Abstract
Background
A novel series of methylene-substituted DIMs (C-DIMs), namely 1,1-bis(3'-indolyl)-1-(p-substituted phenyl)methanes containing t-butyl (DIM-C-pPhtBu) and phenyl (DIM-C-pPhC6H5) groups inhibit proliferation of invasive estrogen receptor-negative MDA-MB-231 and MDA-MB-453 human breast cancer cell lines with IC50 values between 1-5 uM. The main purpose of this study was to investigate the pathways of C-DIM-induced cell death.
Methods
The effects of the C-DIMs on apoptotic, necrotic and autophagic cell death were determined using caspase inhibitors, measurement of lactate dehydrogenase release, and several markers of autophagy including Beclin and light chain associated protein 3 expression (LC3).
Results
The C-DIM compounds did not induce apoptosis and only DIM-C-pPhCF3 exhibited necrotic effects. However, treatment of MDA-MB-231 and MDA-MB-453 cells with C-DIMs resulted in accumulation of LC3-II compared to LC3-I protein, a characteristic marker of autophagy, and transient transfection of green fluorescent protein-LC3 also revealed that treatment with C-DIMs induced a redistribution of LC3 to autophagosomes after C-DIM treatment. In addition, the autofluorescent drug monodansylcadaverine (MDC), a specific autophagolysosome marker, accumulated in vacuoles after C-DIM treatment, and western blot analysis of lysates from cells treated with C-DIMs showed that the Beclin 1/Bcl-2 protein ratio increased.
Conclusion
The results suggest that C-DIM compounds may represent a new mechanism-based agent for treating drug-resistant ER-negative breast tumors through induction of autophagy.
Background
Studies in this laboratory have investigated the mechanisms of cell death induced by a new series of anticancer agents that are derivatives of phytochemicals expressed in crucifers. Indole-3-carbinol is a phytochemical found as a conjugate in cruciferous vegetables, and both indole-3-carbinol and one of its major metabolites, 3,3'-diindolylmethane (DIM), exhibit a broad range of anticancer and antitumorigenic activities against multiple tumor types [1-6]. Epidemiology studies have correlated consumption of cruciferous vegetables with decreased risk for certain types of cancer [7-11], and indole-3-carbinol and DIM may contribute to cancer chemoprevention associated with these vegetables. The mechanisms of growth inhibition induced by DIM have been well-studied and include G0/G1 cell cycle arrest, induction of ER stress, induction of apoptosis, activation of aryl hydrocarbon receptor (AhR)-dependent antiestrogenicity, and downregulation of the androgen receptor (AR) [2,5,12-18]. We also synthesized several DIMs substituted in the indole ring and at the methylene carbon bridge to determine structure-activity relationships.
A novel series of methylene-substituted DIMs (C-DIMs), namely 1,1-bis(3'-indolyl)-1-(p-substituted phenyl)methanes containing t-butyl (DIM-C-pPhtBu) and phenyl (DIM-C-pPhC6H5) groups, activate peroxisome proliferator-activated receptor γ (PPARγ) and induce receptor-dependent and -independent growth inhibitory and pro-apoptotic responses/genes in colon, pancreatic, ovarian, prostate, bladder and breast cancer cells and/or tumors [19-25]. In ER-negative breast cancer cells, the effect of PPARγ-active C-DIMs on the cell cycle, induction of the pro-apoptotic protein NAG-1, and activation of kinases is primarily receptor-independent and effects of C-DIMs on % distribution of MDA-MB-231 and MDA-MB-453 cells in G0/G1, S and G2/M were minimal [26]. Although C-DIMs modulate Bax and Bcl-2 protein expression, PARP is not cleaved, suggesting a caspase-independent form of cell death [26]. Therefore, the mechanism of cell death induced by C-DIMs in breast cancer cells requires further examination.
In the present study, treatment of ER-negative MDA-MB-231 and MDA-MB-453 cells with C-DIMs did not activate caspases or increase Annexin V staining, indicating that apoptotic cell death was not activated [26]. These observations prompted us to examine other cell death pathways including necrosis and autophagy. The latter pathway is important for cellular homeostasis but can also be activated by some anticancer agents. Measurement of LDH release and propidium iodide (PI) staining suggested that necrosis was not the major form of cell death induced in ER-negative breast cancer cells treated with C-DIMs. In contrast, autophagolysosomes were positively stained with monodansylcadaverine (MDC) after treatment with C-DIMs, and there was a significant increase in LC3b and Beclin 1/Bcl-2 protein ratios. In addition, after treatment with C-DIMs, transfected GFP-LC3 localized to autophagosomal membranes of cells. These data support a contributing role of autophagy in the mechanism of action of C-DIMs in ER-negative breast cancer cells.
Methods
Cells, chemicals and other materials
NADH, zVAD-fmk and PI were obtained from Sigma Chemical Co. (St. Louis, MO). MDC was purchased from Fluka (Buchs, Switzerland). The human breast cancer cell lines MDA-MB-231 and MDA-MB-453 were obtained from American Type Culture Collection (Manassas, VA). MDA-MB-231 cells were maintained in DMEM:F-12 supplemented with 0.22% sodium bicarbonate, 10% fetal bovine serum (FBS), and 2 ml/L antibiotic solution (Sigma Chemical Co., St. Louis, MO). MDA-MB-453 cells were maintained in RPMI supplemented with 0.22% sodium bicarbonate, 10% FBS, and 2 ml/L antibiotic solution (Sigma Chemical Co., St. Louis, MO). Cells were grown in 150 cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C and passaged every 5 days. Beclin 1 (H-300) and Bcl-2 (N-19) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The LC3 antibody was purchased from MBL International (Woburm, MA). Horseradish peroxidase substrate for Western blot analysis was purchased from NEN Life Science Products (Boston, MA).
Cell proliferation assay
MDA-MB-231 and MDA-MB-453 cells were seeded at a density of 3-5 × 104/well in 12-well plates and media was replaced the next day with DMEM:F-12 media containing 2.5% charcoal-stripped FBS and pre-treated with 20 μM zVAD-fmk or vehicle control for 30 min. Cells were then co-treated with zVAD-fmk or vehicle control and DMSO or 10 μM DIM-C-pPhCF3, DIM-C-pPhtBu or DIM-C-pPhC6H5 for 48 h. Cells were then counted using a Coulter Z1 cell counter. Each experiment was completed in triplicate and results are expressed as means ± SE for each determination.
