Abstract
Gallic acid, an organic acid, also known as 3,4,5-trihydroxybenzoic acid, is cytotoxic against certain cancer cells, without harming normal cells. The objective of this study is to evaluate whether gallic acid can inhibit glioma cell viability, proliferation, invasion and reduce glioma cell mediated angiogenesis. Treatment of U87 and U251n glioma cells with gallic acid inhibited cell viability in a dose- and time-dependent manner. BrdU and tube formation assays indicated that gallic acid significantly decreased glioma cell proliferation and tube formation in mouse brain endothelial cells, respectively. In addition, gallic acid decreased U87 cell invasion in vitro. Western blot analysis showed that expression of ADAM17, p-Akt and p-Erk was suppressed by gallic acid in both U87 and U251n cell lines. These data suggest that suppression of ADAM17 and downregulation of PI3K/Akt and Ras/MAPK signaling pathways may contribute to gallic acid-induced decrease of invasiveness. Gallic acid may be a valuable candidate for treatment of brain tumor.
Keywords: Gallic acid, glioma, proliferation, angiogenesis, invasion
1. Introduction
The incidence of brain tumors, compared with other cancers, is relatively rare (National Cancer Institute, 2008). Nevertheless, malignant brain tumors have a very high mortality rate. Glioblastoma multiforme is the most frequent primary malignant brain tumor in adults (CBTRUS, 2009). Due to their insidious invasion and extensive neovascularization, glioblastomas are characterized by recurrence (Pilkington, 1997). The diffusing glioma cells invade into the normal brain adjacent to the tumor, which may cause treatment failure by conventional therapy including surgery, radiotherapy, and chemotherapy (Silbergeld and Chicoine, 1997; Bjerkvig et al., 1997). The success of chemotherapy on glioblastoma patients is also hampered by the problem of drug-resistance. Therefore, it is of critical importance that an effective treatment of glioblastoma be developed (Tentori and Graziani, 2009).
Gallic acid is an active component of Chinese gall (Raina et al., 2008; Faried et al., 2007) and is widely distributed in the plant kingdom. Gallic acid can interfere at different stages of tumor development, e.g. it decreases the ornithine decarboxylase response linked to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate (Gali et al., 1991), suppresses tumor angiogenesis (Liu et al., 2006), and inhibits P815 cell metastasis to the liver (Ohno et al., 2001), and activator protein-1 transcriptional activity (Maggi-Capeyron et al., 2001). To date, there are very few papers about the effect of gallic acid in the glioma cells. Therefore, in the present study, we examined the effect of gallic acid in both human glioma U87 and U251n cells. Our results demonstrated that gallic acid reduced glioma cell viability, proliferation and invasion in glioma cells, and tube formation in normal mouse brain endothelial cells. Furthermore, gallic acid suppressed ADAM17 expression which may be associated with the inhibition of invasiveness through the inactivation of PI3K/Akt and Ras/MAPK signaling pathways.
2. Materials and methods
2.1 Materials
Gallic acid was purchased from Tianjin Yi & Fang Best Biotech Company in China. One hundred milligrams of gallic acid was dissolved in 1 ml of dimethyl sulfoxide (DMSO) as stock solution. This stock solution of gallic acid (100 mg/ml or 587.8 mM) was further diluted to appropriate concentrations with cell culture medium immediately before use. Control experiments contained DMSO.
2.2 Cell culture
Human glioblastoma U87, U251n and mouse brain endothelial cells were obtained from American Type Culture Collection (ATCC, Rockville, MD). All cell lines were maintained in DMEM containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, 50 μg/ml streptomycin, and 100 μg/ml amphotericin (Invitrogen). Cell cultures were maintained in 75 cm2 flasks and kept in a humidified atmosphere with 5% CO2 at 37°C.
2.3 MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diplenyltetrazolium bromide) assay
To measure cell viability, cells were seeded into 96-well plates at a density of 1×104 per well. After overnight incubation, the culture medium was removed and cells were rinsed with phosphate buffered saline (PBS) and incubated with different concentrations of gallic acid in complete medium. After 24 h of treatment, MTT was added to each well and incubated for an additional 4 h to allow mitochondrial dehydrogenase to convert MTT into insoluble formazan crystals. The medium was then aspirated, and formazan was solubilized by adding 150 μl of DMSO. The absorption of solubilized formazan was measured at the wavelength of 490 nm by an ELISA plate reader (EL340 microplate reader; Bio-Tek Instruments, Winooske, VT). To measure the dose- and time-dependent effects of gallic acid, one thousand cells were seeded onto 96-well plates and incubated overnight. After removing the culture medium, cells were rinsed with PBS and then treated with different concentrations of gallic acid. The cells were incubated for 24, 48, 72 or 96 h. MTT assay was performed at each time point for three independent experiments.
