Abstract
Regulation of the number of eggs ovulated by different mammalian species remains poorly understood. Here we show that oocyte-specific deletion at the primary follicle stage of core 1 β1,3galactosyltransferase (T-synthase; generates core 1-derived O-glycans), leads to a sustained increase in fertility. T-syn mutant females ovulated 30–50% more eggs and had a sustained increase in litter size compared to controls. Ovarian weights and follicle numbers were greater in mutants but follicular apoptosis was not decreased. The number of follicles entering the growing pool was unaltered and 3 week mutants ovulated less eggs suggesting that increased fertility results from prolonged follicle development. T-syn mutant ovaries also contained numerous multiple-oocyte follicles (MOFs) that appeared to form by adjacent, predominantly preantral, follicles joining - a new mechanism for MOF generation. Ovulation of multiple eggs from MOFs was not the reason for increased fertility based on ovulated egg and corpora lutea numbers. Thus the absence of T-synthase caused modified follicular development leading to the maturation and ovulation of more follicles, to MOF formation at late stages of folliculogenesis, and to increased fertility. These results identify novel roles for glycoprotein(s) from the oocyte as suppressor(s) of fertility and regulator(s) of follicular integrity in the mouse.
Keywords: Multiple-oocyte follicles, T-synthase, ovulation rate
Introduction
The number of eggs ovulated from the ovary limits fertility in mammals by undefined mechanisms. Ovulation is the end point of oogenesis which is initiated when a primordial follicle containing a single egg begins to grow. The pool of primordial follicles in the postnatal ovary is generally believed to be established prior to birth and to remain finite. After proliferation of primordial germ cells during embryogenesis, followed by a period of apoptosis, the development of remaining germ cells is temporarily suspended at the end of meiotic prophase I, a state that may last for weeks or years, depending on the species. After birth, primordial germ cells continuously leave the pool of meiotically quiescent germ cells and resume development until the pool is exhausted. In mice, it takes 2–3 weeks for a primordial follicle to progress through primary, secondary, preantral, antral and preovulatory stages as defined by morphological criteria (1). Some follicles will not complete folliculogenesis and will undergo apoptosis and die. The estrus cycle in the mouse is ~4 days and thus many stages of follicle development are proceeding in the postpubertal ovary at any time.
The number of follicles that attain preovulatory status and subsequently ovulate, is tightly regulated in a species-specific manner, by ill-defined mechanisms. Growth differentiation factor-9 (GDF-9) and bone morphogenetic protein-15 (BMP-15) are oocyte-specific glycoproteins that play a role in the regulation of ovulation rate in sheep. Haploinsufficiency of BMP-15 (2), the BMP-15 receptor BMPR1B, also known as ALK-6 (3–5), or GDF-9 (6) leads to increased fertility in sheep. However, homozygosity for inactivating mutations of BMP-15, GDF-9 or BMPR1B in sheep results in sterility. By contrast, in female mice heterozygosity of either BMP-15 or GDF-9 has no phenotype but, similar to sheep, GDF-9−/− females are infertile and BMP-15−/−females have decreased fertility (7, 8). In this paper we show that a sustained increase in fertility results from removal of core 1-derived O-glycans from oocyte glycoproteins at the primary follicle stage.
Core 1-derived O-glycans are attached to Ser or Thr residues in glycoproteins. They are critical for embryonic development after day 12.5 of gestation (E12.5) (9). The core 1 O-glycan is initiated by the transfer of N-acetylgalactosamine (GalNAc) to Ser or Thr to generate GalNAcα1-Ser/Thr which is extended with Gal by the enzyme core 1 β1,3galactosyltransferase (C1β3GalT-1 or T-synthase) (10, 11) to generate Galβ1-3GalNAcα1-Ser/Thr, also known as the T-antigen (Fig. 1A). Core 2 O-glycans are initiated by the transfer of N-acetylglucosamine (GlcNAc) to core 1 O-glycans (Fig. 1A). We previously generated females lacking core 1-derived O-glycans specifically in oocytes by deletion of T-synthase using a Cre recombinase transgene under the control of the zona pellucida protein 3 (ZP3) promoter (Fig. 1B), and showed that T-synF/F:ZP3Cre females are fertile and produce more pups than controls (12).
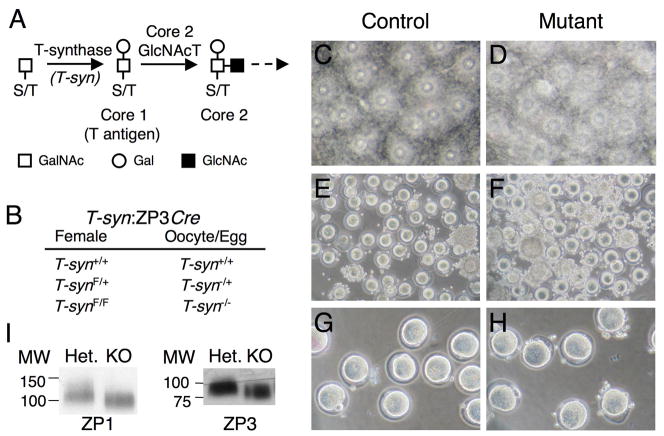
Fig. 1.
Oocyte-specific ablation of T-synthase. (A) Diagram of the synthesis of core 1 and 2 O-glycans. Elimination of T-synthase abrogates the synthesis of both but does not alter the transfer of GalNAc to O-glycan sites on glycoproteins. (B) Relation of genotype of females to genotype of oocytes and ovulated eggs (F=floxed). (C-D) Altered cumulus morphology in the absence of core 1-derived O-glycans. The cumulus-egg mass obtained from superovulated T-synF/F:ZP3Cre females was more dense than controls as seen when allowed to flattened. Both control and mutant images contain approximately the same number of eggs. (E-F) Cumulus cells surrounding mutant eggs were more resistant to removal by hyaluronidase. (G-H) The ZP on mutant eggs appeared thinner as noted previously (12) and confirmed by measurement (see text). (I) Western analysis of 20 egg/cumulus complexes per lane using antibodies to ZP1 and ZP3 showed no decrease in ZP glycoprotein content in mutant eggs. The blot was stripped after ZP1 detection and reprobed for ZP3.
We show here that the fertility of females with oocytes lacking core 1-derived O-glycans is sustained and markedly increased up to at least 6 months of age due to an increase in follicle numbers and the ovulation of more eggs. This is a novel phenotype that is not due to a decrease in apoptosis but appears to reflect prolonged follicular development. In addition, T-syn mutant female ovaries contain multiple-oocyte follicles (MOFs) that are generated late in follicular development; another phenotype that has not previously been reported. The combined data reveal an important role for core 1-derived O-glycans of oocyte glycoprotein(s) in the regulation of follicular development and for the mouse oocyte in the suppression of fertility.