Annexin V staining
MDA-MB-231 and MDA-MB-453 cells were seeded at a density of 2.5-5 × 105/well in 6-well plates and media was replaced the next day with DMEM:F-12 media containing 2.5% charcoal-stripped FBS and DMSO or 10 mM DIM-C-pPhCF3, DIM-C-pPhtBu or DIM-C-pPhC6H5 for 48 h. Cells were also treated for 24 h with 10 mM MG132. Cells were then harvested according to the Annexin-V-FITC protocol provided by BD Biosciences. Briefly, floating cells were transferred to an Eppendorf tube and centrifuged for 2 min at 3000 g. The adherent cells were trypsinized and transferred to the same Eppendorf which was spun and the supernatant was removed. Cells were resuspended in 85 ml of 1× Annexin V binding buffer and 10 ml of PI solution (5 μg/mL). Five ml of Annexin V-FITC conjugate was added to each sample and then incubated in the dark for 15 min before analysis by flow cytometry which may underestimate the number of dead cells due to fragmentation of floating cells. Cells early in apoptosis positively stained for Annexin V, whereas cells in the later stages of apoptosis stained positively for Annexin V and PI due to late apoptotic cells having leaky plasma membranes (magnification = 400×).
PI and Hoechst staining
Vybrant Apoptosis Assay Kit #7 was used from Molecular Probes (Eugene, OR) according to the manufacturer's directions. Briefly, monolayers of cells were cultured for 48 h in 2-well Coverglass Chamber slides. Slides were washed with culture medium without serum or phenol red, and labeled with Hoechst 33343 and propidium iodide at a final concentration of 5 μg/ml and 1.0 μg/ml, respectively. The slides were incubated for 30 min on ice and visualized using a BioRad Radiance 2000 Multiphoton microscope. At least 3 areas per well were analyzed. Two wells were analyzed per treatment and per time point.
LDH assay
MDA-MB-231 and MDA-MB-453 cells were treated for 48 h with DMSO or C-DIMs as indicated. An extra 3 wells were treated for 1 h or until all cells had lysed with 0.3% Triton X in PBS. For each sample, 200 μl of 1.22 mM pyruvate in 50 mM phosphate buffer and 4 μl of 12.4 mg/ml NADH dissolved in 50 mM phosphate buffer were each added to a 96-well plate. Twenty μl of supernatant from treated cells was then added and the plate was incubated at 37°C for 30 min. The LDH concentration was measured at 390 nm and the treated groups were compared to Triton X, which was considered 100% LDH release.
MDC staining
Monolayers of cells were cultured for 48 h in 2-well Coverglass Chamber slides and treated as indicated. Slides were washed with culture medium without serum or phenol red. Cytoplasmic vacuoles were stained with MDC according to the method described [27]. Briefly, cells were exposed to 50 mM of MDC for 10 min at 37°C and visualized using a BioRad Radiance 2000 Multiphoton microscope. At least 3 areas per well were analyzed. Two wells were analyzed per treatment and per time point (magnification = 400×).
Western blots
MDA-MB-231 and MDA-MB-453 cells were seeded in DMEM:F-12 media containing 2.5% charcoal-stripped FBS for 24 h and then treated with either the vehicle (DMSO) or the indicated compounds. In experiments where indicated, cells were pre-treated for 30 min with 10 mM of the proteasome inhibitor MG132. Whole cell lysates were obtained using high salt buffer [50 mM HEPES, 500 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol and 1% Triton X-100 (pH 7.5) and 5 μl/ml of Protease Inhibitor Cocktail]. Protein samples were incubated at 100°C for 2 min, separated on 10-15% SDS-PAGE at 120 V for 3-4 h in 1× running buffer [25 mM Tris-base, 192 mM glycine, and 0.1% SDS (pH 8.3)] and transferred to a polyvinylidene difluoride (PVDF) membrane at 0.9 A for 90 min at 4°C in 1× transfer buffer (48 mM Tris-HCl, 39 mM glycine, and 0.025% SDS). The PVDF membrane was blocked in 5% TBST-Blotto [10 mM Tris-HCl, 150 mM NaCl (pH 8.0), and 0.025% Triton X-100 and 5% non-fat dry milk] with gentle shaking for 30 min and incubated in fresh 5% TBST-Blotto with 1:200-1:1000 primary antibody overnight at 4°C with gentle shaking. After washing with TBST for 10 min, the PVDF membrane was incubated with secondary antibody (1:5000) in 5%TBST-Blotto for 2 h. The membrane was washed with TBST for 10 min and incubated with 10 ml of chemiluminescence substrate for 1.0 min and exposed to Kodak X-OMAT AR autoradiography film. Band intensities were evaluated by scanning laser densitometry (Sharp Electronic Corporation, Mahwah, NJ) using Zero-D Scanalytics software (Scanalytics Corporation, Billerica, MA).
GFP-LC3 localization
Monolayers of cells were cultured for 48 h in 2-well Coverglass Chamber slides and treated as indicated. The GFP-LC3 plasmid was kindly provided by Dr. Tamotsu Yoshimori (Osaka University, Osaka, Japan). MDA-MB-231 cells were transfected with 500-600 ng/well of GFP-LC3 plasmid using Lipofectamine transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. MDA-MB-453 cells were transfected with 600 ng/well of GFP-LC3 plasmid using GeneJuice transfection reagent (EMD Biosciences, Madison, WI) according to the manufacturer's protocol. Cells with GFP-LC3 expression were counterstained with Hoechst DNA dye from Molecular Probes (Eugene, OR). Slides were examined by fluorescence microscopy using a Zeiss Stallion Dual Detector Imaging System (Carl Zeiss Microimaging Inc., Thornwood, NY). The intracellular distribution of GFP-LC3 was evaluated by monitoring GFP-LC3 and Hoechst fluorescence and DIC images throughout the entire thickness of the cell by acquiring optical slices at 0.5 μm intervals using a C-Apochromat 63×, 1.2 NA water immersion lens. Digital images were acquired using Slide Book software (Intelligent Imaging Innovations, Denver, CO). The entire z-stack was subjected to fluorescence deconvolution to remove out-of-plane fluorescence. Cells were examined in more than 5 fields per slide on multiple slides. Data represents the average of all the fields.
Beclin-1 immunohistochemistry in tumors
The generation of tumors was performed as described previously [26] in 4-6 week old female Balb/c athymic nude mice (Nu-/-). All procedures were performed in accordance with the National Institutes of Health "Guide for the Care and Use of Laboratory Animals" and were approved by the Institutional Animal Care and Use Committee of Texas A&M University. Briefly, mice were injected subcutaneously in the left flank with 107 MDA-MB-231 cells in 100 - 200 μl serum-free medium. Groups of mice (12 mice/group) were then treated i.p. with 40 mg/kg DIM-C-pPhC6H5 in 50 μl corn oil, or 50 μl corn oil, every day for 35 total injections starting at day 4 post-tumor inoculations.
