Abstract
Background
The long-term-treatment of glaucoma with topical medications is associated with side effects involving cornea damage. We examined the effect of glaucoma topical medications (bimatoprost, travoprost, latanoprost, timolol, betaxolol, dorzolamide, brinzolamide, brimonidine) on growth of cells of three human epithelial corneal lines.
Methods
The cells were cultured in 8-chamber slides, treated with different concentrations of the medications, and fixed at 24, 48, and 72 h. Cell number on slides to estimate viability and growth curves, frequency of apoptosis (FLICA and caspase-3 activation probes), and proliferation (BrdU incorporation assay) were measured by laser scanning cytometry (LSC).
Results
Depending on concentration all examined medications induced cell necrosis or apoptosis and suppressed proliferation. Significant variability in proliferation and apoptosis was observed within the same cultures depending on local cell density, with cells in high density areas being more resistant. The data indicate that commonly used topical medications exert cytostatic and cytotoxic effects in cultures of corneal cells and suggest that caution should be exercised in their use, particularly, when the corneal diseases are accompanied by cell proliferation and regeneration, in long-term-treatment.
Conclusions
The present approach of using LSC makes it possible to assess and compare cytostatic and cytotoxic effects of different topical medications on the respective target cells.
Key terms: cell proliferation, cell death, cell cycle, apoptosis, antiglaucoma topical medications
Glaucoma is an ocular disease characterized by visual field loss due to optic nerve damage. It is a progressive disease and if left untreated it could lead to blindness. It is also a relatively frequent disease; the frequency of primary open angle glaucoma (the most frequent form of glaucoma) is about 0.5–1.0%. Elevated intraocular pressure (IOP) is the most important risk factor in glaucoma pathogenesis. High IOP not only raises the risk to develop this disease but also worsens prognosis. The only known way of glaucoma treatment is to reduce IOP either with surgery or topical eye drops. Because topical antiglaucoma medications are used for long time periods, side effects are a major concern (1,2). Among them, the local effect on cornea is of major importance. The effects of topical antiglaucoma medications on corneal epithelial cells are well documented in animal models (3,4) as well as in patients (5,6). Corneal toxicities such as kerato-conjunctivitis, keratitis, corneal ulcerations, and corneal opacities have been reported by National Registry of Drug-Induced Ocular Side Effects and WHO (7). Moreover, some of these medications increase central corneal thickness (CCT) (5,6).
There are relatively few publications devoted to the assessment of cytotoxicity of glaucoma topical medications on in vitro models of human epithelial corneal cells. One report presents the evidence of lesser cytotoxicity of travoprost compared to latanoprost (8). In another study, cytotoxicity of eight β-blockers was compared on human cell lines, including the lines of corneal epithelial cells. Among these β-blockers, only one, namely timolol, is currently used in eye drops. Combined with pindolol it shows lowest cytotoxicity and the highest ratio between inhibitory concentration 50% values and β-blocking constant (IC50/KB) (1).
In the present study, we adapted our previously described viability assay of adherent cells to analyze effects of glaucoma topical medications on human corneal cells. This method is based on estimating cells densities as a function of time of incubation, which allows us to generate growth and viability curves, by laser scanning cytometer (LSC) (9). Moreover, the effect of these medications on frequency of apoptosis and cell proliferation rates were measured as well, using FLICA and BrdU incorporation as the respective markers (9).
MATERIALS AND METHODS
Cell Cultures
Three cell lines (HCE-2, 2.040 pRCV-T, and 10.014 pRSV-T) derived from human corneal epithelial cells immortalized by SV-40 virus were obtained from LGL Prochem (former ATCC, Manassas, VA). Cells were cultivated in Keratinocyte-Serum Free Medium supplemented with 0.005 mg/ml insulin, 5 ng/ml epidermal growth factor, 0.05 mg/ml bovine pituitary extract (all from GIBCO, Invitrogen, Grand Island, NY), and 500 ng/ml hydrocortisone (Sigma, St. Louis, MD). All cell lines were maintained in 100% relative humidity, at 37°C, in atmosphere of 5% CO2 in air. The cells grow only in extracellular matrix (ECM) protein pre-coated flask. Eight-chamber slides (Lab-Tek, Nunc, Naperville, IL) or 25 cm2 flask (Nunc, Naperville, IL) were pre-coated with solution of 3.0 mg/ml collagen I (PureCol, Inamed, Fremon, CA), 0.01 mg/ml bovine serum albumin (GIBCO, Invitrogen, Grand Island, NY), and 0.01 mg/ml fibronectin (Invitrogen, Grand Island, NY), for 15 min at room temperature and subsequently dried for a few days. To obtain the optimal growth conditions, 80% of culture medium was daily renewed. Such culture protocol enabled us to obtain corneal cells proliferating at relatively high rate with doubling time about 24–48 h (variable due to cell density). As soon as the 80% of confluence was achieved, the cells were reseeded to maintain them in exponential and asynchronous growth phase. The cells were detached by 0.05% trypsin-EDTA (GIBCO, Invitrogen, Grand Island, NY) short incubation and seeded into the new flasks; sub-seeding ratio was 1:3.
