Abstract
Background: To assess the cost-effectiveness of trabectedin compared with end-stage treatment (EST) after failure with anthracycline and/or ifosfamide in metastatic soft tissue sarcoma (mSTS).
Design: Analysis was carried out using a probabilistic Markov model with trabectedin → EST and EST arms, three health states (stable disease, progressive disease and death) and a lifetime perspective (3% annual discount rate). Finnish resources (drugs, mSTS, adverse events and travelling) and costs (year 2008) were used. Efficacy was based on an indirect comparison of the STS-201 and European Organisation for Research and Treatment of Cancer trials. QLQ-C30 scale scores were mapped to 15D, Short Form 6D and EuroQol 5D utilities. The outcome measures were the cost-effectiveness acceptability frontier, incremental cost per life year gained (LYG) and quality-adjusted life year (QALY) gained and the expected value of perfect information (EVPI).
Results: Trabectedin → EST was associated with 14.0 (95% confidence interval 9.1–19.2) months longer survival, €36 778 higher costs (€32 816 using hospital price for trabectedin) and €31 590 (€28 192) incremental cost per LYG with an EVPI of €3008 (€3188) compared with EST. With a threshold of €50 000 per LYG, trabectedin → EST had 98.5% (98.2%) probability of being cost-effective. The incremental cost per QALY gained with trabectedin → EST was €42 633–47 735 (€37 992–42 819) compared with EST. The results were relatively insensitive to changes.
Conclusion: Trabectedin is a potentially cost-effective treatment of mSTS patients.
Keywords: cancer, economic evaluation, leiomyosarcoma, liposarcoma, quality of life, trabectedin
key findings
Trabectedin improved survival significantly. Trabectedin followed by end-stage treatment (EST) was estimated to result to 14 months of additional survival and 9–10 months of additional quality-adjusted survival [quality-adjusted life years (QALY) gained] compared with EST alone in metastatic soft tissue sarcoma (mSTS) patients who have previously received anthracycline and/or ifosfamide. Trabectedin had an impact on mortality that continued beyond the active trabectedin treatment.
Trabectedin was a potentially cost-effective second-line treatment of mSTS. Trabectedin resulted to €31 590 (€28 192 using hospital price for trabectedin) incremental cost per additional year of life gained and to €42 633–47 735 (€37 992–42 819) cost per additional QALY gained compared with EST. With a threshold of €50 000 per life year gained (LYG), trabectedin had 98.5% (98.2%) probability of being cost-effective.
Results were relatively insensitive to changes in the key parameters. Based on the maximum expected value of perfect information estimate of €3008 (€3188 using the hospital price for trabectedin), the value of additional parameter information is likely to be low.
background
Soft tissue sarcomas (STS) are rare tumours that account for ∼1% of all adult cancers. The annual incidence of STS is 1–3/100 000 [1]. Approximately 50%–80% of STS metastasise [2, 3]. Complete surgical resection is rarely accomplished. Therefore, the main treatment of mSTS is systemic chemotherapy (CT) [1, 2, 4], but the role of (neo)adjuvant CT has remained controversial [2]. The standard first-line treatments for mSTS are anthracycline and ifosfamide alone or in combination (A/I [1, 5]). After progression of STS, there are currently no standard therapies.
Trabectedin (TRA) induces tumour regression and inhibits tumour growth [6]. TRA has produced clinical benefits in ovarian cancer, breast cancer and sarcoma [7]. Recent studies have shown that TRA arrests STS growth in ∼40%–60% of tumours [8–11] and produces significant clinical benefit in patients with STS progression after A/I [9]. At doses used clinically, TRA is well tolerated [12]. The safety and efficacy of TRA shown in clinical trials have been confirmed in compassionate use [8].
The objective of this study was to present the cost-effectiveness of TRA followed by EST (TRA → EST) against EST alone as second-line treatments in patients with STS. The results were presented as incremental cost-effectiveness ratios (ICER), probability of cost-effectiveness and the value of information (VOI). ICER is defined as
where 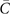 stands for average costs and
stands for average costs and 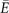 for average health benefits (subscripts indicate treatment; 1 is new and 0 is current). ICER characterises the marginal value of treatments in the form of additional cost per additional unit of health benefit gained or saved during given perspective. In this study, ICERs are produced separately for LYG and QALY gained in a lifelong perspective. The probability of cost-effectiveness was assessed with the cost-effectiveness acceptability frontier (CEAF) and VOI with the expected value of perfect information (EVPI).
for average health benefits (subscripts indicate treatment; 1 is new and 0 is current). ICER characterises the marginal value of treatments in the form of additional cost per additional unit of health benefit gained or saved during given perspective. In this study, ICERs are produced separately for LYG and QALY gained in a lifelong perspective. The probability of cost-effectiveness was assessed with the cost-effectiveness acceptability frontier (CEAF) and VOI with the expected value of perfect information (EVPI).
materials and methods
patients, treatments and timeframe
The modelled study population consisted of adult patients with mSTS who were previously treated with A/I. The population definition followed the indication of TRA.
Because current evidence does not support any other CTs for mSTS, TRA treatment (24-h infusion every 21 days) was compared with EST initiated immediately after failure with A/I. EST comprises multiple treatment alternatives. This approach was supported by oncological experts and the literature [1, 13].