2.4 Sulforhodamine B (SRB) assay
The SRB assay was used for cell viability determination based on the measurement of cellular protein content (Voigt, 2005). Cells were seeded into 96-well plates at a density of 1×104 per well. After overnight incubation, the culture medium was removed and cells were rinsed with PBS followed by treatment with different concentrations of gallic acid. After 24 h of incubation, cells were fixed in 10% trichloroacetic acid for 1 h at 4°C and stained with 0.4% of SRB in 1% acetic acid for 30 min. The excess dye was removed by washing repeatedly with 1% acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution and optical density was determinied at 510 nm using a microplate reader.
2.5 Bromodeoxyuridine (BrdU) proliferation assay
Ten thousand cells in 200 μl complete medium were placed into 8-well chambers. After overnight incubation, the culture medium was removed and cells were rinsed with PBS followed by treatment with various concentrations of gallic acid for 24 h. The cells were incubated with BrdU (40 μg/ml) for 2 h and fixed in 4% paraformaldehyde for 30 min at room temperature. Following fixation, cells were incubated with 50% formaldehyde in 2× SSC at 65°C for 30 min and with 2N HCl at 37°C for 10 min. After incubation with 0.1 M boric acid at room temperature for 3 min, cells were rinsed with PBS and blocked with 1% bovine serum albumin at room temperature for 1 h, followed by incubation with an anti-BrdU antibody overnight at 4°C. Cells were then incubated with a FITC-conjugated secondary antibody to visualize BrdU positive labeled cells. Before mounting with coverslips, cells were incubated with 10 μg/ml of DAPI (4′-6-Diamidino-2-phenylindole, Invitrogen) for 10 min. Four fields of cells were counted randomly in each well under a fluorescent microscope at 10 × magnification. The proliferation rate was expressed as the percentage of BrdU positive labeled cells divided by DAPI labeled cells.
2.6 Wound scratch assay
Cells were seeded at a density of 5×105 per well in 6-well plates in complete medium. After treatment with various concentrations of gallic acid, the monolayers were scratched with a 1-ml plastic pipette tip to create a uniform wound. The wound area was then examined after 8 h of incubation under a phase-contrast microscope at 5× magnification. Photographs of three random fields were taken and the cell migration ability was expressed by the closure of gap distance.
2.7 Invasion assay
BD BioCoat matrigel invasion chambers were used to examine the ability of U87 cells to penetrate the extracellular matrix (ECM). Cells (5×104), after being treated with gallic acid for 24 h, were re-suspended in 500 μl of serum-free medium and added to the upper chamber while the lower chamber was filled with 0.5 ml of complete medium containing FBS, which served as a chemo-attractant. Cells were then incubated for 24 h at 37°C. After removal of cells on the upper surface of the membrane, cells on the lower surface of the membrane were stained with CellTracker™ Green (Molecular Probes, Eugene, OR) for 45 min and fixed in 4% formaldehyde. Four fields of cells were counted randomly in each well under a fluorescent microscope at 200 × magnification. Data was expressed as the percentage of invasive cells as compared with the control. All the experiments were performed in duplicates and results were expressed as mean ± SEM of three independent experiments.
2.8 Tube formation assay
ECM gel (100 μl) was added to each well of a 96-well plate and then incubated for 30 min at 37°C to allow the ECM solution to form a gel. Mouse brain endothelial cells were seeded into the 60 mm dishes at a density of 3×105. After treatment with indicated concentrations of gallic acid for 24 h, 2.5×104 cells were re-suspended in 150 μl of complete medium, seeded onto the solidified ECM gel, and incubated for 2–8 h. The endothelial tubes of five random fields were examined under a phase-contrast microscope, and the extent of tube formation was estimated by inspecting the overall tube length per area.