8-Chamber Slide Experiments
The effect of different commercial available topical antiglaucoma medications on corneal line cells was examined. Travoprost (0.04 mg/ml, Travatan, Alcon, Herts, UK), latanoprost (0.05 mg/ml, Xalatan, Pfizer, Puurs, Belgium), timolol (5 mg/ml, Oftensin, MSD, Whitehouse Station, NJ), betaxolol (5 mg/ml, Betoptic, Alcon, Herts, UK), bimatoprost (0.3 mg/ml, Lumigan, Allergan, Mayo, Ireland), dorzolamide (20 mg/ml, Trusopt, MSD, Whitehouse Station, NJ), brinzolamide (10 mg/ml, Azopt, Alcon, Herts, UK), brimonidine (2 mg/ml, Alphagan, Allergan, Mayo, Ireland) and described previously in experimental model were included in this study (9). Briefly, corneal line cells were seeded into permanox 8-chamner slides in density of ~5000 cells/chamber. Antiglaucoma medications were administrated directly into the chambers to obtain the final concentration as indicated in the text and in the figure captions, as soon as the desired density of cells was obtained. Every day 80% of culture medium with or without adequate amounts of the medications to obtain desired concentrations was renewed. The cultures were terminated after 24, 48, or 72 h. Then, the slides were fixed in cold 1% formaldehyde followed by at least 24 h post-fixation in 70% ethanol (in all but BrdU and confocal microscopy experiments). After rehydration in PBS, the cells were stained by propidium iodide (PI; 10 μg/ml) in the presence of RNase A (100 μg/ml) for 30 min and analyzed by LSC (CompuCyte, Cambridge, MA). Such model of experiment enabled us to count all cells in individual chambers per each time point giving the opportunity to generate growth and viability curves. In most instances, additional staining (FLICA, BrdU, activated caspase-3*) of these slides were also done prior to PI staining. The cultures were run in duplicates and the experiments were at least twice repeated.
FLICA Binding
FLICA experiments were performed as described elsewhere (9–12). Briefly, FAM-VAD-FMK (Immunochemistry Technologies, Bloomington, MN) were administrated directly into the chambers to obtain the final concentration of 10 μM for the final hour of culture. Then the cells were washed twice in warm PBS in Coplin jars and fixed. After storage at −20°C ethanol the cells were rehydrated in PBS, their DNA stained with PI in the presence of RNAse A for 30 min at room temperature, and intensity of red and green fluorescence was measured by LSC.
Incorporation of BrdU
Freshly made solutions of BrdU (Sigma Chemical, St. Louis, MD) were added to culture medium to the final concentration of 10 μM. After 1 h, incubation chambers were removed and cells were fixed in cold 70% ethanol (without formaldehyde preincubation) for at least 2 h. Then, the slides were immersed in 2 M HCl/0.5 Triton X-100 solution for 30 min. Acid was neutralized by incubation in 0.1 M Na2B4O7·10H2O, pH 8.5. Then the cells were permabilized by incubation in 0.5% Tween-20/1% BSA in PBS. Subsequently cells were incubated with 1:20 diluted mAb anti-BrdU conjugated with FITC (BD Bioscience, San Jose, CA) for 30 min at room temperature under the parafilm cover. Then unbound mAbs were washed out and cellular DNA was counterstained with PI in the presence of RNase. Cellular fluorescence was measured by LSC (10).
Detection of Activated Caspase-3
All technical details are described in our previous publications (9–12). Briefly fixed cells were rehydrated in PBS, incubated with 0.2% Triton X-100 for 15 min and incubated with FITC conjugated rabbit anti-caspase 3* mAb (Cell Signaling Technology, Beverly, MA). Cellular DNA was counterstained by PI in the presence of RNase A and green and red fluorescence was measured by LSC (9–12).
