Abstract
Rationale
Mitogen-activated protein kinase (MAPK) pathways provide a critical connection between extrinsic and intrinsic signals to cardiac hypertrophy. Extracellular signal-regulated protein kinase 5 (ERK5), an atypical MAP kinase is activated in the heart by pressure overload. However, the role of ERK5 plays in regulating hypertrophic growth and hypertrophy-induced apoptosis is not completely understood.
Objective
Herein, we investigate the in vivo role and signaling mechanism whereby ERK5 regulates cardiac hypertrophy and hypertrophy-induced apoptosis.
Methods and Results
We generated and examined the phenotypes of mice with cardiomyocyte-specific deletion of the erk5 gene (ERK5cko). In response to hypertrophic stress, ERK5cko mice developed less hypertrophic growth and fibrosis than controls. However, increased apoptosis together with upregulated expression levels of p53 and Bad were observed in the mutant hearts. Consistently, we found that silencing ERK5 expression or specific inhibition of its kinase activity using BIX02189 in neonatal rat cardiomyocytes (NRCMs) reduced myocyte enhancer factor 2 (MEF2) transcriptional activity and blunted hypertrophic responses. Furthermore, the inhibition of MEF2 activity in NRCMs using a non-DNA binding mutant form of MEF2 was found to attenuate the ERK5-regulated hypertrophic response.
Conclusions
These results reveal an important function of ERK5 in cardiac hypertrophic remodeling and cardiomyocyte survival. The role of ERK5 in hypertrophic remodeling is likely to be mediated via the regulation of MEF2 activity.
Keywords: Cardiac hypertrophy, signal transduction, genetically modified mice
Introduction
Cardiac hypertrophy is a virtually universal prerequisite for the development of heart failure. In response to acute or chronic insults the heart initially develops hypertrophic growth. However, sustained stress causes chamber dilation, interstitial fibrosis, and myocyte apoptosis, eventually leading to heart failure or sudden death from arrhythmias. Numerous signaling pathways including Mitogen-Activated Protein (MAP) kinases are implicated in mediating the process of cardiac hypertrophy and hypertrophy-induced cardiomyocyte apoptosis1. In the heart, all four classes of MAP kinases are activated through either mechanical overload or neurohumoral stimulation2. However, our understanding of the critical role of individual MAPK in various aspects of hypertrophic remodeling remains fragmented and in many cases controversial.
Extracellular signal-regulated protein kinase 5 (ERK5) is an atypical MAP kinase, also known as big MAPK (BMK1), as it is more than twice the size of the other MAPKs owing to a very large C-terminal domain3. It is suggested that the role of this unique C-terminal tail is to regulate myocyte enhancer factor 2 (MEF2) transcriptional activity and ERK5 sub-cellular localization3, 4. Upon exposure to various stimuli, ERK5 is activated by phosphorylation downstream of MEK55. An important step in deciphering the function of ERK5 is to identify its downstream targets and MEF2A, C and D are among the best-characterized substrates of ERK54, 5. Analogous to many MAP kinases, ERK5 plays an important role in cell survival, cell proliferation and differentiation. The discovery that ERK5 is an important contributor to cell survival has increased interest in this signaling pathway. In sympathetic neurons, ERK5 is required to mediate the survival response to nerve growth factor by suppressing the expression of Bad and Bim6. The analysis of mice with an endothelial-specific deletion of ERK5 provides physiological evidence that ERK5 is essential for the survival of endothelial cells via the activation of MEF27. Moreover, recent studies have demonstrated that ERK5 activation prevents cardiomyocyte apoptosis, likely through the inhibition of a feedback loop of phosphodiesterase 3A/inducible cAMP early repressor8. Apart from its role in cardiomyocytes survival, ERK5 has also been implicated in regulating cardiac hypertrophy9, 10.
ERK5 can be activated in the heart by pressure overload, and by hypertrophy-stimulating factors including LIF (leukemia inhibitory factor), adrenergic agonists, oxidative and osmotic stresses in cultured cardiomyocytes11, 12. However, previous studies of transgenic mice with cardiac-specific over-expression of activated MEK5 provided conflicting results. Decompensated eccentric hypertrophy with no cardiomyocte apoptosis was observed in MEK5β transgenic mice, which is in stark contrast to the normal cardiac function and morphology found in MEK5α transgenic mice12, 13. Upon hypertrophic stress, MEK5α transgenic mice develop hypertrophic growth to a similar degree to controls but with reduced apoptosis and better cardiac function8. Regarding the capacity of activating ERK5, we have demonstrated that MEK5β is an active enzyme while MEK5α is a stronger activator due to its higher affinity for ERK514. In contrast, Cameron et al. proposed that MEK5β is3 a dominant negative variant that can suppress ERK5 signaling15. Moreover, expression of MEK5α has been suggested to be restricted to brain and liver, whereas MEK5β is ubiquitously expressed16. Given these contradictions emerging from MEK5 studies, it is necessary to investigate the function of ERK5 in the heart using cardiomyocyte-specific ERK5 deletion mice (ERK5cko), a physiologically-relevant model compared to the overexpressing transgenic lines. Hayashi et al. have previously generated and described a cardiomyocyte-specific ERK5 deletion mouse model7. In concordance with their observation, we also found ERK5cko mice (generated in our lab) were viable, fertile and appeared normal, suggesting the specific ablation of erk5 in cardiomyocytes alone does not affect cardiac development. To characterize the role of ERK5 in cardiac hypertrophy we subjected ERK5cko mice to hypertrophic stimuli, the mutant mice developed less hypertrophic growth and fibrosis compared to controls (ERK5f/f), also increased apoptosis with upregulated expression of p53 and Bad were observed in the mutant heart. Consistently, we found that silencing ERK5 expression or specific inhibition of its kinase activity using a novel compound BIX02189 in neonatal rat cardiomyocytes (NRCMs) reduced MEF2 transcriptional activity and blunted the hypertrophic response. Moreover, the inhibition of MEF2 activity in NRCMs using a non-DNA binding mutant form of MEF2C was found to diminish the ERK5-regulated hypertrophic response. These results clearly reveal an important function of ERK5 in stress-induced cardiac hypertrophic remodeling and cardiomyocyte survival.