Tumor Beclin-1 expression was evaluated on 7-μm thick formalin-fixed, paraffin-embedded tissue sections by a standard immunohistochemistry protocol. Briefly, endogenous peroxidase was blocked by the use of 3% hydrogen peroxide in PBS for 10 min. The slides were then washed with distilled water for 2 min and this washing step was repeated twice. Slides were then incubated for 30 min at room temperature with a protein blocking solution (VECTASTAIN Elite ABC kit, Vector Laboratories, Burlingame, CA). Excess blocking solution was drained, and the samples were incubated overnight at 4°C at a 1:100 dilution of anti-rabbit Beclin 1 antibody. Sections were then incubated with biotinylated secondary antibody followed by streptavidin (VECTASTAIN Elite ABC kit, Vector Laboratories, Burlingame, CA). The color was developed by exposing the peroxidase to diaminobenzidine reagent (Vector Laboratories, Burlingame, Ca), and Beclin 1 expression was identified by the brown-colored cytoplasmic staining. Tumors from two different animals per treatment group were evaluated.
Statistical analysis
Statistical differences between different groups were determined by ANOVA followed by Bonferroni post hoc test for multiple comparisons at P < 0.05 using SuperAnova software. The data are presented as mean ± standard error for at least 3 separate determinations for each treatment.
Results
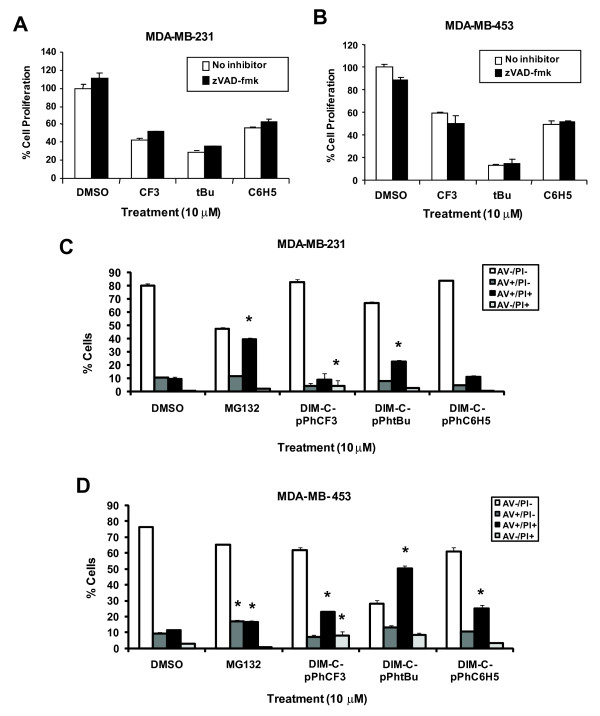
PPARγ-active C-DIMs inhibit growth and activate caspase-dependent apoptosis in colon cancer cells [23]. C-DIMs also inhibit growth of MDA-MB-231 and MDA-MB-453 breast cancer cells; however, this was not accompanied by caspase-dependent PARP cleavage [26]. Figure 1A and 1B show that there was no statistically significant reversal of C-DIM-mediated cell growth inhibition after co-treatment of MDA-MB-231 and MDA-MB-453 cells with the pan-caspase inhibitor zVAD-fmk, suggesting that growth inhibition by the C-DIM compounds was apoptosis-independent.
Figure 1.
Influence of pan-caspase inhibitor zVAD-fmk on growth inhibition induced by C-DIMs. MDA-MB-231 [A] and MDA-MB-453 [B] cells were treated for 48 h with 10 μM DIM-C-pPhCF3 (CF3), DIM-C-pPhtBu (tBu) or DIM-C-pPhC6H5 (C6H5) and with or without 20 μM zVAD-fmk and then counted as described in the Materials and Methods. Results of all proliferation studies are presented as means ± SE for at least three separate determinations for each treatment group. Significantly (p < 0.05) decreased growth (compared to DMSO) in cells treated with C-DIMs alone is indicated (*). FACS analysis of MDA-MB-231 [C] and MDA-MB-453 [D] cells co-stained with Annexin V and PI. Cells were treated for 48 h or 24 h with 10 μM MG132. Floating and adherent cells were incubated with Annexin V and PI and analyzed by flow cytometry as described in the Materials and Methods. Results of all proliferation studies are presented as means ± SE for at least three separate determinations for each treatment group. Significance (p < 0.05) compared to DMSO is indicated (*).
MDA-MB-231 and MDA-MB-453 cells were treated with C-DIMs for 48 h, co-stained with Annexin V-FITC conjugate which is a marker of apoptosis and PI and analyzed by flow cytometry (Figure 1C and 1D). PI is a DNA dye that is impermeable to cells with intact membranes and is a marker of late apoptotic or necrotic cells that have lost membrane integrity. Early apoptotic cells stain positive for Annexin V, whereas cells in the later stages of apoptosis stain positively for both Annexin V and PI. The majority of cells treated with solvent control DMSO clustered in the quadrant negative for both Annexin V and PI. After treatment with the apoptosis-inducing compound MG132, there was a slight increase in MDA-MB-231 (but not MDA-MB-453) cells staining positively for Annexin V alone and a statistically significant increase in cells staining positive for Annexin V and PI in both cell lines, suggesting that cells treated with MG132 were in the late stages of apoptosis. In MDA-MB-231 cells treated with C-DIMs, there was a decrease in the number of cells staining positively for Annexin V alone, and increased staining for Annexin V and PI was observed only for DIM-C-pPhtBu. In both MDA-MB-231 and MDA-MB-453 cells staining for PI alone was observed only after treatment with DIM-C-pPhCF3. In addition, all 3 C-DIM compounds significantly increased Annexin V and PI staining in MDA-MB-453 cells, indicating that these cells exhibited the prototypical characteristics of late stage apoptosis and necrosis (Figure 1D). The failure of C-DIMs to induce Annexin V alone indicates that the ER-negative breast cancer cells do not undergo the early stages of apoptosis and this was consistent with previous studies in these cell lines treated with C-DIMs for only 24 h [26]. The increased number of cells staining positive for both Annexin V and PI are indicative of C-DIM-induced loss of cell membrane integrity but Annexin V/PI staining does not identify which cell death pathways are activated.