LSC Analysis
Fluorescence of cells fixed on chamber slides was measured by LSC. Cellular fluorescence was excited by 488 nm laser and measured by PMTs using standard setting of emission filters. LSC creates gray scale map of entire slide separately for each fluorescence color. It enables not only to measure intensities of maximal pixel and integrated fluorescence for each individual cell, but also to present data as PMT pictures of cells (Fig. 2). Cell fluorescence from each chamber also could be calculated separately. It enables us to count the number of cells attached per chamber per time point. In most experiments red (625 ± 14 nm) PI fluorescence and green florescence (530 ± 15 nm) of FAM-VAD-FMK, BrdU, or FITC-caspase-3* was recorded. These enabled us not only to count all cells in each chamber, but also to measure proliferation or apoptosis of these cells. Because of variability of apoptotic and proliferation indices within single chamber probably due to different cell density all of these indices were estimated based on entire chamber area measurements. Other technical details are presented elsewhere (9,13).
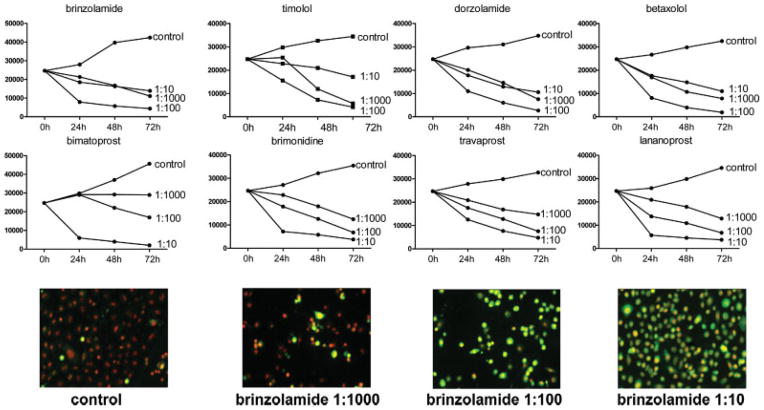
Fig. 2.
Growth curves of human epithelial corneal line cells (10.014pRSV-T) after incubation with topical glaucoma medications. The cells were cultured in the absence or presence of different concentrations of medications in 8-chamber slides, stained with FAM-VAD-FMK and PI at 24 h intervals, and subsequently fixed and analyzed by LSC. Top panels show changes in cell number per culture at given point of culture harvesting. The LSC images of cells treated with brinzolamide for 24 h are shown in the bottom panels. The cells with necrotic appearance are characterized by weak green fluorescence, apoptotic by strong green cytoplasmatic fluorescence and live cells only by nuclear red fluorescence of PI. Cell incubations with 1:10 diluted medications induced necrosis, which was associated with the greater cell attachment to slides than that of apoptotic cells, which predominated in cultures treated with 1:100 diluted medications. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Viability Experiments
The cells were cultured in 8-chamber slide and treated with different concentrations of medications for various time intervals as indicated in the Results section. The chambers were then removed, PI was added to final concentration of 1 μg/ml, slides were covered with cover slips and three random pictures of each chamber were taken by confocal microscope (LSM 5 Pascal, Zeiss) equipped with air objective (X10); fluorescence was exited by 488 nm laser and cells were examined under red fluorescence emission and Nomarski interference contrast illumination. Although the confocal effect was not achieved because of maximally opened pinhole, the measurement enabled us to rapidly estimate viability by measurement frequency of live (PI negative) and dead (PI positive) cells; 500 cells were counted per chamber and viability is expressed as a percentage of dead (PI positive) cells.