Materials and Methods
Mice
All animal studies were approved by the Animal Institute Committee of the Albert Einstein College of Medicine. Oocyte-specific deletion of T-synthase was achieved using females of a mixed genetic background carrying a floxed T-synthase gene, (exons 1 and 2), termed T-synF (9), and a Cre recombinase transgene under the control of the ZP3 promoter (12). Oocyte–specific deletion of T-synF occurs when ZP3 is expressed exclusively by the oocyte early in oogenesis after follicles leave the quiescent pool from the primary stage onwards (13, 14). Homozygous floxed mutant females (T-synF/F:ZP3Cre), heterozygous (T-synF/+:ZP3Cre) and wildtype control females or mutant and control littermates were compared. The ZP3Cre recombinase transgene has no effect on fertility (12, 15) and hence T-syn+/+:ZP3Cre and T-syn+/+,F/+ or F/F were used as controls. Genotyping was performed by PCR of tail genomic DNA (12).
Collection of eggs after administration of exogenous gonadotrophins
To collect ovulated eggs or determine the superovulation rate, females were induced to ovulate by intraperitoneal injection of 5 IU pregnant mare’s serum gonadotrophin (Calbiochem, EMD Chemicals, Inc. San Diego, CA, USA) followed 46–48 h later by 5 IU human chorionic gonadotrophin (hCG; Sigma-Aldrich Corp.). After 14–15 h, oviducts were dissected and placed into M2 medium that had been equilibriated overnight at 37°C with 5% CO2 and air. The oviducts were opened to release the cumulus mass into the medium. The density of the cumulus mass matrix was visualized by removing medium to allow spreading on the dish prior to photography. Cumulus cells were removed from eggs by incubating the cumulus mass in 500 μl M2 medium (Sigma-Aldrich Corp., St. Louis, MO, USA) with 0.3 mg/ml hyaluronidase (Sigma-Aldrich Corp.) containing protease inhibitors (Roche, Indianapolis, IN, USA). During the ~5 min incubation, eggs were gently agitated by pipetting up and down to remove loosely adherent cumulus cells. Eggs were then washed by transferring through three droplets of M2 medium, counted and photographed. The thickness of zona pellucidae was determined using NIH Image J. To convert zona thickness from pixel measurements in Image J to in μm, the average value of the wildtype zona thickness, 13.06 ±1.36 pixels, was ascribed the value of 6.2 μm, the published thickness of mouse zona pellucida (16).
Assessment of fertility
To determine litter size, T-syn+/+:ZP3Cre control and T-synF/F:ZP3Cre mutant females were joined with C57BL/6 males at 6 weeks of age. Each pair of breeding mice remained together for the duration of the breeding experiment. Litter sizes and dates of birth were recorded. Pups were genotyped and weaned prior to delivery of the next litter. At ~6 months, males were removed and females were superovulated (as described above) at ~7 months when their last litter had been weaned. To examine littermates, pairs of control (T-synF/F) and experimental (T-synF/F:ZP3Cre) virgin littermate females (aged 6 weeks to 4.5 months) were joined with a single male per pair. Females were separated into individual cages prior to birth and first litter size was analysed from littermate females that birthed within two days of each other. This ensured that all data were collected from littermate pairs that did not differ by >2 days in age or time spent with the male. The number of eggs naturally ovulated was determined in control and T-synF/F:ZP3Cre mutant females. Since mating only occurs in mice when the female is ovulating, females were joined with C57BL/6 males and checked every morning for a vaginal plug. If a plug was present, females were dissected. Oviducts containing ovulated and potentially fertilized eggs, referred to hereafter as eggs, were removed and the cumulus-egg mass released into a dish containing M2 medium with 0.3 mg/ml hyaluronidase. Cumulus cells were released after ~5 min digestion at room temperature, and eggs counted.
Ovary histology
To examine ovarian morphology, ovaries were collected from unstimulated 3 and 6 week females and weighed before fixation in 10% buffered formalin (Sigma-Aldrich Corp.), 4% paraformaldehyde or Bouin’s fixative for 6–8 h at room temperature. All ovary weights were determined by the same person. A portion of the uterus, the oviduct and the attached ovary were dissected out and transferred into phosphate buffered saline. The ovary was dissected out using a microscope, the ovaries carefully picked up with forceps, fleetingly touched onto tissue to remove excess fluid and weighed on a microbalance. Fixed ovaries were paraffin-embedded, 5 μm sections were cut and stained with Hematoxylin and Eosin (H&E).
Follicle counts
To determine follicle numbers, ovaries from 3 week T-syn mutant and control females of matched body weight were fixed and serially sectioned at 5 μm. All sections were collected, every tenth was stained with H&E, photographed, printed, and follicles counted if the germinal vesicle was visible referring to the original sections when required. To morphologically determine the developmental stage of each follicle the same follicle was identified in several neighboring sections. Once the stage of development had been ascertained, all sections containing the follicle were marked to ensure that that follicle would not be counted again. Follicles were classified based on the Pedersen and Peters morphological criteria (1) as: primary (a complete layer of cuboidal granulosa cells surrounding the oocyte), secondary (two complete layers of granulosa cells), preantral (multiple granulosa cell layers but no antrum), antral (multiple granulosa cell layers with some antral space), atretic preantral and atretic antral (signs of atresia included detached or pycnotic granulosa cells, or oocyte blebbing). In order to avoid potential bias, sections were systematically analysed in order by counting all follicles in one slide from all ovaries before moving onto the second slide from each ovary, and counts were performed blinded. Follicle numbers were corrected to represent the whole ovary by multiplying by 10.
Apoptosis assay
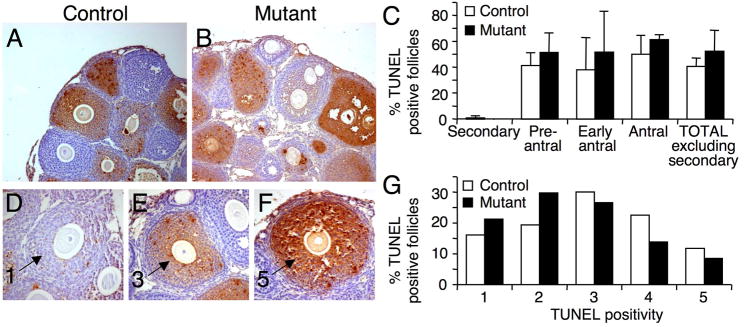
Apoptosis was detected using TUNEL staining (Apoptag kit; Chemicon, Temecula, CA, USA) on 10% buffered formalin fixed ovary sections (6–7 sections per ovary at least 30 μm apart). All follicles where the oocyte was visible were developmentally staged (as described above) and apoptosis was quantified using an arbitrary 1–5 scale. Follicles with the maximum number of TUNEL positive cells combined with morphological abnormalities such as oocyte blebbing and granulosa detachment that still maintained a spherical shape were classified as a ‘5’, and follicles with only a single TUNEL positive cell were classified as a ‘1’. All follicles in between were classified according to the relative number of TUNEL positive cells.
Western analysis
Ovulated eggs were collected after superovulation as described above, SDS-PAGE sample buffer was added, and the lysate separated on a 4–20% Tris gradient gel (Bio-Rad, Hercules, CA, USA) under reducing conditions. Protein was transferred to polyvinylidene fluoride membrane that was probed with antibodies to ZP1 followed by stripping and ZP3 detection, or ZP2 detection as described (15).