Materials and Methods
Induction of Cardiac Hypertrophy
Cardiac hypertrophy was induced by transverse aortic constriction (TAC) or chronic infusion of isoproterenol (ISO, Sigma-Aldrich) at 10mg/ml for 7 days in 8–10 week old male ERK5f/f and ERK5cko mice as previously described17.
An expanded methods section is available in the Supplement Data.
Results
Characterization of ERK5cko Mice
Using α myosin heavy chain (αMHC) promoter driven-Cre transgenic mice, we generated ERK5cko mice. Normal levels of ERK5 mRNA were present in brain and liver, whereas ERK5 mRNA was significantly reduced (approximately 86%) in the mutant ventricle (Supplement Figure IA). Immunoblot analysis of ventricular extracts from ERK5cko mice at 8 weeks old showed more than 80% deletion of ERK5 protein (Supplement Figure IB). It was evident that ablation of ERK5 was specific to the heart since protein levels of ERK5 were equivalent in brain, liver and skeletal muscle from both ERK5cko and ERK5f/f mice (Supplement Figure IB). The absence of ERK5 in the heart did not cause any compensatory changes in the protein levels of other MAPKs, such as ERK1/2, JNK, p38 and MEK5 (Supplement Figure IC). Furthermore, cardiac structure and contractile function were examined by histological analysis and echocardiography, respectively, and no differences were found between the two genotypes (data not shown).
ERK5cko Mice Displayed Less Cardiac Hypertrophic Remodeling Upon Pressure Overload
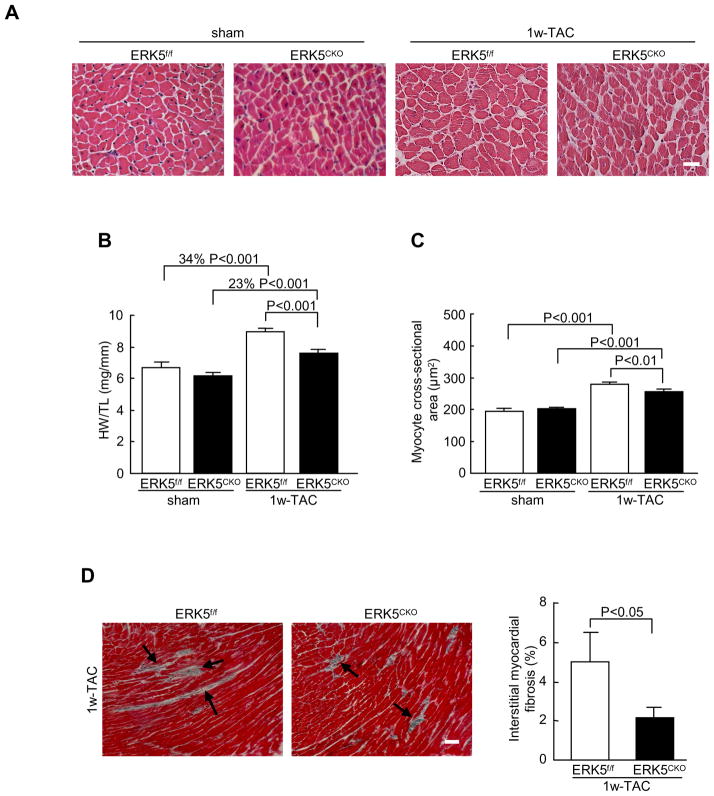
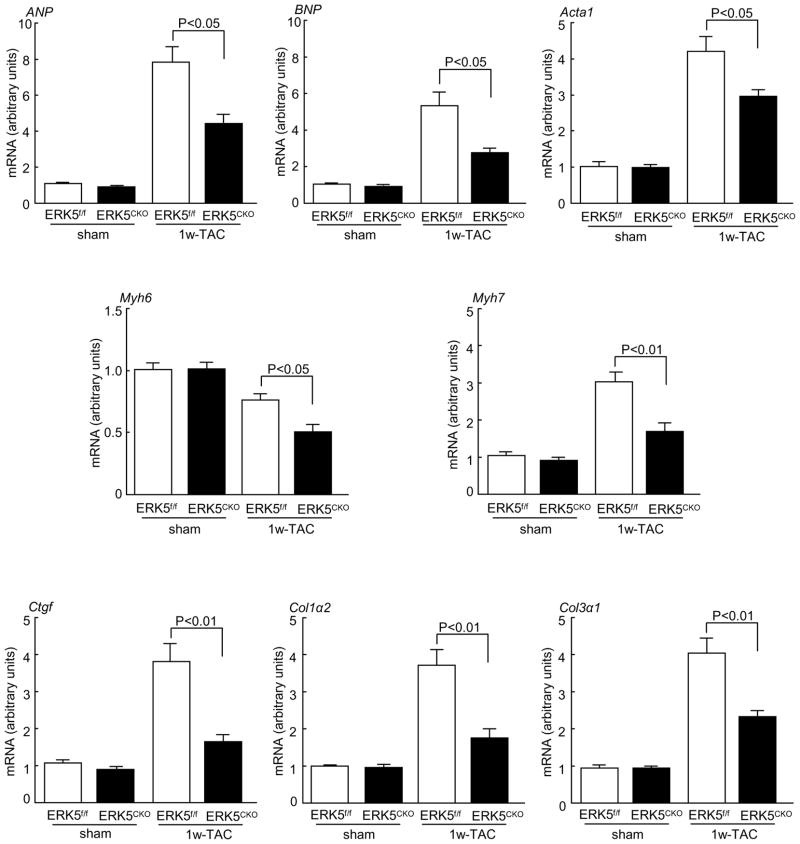
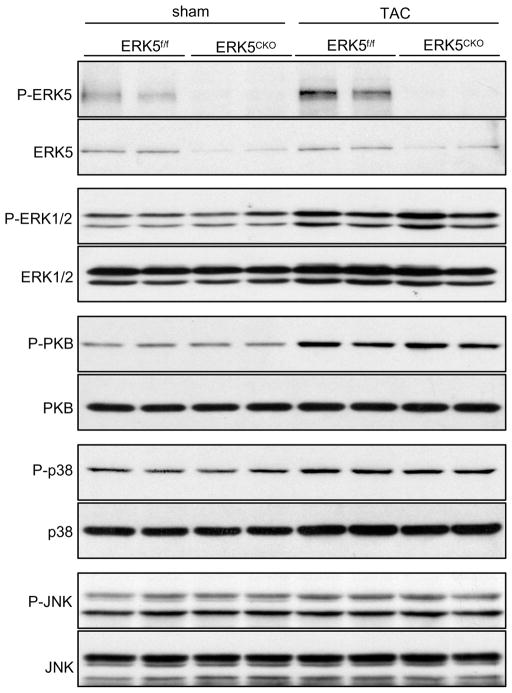
To determine whether ERK5 is required for pressure overload-induced cardiac hypertrophic remodeling, ERK5f/f and ERK5cko mice were subjected to pressure overload stimulation by Following 1 week of TAC, ERK5f/f mice showed a 34% increase in heart weight/tibia length (HW/TL). In contrast, ERK5cko mice showed only a 23% increase in HW/TL (Figure 1A and 1B). Consistent with these results, greater cross-sectional area of cardiomyocytes was observed in ERK5f/f-TAC mice (280.05±6.27 μm2), in comparison to 256.51±8.03 μm2 in ERK5cko-TAC mice (Figure 1C). Pressure overload-induced hypertrophy is often accompanied by interstitial fibrosis. Based on Masson’s trichrome staining, less ventricular fibrosis was seen in ERK5cko-TAC hearts (Figure 1D). Furthermore, cardiac structure and function were assessed by echocardiography. In response to TAC, ERK5cko mice showed a significant reduction in end-diastolic left ventricular posterior wall thickness (dPW) compared with the control group. Meanwhile, we also found in the mutant hearts a trend towards a decrease in fractional shortening (FS %), and a markedly slowed aorta maximum velocity (Ao Vmax), which is a sign of contractile dysfunction (Table 1). Fetal gene activation is a hallmark of hypertrophic remodeling. Shown in Figure 2, upregulation of the transcripts of the hypertrophic gene markers atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), skeletal α-actin (Acta1), and β myosin heavy polypeptide (Myh7) was remarkably blunted in the mutant hearts following TAC; we also found the mRNA level of α myosin heavy polypeptide (Myh6) was compromised in the mutant