2.9 Western blot analysis
U87 and U251n cells (5×105 cells) were seeded onto the 60 mm dishes. After treatment with indicated concentrations for 24 h, cells were harvested and rinsed with PBS followed by extraction in 200 μl RIPA lysis buffer (50 mM Tris HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% SDS and 0.01% sodium azide, pH of 7.4). Equal amounts of proteins, as determined by the BCA protocol (Pierce, Rockford, IL), were run on 10% Tris-Glycine gels (Invitrogen) and then transferred to PVDF membranes (Whatman). The membranes were blocked with 0.1% I-Block (Applied Biosystems, Foster, CA) in PBS-T (0.1% Tween-20) at room temperature for 1 h, followed by incubation with primary antibodies against ADAM17 (Abcam, Cambridge, MA), Akt and p-Akt (Cell signaling, Danvers, MA), Erk, p-Erk and actin (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The membranes were washed with PBS-T and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA). Following washing, the specific proteins were detected using a SuperSignal West Pico chemiluminescent protein detection kit (Pierce). Each experiment was repeated three times. The densities of the bands were analyzed using the Scion image software (Frederick, MD).
2.10 α-Secretase activity assay
ADAM17 possesses α-secretase activity and cleaves amyloid precursor protein at the α-secretase recognition site. An α-secretase activity kit (R&D System) was used to determine ADAM17 proteolytic activity after treatment with gallic acid. Following treatment, U87 cells were collected and lysed with cell extraction buffer. The protein content was determined by BCA protein assay. Total protein (50 μg per sample) was used in the assay, according to the manufacturer’s instructions. Fluorescence was quantified using a Fusion Universal Multi-plate reader (Packard Bioscience, Meriden, CT), with excitation between 335 and 355 nm and emitted light collected between 495 and 510 nm. Data were presented as fluorescence intensity values.
2.11 Statistical analysis
Data were presented as mean and standard error. Statistical significance was analyzed by one-way ANOVA using the GraphPad Prism software (version 4.0). P value smaller than 0.05 (P<0.05) was considered significant.
3. Results
3.1 Gallic acid reduces the viability of glioma cells
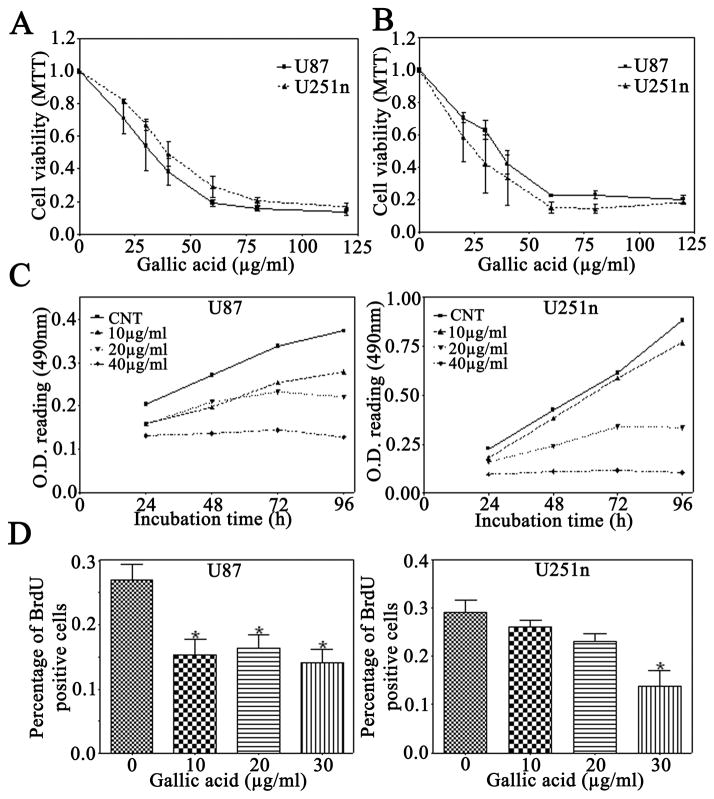
Gallic acid is an effective chemopreventive agent in vivo and in vitro (Agarwal et al., 2006; Faried et al., 2007; Raina et al., 2008). After treatment with gallic acid for 24 h, cell viability was dramatically decreased in both U87 and U251n glioma cells as examined by MTT (Fig. 1A) and SRB assays (Fig. 1B). In comparison with the cytotoxic effect on U87 and U251n glioma cells, gallic acid exhibited less cytotoxicity on normal mouse brain endothelial cells. Gallic acid reduced cell viability to approximately 70%, 54%, and 38% of control in U87 cells and to approximately 81%, 67%, and 49% of the control in U251n cells at the concentrations of 20, 30, and 40 μg/ml, respectively. However, at the same concentrations, gallic acid only reduced the cell viability to approximately 95%, 91%, and 89% of the control, respectively, in mouse brain endothelial cells (data not shown). These results provide evidence that gallic acid has selective dose-dependent cytotoxicity for glioma cells.