Multiple-oocyte follicle (MOF) counts
MOFs were counted in 5 μm serial sections representing 300 μm of ovary starting at a depth of 500 μm. Serial sections were used to identify MOFs as two or more oocytes that enabled identification of each MOF were not always visible in the same section. All 60 ovary sections in the 300 μm were photographed and printed. All follicles were tracked through the 300 μm of ovary looking at the original ovarian sections to ascertain fine detail whenever required. All follicles that contained more than one oocyte were counted and the stage of development noted. Once the presence of a MOF was confirmed, all sections containing that MOF were marked to ensure no MOF was counted more than once.
Ovulation to implantation ratio
To determine if MOFs were ovulating and contributing to elevated fertility, the number of eggs naturally ovulated (obtained on day 1 post-coitum as described above) was compared to the number of follicles that ovulated by counting the number of corpora lutea (CL) on day 6 post-coitum. CL develop from the theca and granulosa cells remaining after ovulation and thus each CL represents an ovulated follicle. To determine CL numbers, females were mated to C57BL/6 males, checked daily for vaginal plugs, dissected on day 6 post-coitum, and the number of CL in the ovary were dissected under a low power microscope and counted. The number of implanted embryos was also noted in the same females on day 6 post-coitum in order to determine the functional competence of the eggs ovulated.
Statistical analyses
All counts were carried out blinded without the observer being aware of genotype. All values are mean ± STDEV. Statistical significance was determined by two-tailed unpaired t-tests using Microsoft Excel Data Analysis Package. Distribution of the TUNEL positive follicles was analysed using a Chi-square test at http://www.graphpad.com/quickcalcs/chisquared2.cfm.
Results
Altered cumulus mass morphology in the absence of core 1-derived O-glycans
Cumulus masses of eggs superovulated from T-synF/F:ZP3Cre females were more dense than those from controls (Fig. 1C–D). This morphological difference was consistent with the resistance of the cumulus mass from T-synF/F:ZP3Cre females to digestion with hyaluronidase (Fig. 1E–F) indicative of an irregularity in cumulus expansion. Prolonged incubation up to 20 min in hyaluronidase did not aid the removal of cumulus cells that remained attached to mutant eggs. However, cumulus mass and egg morphology was not different between 3 week and postpubertal mice of either genotype. The ZP of mutant eggs was thinner as previously noted (12) by ~25% (P<0.0001; 6.2 ±0.6 μm, n=14 versus 4.7 ±0.8 μm, n=31) (Fig. 1G–H). However, western analysis of equal numbers of eggs did not detect any reduction in the amount of ZP1 or ZP3 in eggs lacking T-synthase (Fig. 1I). As expected, ZP1 and ZP3 from mutant eggs migrated faster consistent with the loss of core 1–derived O-glycans (12). ZP2 lacks core 1-O-glycans (17, 18) and thus migration of ZP2 from T-syn mutant eggs was unaltered compared to control (data not shown).
The increased fertility of T-synF/F:ZP3Cre females is sustained
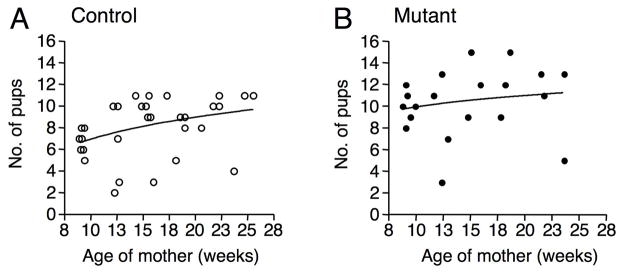
We previously noted that T-syn mutant females had more pups than controls (12). To investigate further, the fertility of T-syn mutant females was assessed for up to 6 months of age. The first litters of ~9 week T-syn mutant females were ~50% larger than controls (P<0.001; Table 1). This was also observed with 7 littermate pairs (P<0.05; Table 1). Consistent with the increased fertility of T-syn mutant females, the number of eggs naturally ovulated was elevated by ~43% compared to controls (P<0.0005; Table 1) which was also observed for mutant versus control littermates (~30%; P<0.0005; Table 1). Furthermore, increased litter size was maintained until at least 6 months of age (Fig. 2A–B). As female mice age, control litter size increases (Fig. 2A). Nevertheless, the elevated fertility of T-syn mutants was maintained with age as females older than 5 months ovulated more eggs than littermate controls (P<0.0005; controls: 12.1 ±1.3 versus mutants: 16.4 ±1.9, n=7 extracted from Table 1). There is a physiological limit of ~20 pups that a female can carry to term. However, the increase in mutant litter size is not tempered by physiological constraints because the number of naturally ovulated mutant eggs was <20. However, the time to first litter (21.1±1.6 days (n=7 controls) versus 21.7±1.4 days (n=6 mutants)) and the time between litters (25.4 ± 8.2 (n=24 controls) versus 28.4 ± 9.6 (n=16 mutants)) did not differ between control and T-syn mutant females, respectively.
Table 1.
T-syn mutant females have increased fertility.
| Control | T-syn Mutant | |
|---|---|---|
| Pups in first litter* | 6.7 ± 1.1a (9.1 ± 0.2; n=7) | 10.0 ± 1.4b (9.2 ± 0.4; n=6) |
| Pups in first litter (littermate pairs) | 7.3 ± 1.3c (16.1 ± 5.3; n=7) | 10.1 ± 2.4d (16.2 ± 5.3; n=7) |
| Eggs naturally ovulated | 8.2 ± 2.3e (9.1 ± 1.2; n=18) | 11.7 ± 1.5f (8.1 ± 2.9; n=10) |
| Eggs naturally ovulated (littermate pairs) | 12.1 ± 1.2g (22.5 ± 5.8; n=10) | 15.7 ± 2.3h (22.5 ± 5.6; n=10) |
Data are mean ± STDEV. Age of females in weeks in parentheses. a versus b; P<0.001, c versus d; P<0.05, e versus f; P<0.0005, and g versus h; P<0.0005.
These data were extracted from accumulated numbers of pups previously published (12).
Fig. 2.
Increased fertility is maintained in T-syn mutant females. (A-B) T-syn+/+:ZP3Cre control (n=7) and T-synF/F:ZP3Cre mutant (n=6) females were mated with C57BL/6 males and pups from all litters produced over the next ~4.5 months were counted (controls, 31 litters; mutants, 20 litters). The trendline is logarithmic.