hearts (Figure 2). Consistent with Masson’s trichrome staining, the fibrosis marker genes connective tissue growth factor (Ctgf), procollagen type I, α2 (Col1α2) and procollagen type III, a3 (Col3α1) were significantly downregulated in ERK5cko-TAC mice compared with the controls (Figure 2). Finally, we investigated the activation of ERK5 and an array of hypertrophic regulators such as ERK1/2, PKB, p38 and JNK after TAC. ERK5 was activated by TAC in ERK5f/f hearts but not in ERK5cko hearts, meanwhile there was no difference observed in the activation of other hypertrophic regulators between the two genotypes although all were activated following TAC (Figure 3). These results suggest that the blunted hypertrophic response in the mutant mice is likely due to the absence of ERK5 in the heart.
Figure 1.
Less response to 1 week TAC induced-hypertrophy in ERK5cko mice. (A) Hematoxylin & eosin staining of heart cross-sections (scale bar: 20μm). (B) HW/TL ratios of ERK5f/f and ERK5cko mice were calculated following TAC or sham operation, n=7 to 9 per group. (C) The mean cross-sectional areas of cardiomyocytes from two genotypes were measured, n=5. (D) Masson’s trichrome-staining of cross-sections shows less interstitial fibrosis in ERK5cko hearts. Arrows indicate areas of fibrosis. Quantification of the relative area of fibrosis is expressed as percentage of the fibrosis area in the microscope views, n=7. Data are presented as mean ± SEM.
Table 1.
Echocardiographic assessment of ERK5f/f and ERK5cko mice after 1 week TAC.
| sham |
TAC |
|||
|---|---|---|---|---|
| ERK5f/f | ERK5CKO | ERK5f/f | ERK5CKO | |
| DPW (mm) | 0.75±0.03 | 0.75±0.06 | 0.99±0.05 | 0.84±0.03* |
| DIVS (mm) | 0.88±0.01 | 0.85±0.04 | 1.13±0.08 | 1.15±0.06 |
| LVEDD (mm) | 4.02±0.04 | 3.88±0.13 | 3.78±0.18 | 3.99±0.17 |
| LVESD (mm) | 2.66±0.04 | 2.69±0.09 | 2.55±0.17 | 2.95±0.24 |
| FS (%) | 34.1±0.5 | 30.4±2.1 | 32.8±1.9 | 27.1±2.9 |
| Ao Vmax [cm/s] | 63.4±5.3 | 62.7±2.7 | 65.7±4.3 | 56.3±2.1* |
| Ratio of E/A-Wave | 1.15±0.09 | 1.26±0.17 | 1.43±0.21 | 1.44±0.13 |
dPW, end-diastolic left ventricular posterior wall thickness; dIVS, end-diastolic interventricular septal wall thickness; LVEDD, diastolic left ventricular internal dimension; LVESD, systolic left ventricular internal dimension; FS, fraction shortening; Ao Vmax, aorta maximum velocity; E/A, ratio of the E/A wave. n=7 to 9 per group, data are presented as mean ± SEM,
P< 0.05, ERK5cko-TAC mice versus ERK5f/f-TAC mice.
Figure 2.
Quantitative real-time PCR analyses of gene markers associated with hypertrophy and fibrosis after 1 week TAC. The data are derived from three independent experiments performed in triplicate and are normalized to the GAPDH content, n=3 to 5 per group. Data are presented as mean ± SEM.
Figure 3.
Analysis of hypertrophic regulators in ERK5cko-TAC hearts. Protein extracts from ERK5f/f and ERK5cko hearts subjected to TAC or sham operation were examined by immunoblotting for total ERK5, ERK1/2, PKB, p38 and JNK expression, as well as their phosphorylation levels using specific antibodies. The results suggest that blunted hypertrophic response in the mutant mice is specifically due to the absence of ERK5 in the heart.