RESULTS
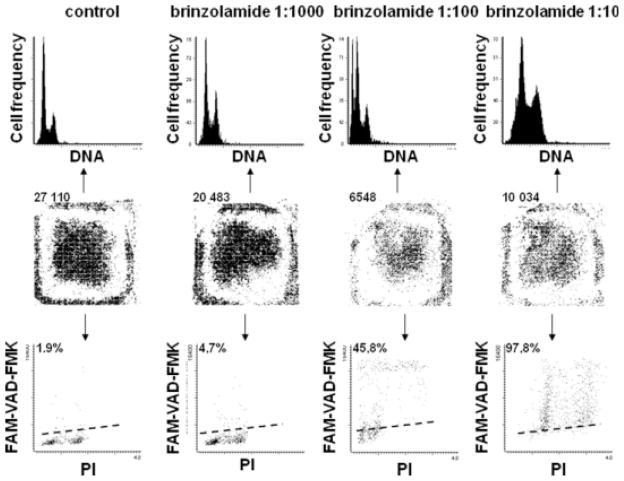
All experiments were carried out on each of the three corneal lines yielding essentially similar results and the representative data are presented in Figures 1–4. All examined medications strongly affected growth of cells maintained at low- and medium-cell density (5–30 × 103 cells/chamber) in time- and dose-dependent manner (Figs. 1 and 2). Both the decrease of proliferation and induction of apoptosis contributed to the suppression of cell growth as represented by the growth curves. As it is evident in Figure 1 in the case of brinzolamide already after 24 h of treatment at 1:1000 concentration a distinct accumulation of cells in G2M, reflecting cell arrest in this phase of the cell cycle, was observed, with relatively minor induction of apoptosis (4.7%). The apoptotic effect was much more pronounced at 1:100 concentration at which 45.8% cells were FAM-VAD-FMK-positive. Induction of apoptosis at that concentration was confirmed by the presence of a distinct sub-G1 cell subpopulation on the cellular DNA content histograms. Even more dramatic effect was seen at 1:10 concentration of the brinzolamide, where nearly all cells had markedly increased intensity of PI fluorescence, up to the level that they appeared to be tetraploid. Nearly all cells (97.8%) from these cultures were FAM-VAD-FMK-positive. It is possible that at this high concentration of brinzolamide (1:10), the cells were undergoing either necrosis or rapid, atypical apoptosis, and did not display all classical features of apoptosis. In the latter mode of cell demise, although certain events of apoptosis can occur (e.g., caspase activation-FAM-VAD-FMK reactivity), other features resemble necrosis; we previously observed such mixed pattern of cell death following treatment with the NF-κB inhibitor sesquiterpene parthenolide (12). In support of the notion that at 1:10 drug concentration the cells were undergoing necrosis or atypical apoptosis is the absence of the sub-G1 subpopulation on the DNA content histogram. Namely, when cells are treated with overdose of a cytotoxic agent and either die by necrosis or rapidly enter the “necrotic” phase of apoptosis their nuclear DNA does not undergo extensive fragmentation (14). Also, the evidence that more cells remained attached at 1:10 dilution (10,034) than at 1:100 dilution (6,548) is consistent with the feature of apoptotic cells that have predilection to detach from the surface on which they grow whereas during rapid cell death including necrosis they remain attached (14). Thus, the rapidly killed cells at high (1:10) drug concentration, unlike dying by typical apoptosis, remained attached to the slide.
Fig. 1.
Corneal epithelial line cells (10.014RSV-T) were cultured in the absence or presence of brinzolamide at different concentration in 8-chamber slides for 24 h, then labeled with FAM-VAD-FMK and PI, and measured by LSC to estimate number of cells in individual chambers (row of central panels; each dot represents a single cell), and to measure frequency of apoptosis (bottom panels) and visualize DNA content histograms (top panels). Number of cells per chamber or percentage of apoptotic (FAM-VAD-FMK-positive) cells are listed above the respective panels.
Fig. 4.
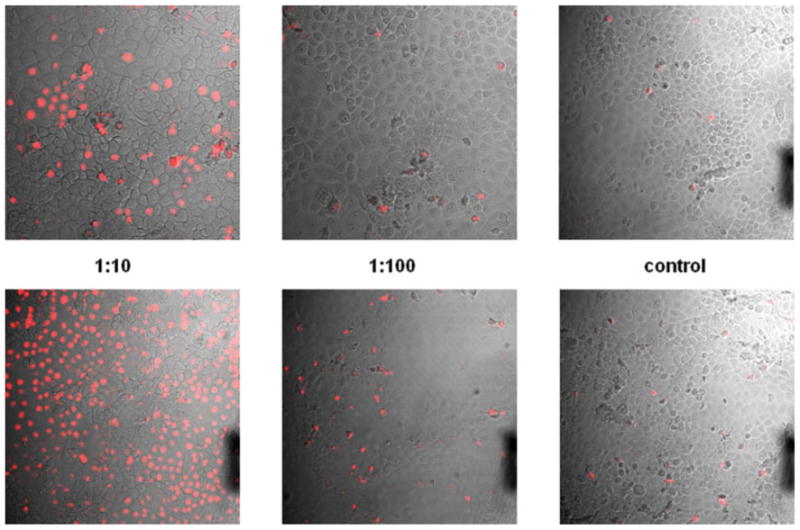
Viability of the untreated and drug-treated corneal cells assessed by PI exclusion test. Corneal line cells (10.014pRSV-T) were incubated for 15 min with different concentration of brimonidine (top panels) or timolol (bottom panels) and then for 1 min with PI. The chambers were then removed, slides were covered by cover glasses and the red fluorescence was examined by confocal microscopy (200×) combined with the Nomarski interference contrast; necrotic and late apoptotic cell nuclei are stained with PI. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