Analysis was carried out from a health care payer perspective (productivity losses, income transfers and value added taxes were excluded) including costs of drugs, mSTS, serious adverse event (SAE) treatment and travelling. The analyses were based on a lifetime duration where the model was run for a total of 60 monthly cycles, i.e. 5 years.
model structure and simulation
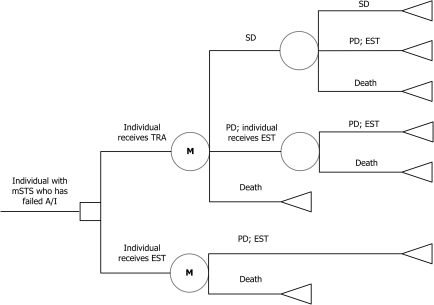
A simplified schematic picture of the model structure is presented in Figure 1. The model started at the point where the treatment with A/I failed. In the model, one cohort was treated with TRA and the other with EST. Patients on TRA could have a treatment response [have a stable disease (SD)], disease progression (PD) or they could die. Patients on EST stayed in PD until death.
Figure 1.
Model structure and health states. A/I, anthracycline and/or ifosfamide; EST, end-stage treatment; M, Markov chain; mSTS, mestastatic soft tissue sarcoma; PD, progressive disease; SD, stable disease; TRA, trabectedin.
The Excel model is based on a probabilistic approach (Monte Carlo simulation [14]) that allows the characterisation of multiple parameter uncertainty (i.e. distributions rather than simple means were modelled). The model applies monthly cycles due to the nature of clinical data. In reality, the treatment cycles of CTs are usually 3 weeks. Half-cycle correction was used (i.e. half of the costs and effects related to the last cycle were accrued, if patient changed health state), but CT-related costs were not corrected as the model assigned all drug costs in the first cycle.
efficacy data
The primary outcome of treatment efficacy was life expectancy. In the model, the efficacy differences were conveyed through transition probabilities. The clinical efficacy, i.e. transition probabilities for staying in a health state or transitioning to another health state, in the TRA arm were taken from STS-201 [9], while the transition probabilities used in the EST arm were obtained from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC STBSG) trial [15, 16] patient level data. The estimation of transition probabilities is presented in Appendix 1.
The use of the historical EORTC STBSG dataset for the EST arm had inherent limitations. However, those studies using ifosfamide [15, 16] were conducted with similar eligibility criteria and efficacy end points as STS-201. Other studies that investigated the efficacy and safety of drugs not approved for mSTS were not considered. Typically, mSTS studies were also hindered by poor follow-up (compare Le Cesne et al. [12]). Since the period of interest was the duration PD → death, poor follow-up could have biased the results. However, among sensitivity analyses, patients receiving active EST (33%) were assumed to experience disease stabilisation for 6 months based on dacarbazine (DAC) results [17]. This seems to be a fair estimate for active EST [2].
probabilistic parameters
The probabilistic approach examines parameter uncertainty using distributions around key parameters [14] in the model and relaxes the assumption of linearity typical for the deterministic (point estimate) approach. Specifically, the probabilistic approach included TRA cycles, TRA dose intensity and transition probabilities. The transition probabilities were averaged >1000 simulations to determine the mean transition probabilities [β, 95% confidence intervals (CIs)] for the TRA → EST arm: remaining SD 0.8476 (0.7741–0.9121), SD → PD 0.1457 (0.0840–0.2159), SD → death 0.0067 (0.0039–0.0100) and PD → death 0.1118 (0.0735–0.1570) and for the EST arm: remaining PD 0.8870 (0.8430–0.9265) and PD → death 0.1118 (0.0735–0.1570).
resource use and costs
Resources and costs are presented in detail in Appendix 2. TRA’s dose intensity was 1.17 mg/m2 in STS-201 and the average body surface area (BSA) was 1.80 m2, which results in 2.10 mg per administration (TRA 1.50 mg/m2 and BSA of 1.70 m2 were assumed in a sensitivity analysis scenario). Given the TRA vial sizes, patients were conservatively assumed to receive two 1-mg vials and one 0.25-mg vial per administration. This included drug wastage (0.15 mg), which can be avoided if infusions are given at the same time for many patients. Average drug cost based on the average of 5.0 TRA cycles in STS-201 was applied equally to all patients in the TRA arm in the first modelling cycle. Conservatively, patients who progress before five cycles with TRA will accrue five cycles worth of costs.
Despite the lack of approved CTs (except TRA) after A/I exhaustion, further CTs are commonly initiated [1, 13]. Conservatively, only 33% of patients were assumed to receive active CT during EST. The type of off-label CT administered at this stage varies based on, e.g., patient toleration and the histology of disease. In the base case, further CT was assumed to be based on etoposide (ETO) and DAC (67% and 33%, respectively). The impacts of these EST assumptions were tested in sensitivity analyses. In the EST arm, the excepted EST CT costs were applied to patients at the beginning of the model and, in the TRA → EST arm, the excepted EST CT costs were applied once patients transited to PD.
After A/I failure, patients consume a variety of resources [13]. There were no Finnish data available on the ongoing costs after A/I failure. Thus, costs of metastatic renal cell carcinoma (mRCC) treatment from two Finnish hospital settings [18] were used because these patients were also without efficient treatments, both mSTS and mRCC have similar progression patterns and imaging examinations and clinician visits of mSTS and mRCC patients seem to be relatively comparable [13, 18]. The sensitivity of this assumption was tested by using UK mSTS costs [13].
