, and this plant also received a small percentage of animals (approximately 1%) from Quebec. In addition to the 5 federally inspected plants there are more than 200 provincially licensed plants that slaughter hogs in Ontario. Although both federal and provincial plants have strict regulated inspection standards, the federally inspected abattoirs are the only ones that can sell outside the province and the country, and their inspection practices have to meet trade requirements (40). Consequently, federal abattoirs may tend to handle more uniform animals with higher health status, compared to provincial plants which are restricted to more local markets. Although federal data appeared to show important disease trends, we think comparisons with provincial data may also be useful because in the case of a disease outbreak, pathological problems may be reflected more markedly in provincially inspected abattoirs. Based on Food Safety Decision Support System data the condemnation rates in provincial abattoirs can be higher (41). It would be important for future studies to compare the relative utility of provincial versus federal abattoir data for disease surveillance based on the quality of the data, its consistency with known disease patterns, and the ability to make inferences to specific spatial locations. The availability of spatial information that reflected the general location of affected farms would enhance the use of these data for surveillance. However, the impact of variation in inspection quality among abattoirs and stable spatial variation in condemnation rates due to environmental and management conditions should be considered when integrating data from several abattoirs (24,42).
Acknowledgments
We thank the abattoir plant for providing the data used in this study. The authors also thank the Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA); the Agriculture and Agri-Food Canada, Food Safety Initiative (FSI); and the Animal Health Strategic Investment (AHSI) for funding. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Christensen J, Ellegaard B, Kirkegaard Petersen B, Willeberg P, Mousing J. Pig health and production surveillance in Denmark: Sampling design, data recording, and measures of disease frequency. Prev Vet Med. 1994;20:47–61. [Google Scholar]
- 2.Brown C. Emerging zoonoses and pathogens of public health significance — An overview. Rev ScTech Off Int Epiz. 2004;23:435–442. doi: 10.20506/rst.23.2.1495. [DOI] [PubMed] [Google Scholar]
- 3.Henning KJ, Mostashari F. Syndromic surveillance. Issues in Science and Technology. 2004;21:10. [Google Scholar]
- 4.Dufour B, Hendrikx P, Toma B. The design and establishment of epidemiological surveillance systems for high-risk diseases in developed countries. Rev Sci Tech Off Int Epi. 2006;25:187–198. doi: 10.20506/rst.25.1.1659. [DOI] [PubMed] [Google Scholar]
- 5.Glickman LT, Moore GE, Glickman NW, Caldanaro RJ, Aucoin D, Lewis HB. Purdue University-Banfield National Companion Animal Surveillance Program for Emerging and Zoonotic Diseases. Vector Borne Zoonotic Dis. 2006;6:14–23. doi: 10.1089/vbz.2006.6.14. [DOI] [PubMed] [Google Scholar]
- 6.Willeberg P, Gerbola M, Petersen BK, Andersen JB. The Danish pig health scheme: Nation-wide computer-based abattoir surveillance and follow-up at the herd level. Prev Vet Med. 1984;3:79–91. [Google Scholar]
- 7.Tuovinen VK, Gröhn Y, Straw BE. Partial condemnations of swine carcasses — A descriptive study of meat inspection findings at Southwestern Finland’s Cooperative Slaughterhouse. Prev Vet Med. 1994;19:69–84. [Google Scholar]
- 8.Davies PR, Bahnson PB, Grass JJ, Marsh WE, Dial GD. Comparison of methods for measurement of enzootic pneumonia lesions in pigs. Am J Vet Res. 1995;56:709–714. [PubMed] [Google Scholar]