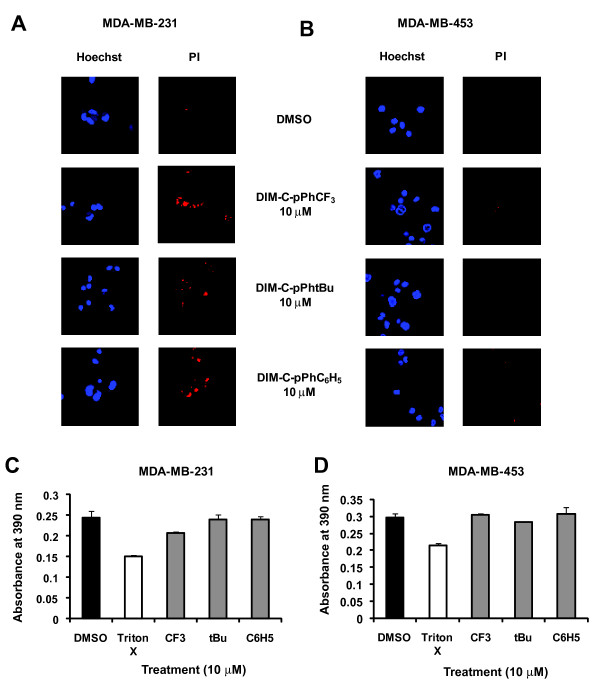
Some MDA-MB-231 cells were stained with PI after treatment with C-DIMs; however, PI staining was not observed in MDA-MB-453 cells treated with C-DIMs (Figure 2A and 2B). LDH release from cells was significantly elevated after treatment with the lytic agent, Triton X in both cell lines; however, among the three PPARγ-active C-DIMs only DIM-C-pPhCF3 significantly increased levels of LDH release in MDA-MB-231 cells, whereas none of the PPARγ-active C-DIMs induced LDH release in MDA-MB-453 cells (Figure 2C and 2D). Thus, only DIM-C-pPhCF3 significantly induced necrosis in one of the two cell lines, suggesting that necrosis was not the predominant death pathway induced by PPARγ-active C-DIMs in ER-negative breast cancer cells.
Figure 2.
Necrosis detection by PI staining and LDH release. MDA-MB-231 [A] and MCF-7 [B] cells were treated for 24 h with DMSO or 10 μM DIM-C-pPhCF3, DIM-C-pPhtBu or DIM-C-pPhC6H5. Two fluorescent DNA dyes Hoechst and propidium iodide, were loaded into cells and incubated on ice for 30 min and visualized using confocal microscopy as described in the Materials and Methods. The same microscopic field of MDA-MB-231 [A] or MDA-MB-453 [B] cells stained with Hoechst and propidium iodide are shown and are representative of other cell areas with the same treatment. At least 3 areas were scanned for each well and two wells were analyzed per treatment and per time point. Width of each field = 100 μm. LDH release after treatment with C-DIMs in MDA-MB-231 [C] and MDA-MB-453 cells [D]. Cells were treated for 48 h or 1 h with 0.1% Triton X and the supernatants were analyzed for LDH as described in the Materials and Methods. Triton X served as a positive for 100% cell lysis and LDH release. Results are presented as the means ± SE for at least three separate determinations for each treatment group. Statistical significance of treatments compared to DMSO (p < 0.05) are represented by an asterisk.
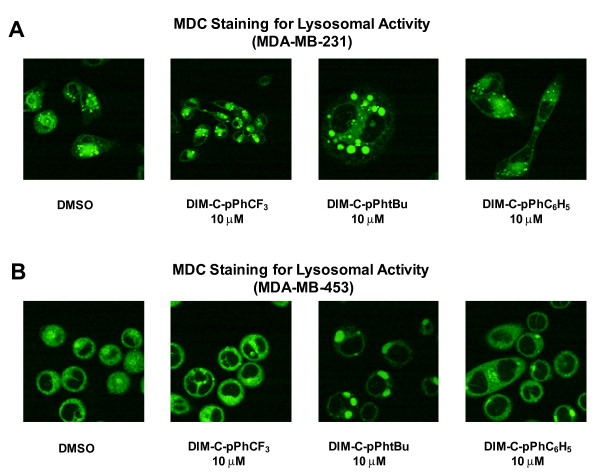
Several drugs that are cytotoxic to cancer cell lines induce autophagic cell death [28-38] and, therefore, the effects of C-DIMs on MDC localization, a positive marker for autophagolysosomes, was investigated. The C-DIMs clearly induced MDC localization in vacuoles in MDA-MB-231 cells, whereas in cells treated with DMSO, a smaller number and size of vacuoles was observed indicating low basal levels of autophagy in these cells (Figure 3A). Thus, treatment with C-DIMs increased the number and/or size of vacuoles in this cell line. MDC was also localized in large vacuoles in MDA-MB-453 cells treated with DIM-C-pPhtBu and DIM-C-pPhC6H5 (Figure 3B), whereas vacuole size and number in cells treated with DIM-C-pPhCF3 resembled the control cells. These results suggest that DIM-C-pPhtBu and DIM-C-pPhC6H5 induced autophagy in MDA-MB-231 and MDA-MB-453 cells.
Figure 3.
Detection of lysosomal activity with monodansylcadaverine (MDC) staining. MDA-MB-231 [A] and MDA-MB-453 cells [B] were treated with DMSO or 10 μM DIM-C-pPhCF3, DIM-C-pPhtBu or DIM-C-pPhC6H5 for 24 h. MDC was loaded into cells and incubated at 37°C for 10 min and visualized using confocal microscopy as described in the Materials and Methods. At least 5 areas were scanned for each well and images represented are characteristic of treatment replicates. Width of each field is 100 μm except for right two panels in [A] which are 50 μm.
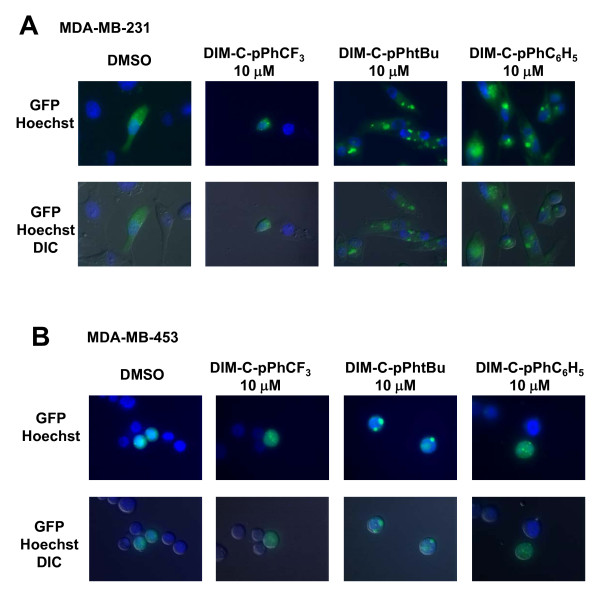
LC3b is critical for autophagosome formation and after induction of autophagy, LC3b-I is lipidated to LC3b-II which localizes to autophagosomal membranes, and the ratio of LC3b-II to LC3b-I protein expression is an indicator of autophagosome formation [39]. Confocal microscopy images of a GFP-LC3 construct transiently transfected into the two cell lines showed that in untreated or DMSO-treated cells, there was a diffuse GFP staining pattern (punctae), whereas after treatment with C-DIMs, GFP-LC3 was primarily localized in vacuoles (Figure 4A and 4B). In MDA-MB-231 cells, there was an increase in the number of vacuoles positively stained for GFP-LC3 after treatment with DIM-C-pPhtBu and DIM-C-pPhC6H5 compared to DMSO (Figure 4A). The effect of DIM-C-pPhCF3 was less pronounced than with the other two compounds in MDA-MB-231 cells and this was consistent with the higher necrotic activity of this compound (Figure 2). In MDA-MB-453 cells, the size of the vacuoles was substantially increased after treatment with DIM-C-pPhtBu, whereas only the number of vacuoles increased in cells after treatment with DIM-C-pPhC6H5 (Figure 4B). These data suggest that C-DIM compounds activated autophagic cell death pathways in MDA-MB-231 and MDA-MB-453 cells.