T-synF/F:ZP3Cre female ovaries contain more follicles
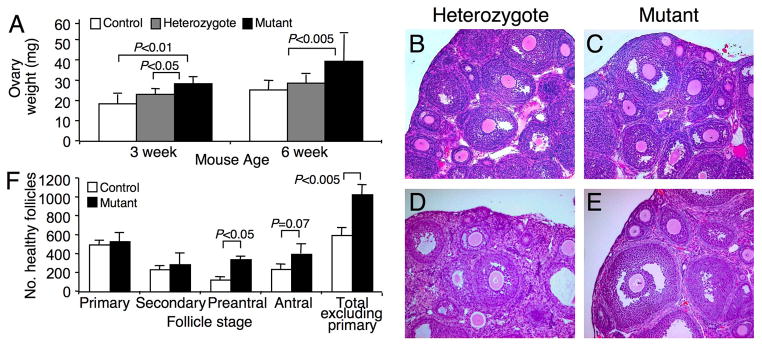
To determine if ovarian morphology and follicle numbers were altered in T-syn mutant females, ovarian weights were determined and follicle numbers counted in ovarian sections. Ovaries were ~55% heavier in T-synF/F:ZP3Cre females at both 3 weeks (prepubertal) and 6 weeks (postpubertal) (Fig. 3A), and as a percentage of body weight which did not differ between genotypes (data not shown). T-syn mutant ovary morphology was grossly normal with follicles present at all expected stages of development at both 3 and 6 weeks of age (Fig. 3B–E). Three week females were used to determine follicle counts because ovarian weights were significantly increased at this age, and synchronisation with exogenous gonadotrophins, which would be required in postpubertal females, did not result in significantly increased numbers of eggs ovulated in contrast to natural ovulation by postpubertal mutant females (see Fig 5A). There were ~70% more morphologically healthy follicles in T-syn mutant ovaries at 3 weeks (Fig. 3F). This was significantly increased for preantral stage follicles and for total follicle counts excluding primary follicles. As expected, no differences were observed at the primary follicle stage when the ZP3Cre recombinase is initially expressed (13) and T-syn is deleted. The total number of morphologically atretic follicles was not different from controls when the number of atretic follicles was expressed as a percentage of the total follicle number (control; 6.83 ±5.25, mutant; 8.13 ±1.74).
Fig. 3.
Ovary morphology in T-synF/F:ZP3Cre females. (A) Ovary weights of unstimulated females at 3 and 6 weeks (n=4–6). (B-C) Ovary sections (5 μm) stained with H&E from 3 week females. (D-E) Ovary sections (5 μm) stained with H&E from 6 week females. (F) The number of morphologically healthy follicles with a visible nucleus were counted in every 10th section of 3 week ovaries and staged (numbers multiplied by 10, n=3 mice).
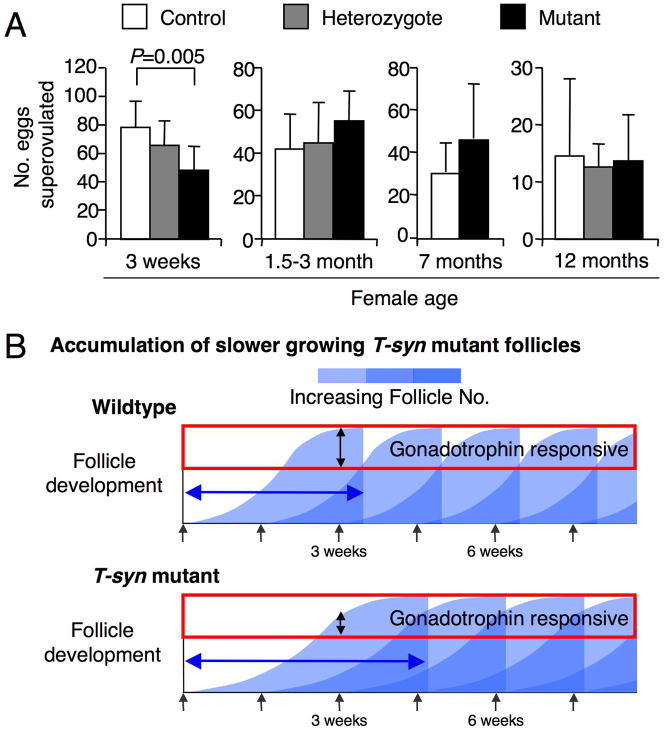
Fig. 5.
Superovulation rates of control, T-synF/+:ZP3Cre (Heterozygote), and T-synF/F:ZP3Cre (Mutant) females at different ages. (A) The number of eggs obtained from mutants after superovulation was not significantly higher than controls in previously bred 7 month (P=0.18; T-syn++:ZP3Cre, n=7; T-synF/F:ZP3Cre, n=6) or 12 month females (control, n=3; T-synF/+:ZP3Cre, n=3, T-synF/F:ZP3Cre, n=4), nor was it different in younger postpubertal T-synF/F:ZP3Cre females (P=0.08; control, n=24; T-synF/+:ZP3Cre, n=13; T-synF/F:ZP3Cre, n=6). However, the number of eggs obtained from prepubertal mutant females was significantly lower than wildtype (wildtype, n=10; T-synF/+:ZP3Cre, n=3; T-synF/F:ZP3Cre, n=6). (B) Proposed model of prolonged T-syn mutant follicle development. Entry of a cohort of primordial follicles into the growing pool (gray arrows beneath the x-axis) remains the same for T-syn mutants and controls. Follicle development (blue curve) in T-syn mutants takes longer (blue arrow). Prolonged follicle development in T-syn mutants results in follicles accumulating, particularly gonadotrophin-responsive follicles. Thus, prolonged follicle development results in less gonadotrophin- responsive follicles at 3 weeks of age but more postpubertally.
Apoptosis is not decreased in T-synF/F:ZP3Cre ovaries
To determine the number of atretic ovarian follicles, apoptosis was examined in 3 week females using the TUNEL assay on 6–7 randomly selected sections per ovary (5 μm) that were greater than 30 μm apart (Fig. 4A–B). The percent of TUNEL-positive apoptotic follicles at each stage of development, or the total follicle number, were not different in T-synF/F:ZP3Cre females compared to controls (controls (n=4) 4 ovaries, 293 follicles; mutants (n=3) 3 ovaries, 263 follicles; Fig. 4C). This was consistent with atretic follicle counts based on morphology. Because the percent of TUNEL-positive follicles at each stage of development in T-syn mutant ovaries was the same, all TUNEL-positive follicles were combined and assessed for TUNEL staining on an arbitrary scale of 1–5 (control; n=93, mutant; n=94, Fig. 4D–F). The distribution of follicles in each category was significantly different by chi-squared test (Fig. 4G, P<0.05). A greater proportion of T-syn mutant follicles had lower levels of apoptosis compared to controls indicating that the rate of follicle death is slowed in T-syn mutant follicles.
Fig. 4.
Apoptosis in ovaries of T-synF/F:ZP3Cre females. (A-B) Follicles undergoing apoptosis with cells staining TUNEL-positive (brown). (C) Percent TUNEL-positive follicles at each stage of development. (D-F) TUNEL-positive follicles were classified according to the number of apoptotic cells on an arbitrary scale of 1–5. Follicles scored as 1, 3 and 5 are shown (arrows). (G) The distribution of TUNEL-positive follicles in each category was significantly different in T-syn mutant females compared to controls (P<0.05).
Superovulatory response
To determine if the additional follicles were able to respond to superovulation, females of different ages were treated with exogenous gonadotrophins. There appeared to be a trend for 1.5–7 month mutant females to ovulate more eggs than controls but this difference was not significant (Fig. 5A). All females that had been previously bred (see above) responded at least as well as controls to superovulation at ~7 months as did 12 month old females (Fig. 5A), demonstrating that fertility of older T-syn mutant females was not decreased by the previous months of high fertility. By contrast, superovulation of 3 week T-syn females produced significantly fewer eggs than controls (P=0.005, Fig. 5A). This indicates that folliculogenesis is modified in prepubertal T-synF/F:ZP3Cre females such that either 48 hours of exogenous gonadotrophin stimulation is inadequate for follicle maturation, or hCG is unable to initiate the ovulation of all follicles. The combined data suggest the model of prolonged folliculogenesis in mutant females shown in Fig 5B and is discussed in the Discussion.