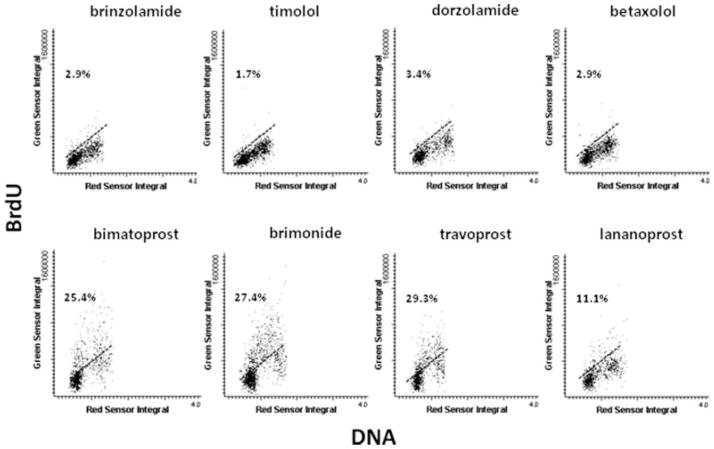
All three methods used to detect apoptosis (FAM-VAD-FMK binding, the presence of activated caspase-3 and sub-G1 cell population) revealed that all presently studied glaucoma medications at 1:1000 and 1:100 concentration were inducing apoptosis. The apoptotic effect was seen after 24 h and was the most pronounced in the case of cells treated with brinzolamide, timolol, dorzolamide, and betaxolol. Cell proliferation as expressed by frequency of cells incorporating BrdU was at the level of 30–40% in the control, untreated cultures. No evidence of BrdU incorporation was seen in cultures treated with the medications at 1:10 concentration at 24 h. At 1:100 concentration, the frequency of cells incorporating BrdU was distinctly reduced compared with control. The reduction was the most pronounced in cultures treated with brinzolamide, timolol, dorzolamide, and betaxolol (Fig. 3). At 1:1000 concentration, the effect of all medications on BrdU incorporation was relatively minor.
Fig. 3.
Scatterplots show proliferation measured by BrdU incorporation. Human epithelial corneal line cells (10.014pRSV-T) were cultured with different dilutions of glaucoma medications, then after 24 h, incubated with BrdU for 1 h, fixed, stained with BrdU mAb and PI, and analyzed by LSC. Influence of topical glaucoma medications, each at 1:100 dilution, on cultured cells was presented.
The summary effects of all glaucoma medications as reflected by the respective growth curves are illustrated in Figure 2. It is quite evident that in all drug-treated cultures with the exception of the cultures treated with bimatoprost, even at the lowest drugs concentration (1:1000) cell growth was totally suppressed. Not only cell proliferation, as measured by growth in cell number, was entirely blocked but actually there was net decline in cell number per culture after 48 and 72 h compared with time 0. At that low drug concentration more pronounced decrease in cell number was observed after treatment with brinzolamide, timolol, dorzolamide, and betaxolol than with bimatoprost, brimonidine, travoprost, and latanoprost. Compared with 1:1000 dilution even more pronounced decline in cell number was seen in cultures treated with the drugs at their 1:100 concentration. Unexpectedly, however, at 1:10 dilutions of brinzolamide, timolol, dorzolamide, and betaxolol more cells remained attached to slides than at 1:100 and 1:1000 dilutions (Fig. 2). Visual inspection of the attached cells at 1:10 drugs concentration revealed their morphology as characteristic of the necrotic dead cells. The low intensity and pattern of FAM-VAD-FMK staining was consistent with their rapid death during which they either reached the “necrotic phase of apoptosis” without displaying typical apoptotic changes or were undergoing straight necrosis (14).
All glaucoma topical medication added to the medium in dilution of 1:10 caused rapid decrease in viability of large majority of corneal cells as measured by their ability to exclude PI. This rapid cytotoxic effect was most pronounced after treatment with brinzolamide, timolol, dorzolamide, and betaxolol when almost no live cells were detected after 15 min of incubation. The medications diluted 1:100 or 1:1000 showed modest or minimal cytotoxic effects, respectively, when tested after short incubations (Fig. 4).
Similar experiments have been carried out on high density corneal cell cultures, with more than 50,000 cells seeded per chamber. Although similar type of effects were observed as in the case of low density cultures (in most cases necrosis at 1:10, apoptosis at 1:100, and suppressed proliferation at 1:1000 dilution) the severity of the cytostatic and cytotoxic effects was generally lower (data not shown).