ERK5cko Cardiomyocytes Were More Vulnerable Under Hypertrophic Stress
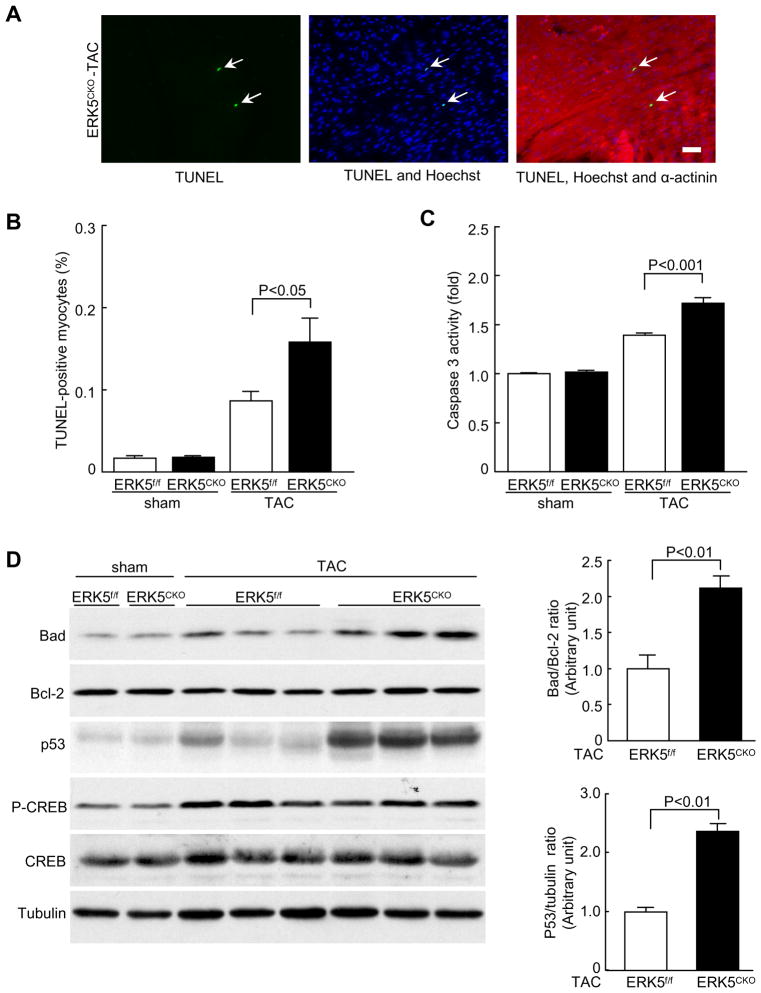
Sustained hypertrophic stress causes cardiomyocyte apoptosis which contributes to the transition of pathological hypertrophy to heart failure. To determine the effect of ERK5 on cardiomyocyte viability under hypertrophic stress TUNEL staining and caspase 3 activity assays were performed to detect cardiomyocyte apoptosis. We observed increased TUNEL-positive nuclei along with enhanced caspase 3 activity in the mutant hearts following TAC (Figure 4A–C). In accordance with this finding, ERK5cko-TAC mice showed augmented protein levels of Bad and p53, whereas the expression level of Bcl-2 was comparable in both genotypes (Figure 4D). A recent study demonstrated a protective role of ERK5 in sympathetic neurons through the phosphorylation of Ca2+/cAMP response element-binding protein (CREB), thereby suppressing the transcription of Bad6. However, we did not observe any change in the protein level and phosphorylation status of CREB in the mutant hearts (Figure 4D). Furthermore, in the two sham groups, no difference was detected in expression levels of these apoptosis-associated proteins (Supplement Figure II). Together, these data suggest that ERK5 is required for cardiomyocyte survival in response to pressure overload by suppressing the mitochondrion-dependent apoptotic pathway.
Figure 4.
ERK5 is required for cardiomyocyte survival in response to hypertrophic stress. (A) Increased apoptosis in ERK5cko ventricular myocardium after 1 week TAC was detected by TUNEL assay (scale bar: 50μm). Triple staining was performed: TUNEL (green), Hoechst (blue), α-actinin (red). Arrows point to TUNEL-positive nuclei. (B) The bar graph summarizes the percentage of TUNEL-positive nuclei in ERK5cko hearts compared with that in control hearts, n=7 to 11 mice per group. (C) Caspase 3 activity was measured by caspase activity assay after TAC, n=7. (D) Immunoblot analyses of protein levels of Bad, Bcl-2, p53, CREB and phosporylation of CREB by specific antibodies. Tubulin expression is the protein loading control. The ratios of Bad/Bcl-2 and p53/tubulin are represented by the bar graphs, n=4 per group. Data are mean ± SEM.
Prolonged Pressure Overload Caused Cardiac Dysfunction in ERK5cko Mice
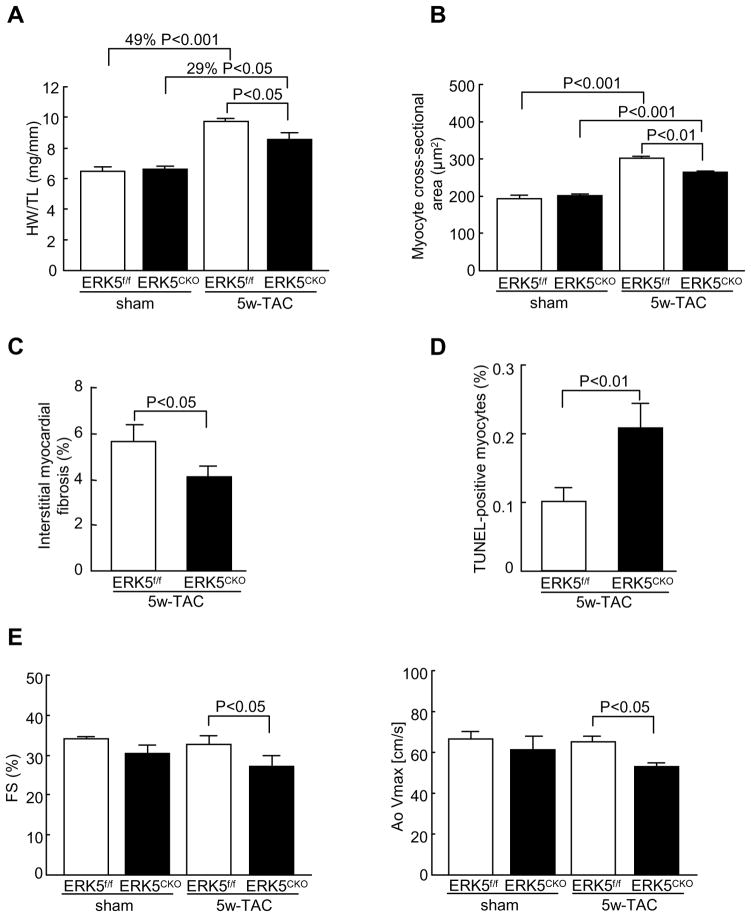
To further examine whether the lack of ERK5 in cardiomyocytes predisposes mice to heart failure following long-term pressure overload stimulation, ERK5f/f and ERK5cko mice were subjected to 5 weeks of TAC. Likewise, 5 weeks of TAC also caused compromised HW/TL ratio, smaller myocyte cross-sectional areas, and less fibrosis in ERK5cko mice compared with the controls (Figure 5A–C). Analysis of gene expression profile showed that mRNA levels of ANP, BNP, Myh7, Acta1, Ctgf, Col1α2 and Col3α1 were significantly lower in ERK5cko-TAC hearts (Supplement Figure III). Furthermore, a much more pronounced apoptosis was detected in ERK5cko cardiomyocytes (Figure 5D). Interestingly, we observed detrimental changes in ERK5cko cardiac function after 5 weeks of TAC, which was evident by significant decreases in FS % and AoVmax (Figure 5E). Collectively, these data demonstrate the functional importance of ERK5 in protecting the heart from failure following chronic pressure overload stimulation.