Finnish unit costs [19] indexed to the year 2008 using official health care index were used to estimate the administration costs of TRA and treatment of SAEs. Conservatively, all TRA patients were assumed to be treated on an inpatient basis in cancer clinic (outpatient administration is presented as a sensitivity analysis scenario) and the administration costs were applied in the first cycle of the model. Though TRA was well tolerated in STS-201, the model accounts for the cost impact of seven SAEs related to drugs that led to hospitalisation. Since vomiting and nausea were common, it was assumed that the cost of inpatient treatment of gastroenteritis was an appropriate proxy for the SAE treatment cost. No costs for treating any SAEs were applied in the EST arm and no death costs were assumed. The impacts of death and TRA SAE costs were explored among sensitivity analyses.
utilities
Incremental cost per LYG does not include the quality of survival, and thus, it falls short of a measure that can be more readily assessed against other health care interventions, such as the cost per QALY gained. A systematic search of the literature yielded no data on the generic quality of life (QoL) associated with the STS health states used in the model. Thus, the disease-specific scale score results of EORTC QoL questionnaire (QLQ-C30) by Poveda et al. [20] in mSTS population were mapped to 15D [21], Short Form 6D (SF-6D [22]) and EuroQol 5D (EQ-5D [23]) generic QoL index values using the ordinary least-squares regression equations on Kontodimopoulos et al. [24]. The numbers used in the estimation are presented in Appendix 3.
The average expected QoL indexes based on 15D, SF-6D and EQ-5D were 0.736, 0.668 and 0.654, respectively. QoL was set to 0 for death. SAEs produced by TRA were taken into account by assuming a disutility (QoL decrease) of 0.200 per SAE. No disutilities due to EST SAEs were included.
sensitivity analysis
As the base case analysis was probabilistic and explored the effect of multivariate parameter uncertainty, figures presenting simulated ICERs as cost-effectiveness plane (CEP) and CEAF based on the probabilistic sensitivity analysis were appealing. Percentile method was used to derive CIs from the 1000 probabilistic simulation results. CEAF illustrates the sensitivity of the conclusion of cost-effectiveness to various thresholds of acceptability that a payer may hold, recognises the uncertainty of the generated cost-effectiveness estimate and presents the optimal treatment options [25] that have the highest expected net monetary benefit (NMB = E × willingness to pay − C).
CEAF does not consider the consequences of a wrong decision or VOI. Thus, per patient EVPI was estimated [14, 25], which combines both the probability of wrong decision and the consequences of that wrong decision as NMB forgone. The EVPI estimate also represents the value of parameter uncertainty that could be resolved by acquiring additional research evidence for model parameters (i.e. how much would be reasonable to invest for a new study at patient level given the willingness to pay, if parameter uncertainty needs to be decreased and willingness to pay means the acceptable cost for benefit).
In addition, the following scenarios were simulated
Discounting not employed
Ongoing costs based on UK mSTS cost data from Judson et al. [13]: €2320.26 (€2511.76 in 2008 value) for 119 days (€633.22 per cycle in 2008 value, £1 = €1.153245)
Death costs included based on Judson et al. [13] mSTS cost data: €566.70 (€618.27 in 2008 value) per patient
0%, 20% or 50% receive active EST
TRA wholesale/hospital price (in hospital €1994.00 per 1 mg and €530.00 per 0.25 mg)
TRA as outpatient (ambulatory pumps)
TRA dose intensity of 1.50 mg/m2 and BSA of 1.70 m2
TRA SAE costs doubled
ETO monotherapy 120 mg/m2 days 1, 3 and 5; every 21 days as EST
DAC monotherapy 250 mg/m2 days 1–5; every 21 days as EST
IADIC; doxorubicin 50 mg/m2 day 1, ifosfamide 1 g/m2 with mesna 40 mg/kg days 1–5 and DAC 250 mg/m2 days 1–5; very 21 days as EST
IE; ifosfamide 1 g/m2 with mesna 40 mg/kg days 1–5, ETO 100 mg/m2 days 1, 3, 5, phenobarbital 100 mg p.o. days 0–6 and growth hormone; every 21 days as EST
IMVP-a6; ifosfamide 1 g/m2 with mesna 40 mg/kg days 1–5, ETO 100 mg/m2 days 1–3, methotrexate 30 mg/m2 days 3 and 10; every 21 days as EST
Patients on active EST (33%) experience disease stabilisation for 6 months [17] in both EST groups or in EST-only group
All patients treated with EST receive active treatment and EST alone patients experience disease stabilisation for 6 months [17]. Actually, this is TRA versus ETO/DAC setting, which conservatively assumes no additional benefit with active EST after TRA.
results
According to the STS-201 and EORTC STBSG data analysis, the transition probability of PD → death in TRA naive patients was higher than that of death for those patients who were treated with TRA. Once a TRA-treated patient progressed after SD, the probability of death increased markedly, but it remained lower than for those patients who were TRA naive.
effectiveness and costs
TRA → EST had both a statistically and clinically significant advantage over EST in terms of mean overall survival (OS): 21.1 (95% CI 16.2–26.4) versus 7.2 (95% CI 6.4–8.1) months, respectively, in mSTS patients who received priory A/I (Table 1). The extra months TRA → EST patients survived compared with EST patients were 14.0 (95% CI 9.1–19.2), i.e. 1.16 (95% CI 0.76–1.60) LYGs.
Table 1.