DISCUSSION
The present data show that the glaucoma topical medications exert distinct cytostatic and cytotoxic effects on human corneal lines cells in vitro. The growth curves revealed the net loss of cells in cultures after 48 and 72 h of culturing at all three concentrations of each medication, with the exception of 1:1000 bimatoprost. The decline in cell number was a consequence of both cell death and suppression of proliferation. At high drug concentration (1:10), large majority of cells exhibited markedly increased intensity of PI fluorescence (Fig. 1). Most likely their chromatin structure was altered in such way that DNA became more accessible to PI. It has been reported that only a fraction of DNA in the nucleus is accessible to the intercalating dyes such as PI, acridine orange, or actinomycin D and dissociation of histones from DNA is required to increase accessibility of DNA to these dyes, which is then reflected by the enhanced intensity of nuclear fluorescence (15). The death of most cells in these cultures was fast and the mode of death resembled either necrosis or rapidly progressing apoptosis when shortly after the treatment cells enter the necrotic phase of apoptosis. Many of such cells remained attached to the culture slides, their DNA was not extensively fragmented and binding of the activated caspases reporter FAM-VAD-FMK was less intense than in the case of genuine apoptotic cells.
Apoptosis, as evidenced by DNA fragmentation, strong FAM-VAD-FMK binding, presence of activated caspase-3*, and detachment from cultures, was the mode of cell death at moderate drug concentrations (1:100). Also apparent were the cycle effects, namely the arrest in G2M phase of the cell cycle (Fig. 1) and decreased incorporation of BrdU, the latter reflected by both, the reduced frequency of BrdU-incorporating cells and lower intensity of BrdU fluorescence of the S-phase cells (Fig. 3). Even at the lowest (1:1000) concentration the growth was negative, most likely a consequence of apoptosis and reduced rate of proliferation, which although not extensive when measured after 24 h, could contribute to the overall negative effect on growth curves.
Human corneal line cells are difficult to culture. They need ECM protein stimulation as well as cell–cell contact, which is reflected by the characteristic pattern of more extensive growth in center or in peripheral part of chambers where initial density of cells is higher (Fig. 1). We observed that the cultures of higher density were more resistant for topical glaucoma medications treatment. It is possible that intercellular interactions may provide cell resistance to the cytotoxic agents. Furthermore, the rate of cell proliferation was reduced in high density areas, with about 5% cells incorporating BrdU as compared with 30–40% at low cell density. It is known that nonproliferating, quiescent cells are generally more resistant to exogenous cytotoxins than the proliferating ones. In fact, we observed large variability in proliferation and frequency of apoptosis in different local areas within the same chamber, in some cases reaching up to 20-fold compared to other areas, and there was a distinct correlation between frequency of apoptosis and BrdU-incorporating cells in the drug-treated cultures, with higher apoptotic index in the areas of high proliferation.
It was unexpected to observe such cytostatic and cytotoxic effects of glaucoma medications, which are being widely used by patients with no apparent harm. Human corneal line cell cultures (especially at low- and medium-cell density), thus, seem to be more sensitive to topical glaucoma medications than normal human cornea cells. Several explanations can account for the finding. Cells in the cornea probably receive a stronger positive stimulation from other cells, ECM proteins and growth factors than cells cultured in artificial in vitro conditions (even although flasks were ECM protein coated). Integrin ECM protein receptors should better recognize (providing stronger stimuli) antigen motives of human ECM of corneal basement membrane than bovine ECM antigen used in this study. Moreover, eye drop concentration in lacrimal sac is rapidly reduced due to lacrima production. Although the initial glaucoma medication concentrations when applied to eye are generally higher than used in this study, their effect may lasts shorter time period than in long-term cultures. However, we observed that even short incubation (15 min) with high 1:10 concentration of the medication exerted the cytotoxic effect (Fig. 4). It is also worth emphasizing that the cells were cultured in serum-free conditions what makes them more sensitive to any stress, as the serum or plasma provides resistance to various agents that target plasma membrane. It was observed before that corneal line cells (10.014 pRSV-T) cultured without fetal calf serum are about four times more sensitive for timolol stimulation than corneal line cells (HCEpiC) cultured with addition of FCS (1).