- 9.Jackowiak J, Kiermeier A, Kolega V, Missen G, Reiser D, Pointon AM. Assessment of producer conducted antemortem inspection of market pigs in Australia. Aust Vet J. 2006;84:351–357. doi: 10.1111/j.1751-0813.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter JA, Scorgie A, Josephson G. Leptospira interrogans serovar Pomona infection associated with carcass condemnation of swine at slaughter. J Swine Health Prod. 2006;14:145–148. [Google Scholar]
- 11.Heinonen M, Gröhn YT, Saloniemi H, Eskola E, Tuovinen VK. The effects of health classification and housing and management of feeder pigs on performance and meat inspection findings of all-in-all-out swine-finishing herds. Prev Vet Med. 2001;49:41–54. doi: 10.1016/s0167-5877(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 12.Poljak Z, Friendship RM, Carman S, McNab WB, Dewey CE. Investigation of exposure to swine influenza viruses in Ontario (Canada) finisher herds in 2004 and 2005. Prev Vet Med. 2008;83:24–40. doi: 10.1016/j.prevetmed.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Delay J, McEwen B, Carman S, van Dreumel T, Fairles J. Porcine circovirus type 2-associated disease is increasing. AHL Newsletter, University of Guelph, Laboratory Services. 2005 Sep;9(3):22. [Google Scholar]
- 14.Carman S, McEwen B, Josephson G, Fairles J. PRRSV outbreak in southwestern Ontario. AHL Newsletter, University of Guelph, Laboratory Services. 2005 Mar;9(1):6. [Google Scholar]
- 15.Van Dreumel T, Josephson G, Lusis P. Porcine circovirus type 2-associated conditions in pigs. AHL Newsletter, University of Guelph, Laboratory Services. 2005 Mar;9(1):5. [Google Scholar]
- 16.Carman S, Cai HY, DeLay J, et al. The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease — 2004–2006. Can J Vet Res. 2008;72:259–268. [PMC free article] [PubMed] [Google Scholar]
- 17.Carman S, McEwen B, DeLay J, Cai H, Fairles J, van Dreumel T. Porcine circovirus 2 — associated disease diagnosis decline in 2007 and 2008. AHL Newsletter, University of Guelph, Laboratory Services September. 2008;12:22. [Google Scholar]
- 18.Carman S, McEwen B, DeLay J, et al. Porcine circovirus-2 associated disease in swine in Ontario (2004 to 2005) Can Vet J. 2006;47:761–762. [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnon CA, Tremblay D, Tijssen P, Venne MH, Houde A, Elahi SM. The emergence of porcine circovirus 2b genotype (PCV-2b) in swine in Canada. Can Vet J. 2007;48:811–819. [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana J, Segalés J, Rosell C, et al. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet Rec. 2001;149:357. doi: 10.1136/vr.149.12.357. [DOI] [PubMed] [Google Scholar]
- 21.Cardinal F, Jones B. PCVAD Vaccine results in grower-finisher units: Practical evaluation and considerations. Proc of the 2008 Banff Pork Seminar: Advances in Pork Production. 2008:197–203. [Google Scholar]
- 22.McEwen B, Carman S, Fairles J, Slavic D, Cai H. Porcine circovirus type 2-associated disease and co-infections. AHL Newsletter, University of Guelph, Laboratory Services March. 2007;11:8. [Google Scholar]
- 23.Elbers ARW, Tielen MJM, Snijders JMA, Cromwijk WAJ, Hunneman WA. Epidemiological studies on lesions in finishing pigs in the Netherlands. I. Prevalence, seasonality and interrelationship. Prev Vet Med. 1992;14:217–231. [Google Scholar]
- 24.Martinez J, Jaro PJ, Aduriz G, Gomez EA, Peris B, Corpa JM. Carcass condemnation causes of growth retarded pigs at slaughter. Vet J. 2007;174:160–164. doi: 10.1016/j.tvjl.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Van Dreumel T, Vilaca K, McRaild P, Cai H, DeLay J. Mycoplasma hyorhinis arthritis in organically raised pigs. AHL Newsletter, University of Guelph, Laboratory Services. 2008;12:31. [Google Scholar]
- 26.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiology Research. AVC Inc; Charlottetown, Prince Edward Island, Canada: 2003. Modeling count and rate data. [Google Scholar]