Results of base case cost-effectiveness, sensitivity and CUA
| Cost-effectiveness scenarios | Costs (€) |
Life years |
ICER | ||||
| TRA | EST | Incr. | TRA | EST | Incr. | ||
| Base case, TRA’s retail price (SE) | 44 346 (11 324) | 7568 (525) | 36 778 (11 431) | 1.760 (0.438) | 0.596 (0.072) | 1.164a (0.429) | 31 590 (13 444) |
| Base case, TRA’s hospital price (SE) | 40 384 (9676) | 7568 (525) | 32,816 (9695) | 1.760 (0.438) | 0.596 (0.072) | 1.164a (0.429) | 28 192 (11 591) |
| Undiscounted | 44 349 | 7618 | 36 732 | 1.795 | 0.603 | 1.192 | 30 807 |
| UK mSTS ongoing cost data | 44 657 | 7706 | 36 950 | 1.755 | 0.598 | 1.156 | 31 951 |
| Death cost data from UK included | 44 764 | 8138 | 36 626 | 1.764 | 0.595 | 1.169 | 31 330 |
| EST: 0% seek EST CT | 40 454 | 4106 | 36 347 | 1.764 | 0.598 | 1.166 | 31 162 |
| EST: 20% seek EST CT | 42 449 | 6185 | 36 264 | 1.753 | 0.597 | 1.156 | 31 363 |
| EST: 50% seek EST CT | 46 180 | 9295 | 36 885 | 1.760 | 0.595 | 1.165 | 31 653 |
| TRA: wholesale/hospital price | 40 384 | 7563 | 32 821 | 1.756 | 0.595 | 1.161 | 28 273 |
| TRA: given as outpatient | 39 033 | 5475 | 33 558 | 1.754 | 0.597 | 1.157 | 29 005 |
| TRA: 1.5 mg/m2, BSA 1.7 m2 | 49 589 | 7568 | 42 021 | 1.768 | 0.596 | 1.172 | 35 849 |
| TRA: SAE costs doubled | 44 526 | 7571 | 36 955 | 1.766 | 0.597 | 1.169 | 31 602 |
| EST: ETO monotherapy | 45 097 | 8420 | 36 677 | 1.765 | 0.599 | 1.166 | 31 442 |
| EST: DAC monotherapy | 42 201 | 5912 | 36 289 | 1.768 | 0.598 | 1.170 | 31 003 |
| EST: IADIC | 45 903 | 9157 | 36 746 | 1.758 | 0.593 | 1.165 | 31 550 |
| EST: IE | 47 822 | 10 991 | 36 831 | 1.765 | 0.597 | 1.168 | 31 539 |
| EST: IMVP-6a | 45 384 | 8703 | 36 681 | 1.767 | 0.596 | 1.171 | 31 322 |
| EST: on average 2 month disease stabilisation in both EST groups | 44 581 | 7775 | 36 806 | 1.840 | 0.629 | 1.211 | 30 402 |
| EST alone: on average 2 month disease stabilisation | 44 393 | 7787 | 36 606 | 1.766 | 0.631 | 1.135 | 32 241 |
| EST: 100% active EST; on average 6 month stabilisation in EST alone | 51 619 | 15 263 | 36 357 | 1.772 | 0.697 | 1.075 | 33 830 |
| CUA: 15D, TRA’s retail price (SE) | 44 395 (11 087) | 7559 (509) | 36 835 (10 868) | 1.302b (0.332)b | 0.438b (0.051)b | 0.864a,b (0.281)b | 42 633c (18 521) |
| CUA: 15D, TRA’s hospital price (SE) | 40 384 (9676) | 7559 (509) | 32 825 (9695) | 1.302b (0.332)b | 0.438b (0.051)b | 0.864b (0.281)b | 37 992c (15 786) |
| CUA: SF-6D, TRA’s retail price | 44 473 | 7568 | 36 905 | 1.175b | 0.398b | 0.777b | 47 523c |
| CUA: SF-6D, TRA’s hospital price | 40 384 | 7568 | 32 816 | 1.175b | 0.398b | 0.777b | 42 234c |
| CUA: EQ-5D, TRA’s retail price | 44 130 | 7585 | 36 545 | 1.157b | 0.392b | 0.766b | 47 735c |
| CUA: EQ-5D, TRA’s hospital price | 40 384 | 7585 | 32 799 | 1.157b | 0.392b | 0.766b | 42 819c |
Pharmacy retail prices without value added tax assumed for trabectedin, if not otherwise stated.
Significant amount of LYGs or QALYs.
QALY.
Incremental cost-utility ratio.
BSA, body surface area; CT, chemotherapy; CUA, cost-utility analysis; DAC, dacarbazine; EQ-5D, EuroQol 5D; EST, end-stage treatment; ETO, etoposide; Incr., increment; IADIC, doxorubicin, ifosfamide, mesna and DAC; ICER, incremental cost-effectiveness ratio; IE, ifosfamide, mesna, ETO, phenobarbital and growth hormone; IMVP-6a, ifosfamide, mesna, ETO and methotrexate; LYGs, life year gained; mSTS, metastatic soft tissue sarcoma; SAE, serious adverse event; SE, standard error; SF-6D, Short Form 6D; QALY, quality-adjusted life year; TRA, trabectedin.
Based on the QoL tool, TRA → EST resulted to the quality-adjusted survival of 1.16–1.30 QALYs and the respective QALY amount due to EST was 0.39–0.44 QALYs (Table 1). Consequently, the lowest QALYs gained due to TRA → EST compared with EST was 0.77 (95% CI 0.55–1.04) based on EQ-5D and the highest 0.86 (95% CI 0.61–1.16) based on 15D. The 15D results were also most credible based on Kontodimopoulos et al. [24].