Commercially available antiglaucoma medications containing all components including preservatives rather than their active components were used in this study. We were motivated to carry out studies on their cytotoxicity as more relevant to the patients’ exposure compared to the analysis of isolated refined active components. It is likely, however, that at least part of the observed effects is due to preservatives. There is strong evidence in the literature that preservatives (especially benzalkonium chloride-BAC) and commercial solutions of glaucoma medications with preservatives on eye anterior segment cells exert cytotoxic, plasma membrane-permeabilizing, anti-proliferative, and/or proapoptotic effects detected in both in animal or in vitro models (16–25). Some evidence, however, is also to the contrary (26). Other data suggests protective role of antiglaucoma free component (latanoprost, travoprost) against conservative cytotoxic effect (27,28). Moreover, it seems that latanoprost, travoprost, bimatoprost do not induce direct stimulation of inflammation (25). It is worth emphasizing that majority of these studies are done on commercial solutions and only few on their active components (1,16,20). In this study, we included the largest number of antiglaucoma medication examining their influence on growth, viability, apoptosis, and proliferation of epithelial corneal human line cells.
Epithelial corneal human cells as first line barrier cells of eye, are the most exposed to toxic effects of topical treatment. There are only two other articles assessing their influence on epithelial corneal line cells (1,8). The authors examined timolol, latanoprost, and travoprost showing results consistent with ours. However, because of long-term-treatment model used in our studies, we were able for the first time to detect the drug-induced changes in the cell cycle. Furthermore, the measurements of the whole chamber areas by LSC allowed us also observe the variability and relationship of apoptosis and proliferation, dependent on local density within the same cultures.
In conclusion, our observations on the cytostatic and cytotoxic properties of the glaucoma topical medications call for a caution in their long-term application. Especially in the instances when the corneal diseases are accompanied by cell proliferation and regeneration, at least temporary usage of preservative free or less cytotoxic topical medication should be considered.
Acknowledgments
Grant sponsor: Polish Ministry of Science; Grant number: 2P05B08828; Grant sponsor: NCI; Grant number: RO1 28 704.
The authors thank Dr. R. Paduch for his help in cell cultures and Dr. S. Radej for confocal microscopy assistance.
LITERATURE CITED
- 1.Cheong H, Johnson J, Cormier M, Hosseini K. In vitro cytotoxicity of eight β-blockers in human corneal epithelial and retinal pigment epithelial cell lines: Comparison with epidermal keratinocytes and dermal fibroblasts. Toxicol In Vitro. 2008;22:1070–1076. doi: 10.1016/j.tiv.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Markstein R. Physiological aspects in the transfer of topical drugs into, within, and out of the eye. In: Orgül S, Flammer J, editors. Pharmacotherapy in Glaucoma. Bern: Verlag Hans Huber; 2000. pp. 55–63. [Google Scholar]
- 3.Okada Y. Effect of topical antiglaucoma medictions on corneal epithelium as evaluation by gene expression patterns. Cornea. 2007;26 (Suppl 1):S46–S54. doi: 10.1097/ICO.0b013e31812f6a71. [DOI] [PubMed] [Google Scholar]
- 4.Noecker RJ, Herrygers LA, Anwaruddin R. Cornea and conjunctival changes caused by commonly used glaucoma medication. Cornea. 2004;23:490–496. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Okugawa K, Oshika T, Amano S. Influence of dorzolamide on corneal endotherium. Jpn J Ophthalmol. 2003;47:129–133. doi: 10.1016/s0021-5155(02)00667-6. [DOI] [PubMed] [Google Scholar]
- 6.Harasymowycz P, Papamatheakis D, Ennis M, Brady M, Gordon K. Relation between travoprost and central corneal thickness in ocular hypertension and open-angle glaucoma. Cornea. 2007;26:34–41. doi: 10.1097/ICO.0b013e31802e3ce4. [DOI] [PubMed] [Google Scholar]
- 7.Fraunfelder FW. Corneal toxicity from topical ocular and systemic medication. Cornea. 2006;25:1133–1138. doi: 10.1097/01.ico.0000240084.27663.fd. [DOI] [PubMed] [Google Scholar]
- 8.Yee RW, Norcom EG, Zhao XC. Comparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human corneal epithelial cell culture system. Adv Ther. 2006;23:511–519. doi: 10.1007/BF02850039. [DOI] [PubMed] [Google Scholar]