Figure 5.
Prolonged pressure overload caused diminished hypertrophic response and cardiac dysfunction in ERK5cko mice. (A) HW/TL ratios of ERK5f/f and ERK5cko mice following 5 weeks TAC or sham operation. (B) Quantification of the mean cross-sectional areas of cardiomyocytes from two genotypes. (C) Quantification of interstitial fibrosis induced by TAC. (D) TUNEL-positive nuclei in ERK5cko hearts were significantly more than that in the controls after 5 weeks of TAC. (E) Echocardiography assessment (FS %, AoVmax) demonstrated impaired cardiac function in ERK5cko mice. n=7 to 9 per group. Data are presented as mean ± SEM.
ERK5cko Mice Showed an Attenuated Cardiac Hypertrophic Response to Chronic β-adrenergic Stimulation
We next investigated whether ERK5 is required for cardiac hypertrophic remodeling in response to chronic β-adrenergic stimulation. After 7 days administration of isoproterenol, ERK5cko mice developed blunted hypertrophy reflected by a 32% increase in HW/TL, compared with the controls (48%). Consistently, ERK5cko mice showed a reduction in cross-sectional area (254.35±3.37 μm2), compared with ERK5f/f mice whose cardiomyocyte cross-sectional area was 306.7±5.4 μm2 after the ISO-treatment (Supplement Figure IV). Interstitial fibrosis was also more noticeable in the control hearts (Supplement Figure IV). Furthermore, the transcript levels of ANP and BNP were found to be remarkably elevated in the ERK5f/f hearts, compared with the mutant hearts (Supplement Figure IV). These results indicate that ERK5 regulates cardiac hypertrophic remodeling not only by pressure overload, but also by chronic β-adrenergic stimulation.
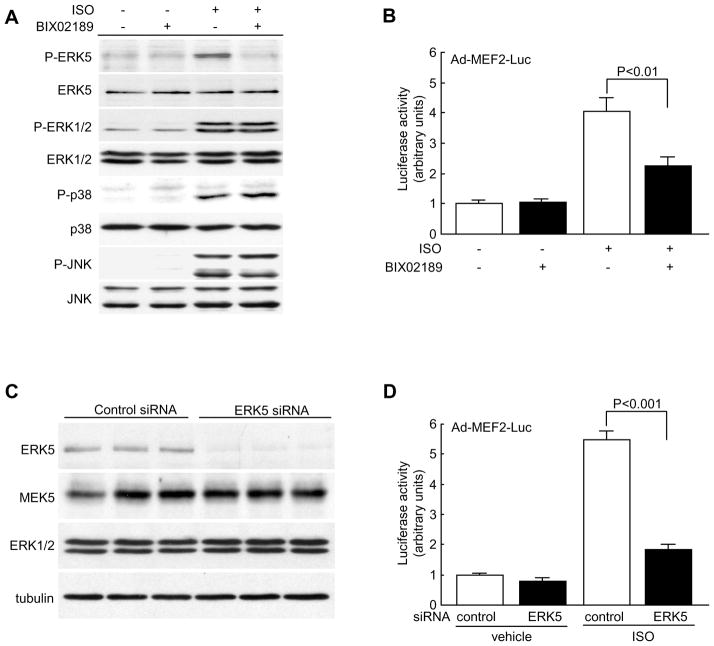
The Knockdown of ERK5 or Inhibition of Its Activation Suppressed MEF2 Transcriptional Activity and Promoted Apoptosis in NRCMs
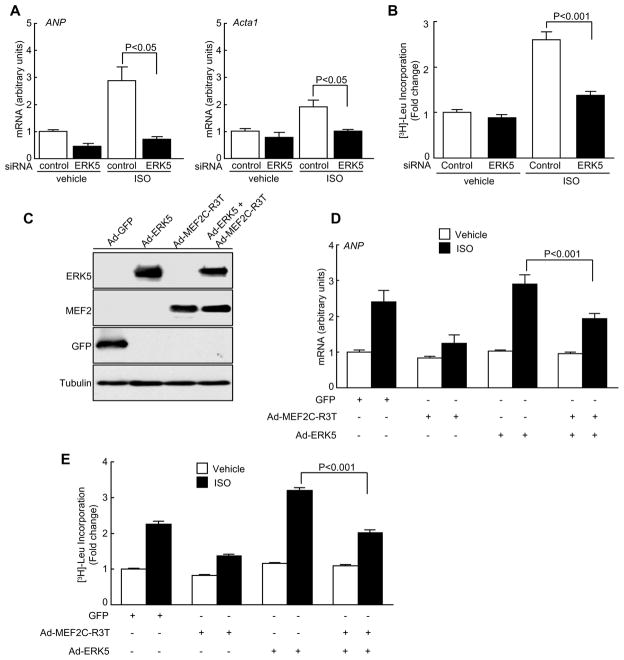
MEF2 transcription factor is a key regulator of pathological cardiac hypertrophy18. Led by the result that the transcript levels of Ctgf, Myh6 and Acta1 (known to be downstream targets of MEF218–20) were compromised in ERK5cko-TAC hearts we further examined whether MEF2 transcriptional activity was mediated by ERK5 in cardiomyocytes in response to hypertrophic stimuli. Using the MEK5/ERK5 selective inhibitor (BIX02189, 10μM), ERK5 phosphorylation was specifically inhibited which led to attenuated MEF2 activity in NRCMs following ISO treatment (Figure 6A, 6B). To corroborate this result, NRCMs were transfected with the reporter plasmid pG5E1bLuc together with a construct encoding Gal4, Gal4-MEF2A, or Gal4-MEF2D. As expected, reduced MEF2 transcriptional activity was detected in BIX02189-treated NRCMs after ISO stimulation (Supplement Figure V). In parallel, using an siRNA method we specifically suppressed endogenous ERK5 expression by 83% in NRCMs; a similar inhibitory effect on MEF2 transcriptional activity was also found (Figure 6C, 6D and Supplement Figure V). Furthermore, siERK5-treated NRCMs showed an impaired hypertrophic response which was evident by decreased mRNA levels of ANP and Acta1, and diminished protein synthesis (Figure 7A, 7B). To further critically examine the effect of MEF2 on ERK5 mediated-hypertrophic response, an adenovirus containing a non-DNA binding mutant form of MEF2C (Ad-MEF2C-R3T) was used to inhibit MEF2 activity8, 21. After ISO stimulation, we observed an increased rate of protein synthesis and elevated transcript level of ANP in Ad-ERK5-infected NRCMs; however, such hypertrophic increases were appreciably blunted by the co-infection of Ad-MEF2C-R3T (Figure 7C–E). Combined, these data strongly suggest that MEF2 acts downstream of ERK5 to promote a hypertrophic response. Lastly, we evaluated the effect of ERK5 knockdown or the inhibition of its kinase activation on apoptosis in NRCMs. Consistent with apoptosis results obtained from ERK5cko-TAC mice, increased apoptosis was detected in siERK5-treated or BIX02189-treated NRCMs upon exposure to the proapoptotic agent sorbitol, which confirms the protective role of ERK5 in cardiomyocytes (Supplement Figure VI).
Figure 6.