The absence of core 1-derived O-glycans in the oocyte leads to multiple-oocyte follicles
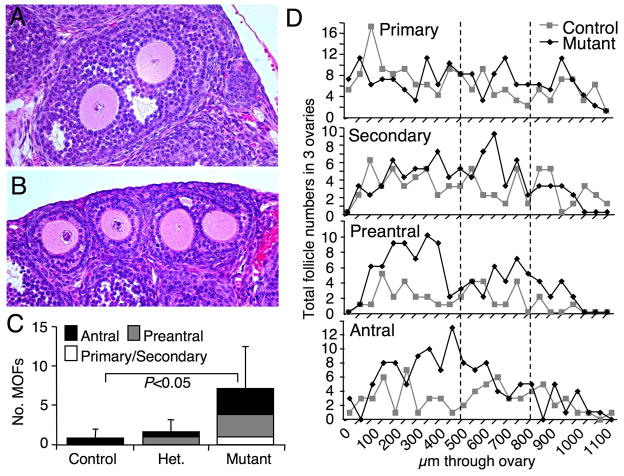
In examining ovarian morphology, multiple-oocyte follicles (MOFs) were observed in both 3 and 6 week T-synF/F:ZP3Cre females (Fig. 6A–B), a phenomenon rarely seen in wildtype ovaries. MOFs were counted and their stage of development determined in 60 consecutive 5 μm sections representing 300 μm of ovarian tissue from 3 week females. Ovaries from T-synF/F:ZP3Cre females had ~9 times as many MOFs as control ovaries (7.17 ± 5.38, n=6 versus 0.83 ± 1.17, n=6 (P<0.05); Fig. 6C). Because the presence of MOFs can vary with genetic background (19), MOFs were also counted in littermate pairs. Additional T-synF/F:ZP3Cre mutants aged 3 to 8.5 weeks contained ~8 times the MOFs present in littermate controls (1.60 ± 1.95 versus 0.20 ± 0.45; n=5). The reduced overall number of MOFs in littermate ovaries compared to 3 week ovaries was due to the presence of corpora lutea and less total follicles in older females (4 of 5 pairs). The one 3 week littermate pair contained 1 MOF in control and 5 MOFs in mutant ovary.
Fig. 6.
Multiple-oocyte follicles (MOFs) in T-synF/F:ZP3Cre female ovaries. (A-B) Examples of MOFs present in H&E stained ovarian sections from T-synF/F:ZP3Cre females including an early antral MOF (A), and a secondary stage MOF beside two separate secondary stage follicles (B). (C) MOFs were counted and staged in 60 consecutive 5 μm sections of 3 week ovaries (control, n=6; T-synF/+:ZP3Cre, n=3; T-synF/F:ZP3Cre, n=6) starting at a depth of 500 μm (the area between the two broken lines in D). (D) Total number of follicles at each stage counted in every 10th section of a subset of 3 ovaries from 3 females used for MOF counts in C (presented as total ovary follicle counts in Fig. 3H). Follicle numbers (D) indicate that all stages of development are equally present in the sections analysed for MOFs (area between the two broken lines).
MOF numbers in heterozygous T-synF/+:ZP3Cre 3 week ovaries lay between the numbers in control and T-synF/F:ZP3Cre ovaries (1.67 ± 1.53, n=3) as observed for ovarian weights (Fig. 3A) and numbers of eggs after superovulation (Fig. 5A). These data imply that heterozygosity of the T-synthase enzyme results in a weaker version of the phenotype observed with complete ablation of T-synthase which is unusual for heterozygous expression of a glycosyltransferase (20, 21).
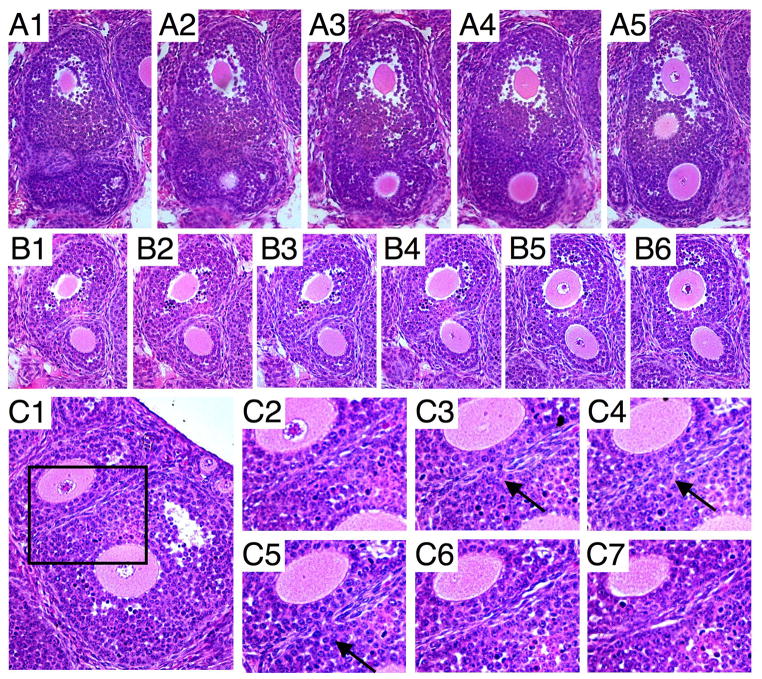
Surprisingly, the vast majority of MOFs were well-developed with multiple layers of granulosa cells or containing an antrum (Fig. 6C), indicating that MOFs were being generated late in follicle development from adjacent follicles. This is in contrast to MOF generation due to aberrant follicle nest breakdown, the only mechanism currently described for MOF formation (22). Follicles of all stages of development were equally represented in the 3 week ovary sections examined, eliminating the possibility that more antral MOFs were counted due to an uneven distribution of follicle stages (Fig. 6D). However, being adjacent is not all that is required as seen in Fig. 6B which contains a secondary stage MOF and two secondary stage follicles that are adjacent but clearly not joined. Some MOFs appeared to be mainly spherical or oval with a continuous follicle wall, as seen in follicles with a single oocyte (Fig. 6A–B). On the other hand, some MOFs appeared as two separate spheres joined together (Fig. 7A5, B6). Indeed, sequential sections revealed some MOFs with very irregular follicle boundaries (Fig. 7A–B), and two follicles for which the adjoining boundary was only just breached (Fig. 7C1–7). In addition, it appears that follicles do not need to be at the same stage of development for MOFs to be generated as seen in Fig. 7B6 where a secondary follicle is joined with a preantral follicle.
Fig. 7.
Generation of MOFs at later stages of follicle development in T-synF/F:ZP3Cre females is shown using serial ovarian sections stained with H&E. (A1-5, B1-6) Examples of two MOFs with irregular boundaries indicative of follicles joining. (C1-7) Serial sections of two adjacent follicles with a breached barrier showing mixing of the granulosa cells (arrows).