Figure 4.
Translocation of GFP-LC3 after treatment with C-DIMs. Translocation of transiently transfected GFP-LC3 plasmid. GFP-LC3 was transiently transfected into MDA-MB-231 [A] and MDA-MB-453 [B] and cells were treated with DMSO or 10 μM C-DIMs as outlined in the Materials and Methods. Confocal microscopy images of live cells were captured after treatment with C-DIMs for 24 h and differential interference contrast (DIC) and DIC/GFP overlay images of the same microscopic field are illustrated. At least 3 areas were scanned per well and the images depicted are representative of treatment replicates. Width of each field is 100 μm.
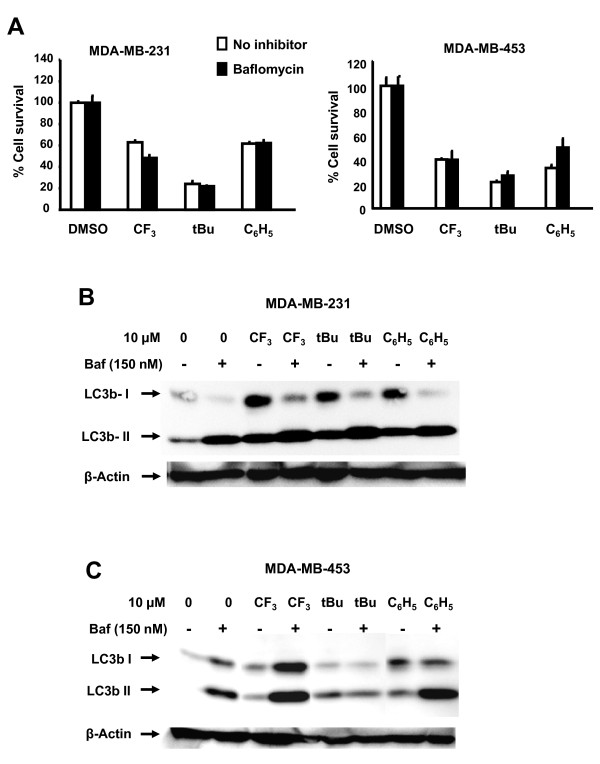
Bafilomycin A1 inhibits maturation of autophagosomes into autolysosomes by inhibiting fusion between autophagosomes and lysosomes [40] and this compound was used to further investigate the role of autophagy in C-DIM-induced growth inhibition. Figure 5A shows that DIM-C-pPhCF3, DIM-C-pPhtBu and DIM-C-pPhC6H5 alone inhibit growth of MDA-MB-231 and MDA-MB-453 cells. Their growth inhibitory effects were not affected after treatment in combination with bafilomycin and similar effects were observed with 3-methyladenine (data not shown). These results indicate that induction of autophagy by C-DIMs is not important for their inhibition of cell growth. In untreated MDA-MB-231 cells, both LC3b-I and LC3b-II proteins are expressed; treatment with DIM-C-pPhCF3, DIM-C-pPhtBu and DIM-C-pPhC6H5 increased expression of both proteins. Bafilomycin alone increased accumulation of LC3b-II protein and in combination with the C-DIM compounds, LC3b-II levels were further increased compared to bafilomycin alone, suggesting activation of autophagy by the C-DIMs in this cell line. With the exception of DIM-C-pPhtBu, similar results were observed in MDA-MB-453 cells treated with bafilomycin alone or in combination with C-DIMs. It is possible that DIM-C-pPhtBu may affect clearance of the autophagic vesicles.
Figure 5.
Effects of bafilomycin on C-DIM-induced responses. [A] Growth inhibition in breast cancer cells. MDA-MB-231 and MDA-MB-453 cells were treated with DMSO, C-DIMs alone (10 μm), or in combination with bafilomycin (150 nM) for 48 hr, and cells were counted as described in the Materials and Methods. Results are expressed as means ± SD for replicate (3) determinations. Effects of bafilomycin on LC3b protein in MDA-MB-231 [B] and MDA-MB-453 [C] cells. Cells were treated with DMSO, C-DIMs alone, or in combination with bafilomycin for 48 hr, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods.