ERK5 is required for mediating MEF2 activity in hypertrophic cardiomyocytes. (A) Immunoblot analyses of expression and phosphorylation levels of ERK5, ERK1/2, p38 and JNK in BIX02189 treated-NRCMs. (B) NRCMs were infected with Ad-MEF2-Luc for 24h prior to the incubation of BIX02189. After 2h incubation with the inhibitor, the MEF2 transcriptional activity was measured by the luciferase reporter assay system following ISO stimulation. (C) NRCMs were transfected with ERK5 siRNA or negative control siRNA. At 72h after transfection, cell lysates were prepared for analysis of ERK5 protein level by immunoblotting. MEK5 and ERK1/2 protein levels were also examined to determine the specificity of ERK5 knockdown. (D) The MEF2 transcriptional activity was measured in siRNA-treated NRCMs following ISO treatment. n=6 per group. Data are mean ± SEM.
Figure 7.
The critical role of MEF2 in ERK5-mediated hypertrophy. (A) Quantitative real-time PCR analyses of hypertrophic gene markers in siRNA-treated NRCMs after ISO stimulation. The data are derived from three independent experiments performed in triplicate and are normalized to the GAPDH content, n=3. (B) [3H] Leucine incorporation was measured in siRNA-treated NRCMs after ISO treatment, n=6. (C) Immunoblot analyses of ERK5, MEF2C and GFP expression in adenovirual infected NRCMs. (D) NRCMs were infected with Ad-MEF2C-R3T, Ad-ERK5 or Ad-ERK5 together with Ad-MEF2C-R3T. Transcript level of ANP was measured after ISO treatment. (E) [3H] Leucine incorporation was measured in NRCMs infected with indicated adenoviruses before and after ISO stimulation, n=6. Data are mean ± SEM.
Discussion
The major finding of this study is that ERK5 plays an important role in promoting hypertrophic remodeling and cardiomyocyte survival. In the absence of ERK5, hypertrophic growth, interstitial fibrosis and fetal gene reactivation were profoundly compromised; meanwhile increased cardiomyocyte death was seen following hypertrophic stimuli. Further studies in NRCMs treated with either siERK5 or a novel ERK5 kinase inhibitor (BIX02189) have provided clear evidence that MEF2 is a critical component downstream of the ERK5 signaling pathway in regulating hypertrophic response.
The role of ERK5 in hypertrophic remodeling
ERK5 is implicated in the induction of cardiac hypertrophy and heart failure in humans10, 22; however, our understanding of its involvement in various aspects of cardiac remodeling is limited, and in some cases controversial. For example, previous studies using transgenic mice with cardiac-specific overexpression of activated MEK5 provided conflicting results8, 12, 13, which can be partially explained by the fact that different isoforms (α, β form) of MEK5 were used to generate the transgenic lines. An additional explanation is the transgenic approach, as phenotypes observed from transgenic mouse models are largely associated with the copy number of overexpressed genes. With respect to the fact that ERK5 may exert actions independent of its kinase activity23, it was hoped that the analysis of ERK5cko mice would generate more direct information on the specific contribution of ERK5 in the heart. In the present study, phenotypes observed from ERK5cko mice were reminiscent of observations in MEF2D-null mice19; they also demonstrated blunted hypertrophic growth and fibrosis, together with the downregulation of mRNA levels of MEF2-regulated genes. In line with these observations, enhanced hypertrophy was seen in mice with cardiac-specific overexpression of MEF2A after TAC20. Interestingly, ERK5 and p38 MAPK are able to phosphorylate and activate MEF2A and MEF2C, whereas MEF2D is a specific substrate of ERK53, 5. Of note, the major MEF2 isoforms in the adult heart are MEF2A and MEF2D, which participate in mediating different aspects of pathological cardiac signaling19, 20. Recent studies in conventional and cardiomyocyte-specific p38 knockout mouse models suggest that p38 does not promote cardiac hypertrophy and may, in fact, prevent it24, 25. Considering the above evidence, MEF2 is most likely to perform as a hypertrophic player downstream of ERK5, rather than p38. Furthermore, responding to isoproterenol stimulation, a diminution was found in MEF2 transcriptional activity in NRCMs in which ERK5 was exclusively knocked down or its kinase activation was specifically inhibited. In addition to this, reduced protein synthesis and downregulated mRNA levels of ANP and Acta1 were detected in siERK5-treated NRCMs following isoproterenol treatment. Moreover, the finding that the inhibition of MEF2 activity was able to attenuate the ERK5-regulated hypertrophic response strengthens the notion that MEF2 acts downstream of ERK5 in regulating hypertrophy.