Expected lifetime costs for TRA → EST were €44 346 (95% CI €34 073–56 269) assuming the pharmacy retail price for TRA and €40 384 (95% CI €31 282–50 247) assuming the hospital price for TRA. EST resulted in €7568 (95% CI €7129–8158) lifetime costs (hospital prices assumed). Consequently, the incremental cost of TRA → EST compared with EST was €36 778 (95% CI €26 397–48 801) assuming the pharmacy retail price for TRA and €32 816 (95% CI €23 315–42 317) assuming the hospital price for TRA.
cost-effectiveness
The use of TRA → EST in mSTS patients was associated with an incremental cost of €31 590 (95% CI €21 279–47 630) per LYG compared with EST when the pharmacy retail price for TRA was assumed. The correspondent result was €28 192 (95% CI €16 833–39 551) with the wholesale/hospital price for TRA. The cost for a QALY gained with TRA → EST compared with EST was €42 633–47 735 based on the pharmacy retail price of TRA and €37 992–42 819 based on the hospital price for TRA, depending on the utility tool. The cost-utility based on SF-6D and EQ-5D was almost equivalent, whereas the 15D-based results were €5000 lower (Table 1).
sensitivity analysis
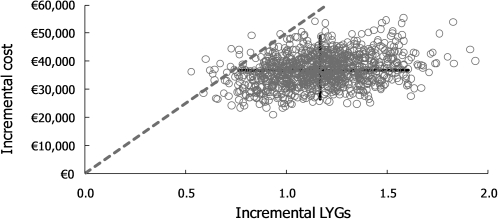
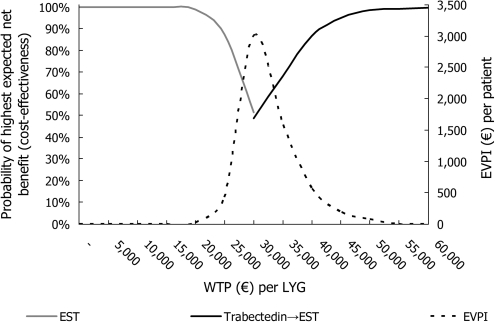
CEP is presented in Figure 2 and CEAF in Figure 3. According to CEP, TRA provides significant gain in life years with significantly higher costs. However, the uncertainty related to ICER is not significant with the generally approved ICER thresholds: according to CEAF, the 50%, 75%, 90% and 95% probabilities of cost-effectiveness for TRA → EST were obtained with the willingness to pay (WTP) levels of €31 797, €36 445, €41 534 and €44 967 per LYG when TRA had the pharmacy retail price (€28 464, €33 461, €38 755 and €43 058 per LYG with the hospital price for TRA), respectively.
Figure 2.
The cost-effectiveness plane (CEP) represents the results of 1000 simulations for incremental lifetime costs (the pharmacy retail price for trabectedin was assumed) and life years gained (LYG) for TRA → end-stage treatment (EST) versus EST (EST is fixed in the origin of the CEP). 95% confidence intervals for incremental costs and effects, and a dashed line showing where the threshold for €50 000 per LYG stands are shown in the CEP; all points under this line were associated with an incremental cost-effectiveness ratio of <€50 000 per LYG. This characterised 98.5% of the simulations.
Figure 3.
Cost-effectiveness acceptability frontier (solid curves), which presents the optimal treatment options, together with expected value of perfect information (EVPI, dashed curve) frontier. The pharmacy retail prices were assumed for trabectedin. EST, end-stage treatment; LYG, life years gained.
The highest EVPI estimate was obtained with the ICER value, which was equivalent to the change of optimal treatment in the CEAF from EST to TRA → EST (Figure 3). With the WTP of €31 590 (€28 192 assuming the hospital price for TRA) per LYG, the EVPI estimate was €3008 (€3188). With WTP levels of €30 000, €40 000 and €50 000 per LYG, the respective EVPI estimates were €2101, €566 and €75 (€2328, €374 and €59 with the hospital price for TRA).
Generally, the results were robust to changes in the key parameters. For example, when all patients treated with EST were assumed to receive off-label active CT treatment and EST alone patients were assumed to experience disease stabilisation for 6 months (TRA versus ETO/DAC setting), the result was €33 830 (95% CI €22 237–55 822) per LYG. Yet, the results were somewhat sensitive to the administered dose and price of TRA (Table 1). The increase of TRA to the recommended dose in place of that actually observed in STS-201 resulted to €35 849 (95% CI €26 479–52 164) per LYG (TRA’s pharmacy retail price assumed). This was unrealistic, as the base case scenario accounted for dose reductions and withdrawals, which were expected with CTs. When the pharmacy premium was excluded from the price of TRA and the hospital/wholesale price was used, the incremental cost per LYG decreased to €28 192. This can be more readily applicable in countries where the pharmacy premium is low or when TRA is used in the public hospital settings in Finland.
discussion
TRA → EST results in a significantly higher OS than EST, but with higher costs. Thus, we asked whether TRA → EST is a cost-effective second-line treatment of mSTS patients.
TRA slowed progression and had an impact on mortality that continued beyond the TRA treatment. The incremental cost per LYG and QALY gained were €31 590 and €42 633–47 735 for TRA → EST versus EST when the pharmacy retail price for TRA was assumed, respectively, which indicate that TRA → EST was potentially cost-effective. The results were in line with the previous cost-effectiveness results obtained in the Finnish setting [17, 26, 27]. When the wholesale/hospital price was assumed for TRA, the incremental cost per LYG and QALY gained was €28 192 and €37 992–42 819, which are more applicable in countries with lower pharmacy premium (e.g. Sweden) or in the Finnish public hospital setting. Also the highest EVPI estimate per patient was relatively low €3008 (€3188 assuming the hospital price for TRA) compared with the total per patient costs, meaning that gathering additional information for model parameters based on the setting presented in this study may have little impact on the results.