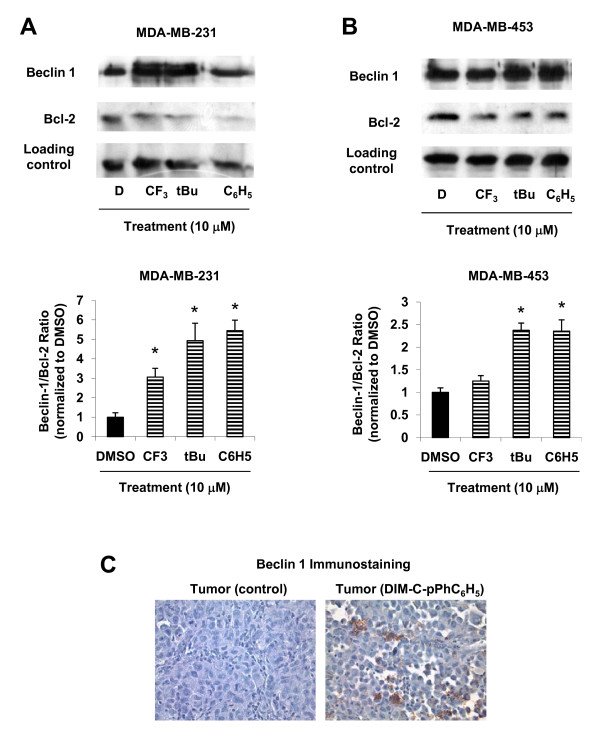
Although the same autophagy-related genes (Atg5, Atg6, Atg7) play a role in autophagy and autophagic cell death, both Atg5 and Atg6/Beclin 1 are upregulated in autophagic cell death and remain low in autophagy [41]. The anti-apoptotic protein Bcl-2 has an inhibitory action on Beclin 1 and the subsequent induction of autophagy [42] and, therefore, the effects of C-DIMs on expression of these proteins were further investigated. Treatment of MDA-MB-231 cells with C-DIMs increased Beclin 1 and decreased Bcl-2 protein levels (Figure 6A), and in MDA-MB-453 cells treated with C-DIMs, Beclin 1 protein levels increased only after treatment with DIM-C-pPhtBu and DIM-C-pPhC6H5 and Bcl-2 protein was decreased (Figure 6B). Densitometric analyses of these proteins showed that C-DIMs increased Beclin 1/Bcl-2 ratios in MDA-MB-231 compared to that observed for DMSO (Figure 6A); however, a statistically significant increase in Beclin 1/Bcl-2 protein ratios was observed only in MDA-MB-453 cells treated with DIM-C-pPhtBu and DIM-C-pPhC6H5 (Figure 6B). In vivo studies showed that DIM-C-pPhC6H5 inhibited growth of tumors in nude mice bearing MDA-MB-231 cells as xenografts [26]. We analyzed induction of autophagy in these tumors after treatment with DIM-C-pPhC6H5 and the results (Figure 6C) show enhanced staining of Beclin 1 in tumors from treated animals versus mice receiving only corn oil (vehicle control). These results suggest that C-DIMs induce cell death (Figure 1) in ER-negative breast cancer cells and this may be partially due to activation of autophagy and this demonstrates the versatility of this novel class of anticancer drugs which activate multiple receptor-independent cell death pathways in different cancer cell lines.
Figure 6.
Modulation of autophagy-related gene Beclin 1 and Bcl-2 protein expression. MDA-MB-231 [A] and MDA-MB-453 [B] cells were treated for 24 h with DMSO or C-DIMs, and whole cell lysates were isolated and analyzed by Western blot analysis according to procedures outlined in the Materials and Methods. Densitometric analyses of Beclin 1 and Bcl-2 protein expression in MDA-MB-231 and MDA-MB-453 cells was carried as described in the Materials and Methods. Results are expressed as means ± SE for three replicate determinations for each treatment group. * Significantly different from DMSO (p < 0.05). [C] Enhanced tumor staining of Beclin 1 after treatment with DIM-C-pPhC6H5 in nude mice bearing MDA-MB-231 cells as xenografts. Fixed tumor tissue from corn oil and DIM-C-pPhC6H5-treated mice [26] were stained with Beclin 1 as described in the Materials and Methods. Results are representative of tumors from two different animals per treatment group. Width of each field is 230 μm.
Discussion
C-DIMs inhibit ER-negative MDA-MB-231 and MDA-MB-453 breast cancer cell proliferation and tumor growth in athymic nude mice bearing MDA-MB-231 cells as xenografts. C-DIMs represent a novel series of compounds that exert their growth inhibitory and antitumorigenic effects in a cell context-dependent manner. C-DIMs activate PPARγ in a number of cancer cell types including ovarian, bladder, colon, pancreatic and ER-negative and ER-positive breast cancer cells [19-22,24,25]. PPARγ agonists inhibit cancer growth by molecular mechanisms that include G1 cell cycle arrest, induction of apoptosis and terminal differentiation [43-46]. PPARγ-active C-DIMs and other PPARγ agonists also activate PPARγ-independent growth inhibitory pathways including induction of NAG-1, ER stress and apoptosis [20,47-52]. C-DIMs induce NAG-1 which is proapoptotic in some cancer cell lines; however, in MDA-MB-231 and MDA-MB-453 cells, these compounds induced NAG-1 but not apoptosis. In addition, induction of other receptor-dependent and -independent pro-apoptotic and differentiation pathways such as ER stress and caveolin 1 was not observed in ER-negative breast cancer cells. Thus, the precise mechanism of C-DIM-induced cell death in ER-negative breast cancer cells is unclear and multiple pathways may be involved.
EB1089 is a vitamin D analog that induces caspase-independent cell death in ER-positive MCF-7 and ER-negative MDA-MB-231 and MDA-MB-453 breast cancer cells, and similar results were observed for C-DIMs which also induced caspase-independent growth inhibition which could not be reversed after co-treatment with the pan-caspase inhibitor zVAD-fmk (Figure 1). We also confirmed that treatment with C-DIMs for 48 h did not induce Annexin V staining indicative of early apoptotic cells in ER-negative breast cancer cells, whereas the pro-apoptotic agent MG132 induced cells staining with Annexin V staining in cells (Figure 1). These results coupled with previous studies showing that treatment with C-DIMs for 24 h did not induce caspase-dependent PARP cleavage in these cell lines [26] confirm that these compounds primarily induce apoptosis-independent cell death. Other chemotherapeutic agents such as the cytotoxic drug paclitaxel and related analogs induced cell death in breast cancer cells; however, only a small percentage of cells were undergoing apoptosis [29]. Thus, the current studies suggest that an alternative form of cell death is induced by C-DIMs in ER-negative breast cancer cells and this contrasts with the induction of caspase-dependent apoptosis in ER-positive MCF-7 cells treated with C-DIMs [25].
Necrosis is an alternative form of cell death and although initially considered an uncontrolled form of cell demise, there is increasing evidence supporting the concept of programmed necrosis [53]. For instance, DNA damaging agents induced programmed cell death in Bax-/-Bak-/- cells but only in actively proliferating cells and these observations suggested an intrinsic cellular control point that decides cellular fate [54]. This form of cell death is particularly appealing for chemotherapeutic agents since many tumor cells have dysfunctional apoptotic pathways and, therefore, apoptosis-inducing agents are not always effective for treating cancer [53]. In MDA-MB-231 cells, minimal staining of PI and a statistically significant release of LDH was observed only after treatment with DIM-C-pPhCF3; signs of necrosis (PI staining) were also observed in MDA-MB-231 cells treated with DIM-C-pPhtBu or DIM-C-pPhC6H5 (Figure 2A and 2C). The lack of LDH release and PI staining in MDA-MB-453 cells treated with C-DIMs (Figure 2B and 2D) suggested that necrosis was not significantly induced in this cell line. These results also indicate some structure-dependent differences between C-DIMs and also with the structurally-related ring-substituted DIMs. For example, 5,5'-dibromoDIM induced necrotic cell death in ER-negative MDA-MB-453 cells, whereas DIM-C-pPhtBu and DIM-C-pPhC6H5 did not activate this cell death pathway [12].