CTGF is a key mediator of tissue fibrosis, which is predominantly expressed in fibroblasts; however, during cardiac remodeling it is also secreted by cardiomyocytes26, 27. Several lines of evidence indicate the important role of MEF2 in regulating CTGF expression19, 20, 28. In the present study, the decreased Ctgf mRNA level along with less interstitial fibrosis were seen in ERK5cko-TAC hearts, thus it is plausible to propose that absence of ERK5 in cardiomyocytes is likely responsible for such phenotypes via downregulated MEF2 activity. Taken together, these data provide unequivocal evidence that ERK5 is responsible for controlling MEF2 activity in different aspects of hypertrophic remodeling.
In parallel, we also investigated the activation of a number of hypertrophic regulators following TAC stress, including ERK1/229, p3824, JNK17 and PKB30. However, we did not observe any difference in their activities between the two genotypes; this underpins the notion that ERK5 is an important character in mediating hypertrophic remodeling. It is worth noting that residual hypertrophic growth seen in ERK5cko-TAC mice is likely due to the activation of ERK1/2 and PKB, which are known as promoters of hypertrophy29, 30.
ERK5 is required for cardiomyocyte survival
Apart from the altered hypertrophic response, we also detected an increase in apoptotic cardiomyocytes with enhanced Bad and p53 protein levels in TAC treated-mutant hearts, indicating that ERK5 prevents hypertrophy induced-cardiomyocyte loss. The ability of ERK5 to protect endothelial cells from shear stress via inhibition of Bad activation was established by Pi et al31. Furthermore, evidence has been provided that ERK5 is required to mediate neuronal survival by suppressing Bad expression via a mechanism dependent on CREB transcriptional activity6. Consistent with this study, we also found upregulated Bad expression in ERK5cko-TAC cardiomyocytes, although we did not observe any change in the protein level and phosphorylation status of CREB. CREB is a pro-survival transcription factor which either acts as an activator for increasing Bcl-2 expression or as a repressor of Bad expression6, 32. Interestingly, cardiomyocyte-specific inactivation of CREB did not cause any increase in apoptosis at basal level32; nevertheless, further studies are required to address the role of CREB in protecting cardiomyocytes following stress. Prolonged pressure overload is known to cause an accumulation of p53, which is a pivotal mediator of apoptosis33, 34. It has been demonstrated that p53 is able to induce a number of Bcl-2 family genes, including Bad34, 35. Thus, the upregulated p53 expression found in ERK5cko-TAC hearts is proposed to be responsible for the increased level of Bad. It is also worth noting that impaired contractility seen in the mutant hearts after TAC is very likely attributed to the increased apoptosis. Although the actual rate of apoptosis is low, it is, however, reasonable to believe that this level of apoptosis is sufficient to cause cardiac dysfunction36. As discussed, it is suggested that ERK5 is necessary for protecting cardiomyocyte against hypertrophic stress and for determining cardiac function during hypertrophic remodeling.
Novel pharmacological inhibitors of the MEK5/ERK5 pathway
ERK5 is one of the least studied MAPK subfamilies due, in part, to the lack of biological/pharmacological tools, including specific inhibitors. Recently, the MEK5/ERK5 selective inhibitors, BIX02188 and BIX02189 have been identified. BIX02189 is demonstrated to have greater potency in inhibiting ERK5 activity than BIX0218837, 38. In the present study, by applying BIX02189 to NRCMs, we detected that ERK5 phosphorylation was specifically blocked without affecting the activation of other MAP kinases; MEF2 transcriptional activity was also impaired. Thus, for the first time, our results provide important information regarding the effect of BIX02189 in cardiomyocytes. It has been demonstrated that ERK5 is required for tumor growth due to its essential role in the development of tumor vasculature39. Selectively targeting the ERK5 pathway by pharmacological compounds is thought to be a promising approach for treating cancers. Although, the availability of selective MEK5/ERK5 inhibitors may shed light in developing such a therapeutic strategy, it must nevertheless be considered that ERK5 has a role in many other tissues. Further investigation of these MEK5/ERK5 inhibitors in vivo under basal and various stress conditions is certainly required.
In conclusion, the present study provides convincing evidence that clarifies the in vivo role of ERK5 in the heart. ERK5 regulates hypertrophic remodeling and protects cardiomyocyes from hypertrophic stress mostly likely by regulating the MEF2 transcription activity. The recognition of the functional importance of ERK5 in the heart provides invaluable information regarding the possible beneficial or adverse effects of new therapeutic strategies.
An expanded discussion is available in online data.
Supplementary Material
Acknowledgments
We are indebted to Dr. Roger Snow (Department of Medical Chemistry, Boehringer Ingelheim Pharmaceuticals, Inc) for kindly providing us with the ERK5 inhibitor BIX02189.
Sources of Funding
This work was supported by the British Heart Foundation (PG/07/055/23144) to XW, EJC and AHW.