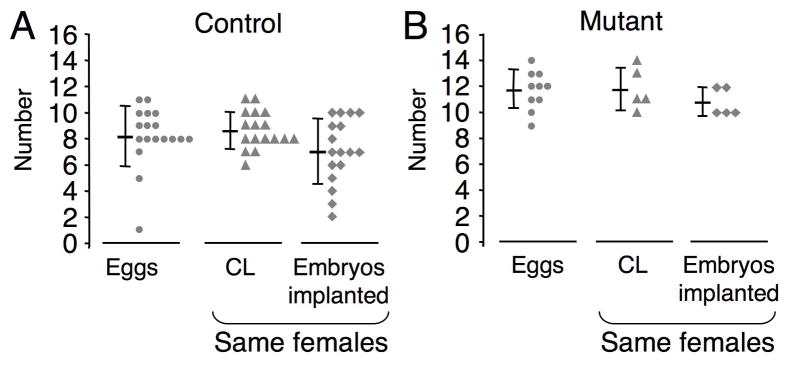
MOFs do not contribute to increased fertility
To determine if MOFs were contributing to the elevated fertility of T-synF/F:ZP3Cre females, the number of eggs naturally ovulated was determined by counting eggs the morning after ovulation (Table 1), and by counting CL and implanted embryos on day 6 post-coitum. CL develop from the theca and granulosa cells that remain after ovulation and thus each CL represents an ovulated follicle. In T-synF/F:ZP3Cre females the number of eggs ovulated was no more than the number of CL, and the number of implanted embryos was approximately equal to the number of CL (Fig. 8A–B). The ratio of implanted embryos to CL was the same for mutant (0.93 ± 0.12, n=5) and control females (0.81 ± 0.23, n=17), despite the increased ovulation rate in mutants. In addition, the number of implantation sites reflected litter size (Table 1 and Fig. 2A–B), demonstrating that ovulated mutant eggs are developmentally competent. Fertilization efficiency is also not decreased in T-syn mutant eggs since the ratio of implantation sites to eggs is equal for controls versus mutants (0.861 versus 0.874). Therefore ovulation of two or more eggs from a single follicle is not the reason for the elevated fertility in these females. The possibility exists that ovulation of an incompletely joined MOF in T-synF/F:ZP3Cre females might result in more than one CL. However, (i) the majority of MOFs were well joined (see Fig. 6A–B versus Fig. 7C; (ii) it is unlikely that partially joined MOFs (Fig. 7A–B) would form more than one CL as this would involve separation of follicle remnants after ovulation; and (iii) the increase in follicle numbers (Fig. 3F) do not support the likelihood that MOFs alone caused the increased fertility of T-syn mutant females.
Fig. 8.
Elevated fertility in T-synF/F:ZP3Cre females is not due to the ovulation of multiple eggs from multiple-oocyte follicles (MOFs). (A-B) Number of eggs naturally ovulated counted on the day post-coitum, and number of CL, which reflect the number of follicles ovulated, and the number of implanted embryos counted on day 6 post-coitum. The bar represents mean ± STDEV and each triangle, circle or diamond represents a data point from a single female. Eggs were collected at day 1 post-coitum (controls, 9.1 ± 1.2 weeks, n=18; T-synF/F:ZP3Cre, 8.1 ± 2.9 weeks, n=10), and CL and implanted embryo numbers were collected from the same mice on day 6 post-coitum (control, 7.7 ±1.7 weeks, n=17; T-synF/F:ZP3Cre, 6.8 ±0.6 weeks, n=5). Control CL versus mutant: P<0.0005, control eggs versus mutant: P<0.0005, control implanted embryos versus mutant: P=0.01.
Discussion
Female fertility in mammals is tightly regulated in a species-specific manner reflected in the number of eggs ovulated. In this paper we show that female mice with oocyte-specific deletion of T-Syn (12) have more functional follicles in the ovary and naturally ovulate ~30–50% more eggs than controls. Mutant eggs are developmentally competent resulting in larger litters. Enhanced fertility is maintained until at least 7 months of age. The absence of core 1-derived O-glycans in oocytes also leads to the generation of MOFs that appear to be formed by the joining of follicles predominantly at the preantral stage. However, the increase in fertility is not due to ovulation of MOFs because approximately equal numbers of CL, eggs and implanted embryos were observed in T-synF/F:ZP3Cre females. These data reveal novel roles for core 1-derived O-glycans on oocyte glycoproteins in female reproduction - firstly as suppressors of fertility and secondly as regulators of follicular integrity.
The increase in fertility in T-syn mutant mice suggests that one or more oocyte glycoprotein(s), that would normally possess core 1-derived O-glycans, have a role in regulating oogenesis and ovulation rate in the mouse. Other mutations that affect glycan synthesis do not lead to increased fertility. Oogenesis in mice lacking α1,3galactosyltransferase results in moderately altered O- and N-glycans, but the lack of the Galα1→3Gal epitope does not alter fertility (23). Mice lacking core 2 β-1,6-N-acetylglucosaminyltransferase type L do not synthesize core 2 O-glycans and are fertile (24). Females with oocyte-specific deletion of complex and hybrid N-glycans (15) or glycosylphosphatidylinositol anchors (25) have decreased fertility.
Increased female fertility has been observed in a few other mouse models but none exhibit a sustained elevation of fertility. Immature females lacking tumor necrosis factor receptor type 1 produce an increased number of eggs in response to superovulation, but this response declines to control levels at 8 weeks and falls below controls with increased age (26). This phenotype has been attributed to precocious follicular development and is the opposite phenotype to T-synF/F:ZP3Cre mice in which immature females have a decreased superovulation rate at 3 weeks but sustained elevated fertility post-pubertally. Young females overexpressing Bcl-2 also have higher ovulation rates and increased litter size, but this is due to decreased oocyte apoptosis (27). T-synF/F:ZP3Cre females exhibit no decrease in the numbers of follicles undergoing apoptosis. Older Bcl-2 transgenic females have an increased susceptibility to ovarian germ cell tumorigenesis (27) which was not seen in T-syn mutants. Female mice overexpressing growth hormone can have an elevated ovulation rate (28, 29) but more frequently are infertile (30). Female mice lacking anti-mullerian hormone have increased ovarian weight at 4 months due to increased numbers of developing follicles, but they exhibit no increase in ovulation rate (31). Therefore, of the mouse models generated to date, none have increased fertility that is maintained with age. In addition, none of the previously targeted proteins are expressed in the oocyte and therefore cannot be the basis for the elevated fertility of T-syn mutant females.