Autophagic cell death is an alternative form of programmed cell death where cells lack the hallmarks of apoptosis but there is an accumulation of autophagic vacuoles in the cytoplasm [55], and several structurally diverse chemotherapeutic agents induce autophagic cell death in various cancer cell lines [28-30,32-34]. For example, the PPARγ agonist prostaglandin J2 induced autophagic cell death in prostate cancer cells [34] and the phytochemical sulforaphane induced autophagic cell death in prostate cancer cells [28]. Therefore, we also examined the effects of C-DIMs on activation of autophagy in MDA-MB-231 and MDA-MB-453 breast cancer cells. There are several biochemical methods for detecting autophagic activity including acidic dyes that label vacuoles which exhibit lysosomal activity [27]. Autophagic cell death in prostate cancer cells treated with sulforaphane exhibited lysosomotropic staining of cytoplasmic vacuoles with acridine orange [28]. Other chemotherapeutic candidate drugs induce signs of autophagy in breast cancer cells [29,32,37,56]. Prenylated flavones inhibited cell growth and induced autophagy in both ER-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells and this response was typified by the formation of cytoplasmic vacuoles that stained with the autophagic marker MDC [56]. The vitamin D analog EB1089 also inhibited cell growth in MCF-7 breast cancer cells and increased uptake of MDC into cytoplasmic vacuoles [37]. These observations were similar to effects of C-DIMs on ER-negative breast cancer cells which also exhibited increased uptake of MDC into vacuoles (Figure 3), suggesting that these compounds induce autophagy. Therapeutic agents such as EB1089 and prenylated flavones typically induce punctate lysosomotropic staining, whereas treatment with DIM-C-pPhtBu in particular induced formation of very large MDC-stained vacuoles (Figure 3). These differences in staining may be due, in part, to differences in the size of autophagosomes in various cancer cell lines [57] caused by differences in autophagic flux.
LC3b, a critical protein involved in the early stage of autophagosome formation, becomes lipidated upon induction of autophagy [58]. Induction of LC3-II protein expression, an increase in the ratio of LC3b-II/LC3b-I expression, and translocation of LC3 to autophagosomal membranes are diagnostic molecular markers indicative of autophagy [39]. For example, treatment of breast cancer cells with camptothecin or paclitaxel analogs increase the ratio of LC3b-II/LC3b-I protein expression [29,59]. With the exception of DIM-C-p-PhCF3 in MDA-MB-453 cells, treatment with C-DIMs induced translocation of GFP-LC3 from the cytoplasm to autophagosomal membranes (Figure 4A and 4B). Chemotherapeutic agent EB1089 or radiation that induce autophagic cell death typically induce GFP-LC3 staining patterns in breast cancer cells similar to those observed in this study with C-DIMs [33,37]. Bafilomycin blocks maturation of autophagosomes and in combination with C-DIMs did not inhibit or enhance their growth inhibitory effects in MDA-MB-231 and MDA-MB-453 cells (Figure 5A). However, bafilomycin alone increased accumulation of LC3b-II in both cell lines (Figure 5B and 5C) and with the exception of DIM-C-pPhtBu (MDA-MB-453 cells), the C-DIM compounds further enhanced bafilomycin-induced LC3b-II levels, suggesting activation of autophagy in these cells.
An important difference between autophagy as a survival mechanism versus autophagic cell death involves the Atg5 and Atg6/Beclin 1 genes, which are upregulated in autophagic cell death [41]. The anti-apoptotic protein Bcl-2 is also critically involved as a negative regulator of the induction of autophagy by Beclin 1 [42]. Ceramide [60], camptothecin [61], and EB1089 [37] induce autophagic cell death in breast cancer cells and all of these agents increase Beclin 1 protein expression. These observations are similar to the significant increase of Beclin 1 protein levels and Beclin 1/Bcl-2 ratios in MDA-MB-231 and MDA-MB-453 cells after treatment with C-DIMs (Figure 6A and 6B) and also in tumors from mice bearing MDA-MB-231 cells as xenografts and treated with DIM-C-pPhC6H5 (Figure 6C). While further studies that explore autophagic flux and the actions of other autophagy inhibitors on C-DIM growth inhibition are warranted, these findings represent the first report to document the activation of an autophagic program in ER-negative breast cancer cells with C-DIMs.
Conclusions
In summary, results of this study demonstrate that C-DIM compounds clearly decrease ER-negative breast cancer cell proliferation and like some other cancer chemotherapeutic drugs induce autophagy. These results are in contrast to the mechanisms of action of C-DIMs in colon, pancreatic, ovarian, prostate and bladder cancer cell lines where these same compounds induce both PPARγ-dependent and -independent growth inhibitory and pro-apoptotic responses [19,20,22-25,47]. The mechanisms associated with the cell context-dependent differences in the anticancer activities of C-DIMs and their mechanisms of growth inhibition and cell death in ER-negative breast cancer cells are currently being further investigated. The unusual activity of C-DIMs is also being exploited for the clinical treatment of ER-negative/highly invasive breast tumors that normally only respond to highly cytotoxic drugs which induce serious toxic side effects.
Abbreviations
AHR: aryl hydrocarbon receptor; AR: androgen receptor; C-DIMS: methylene-substituted DIMs; DIM: 3,3'-diindolylmethane; DIM-C-PPHC6H5: 1,1-bis(3'-indolyl)-1-(p-phenyl)methane; DIM-C-PPHTBU: 1,1-bis(3'-indolyl)-1-(p-t-butyl)methane; FBS: fetal bovine serum; MDC: monodansylcadaverine; PI: propidium iodide; PPARγ: peroxisome proliferator-activated receptor γ; PVDF: polyvinylidene difluoride
Competing interests
The authors reports no conflicts of interest in this work; however, the C-DIM compounds have been licensed from Texas A&M University by Plantacor (College Station, TX).
Authors' contributions
KV carried out the majority of the in vitro studies, analyzed and summarized the results, and drafted the manuscript. YS and AF carried out in vitro studies and were involved in the design and implementation of the anticancer activities of C-DIMs. RCB and RB collaborated in all of the imaging studies. GC and IJ carried out the studies with bafilomycin and 3-methyladenine. SS coordinated the study, synthesized the compounds, and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Kathy Vanderlaag, Email: kathy_vanderlaag@hotmail.com.
Yunpeng Su, Email: ysu@swmail.sw.org.
Arthur E Frankel, Email: afrankel@swmail.sw.org.
Robert C Burghardt, Email: rburghardt@cvm.tamu.edu.
Rola Barhoumi, Email: rmouneimne@cvm.tamu.edu.
Gayathri Chadalapaka, Email: gchadalapaka@cvm.tamu.edu.
Indira Jutooru, Email: ijutooru@cvm.tamu.edu.
Stephen Safe, Email: ssafe@cvm.tamu.edu.