The results were most sensitive to the administered dose and price of TRA. None of the other sensitivity analyses showed a marked effect on the cost-effectiveness and probabilistic approach took into account the relatively small sample sizes of STS-201 and EORTC STBSG. mSTS also lacked generic QoL data. Given STS is an orphan disease, however, this is unsurprising. Though utilities were estimated using mapping and the impact of QoL was tested using different tools, these analyses were subject to uncertainty and were conservative for TRA (i.e. usually SD utility > PD utility, but here, SD utility = PD utility [17, 26, 27]). Nonetheless, TRA demonstrated potential cost-utility, which could also be acceptable, e.g. in the UK.
A modelled economic evaluation was essential to capture the potential costs and benefits associated with TRA → EST and EST in a therapeutic setting outside the STS-201 [9] and EORTC STBSG [15, 16]. The STS-201 was aimed at assessing the efficacy of two different TRA doses. For both STS-201 regimes, the 6-month PFS rates were considerably greater than, e.g., the 6-month PFS of 14% reported by van Glabbeke and Verweij [28] for active regimes in pre-treated patients with STS, and the TRA results generally [8, 10–12, 29] were consistent. Also the ifosfamide-based EORTC STBSG [15, 16] data used for the EST arm were in line with DAC and ETO results [17, 30].
The primary limitation was the use of historical data for EST arm rather than head-to-head data. Acknowledging the limitations of historical comparisons, STS-201 survivals compare favourably with survivals reported for patients after failure of second-line ifosfamide [15, 16] using the correspondent eligibility criteria as in STS-201 and for patients who received DAC [17] or ETO [30] after the failure on standard CT. Also, the trial-based efficacy and safety of TRA has been recently confirmed by a compassionate use study [8], and TRA trials have been found to be well designed [12], which improved the feasibility of this approach. A randomised data of TRA against current clinical practice was not available for such a late cancer stage due to ethical concerns and a lack of appropriate comparators.
The economic evaluation illustrated the incremental cost-effectiveness associated with treating mSTS patients who have failed A/I. The results were within the bounds of what would usually be considered as good value for money for a cancer treatment. Before TRA, for mSTS patients no significant therapeutic improvements had occurred over three decades.
conclusions
TRA was a potentially cost-effective treatment of mSTS patients who have received priory A/I. In fact, the cost-effectiveness of TRA was comparable with or superior to many other cancer drugs for nonorphan conditions. The value of additional parameter information using equivalent setting and parameter definitions is likely to be low.
funding
Oy Swedish Orphan Ab, Espoo, Finland.
disclosure
E.J.O.S. is employee and shareholder of ESiOR Oy, commissioned by Oy Swedish Orphan Ab to perform this study; ESiOR carries out commissioned studies and health economic analysis for several pharmaceutical companies, food industry companies, and hospitals. B.G.S.A. is employee of PharmaMar and T.J. is employee and joint owner of Docrates. All authorship decisions were made on the basis of scientific consideration.
Acknowledgments
The authors wish to thank Mrs Taru Hallinen and anonymous referees for comments during the manuscript phase of the study, and docent, David Laaksonen for the language revision. Previous presentation: Selected results of this study were presented previously as a poster at the ISPOR 12th Annual European Congress, Paris, France, 2009.
appendix 1. The estimation of transition probabilities
Transition probabilities SD → PD treated with TRA, PD → death after TRA and PD → death with EST were estimated using the same principle: the number of months that it took for ≥20% of the population to remain in health state (SD or PD) was used to calculate the per cycle probabilities of progression to PD or death. This conservative cut-off ensured that only the most robust data were used. The outliers who took an unusually long time to SD → PD relative to the rest of the TRA population were excluded because the inclusion of these outliers would distort the transition probabilities and boost the cost-effectiveness associated with TRA.
TRA → EST arm transition probabilities per cycle (STS-201 24-h q3wk regime)
Total SD → PD; for 9 months ≥20% remained SD: 0.1578 (i.e. 1 −[(1 − 0.787) (1/9)] = 0.1578; compare Fleurence and Hollenbeak [1] for the equation). This comprised SD → PD and SD → death
SD → death; proportion of patients for whom TTP = OS multiplied by SD → PD: 0.0070
SD → PD; SD → death was subtracted from the total SD → PD: 0.1508
Remaining PF; complement of the total SD → PD: 0.8422
PD → death; for 26 months ≥20% remained PD: 0.0573.
EST arm transition probabilities per cycle (EORTC STBSG)
PD → death; for 11 months ≥20% remained PD: 0.1147
Remaining PD; complement of PD → death: 0.8853.
Source for Appendix 1
[1] Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics 2007; 25: 3–6.