Figure 1.
Effect of gallic acid on cell viability and proliferation of glioma cells. A: MTT assay of U87 and U251n glioma cells treated with various concentrations of gallic acid. B: SRB assay of U87 and U251n glioma cells treated with the same concentrations. C: U87 and U251n were treated with the indicated concentration of gallic acid for up to 96 h in complete medium. MTT assay was used to assess growth of cells in culture. D: BrdU proliferation assay of U87 and U251n cells treated with different doses of gallic acid for 24 h. The cell nuclear incorporation of BrdU was measured. The proliferation rate was presented as mean ± SEM value of percentage from BrdU-labeled cells vs. DAPI-labeled cells (data obtained from three independent experiments). *P<0.01, control vs. treated groups.
3.2 Gallic acid inhibits proliferation of glioma cells
To determine anti-proliferative effect of gallic acid on glioma cells, U87 and U251n cells were treated with gallic acid at the concentrations of 0, 10, 20, or 40 μg/ml, and MTT assay was performed at 24, 48, 72, and 96 h (Fig. 1C). Compared with control groups at 0 μg/ml, gallic acid elicited significant inhibition in the proliferation of both U87 and U251n cells in a dose-dependent manner, but did not significantly and time-dependently inhibit proliferation of both glioma cells. U87 cells were more sensitive to gallic acid than U251n cells at the concentration of 10 μg/ml.
To further determine the effect of gallic acid on cell proliferation, U87 and U251n cells were treated with 10, 20 and 30 μg/ml of gallic acid for 24 h and BrdU assay was performed (Fig. 1D). The percentage of BrdU positive U87 cells was reduced from 26% of control group to 14% after treatment with gallic acid at the concentration of 30 μg/ml. Similarly, the percentage of BrdU positive U251n cells was reduced from 28% of control group to 15% after treatment with gallic acid at the same concentration. Similar to the results in Fig. 1C, U87 cells were more sensitive to gallic acid than u251n cells at low concentrations. These results suggest that gallic acid inhibits glioma cell proliferation.
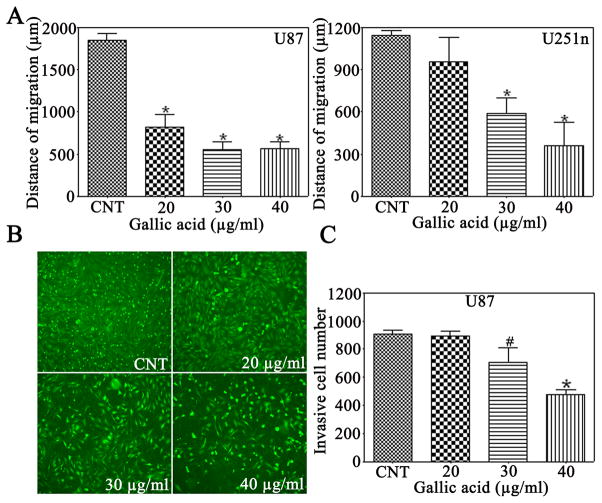
3.3 Gallic acid reduces glioma cell migration and invasion
Gliomas are characterized by insidious invasion to adjacent normal brain tissue (Prados and Levin, 2000). To investigate the effect of gallic acid on glioma cell migration and invasion ability, a wound-scratch assay and transwell inserts coated with Matrigel were used. Different cell lines showed different sensitivity to gallic acid. As compared with the control group, the gap distance was significantly reduced in U87 cells by gallic acid at the concentration of 20 μg/ml (Fig. 2A). However, the gap distance was not significantly decreased at the same concentration in U251n cells. At the concentrations of 30 and 40 μg/ml, gallic acid dramatically inhibited cell migration to 29% and 30% of the control in U87 cells, and 51% and 31% of the control in U251n cells, respectively. Invasiveness is an important characteristic of glioma cells and target of anti-cancer agent development (Pervaiz, 2002). As shown in Figure 2B & 2C, gallic acid caused a significant reduction of invasiveness (P<0.05) to approximately 50% of the control of U87 at a concentration of 40 μg/ml.
Figure 2.