Acknowledgements
This work was supported by the National Institutes of Health (R01CA108718) and Texas AgriLife Research.
References
- Bradlow HL, Michnovicz JJ, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- Grubbs CJ, Steele VE, Casebolt T, Juliana MM, Eto I, Whitaker LM, Dragnev KH, Kelloff GJ, Lubet RL. Chemoprevention of chemically-induced mammary carcinogenesis by indole-3-carbinol. Anticancer Res. 1995;15:709–716. [PubMed] [Google Scholar]
- Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane (DIM) induces a G1 cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21WAF1/CIP1 expression. Carcinogenesis. 2002;23:1297–1305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kojima T, Morishita Y, Mori H. Inhibitory effects of the natural products indole-3-carbinol and sinigrin during initiation and promotion phases of 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Jpn J Cancer Res. 1992;83:835–842. doi: 10.1111/j.1349-7006.1992.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–81. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW. et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer. 2001;41:17–28. doi: 10.1207/S15327914NC41-1&2_2. [DOI] [PubMed] [Google Scholar]
- Zhang SM, Hunter DJ, Rosner BA, Giovannucci EL, Colditz GA, Speizer FE, Willett WC. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin's lymphoma among women. Cancer Epidemiol Biomarkers Prev. 2000;9:477–485. [PubMed] [Google Scholar]
- Vanderlaag K, Samudio I, Burghardt R, Barhoumi R, Safe S. Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3'-indolyl)methane (DIM) and 5,5'-dibromoDIM. Cancer Lett. 2005;236:198–212. doi: 10.1016/j.canlet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3'-Diindolylmethane (DIM) and derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Gene expression profiling revealed survivin as a target of 3,3'-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006;66:4952–4960. doi: 10.1158/0008-5472.CAN-05-3918. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Park SY, Shin HK, Kwon DY, Surh YJ, Park JH. Activation of caspase-8 contributes to 3,3'-Diindolylmethane-induced apoptosis in colon cancer cells. J Nutr. 2007;137:31–36. doi: 10.1093/jn/137.1.31. [DOI] [PubMed] [Google Scholar]
- Hong C, Firestone GL, Bjeldanes LF. Bcl-2 family-mediated apoptotic effects of 3,3'-diindolylmethane (DIM) in human breast cancer cells. Biochem Pharmacol. 2002;63:1085–1097. doi: 10.1016/S0006-2952(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARγ-dependent and PPARγ-independent pathways. Mol Cancer Ther. 2006;5:1362–1370. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- Lei P, Abdelrahim M, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. Mol Cancer Ther. 2006;5:2324–2336. doi: 10.1158/1535-7163.MCT-06-0184. [DOI] [PubMed] [Google Scholar]
- Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor γ-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;145:5774–5785. doi: 10.1210/en.2004-0686. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Smith R III, Samudio I, Zhang W, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor γ-mediated growth inhibition, transactivation and differentiation markers in colon cancer cells. Cancer Res. 2004;64:5994–6001. doi: 10.1158/0008-5472.CAN-04-0399. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Res. 2006;66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R III, Phillips T, Abdelrahim M, Samudio I, Safe S. A new class of peroxisome proliferator-activated receptor γ (PPARγ) agonists that inhibit growth of breast cancer cells: 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes. Mol Cancer Therap. 2004;3:247–259. [PubMed] [Google Scholar]
- Vanderlaag K, Su Y, Frankel AE, Grage H, Smith R, Khan S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer Res Treat. 2008;109:273–283. doi: 10.1007/s10549-007-9648-y. [DOI] [PubMed] [Google Scholar]
- Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- Gorka M, Daniewski WM, Gajkowska B, Lusakowska E, Godlewski MM, Motyl T. Autophagy is the dominant type of programmed cell death in breast cancer MCF-7 cells exposed to AGS 115 and EFDAC, new sesquiterpene analogs of paclitaxel. Anticancer Drugs. 2005;16:777–788. doi: 10.1097/01.cad.0000171514.50310.85. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, Walker R, Hermann RS. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-Δ(12,14)-prostaglandin J2. Cell Growth Differ. 2000;11:49–61. [PubMed] [Google Scholar]
- Kuo PL, Hsu YL, Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 2006;5:3209–3221. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang L, Feng C, Wen LP. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophys Res Commun. 2005;337:52–60. doi: 10.1016/j.bbrc.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. Terminal differentiation of human breast cancer through PPARγ. Mol Cell. 1998;1:465–470. doi: 10.1016/S1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura W, Okumura T, Takahashi N, Obara T, Kohgo Y. Activation of peroxisome proliferator-activated receptor gamma by troglitazone inhibits cell growth through the increase of p27KiP1 in human pancreatic carcinoma cells. Cancer Res. 2000;60:5558–5564. [PubMed] [Google Scholar]
- Clay CE, Namen AM, Atsumi G, Willingham MC, High KP, Kute TE, Trimboli AJ, Fonteh AN, Dawson PA, Chilton FH. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and NAG-1. Mol Pharmacol. 2005;68:1782–1792. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, Murthy R, You YJ, Safe S. 2-Cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor γ in colon and pancreatic cancer cells. Carcinogenesis. 2007;28:2337–2346. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- Ray DM, Akbiyik F, Phipps RP. The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol. 2006;177:5068–5076. doi: 10.4049/jimmunol.177.8.5068. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Thomas DM, Thompson EW, Williams ED. PPARgamma-independent induction of growth arrest and apoptosis in prostate and bladder carcinoma. BMC Cancer. 2006;6:53. doi: 10.1186/1471-2407-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, Huang JW, Chen CS. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005;65:1561–1569. doi: 10.1158/0008-5472.CAN-04-1677. [DOI] [PubMed] [Google Scholar]
- Huang JW, Shiau CW, Yang YT, Kulp SK, Chen KF, Brueggemeier RW, Shapiro CL, Chen CS. Peroxisome proliferator-activated receptor gamma-independent ablation of cyclin D1 by thiazolidinediones and their derivatives in breast cancer cells. Mol Pharmacol. 2005;67:1342–1348. doi: 10.1124/mol.104.007732. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- Pedro M, Lourenco CF, Cidade H, Kijjoa A, Pinto M, Nascimento MS. Effects of natural prenylated flavones in the phenotypical ER (+) MCF-7 and ER (-) MDA-MB-231 human breast cancer cells. Toxicol Lett. 2006;164:24–36. doi: 10.1016/j.toxlet.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M. et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N. et al. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- Lamparska-Przybysz M, Gajkowska B, Motyl T. Cathepsins and BID are involved in the molecular switch between apoptosis and autophagy in breast cancer MCF-7 cells exposed to camptothecin. J Physiol Pharmacol. 2005;56(Suppl 3):159–179. [PubMed] [Google Scholar]