In T-syn mutant females, the number of follicles that resume meiosis and enter the growing pool is unchanged (Fig. 3F) as expected because deletion of T-syn does not occur until the primary stage, after follicles have resumed growth. Therefore the increase in follicle numbers in T-syn mutants must be due to altered follicular dynamics post-recruitment. A moderate prolonging of follicle development would cause an accumulation of follicles, potentially at the preantral stage (Fig. 3F), so that more would be available for subsequent ovulation. This is supported by a slowed rate of T-syn mutant follicle death since atretic follicles in T-syn mutant ovaries have lower numbers of apoptotic cells than controls (Fig. 4G). If the time for follicle development is prolonged, the number of follicles able to respond to exogenous gonadotrophins would be expected to be decreased early in ovarian development and indeed, prepubertal 3 week T-syn mutant females have a lower superovulation rate than controls (Fig. 5A). We propose that the absence of core 1-derived O-glycans on oocyte glycoproteins early in oogenesis leads to prolonged follicular development, allowing follicles to accumulate prior to ovulation (Fig. 5B). Prolonged growth of mutant follicles results in more follicles becoming follicle stimulating hormone (FSH)-independent and ovulating, leading to the observed increase in naturally ovulated eggs and litter size in T-syn mutant females. It should be noted that the number of preantral and antral follicles was not decreased in 3 week females, yet the response to exogenous gonadotrophins was lower than controls and therefore follicle function as well as rate of growth may be modified by the presence of a mutant oocyte.
Another novel feature of the T-syn mutant phenotype is the presence of MOFs. Mouse models that generate MOFs have been previously described but all have decreased fertility - the opposite to T-syn mutant females. MOFs are also not the reason for the enhanced fertility in T-syn mutant females, since we have shown that the number of eggs ovulated is approximately equal to the number of CL. MOFs have been observed in female mice treated neonatally with testosterone (32), diethylstilbestrol, estradiol (33) or the phytoestrogen genistein (34). In addition, a number of mouse mutants generate MOFs including BMP-15−/−, BMP-15−/−/GDF-9+/− (8), GCNF−/− (germ cell nuclear factor) (35), a negative repressor of BMP-15 and GDF-9), Cpeb (a sequence-specific RNA-binding protein) knockdown oocytes which results in reduced GDF-9 expression (36), Ahch−/− (Dax1) (37), FSH−/−/inhibin−/− (38), inhibin α-subunit overexpression (39), FSH-R+/− (FSH receptor) but not FSH-R−/− (40, 41), Lfng−/− (Lunatic Fringe) (42), and GHR/GHBP−/− (growth hormone receptor and binding protein) (43). Of these genes, only GCNF (44), Cpeb (36), BMP-15 (45), GDF-9 (46), and FSH-R (47) are known to be expressed in the oocyte, and only the latter three are glycoproteins that might carry O-glycans. Decreased function of the FSH-R is unlikely to be responsible for the T-syn mutant phenotype because, although ovaries from FSH-R+/− females have MOFs, these females have decreased fertility (48). In addition, a function for FSH-R in oocytes has not been described. The T-syn mutant phenotype cannot be due to inactive BMP-15 or GDF-9 because BMP-15−/− females have decreased fertility (8) and GDF-9 null mice are infertile (7). Furthermore, increased expression of BMP-15 and GDF-9 induced by deletion of GCNF also leads to decreased fertility (35). On the other hand, heterozygosity of GDF-9 or BMP-15, or the BMP-15 receptor, ALK-6, does lead to increased fertility in sheep (2–6). It is possible therefore that if mouse GDF-9 and/or BMP-15 are found to carry core 1-derived O-glycans, their modification in T-syn mutant oocytes may alter folliculogenesis leading to an increase in fertility as observed in sheep. In vitro assays of follicle growth and development provide an approach to identify oocyte glycoproteins responsible for the altered folliculogenesis in T-syn mutant females.
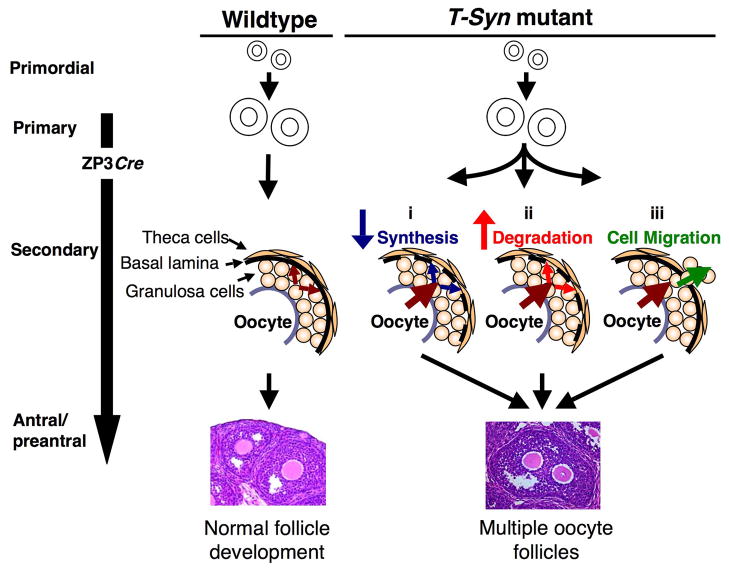
MOF generation has been attributed to aberrant breakdown of germ cell nests that normally occurs within a few days of birth (30, 49) and is associated with decreased fertility in mice. However, in the current study, deletion of T-syn occurs after the initiation of folliculogenesis when the ZP3 promoter becomes active, potentially months after the breakdown of germ cell nests. In fact, the majority of MOFs in T-synF/F:ZP3Cre ovaries exist at later stages of development and therefore the mechanism for the formation of MOFs appears to be the joining of adjacent follicles. Maturing follicles with breaches in the follicle wall have not previously been reported and support this hypothesis (Fig. 7C1–7). Three possibilities are proposed for MOF formation in Fig. 9: (i) decreased synthesis of extracellular matrix proteins that comprise the basal lamina, (ii) increased degradation of the basal lamina in excess of that required for normal remodeling during follicle growth, and (iii) aberrant initiation of cellular invasion by the granulosa cells resulting in destruction of the basal lamina and joining of adjacent follicles. The cells that secrete the extracellular matrix that comprises the basal lamina and the mechanisms that regulate generation of the basal lamina have not been elucidated (50), and if mechanisms (i) or (ii) operate, a role for oocyte glycoprotein(s) in basal lamina generation would be identified. Mechanism (iii) represents a new model for the study of cellular invasion that may be relevant to pathological states of tissue remodeling. Irrespective of the mechanism, it is clear that MOF formation later in folliculogenesis is not detrimental to fertility, unlike early MOF generation in other mouse models.
Fig. 9.
Models of MOF generation in T-synF/F:ZP3Cre females. Follicle development is normal until ZP3 promoter activation at the primary stage which results in deletion of T-syn by the ZP3Cre recombinase transgene. After deletion of T-syn, MOFs may form due to breaching of the basal lamina by: (i) glycoprotein changes in the oocyte leading to decreased secretion of ECM proteins either directly from the oocyte or from granulosa or theca cells stimulated by glycoprotein(s) of the oocyte; (ii) glycoprotein changes in the oocyte leading to increased breakdown of basal lamina that normally occurs during follicle growth by secreted proteases either directly from the oocyte or from granulosa or theca cells stimulated by glycoprotein(s) of the oocyte; or (iii) glycoprotein changes in the oocyte leading either directly or indirectly to aberrant initiation of granulosa cell invasion and destruction of the basal lamina.