- 27.Kulldorff M. SaTScan User Guide (version 7.0). c. 2006. 2006. [Last accessed November 8, 2010]. Available from http://www.satscan.org/
- 28.Carman S, McEwen B, DeLay J, Cai H, Fairles J. Porcine circovirus 2-associated disease diagnoses decline in the later part of 2006. AHL Newsletter, University of Guelph, Laboratory Services December. 2006;10:30. [Google Scholar]
- 29.Carman S, McEwen B, Fairles J. PRRSV infection is ongoing in Ontario swine herds. AHL Newsletter, University of Guelph, Laboratory Services September. 2007;11:26. [Google Scholar]
- 30.Corzo CA, Friendship RM, Dewey CE, Blackwell T. Sero-prevalence of Lawsonia intracellularis in Ontario swine herds. J Swine Health Prod. 2005;13:314–317. [Google Scholar]
- 31.Farzan A, Friendship RM, Dewey CE, Poppe C, Funk J, Muckle CA. A longitudinal study of the Salmonella status on Ontario swine farms within the time period 2001–2006. Food Borne Path Dis. 2008;5:579–588. doi: 10.1089/fpd.2007.0074. [DOI] [PubMed] [Google Scholar]
- 32.Drolet R, D’Allaire S, Larochelle R, Magar R, Ribotta M, Higgins R. Infectious agents identified in pigs with multifocal interstitial nephritis at slaughter. Vet Rec. 2002;150:139–143. doi: 10.1136/vr.150.5.139. [DOI] [PubMed] [Google Scholar]
- 33.van Dreumel T, Josephson G, Lusis P. Porcine circovirus type 2-associated conditions in pigs, AHL Newsletter, University of Guelph Laboratory Services. 2005;9:22. [Google Scholar]
- 34.Cooper VL, Hesse RA, Doster AR. Renal lesions associated with experimental porcine reproductive and respiratory syndrome virus (PRRSV) infection. J Vet Diag Inv. 1997;9:198–201. doi: 10.1177/104063879700900216. [DOI] [PubMed] [Google Scholar]
- 35.Baker TF, McEwen SA, Prescott JF, Meek AH, Waltnertoews D. The prevalence of Leptospirosis and its association with multifocal interstitial nephritis in swine at slaughter. Can J Vet Res. 1989;53:290–294. [PMC free article] [PubMed] [Google Scholar]
- 36.Elbers ARW, de Jong MF, Wellenberg GJ. Risk factors for clinical signs of PMWS and PDNS in pigs in The Netherlands: A case-control study. Tijdschrift voor diergeneeskunde. 2006;131:318–325A. bst. [PubMed] [Google Scholar]
- 37.Davies PR, Bahnson PB, Marsh WE, Dial GD. National Pork Producers Council, 1995 Research Investment Report. Des Moines, IA: National Pork Producers Council; Prevalence of gross lesions in slaughtered pigs — the PigMON Database 1990–1993. [Google Scholar]
- 38.Shadbolt PV, Mitchell WR, Blackburn DJ, Meek AH, Friendship RM. Perceived usefulness of the collection of subclinical and other disease entities detected at slaughter. Can Vet J. 1987;28:439–445. [PMC free article] [PubMed] [Google Scholar]
- 39.Hurnik D, Dohoo IR, Donald A, Robinson NP. Factor analysis of swine farm management practices on Prince Edward Island. Prev Vet Med. 1994;20:135–146. [Google Scholar]
- 40.Ontario Ministry of Agriculture and Food. What you should know if you raise, deal, handle or purchase livestock or poultry for slaughter. Your responsibilities under the meat inspection Act (Ontario) [monograph on the Internet]. c. 1994. [Last accessed November 8, 2010]. Available from https://ospace.scholarsportal.info/bitstream/1873/4777/2/10308452.pdf.
- 41.Pearl DL. Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) report. March 12. 2008. Final report on cluster analyses of Food Safety Decision Support System data using market hog data from provincially inspected abattoirs in Ontario (2001–2005) [Google Scholar]
- 42.Hill JR, Jones JET. An investigation of the causes and of the financial loss of rejection of pig carcasses and viscera unfit for human consumption. I. Studies at seven abattoir. British Vet J. 1984;140:450–457. doi: 10.1016/0007-1935(84)90039-3. [DOI] [PubMed] [Google Scholar]