appendix 2. Resources, unit costs and resource use
| Resource | Unit cost (2008 €) | Source | Units/cycle | Cycles |
| TRA treatment | ||||
| Yondelis 2 × 1 mg + 1 × 0.25 mg; dexamethasone, pharmacy Or hospital price for above (optional base case scenario) | 5236.47 4528.68 | [1] | 1 | 5 |
| Administration; laboratory tests; travellinga | 751.14 | [2]b | 1 | 5 |
| ETO EST (120 mg/m2) | ||||
| Etoposid 20 mg/ml, 3 × 5 ml | 60.00 | [1] | 3 | 6 |
| Administration | 679.42 | [2]b | 3 | 6 |
| Laboratory tests and travellinga | 66.90 | [2]b | 1 | 6 |
| DAC EST (250 mg/m2) | ||||
| Dadatic 2 × 200 mg + 1 × 100 mg | 53.93 | [1] | 5 | 6 |
| Administration | 679.42 | [2]b | 5 | 6 |
| Laboratory tests and travellinga | 108.62 | [2]b | 1 | 6 |
| IADIC EST | ||||
| Adriamycin 2 mg/ml, 2 × 25 ml; Haloxan 1 g/m2, 2 g; Uromitexan 100 mg/ml, 40 mg/kg, 2 × 10 ml + 1 × 4 ml; Dadatic 2 × 200 mg + 1 × 100 mg | 231.75 | [1] | 1 | 6 |
| Administration | 679.42 | [2]b | 5 | 6 |
| Laboratory tests and travellinga | 108.62 | [2]b | 1 | 6 |
| IE EST | ||||
| Haloxan 1 g/m2, 2 g; Uromitexan 100 mg/ml, 40 mg/kg, 2 × 10 ml + 1 × 4 ml | 42.58 | [1] | 5 | 6 |
| Etoposid 100 mg/m2, 20 mg/ml, 2 × 5 ml | 40.00 | [1] | 3 | 6 |
| Luminaletten 100 mg, 7 × 15 mg | 0.85 | [1] | 6 | 6 |
| Neulasta 6 mg | 1298.08 | [1] | 1 | 6 |
| Administration | 679.42 | [2]b | 5 | 6 |
| Laboratory tests and travellinga | 66.90 | [2]b | 1 | 6 |
| IMVP-a6 EST | ||||
| Haloxan 1 g/m2, 2g; Uromitexan 100 mg/ml, 40 mg/kg, 2 × 10 ml + 1 × 4 ml | 42.58 | [1] | 5 | 6 |
| Etoposid 100 mg/m2, 20 mg/ml, 2 × 5 ml | 40.00 | [1] | 3 | 6 |
| Methotrexate 30 m2, 25 mg/ml, 3 × 1 ml | 8.73 | [1] | 2 | 6 |
| Administration | 679.42 | [2]b | 7 | 6 |
| Laboratory tests and travelling | 33.87 | [2]b | 1 | 6 |
| Travelling to administration | 33.03 | [2]b | 2 | 6 |
| SAE related to TRA | ||||
| Treatment and travelling | 1472.90 | [2]b | 0.054 | 1 |
| after A/I failure | ||||
| Ongoing treatment | 607.32 | [3]c | 1 | |
Travelling to administration (€33.03) and to laboratory (€6.49).
Indexed from 2006 to 2008 price level with the factor of 1.0951 obtained from the Official Statistics Finland.
Indexed from 2005 to 2008 price level (€3042.44 during 4.98 months; €607.32 per cycle).
A/I = anthracycline and/or ifosfamide; DAC, dacarbazine; EST, end-stage treatment; ETO = etoposid; IADIC, doxorubicin, ifosfamide, mesna and DAC; IE, ifosfamide, mesna, ETO, phenobarbital and growth hormone; IMVP-6a, ifosfamide, mesna, ETO and methotrexate; SAE = serious adverse event; TRA, trabectedin.
Sources for Appendix 2
[1] Finnish Medicine Tariff. Helsinki, 1/2010.
[2] Hujanen T, Kapiainen S, Tuominen U et al. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006, Helsinki: Stakes, 2008.
[3] Purmonen T, Martikainen JA, Soini EJ et al. Economic Evaluation of Sunitinib Malate in Second-Line Treatment of Metastatic Renal Cell Carcinoma in Finland. Clin Ther 2008; 30: 382–392.
appendix 3. Quality of life estimation
Disease-specific EORTC QLQ-C30 scale values [1] were mapped to 15D, SF-6D and EQ-5D generic quality of life values (utilities) by multiplying them with coefficients obtained from the regression models [2]
| Tool | QLQ-C30 predictor | Scale valuea | Multiplierb | Outcomec |
| 15D | Physical functioning | 63.477 | 0.00299 | 0.18980 |
| Global health status | 53.526 | 0.00262 | 0.14024 | |
| Insomnia | 27.084 | −0.00096 | −0.02600 | |
| Cognitive functioning | 86.409 | 0.00198 | 0.17109 | |
| Constant | 1.000 | 0.26114 | 0.26114 | |
| Utilityd | 0.73626 | |||
| SF-6D | Social functioning | 67.451 | 0.00082 | 0.05531 |
| Global health status | 53.526 | 0.00085 | 0.04550 | |
| Emotional functioning | 70.577 | 0.00167 | 0.11786 | |
| Pain | 28.972 | −0.00122 | −0.03535 | |
| Constipation | 17.019 | −0.00110 | −0.01872 | |
| Dyspnoea | 8.481 | −0.00064 | −0.00543 | |
| Constant | 1.000 | 0.50842 | 0.50842 | |
| Utilityd | 0.66760 | |||
| EQ-5D | Physical functioning | 63.477 | 0.00508 | 0.32246 |
| Emotional functioning | 70.577 | 0.00313 | 0.22091 | |
| Global health status | 53.526 | 0.00546 | 0.29225 | |
| Constant | 1.000 | −0.18143 | −0.18143 | |
| Utilityd | 0.65419 |
Weighted means from Table III in [1].
Bs from Table 3 in [2].
Outcome = scale value × multiplier.
Sum.
EORTC, European Organisation for Research and Treatment of Cancer; EQ-5D, EuroQol 5D; SF-6D, Short Form 6D.
Sources for Appendix 3
[1] Poveda A, López-Pousa A, Martín J et al. Phase II Clinical Trial With Pegylated Liposomal Doxorubicin (CAELYX(R)/Doxil(R)) and Quality of Life Evaluation (EORTC QLQ-C30) in Adult Patients With Advanced Soft Tissue Sarcomas: A study of the Spanish Group for Research in Sarcomas (GEIS). Sarcoma 2005; 9: 127–132.
[2] Kontodimopoulos N, Aletras VH, Paliouras D et al. Mapping the Cancer-Specific EORTC QLQ-C30 to the Preference-Based EQ-5D, SF-6D, and 15D Instruments. Value Health 2009; 12: 1151–1157.