In summary, oocyte-specific deletion of T-synthase generates a novel female fertility phenotype. By precluding the generation of core 1-derived O-glycans early in folliculogenesis we have revealed a regulatory role for core 1-derived O-glycans in follicular development. The lack of core 1-derived O-glycans in the oocyte leads to the ovulation of an increased number of eggs and to the generation of MOFs. However, the increase in egg number is not due to ovulation of MOFs nor to a decrease in apoptosis of follicles, but rather to an increased number of mature follicles at ovulation, most likely due to a prolonged rate for follicle development allowing accumulation of follicles. The eggs produced are all fertilized since the ovulation rate is equivalent to the number of implanted embryos or pups delivered. Thus, removal of core 1-derived O-glycans from the oocyte has revealed a new mechanism for the regulation of fertility in mice that may be relevant in other species.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Wen Dong, help from Jason Aglipay, advice from Radma Mahmood and Mimi Kim, discussions with Mark Stahl and comments on the manuscript from Anthony Michael and Paula Cohen. This work was supported by NCI grant RO1 30645 to PS and partial support was provided by the Einstein Cancer Center grant PO1 13330.
Abbreviations
- ZP
Zona pellucida
- FSH
follicle stimulating hormone
- MOF
multiple-oocyte follicles
- CL
corpora lutea
- GDF
growth differentiation factor
- BMP
bone morphogenetic protein
References
- 1.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 2.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 3.Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, Pisselet C, Riquet J, Monniaux D, Callebaut I, Cribiu E, Thimonier J, Teyssier J, Bodin L, Cognie Y, Chitour N, Elsen JM. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes. Proc Natl Acad Sci U S A. 2001;98:5104–5109. doi: 10.1073/pnas.091577598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza CJ, MacDougall C, MacDougall C, Campbell BK, McNeilly AS, Baird DT. The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J Endocrinol. 2001;169:R1–6. doi: 10.1677/joe.0.169r001. [DOI] [PubMed] [Google Scholar]
- 5.Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan JC, O’Connell AR, McNatty KP, Montgomery GW. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod. 2001;64:1225–1235. doi: 10.1095/biolreprod64.4.1225. [DOI] [PubMed] [Google Scholar]
- 6.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 8.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 9.Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 12.Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J Cell Sci. 2007;120:1341–1349. doi: 10.1242/jcs.004291. [DOI] [PubMed] [Google Scholar]
- 13.Philpott CC, Ringuette MJ, Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol. 1987;121:568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- 14.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 15.Shi S, Williams SA, Seppo A, Kurniawan H, Chen W, Ye Z, Marth JD, Stanley P. Inactivation of the Mgat1 gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol Cell Biol. 2004;24:9920–9929. doi: 10.1128/MCB.24.22.9920-9929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassarman PM, Qi H, Litscher ES. Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proc Biol Sci. 1997;264:323–328. doi: 10.1098/rspb.1997.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagdas SK, Araki Y, Chayko CA, Orgebin-Crist MC, Tulsiani DR. O-linked trisaccharide and N-linked poly-N-acetyllactosaminyl glycans are present on mouse ZP2 and ZP3. Biol Reprod. 1994;51:262–272. doi: 10.1095/biolreprod51.2.262. [DOI] [PubMed] [Google Scholar]
- 18.Boja ES, Hoodbhoy T, Fales HM, Dean J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J Biol Chem. 2003;278:34189–34202. doi: 10.1074/jbc.M304026200. [DOI] [PubMed] [Google Scholar]
- 19.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Stanley P. Roles of O-fucose glycans in notch signaling revealed by mutant mice. Methods Enzymol. 2006;417:127–136. doi: 10.1016/S0076-6879(06)17010-X. [DOI] [PubMed] [Google Scholar]
- 21.Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 22.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 23.Thall AD, Maly P, Lowe JB. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 1995;270:21437–21440. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- 24.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 25.Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J Cell Sci. 2003;116:2149–2155. doi: 10.1242/jcs.00430. [DOI] [PubMed] [Google Scholar]
- 26.Roby KF, Son DS, Terranova PF. Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol Reprod. 1999;61:1616–1621. doi: 10.1095/biolreprod61.6.1616. [DOI] [PubMed] [Google Scholar]
- 27.Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137:4837–4843. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- 28.Naar EM, Bartke A, Majumdar SS, Buonomo FC, Yun JS, Wagner TE. Fertility of transgenic female mice expressing bovine growth hormone or human growth hormone variant genes. Biol Reprod. 1991;45:178–187. doi: 10.1095/biolreprod45.1.178. [DOI] [PubMed] [Google Scholar]
- 29.Thomas AD, Murray JD, Famula TR, Oberbauer AM. Growth hormone and fertility in oMt1a-oGH transgenic mice. Reproduction. 2001;122:537–544. doi: 10.1530/rep.0.1220537. [DOI] [PubMed] [Google Scholar]
- 30.Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12:537–555. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- 31.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti- Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 32.Iguchi T, Todoroki R, Takasugi N, Petrow V. The effects of an aromatase inhibitor and a 5 alpha-reductase inhibitor upon the occurrence of polyovular follicles, persistent anovulation, and permanent vaginal stratification in mice treated neonatally with testosterone. Biol Reprod. 1988;39:689–697. doi: 10.1095/biolreprod39.3.689. [DOI] [PubMed] [Google Scholar]
- 33.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal Exposure to Estrogens Suppresses Activin Expression and Signaling in the Mouse Ovary. Endocrinology. 2007;148:1968–1976. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 34.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 35.Lan ZJ, Gu P, Xu X, Jackson KJ, DeMayo FJ, O’Malley BW, Cooney AJ. GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility. Embo J. 2003;22:4070–4081. doi: 10.1093/emboj/cdg405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racki WJ, Richter JD. CPEB controls oocyte growth and follicle development in the mouse. Development. 2006;133:4527–4537. doi: 10.1242/dev.02651. [DOI] [PubMed] [Google Scholar]
- 37.Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- 38.Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 39.McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- 40.Danilovich N, Sairam MR. Haploinsufficiency of the follicle-stimulating hormone receptor accelerates oocyte loss inducing early reproductive senescence and biological aging in mice. Biol Reprod. 2002;67:361–369. doi: 10.1095/biolreprod67.2.361. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Balla A, Danilovich N, Sairam MR. Developmental and molecular aberrations associated with deterioration of oogenesis during complete or partial follicle-stimulating hormone receptor deficiency in mice. Biol Reprod. 2003;69:1294–1302. doi: 10.1095/biolreprod.103.015610. [DOI] [PubMed] [Google Scholar]
- 42.Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- 43.Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–532. doi: 10.1530/rep.1.00946. [DOI] [PubMed] [Google Scholar]
- 44.Chen F, Cooney AJ, Wang Y, Law SW, O’Malley BW. Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Mol Endocrinol. 1994;8:1434–1444. doi: 10.1210/mend.8.10.7854358. [DOI] [PubMed] [Google Scholar]
- 45.Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 46.McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 47.Patsoula E, Loutradis D, Drakakis P, Kallianidis K, Bletsa R, Michalas S. Expression of mRNA for the LH and FSH receptors in mouse oocytes and preimplantation embryos. Reproduction. 2001;121:455–461. doi: 10.1530/rep.0.1210455. [DOI] [PubMed] [Google Scholar]
- 48.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- 49.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 50.Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24:195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]