- 9.Pozarowski P, Huang X, Gong RW, Priebe W, Darzynkiewicz Z. Simple, semi-automatic assay of cytostatic and cytotoxic effects of anti-tumor drugs by laser-scanning cytometry (LSC): Effects of the bisintercalator WP631 on growth and cell cycle of T-24 cells. Cytometry Part A. 2004;57A:113–119. doi: 10.1002/cyto.a.10121. [DOI] [PubMed] [Google Scholar]
- 10.Pozarowski P, Grabarek J, Darzynkiewicz Z. Flow cytometry of apoptosis. In: Robinson JP, Darzynkiewicz Z, Dean P, Hibbs A, Orfao A, Rabinovitch P, Wheeless L, editors. Current Protocols In Cytometry. New York: John Wiley and Sons; 2003. pp. 1–33. [Google Scholar]
- 11.Pozarowski P, Huang X, Halicka D, Lee B, Johnson G, Darzynkiewicz Z. Interaction of fluorochrome-labeled caspase inhibitors with apoptotic cells. A caution In data interpretation. Cytometry Part A. 2003;55A:50–60. doi: 10.1002/cyto.a.10074. [DOI] [PubMed] [Google Scholar]
- 12.Pozarowski P, Halicka DH, Darzynkiewicz Z. NF-κB inhibitor sesquiterpene parthenolide induces concurrently atypical apoptosis and necrosis: Difficulties in identification of dead cells in such cultures. Cytometry Part A. 2003;54A:118–124. doi: 10.1002/cyto.a.10057. [DOI] [PubMed] [Google Scholar]
- 13.Pozarowski P, Holden E, Darzynkiewicz Z. Laser scanning cytometry: Principles and applications. Methods Mol Biol. 2006;319:165–192. doi: 10.1007/978-1-59259-993-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology. Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 15.Darzynkiewicz Z, Traganos F, Kapuscinski J, Staiano-Coico L, Melamed MR. Accessibility of DNA in situ to various fluorochromes: Relationship to chromatin changes during erythroid differentiation of Friend leukemia cells. Cytometry. 1984;5:355–363. doi: 10.1002/cyto.990050411. [DOI] [PubMed] [Google Scholar]
- 16.Hamard P, Blondin C, Debbash C, Warnet JM, Baudouin C, Brignole F. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241:1037–1043. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Baudouin C, Pauly A, Bringnole-Baudouin F. Conjunctival an corneal reaction in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008;92:1275–1282. doi: 10.1136/bjo.2008.138768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarey B, Edekhaser H. In vivo corneal permeability following treatment with prostaglandin analogs (correction of analogs) with or without benzalonium chloride. J Ocul Pharmacol Ther. 2007;23:445–451. doi: 10.1089/jop.2007.0024. [DOI] [PubMed] [Google Scholar]
- 19.Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea. 2008;27:339–343. doi: 10.1097/ICO.0b013e31815cf651. [DOI] [PubMed] [Google Scholar]
- 20.De Saint Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr Eye Res. 2000;20:85–94. doi: 10.1076/0271-3683(200002)20:2;1-d;ft085. [DOI] [PubMed] [Google Scholar]
- 21.Brasnu E, Brignole-Baudouin F, Riancho L, Warnet JM, Baudouin C. Comparative study on the cytotoxic effect of benzalkonium chloride on the Wong-Kilboure derivative od Chang conjunctival and IOBA-NHC cell lines. Mol Vis. 2008;14:394–402. [PMC free article] [PubMed] [Google Scholar]
- 22.Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: Travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48:4123–4128. doi: 10.1167/iovs.07-0266. [DOI] [PubMed] [Google Scholar]
- 23.Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: A review. Curr Opin Ophthalmol. 2007;18:134–139. doi: 10.1097/ICU.0b013e328089f1c8. [DOI] [PubMed] [Google Scholar]
- 24.Wu KY, Wang HZ, Hong SJ. Effects of antiglaucoma drugs on cellular proliferation in cultured human corneal keratocytes. Kaohsiung J Med Sci. 2006;22:120–125. doi: 10.1016/S1607-551X(09)70231-1. [DOI] [PubMed] [Google Scholar]
- 25.Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:244–2450. doi: 10.1167/iovs.04-1331. [DOI] [PubMed] [Google Scholar]
- 26.Wu KY, Wang HZ, Hong SJ. Cellular cytotoxicity of antiglaucoma drugs in cultured corneal endothelium cells. Kaohsiung J Med Sci. 2007;23:105–111. doi: 10.1016/S1607-551X(09)70384-5. [DOI] [PubMed] [Google Scholar]
- 27.Guenoun J-M, Baudouin C, Rat P, Pauly A, Warnet J-M, Brignole-Baudouin F. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatopros on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4594–4599. doi: 10.1167/iovs.05-0776. [DOI] [PubMed] [Google Scholar]
- 28.Pisella PJ, Debbasch C, Hamard P, Creuzot-Garcher C, Rat P, Brignole F, Baudouin C. Conjunctival proinflammatory and proapoptotic effect of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45:1360–1368. doi: 10.1167/iovs.03-1067. [DOI] [PubMed] [Google Scholar]