Gallic acid inhibits U87 and U251n cell migration and reduces invasiveness of U87 cells. A: In the wound scratch assay, the migration ability was presented as mean ± SEM of the migration distance. B: Microscopy images of detected cells that migrated into the lower chamber (magnification ×200). C: The cell migration was quantified by the cell number from treated groups versus control group. #P<0.05, *P<0.01, as compared with the control group.
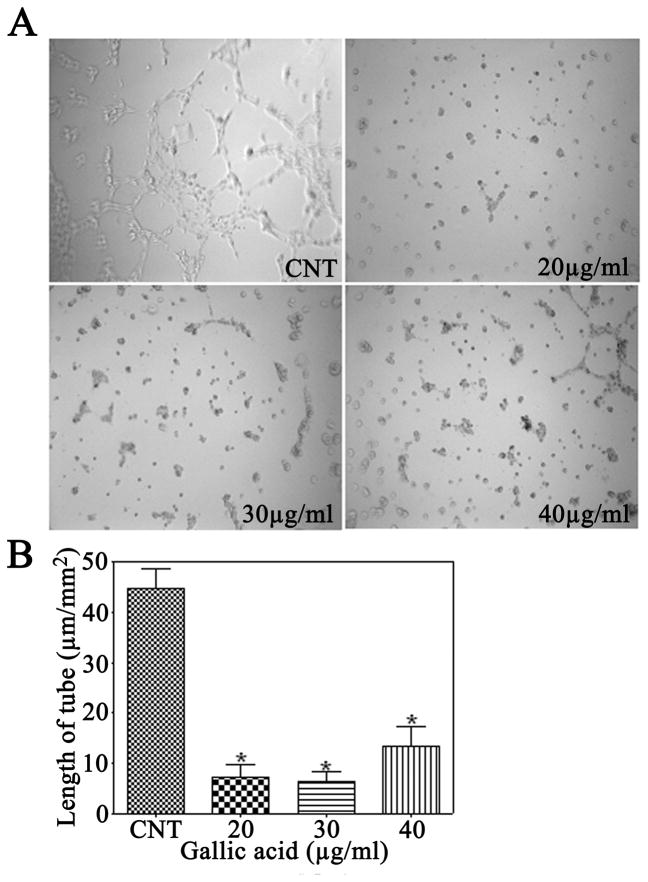
3.4 Gallic acid inhibits tube formation
Glioma induces extensive neovascularization in adjacent tissue (Prados and Levin, 2000); we, therefore, investigated whether gallic acid had the ability to inhibit tube formation in mouse brain endothelial cells treated for 24 h. Control groups treated with medium only were composed of multiple cells that gathered together and adhered to each other. However, gallic acid inhibited the elongation of tubes at all concentrations of 20, 30, and 40 μg/ml, and the tube length per area was 8.7%, 7.5%, and 15.9% of the control group, respectively (Fig. 3).
Figure 3.
Effect of gallic acid on mouse brain endothelial cell tubulogenesis in vitro. A: Representative photomicrographs during the tube formation of mouse brain endothelial cells pretreated with indicated concentrations of gallic acid for 24 h. B: The ability to form tubes was expressed as ratios of length of formed tubes per picture field. *P<0.01, as compared with the control group.
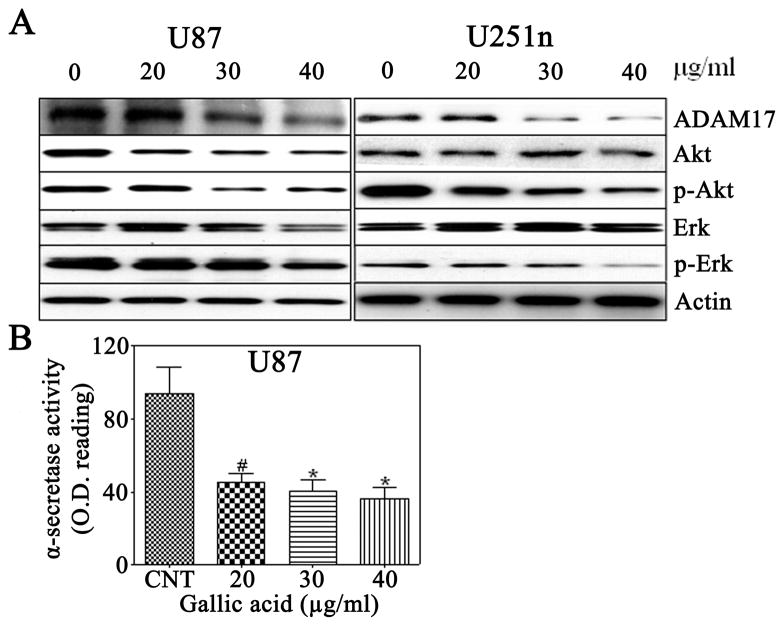
3.5 Gallic acid suppresses ADAM17 activity and expression in glioma cells
Previous studies have reported that ADAM17 contributes to cancer cell invasiveness through activation of PI3K/Akt (Zheng et al., 2007; Zheng et al., 2008) and Ras/MAPK/Erk signal transduction pathways (Tan et al., 2005) To determine whether gallic acid inhibits glioma cell invasion through the reduction of ADAM17, the expression of ADAM17, Erk/p-Erk, and Akt/p-Akt was analyzed by Western blotting in U87 and U251n glioma cells treated with gallic acid (20, 30, 40 μg/ml) for 24 h. In both U87 and U251n cells, ADAM17, p-Erk, and p-Akt were significantly reduced by gallic acid (Fig. 4A). To further confirm the effect of gallic acid on ADAM17, ADAM17 activity was measured with an α-secretase activity assay kit. Treatment of U87 cells with 20, 30, and 40 μg/ml of gallic acid for 24 h decreased ADAM17 activity to 47.9% (p<0.05), 43.5% (p<0.01), and 38.9% (p<0.01) of the control, respectively (Fig. 4B). These results suggest that gallic acid may reduce tumor invasiveness through downregulation of ADAM17, p-Erk, and p-Akt.
Figure 4.
A: Western blot analysis of expression of ADAM17, Erk/p-Erk and Akt/p-Akt in U87 and U251n cells. The cells were treated with 20, 30, and 40μg/ml gallic acid for 24 h and subjected to immunoblotting with antibodies against ADAM17, Erk/p-Erk, and Akt/p-Akt. Actin was used as sample loading controls. B: Effect of gallic acid on ADAM17 activity in U87 cells. #P<0.05, *P<0.01 as compared with the control cells.