Non-standard Abbreviations and Acronyms
- αMHC
α myosin heavy chain
- Actal
skeletal α-actin
- Ad
adenovirus
- ANP
atrial natriuretic peptide
- Ao Vmax
, aorta maximum velocity
- BMK1
big mitogen-activated protein kinase1
- BNP
brain natriuretic peptide
- Camp
cyclic adenosine monophosphate
- Col1α2
procollagen type I, α2
- Col3α1
procollagen type III, α3
- CREB
Ca2+/cAMP response element-binding protein
- Ctgf
connective tissue growth factor
- dIVS
end-diastolic interventricular septal wall thickness
- dPW
end-diastolic left ventricular posterior wall thickness
- ERK5
extracellular signal-regulated protein kinase 5
- FS
fractional shortening
- HIF-1α
hypoxia inducible factor 1α
- HW/TL
heart weight/tibia length
- ISO
isoproterenol
- LIF
leukemia inhibitory factor
- LVEDD
diastolic left ventricular internal dimension
- LVESD
systolic left ventricular internal dimension
- MAPK
mitogen-activated protein kinase
- MEF2
myocyte enhancer factor 2
- MEK5
MAPK/ERK kinase 5
- Myh6
α myosin heavy polypeptide
- Myh7
β myosin heavy polypeptide
- NRCM
neonatal rat cardiomyocyte
- TAC
transverse aortic constriction
- TUNEL
Terminal-Deoxynucleotidyl-Transferase-Mediated-dUTP-Nick-End-Labeling
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None.
References
- 1.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Kondoh K, Terasawa K, Morimoto H, Nishida E. Regulation of nuclear translocation of extracellular signal-regulated kinase 5 by active nuclear import and export mechanisms. Mol Cell Biol. 2006;26:1679–1690. doi: 10.1128/MCB.26.5.1679-1690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Merritt AJ, Seyfried J, Guo C, Papadakis ES, Finegan KG, Kayahara M, Dixon J, Boot-Handford RP, Cartwright EJ, Mayer U, Tournier C. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegan KG, Wang X, Lee E-J, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 2009;16:674–683. doi: 10.1038/cdd.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe J. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kacimi R, Gerdes AM. Alterations in G protein and MAP kinase signaling pathways during cardiac remodeling in hypertension and heart failure. Hypertension. 2003;41:968–977. doi: 10.1161/01.HYP.0000062465.60601.CC. [DOI] [PubMed] [Google Scholar]
- 10.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 11.Nadruz W, Kogarg CB, Jr, Constancio SS, Corat PDC, Franchimi KG. Load-induced transcriptional activation of c-jun in rat myocardium regulated by myocyte enhancer factor 2. Circ Res. 2003;92:243–251. doi: 10.1161/01.res.0000053184.94618.97. [DOI] [PubMed] [Google Scholar]
- 12.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron SJ, Itoh S, Baines CP, Zhang C, Ohta S, Che W, Glassman M, Lee JD, Yan C, Yang J, Abe J. Activation of big MAP kinase 1 (BMK/ERK5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Letters. 2004;566:255–260. doi: 10.1016/j.febslet.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 14.Seyfried J, Wang X, Kharebava G, Tournier C. A novel mitogen-activated protein kinase docking site in the N terminus of MEK5α organizes the components of the extracellular signal-regulated kinase 5 signaling pathway. Mol Cell Biol. 2005;25:9820–9828. doi: 10.1128/MCB.25.22.9820-9828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron SJ, Abe J, Malik S, Che W, Yang J. Differential role of MEK5alpha and MEK5beta in BMK1/ERK5 activation. J Biol Chem. 2004;279:1506–1512. doi: 10.1074/jbc.M308755200. [DOI] [PubMed] [Google Scholar]
- 16.English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH. Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270:28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Zi M, Jin JW, Prehar S, Oceandy D, Kimura TE, Lei M, Neyses L, Weston AH, Cartwright EJ, Wang X. Cardiac-Specific Deletion of Mkk4 Reveals Its Role in Pathological Hypertrophic Remodeling, but Not in Physiological Cardiac Growth. Circ Res. 2009;104:905–914. doi: 10.1161/CIRCRESAHA.108.188292. [DOI] [PubMed] [Google Scholar]
- 18.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–1088. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Phan D, van Rooij E, Wang DZ, McAnally J, Qi X, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. The MEF2D transcription factor mediates stress-dependent cardiac remodelling in mice. J Clin Invest. 2008;118:124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD. Myocyte enhance factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J Biol Chem. 2006;281:9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 21.Molkentin JD, Black BL, Martin JF, Olson EN. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol Cell Biol. 1996;16:2627–2636. doi: 10.1128/mcb.16.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal s6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 23.Kasler HG, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevisky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. p38α Mitogen-Activated Protein Kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 27.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of MicroRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 28.Van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, Doevendans PA, Schneider MD, van Echteld CJA, De Windt LJ. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114:298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 30.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. AKT/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pi X, Yan C, Berk BC. Big Mitogen-Activated Protein Kinase (BMK1)/ERK5 protests endothelial cells from apoptosis. Circ Res. 2004;94:362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 32.Matus M, Lewin G, Stümpel F, Buchwalow IB, Schneider MD, Schütz G, Schmitz W, Müller FU. Cardiomyocyte-specific inactivation of transcription factor CREB in mice. FASEB J. 2007;21:1884–1892. doi: 10.1096/fj.06-7915com. [DOI] [PubMed] [Google Scholar]
- 33.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahar T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. P53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 34.Jiang P, Du W, Wu M. P53 and Bad: remote strangers become close friends. Cell Res. 2007;17:283–285. doi: 10.1038/cr.2007.19. [DOI] [PubMed] [Google Scholar]
- 35.Jiang P, Du W, Heese K, Wu M. The Bad cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26:9071–9082. doi: 10.1128/MCB.01025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foo RS-Y, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatake RJ, O’Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler SZ, Kashem MA, Kaplita P, Snow RJ. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun. 2008;377:120–125. doi: 10.1016/j.bbrc.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Tatake RJ, Natarajan K, Taba Y, Garin G, Tai C, Leung E, Surapisitchat J, Yoshizumi M, Yan C, Abe J, Berk BC. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun. 2008;370:159–163. doi: 10.1016/j.bbrc.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi M, Fearns C, Eliceiri B, Yang Y, Lee JD. Big Mitogen-Activated Protein Kinase 1/Extracellular Signal-Regulated Kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res. 2005;65:7699–7706. doi: 10.1158/0008-5472.CAN-04-4540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.