References
- 1.Leyvraz L. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2007;18:ii74–ii76. doi: 10.1093/annonc/mdm046. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Sajeevan KV, Babu KG, et al. Chemotherapy in adult soft tissue sarcoma. Indian J Cancer. 2009;46:274–287. doi: 10.4103/0019-509X.55547. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MF, Alektiar KM, Maki RG. Soft tissue sarcoma. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 1841–1890. [Google Scholar]
- 4.Verweij J, Mouridsen HT, Nielsen OS, et al. The present state of the art in chemotherapy for soft tissue sarcomas in adults: the EORTC point of view. Crit Rev Oncol Hematol. 1995;20:193–201. doi: 10.1016/1040-8428(94)00146-K. [DOI] [PubMed] [Google Scholar]
- 5.Clark M, Fisher C, Judson I. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 6.Soares DG, Escargueil AE, Poindessous V, et al. Replication and homologous recombination repair regulate DNA double-strand break formation by the antitumor alkylator ecteinascidin 743. Proc Natl Acad Sci U S A. 2007;104:13062–13067. doi: 10.1073/pnas.0609877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganjoo KR, Patel SR. Trabectedin: an anticancer drug from the sea. Expert Opin Pharmacother. 2009;10:2735–2743. doi: 10.1517/14656560903277236. [DOI] [PubMed] [Google Scholar]
- 8.Fayette J, Boyle H, Chabaud S, et al. Efficacy of trabectedin for advanced sarcomas in clinical trials versus compassionate use programs: analysis of 92 patients treated in a single institution. Anticancer Drugs. 2010;21:113–119. doi: 10.1097/CAD.0b013e328333057b. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 10.Kasper B, Schmitt T, Wuchter P, et al. The use of positron emission tomography in soft tissue sarcoma patients under therapy with trabectedin. Mar Drugs. 2009;7:331–340. doi: 10.3390/md7030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sessa C, Perotti A, Noberasco C, et al. Phase I clinical and pharmacokinetic study of trabectedin and doxorubicin in advanced soft tissue sarcoma and breast cancer. Eur J Cancer. 2009;45:1153–1161. doi: 10.1016/j.ejca.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Le Cesne A, Domont J, Gioffi A, et al. Mapping the literature: role of trabectedin as a new chemotherapy option in advanced pretreated soft tissue sarcoma. Drugs Today. 2009;45:403–421. doi: 10.1358/dot.2009.45.6.1378934. [DOI] [PubMed] [Google Scholar]
- 13.Judson I, Al-Muderis O, Scott D, et al. Cost of Management of Metastatic Soft Tissue Sarcoma. Birmingham, UK: NCRI Cancer Conference; 2007. (poster) [Google Scholar]
- 14.Briggs A, Claxton K, Schulpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 15.van Oosterom A, Mouridsen H, Nielsen O, et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 2002;38:2397–2406. doi: 10.1016/s0959-8049(02)00491-4. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen O, Judson I, van Hoesel Q, et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2000;36:61–67. doi: 10.1016/s0959-8049(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 17.Buesa J, Lopez-pousa A, Martin J, et al. Phase II trial of first-line high-dose ifosfamide in advanced soft tissue sarcomas of the adult: a study of the Spanish Group for Research on sarcomas (GEIS) Ann Oncol. 1998;9:871–876. doi: 10.1023/a:1008474802882. [DOI] [PubMed] [Google Scholar]
- 18.Purmonen T, Martikainen JA, Soini EJ, et al. Economic evaluation of sunitinib malate in second-line treatment of metastatic renal cell varcinoma in Finland. Clin Ther. 2008;30:382–392. doi: 10.1016/j.clinthera.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hujanen T, Kapiainen S, Tuominen U, et al. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Helsinki, Finland: Stakes; 2008. [Google Scholar]
- 20.Poveda A, López-Pousa A, Martín J, et al. Phase II Clinical Trial With Pegylated Liposomal Doxorubicin (CAELYX(R)/Doxil(R)) and Quality of Life Evaluation (EORTC QLQ-C30) in Adult Patients With Advanced Soft Tissue Sarcomas: a study of the Spanish Group for Research in Sarcomas (GEIS) Sarcoma. 2005;9:127–132. doi: 10.1080/13577140500287024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33:328–336. doi: 10.3109/07853890109002086. [DOI] [PubMed] [Google Scholar]
- 22.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 23.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 24.Kontodimopoulos N, Aletras VH, Paliouras D, et al. Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15D instruments. Value Health. 2009;12:1151–1157. doi: 10.1111/j.1524-4733.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 25.Barton G, Briggs A, Fenwick E. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11:886–897. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Soini EJ, Martikainen JA, Nousiainen T. Economic evaluation of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy regime with or without Rituximab (R) in the 2nd line treatment of follicular non-Hodgkin lymphoma. Value Health. 2008;11:A470. (Abstr) [Google Scholar]
- 27.Martikainen J, Kivioja A, Hallinen T, et al. Economic evaluation of temozolomide in the treatment of recurrent glioblastoma multiforme. Pharmacoeconomics. 2005;23:803–816. doi: 10.2165/00019053-200523080-00006. [DOI] [PubMed] [Google Scholar]
- 28.van Glabbeke M, Verweij J. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 30.Keizer H, Crowther D, Nielsen O, et al. EORTC Group phase II study of oral etoposide for pretreated soft tissue sarcoma. Sarcoma. 1997;1:99–101. doi: 10.1080/13577149778371. [DOI] [PMC free article] [PubMed] [Google Scholar]