4. Discussion
Our study has shown that gallic acid significantly reduces U87 and U251n glioma cell viability after 24 h of treatment in a dose-dependent manner, as examined by MTT and SRB assays. As compared with the cytotoxic effect on glioma cells, gallic acid shows a substantially reduced toxicity on normal mouse brain endothelial cells at the same concentrations. These results suggested that gallic acid has selective cytotoxicity in glioma. Our data provide direct evidence that gallic acid inhibits cell proliferation in glioma cells. There is a correlation between the MTT cell viability assay and the BrdU incorporation assay. U87 cells were more severely affected by gallic acid at 10 μg/ml than U251n cells. p53 increases the sensitivity of human glioblastoma cells to chemo-therapeutic drug celecoxib and proliferation of U87 cells could be significantly inhibited more than U251n (Kang et al., 2006, 2009). It has been reported that U87 contains wild-type p53 while U251n contains the mutant form (Cerrato et al., 2001; Lee et al., 2006). This suggested that p53 might play a role in the different cellular response to gallic acid. Previous studies have reported that gallic acid induces apoptosis in human prostate cancer cells (Agarwal et al., 2006), esophageal cancer cells (Faried et al., 2007), and human HL-60 promyelocytic leukemia cells (Madlener et al., 2007). By employingHoechst 33342 staining, flow cytometry, and caspase-3 activity assay, gallic acid did not induce significant apoptosis after 24 h treatment on glioma cells (data not shown).
Neoangiogenesis, the growth of new capillaries in response to pro-angiogenic factors secreted by glioma cells due to a lack of oxygen and nutrients, plays a crucial role in tumor growth (Chi et al., 2007; Lamszus et al., 2005). Gallic acid has been reported to be partially responsible for the anti-angiogenesis activities of Rubus leaf extract in vitro (Liu et al., 2006). A human placental vein angiogenesis model assay showed that gallic acid displays a dose-dependent inhibition of human angiogenic initiation and neovessel growth. Moreover, serum from rats with i.p. administration of 250 mg Rubus leaf extract significantly inhibited angiogenic initiation and subsequent neovessel growth that had initiated an angiogenic response(Liu et al., 2006). However, serum of the rats treated orally with the extract did not significantly inhibit angiogenic initiation and neovessel growth. Therefore, we selected an in vitro tube formation assay for the angiogenesis study. Cell viability of normal brain endothelial cells is reduced less by gallic acid than in U87 and U251n cells at the concentrations of 20, 30, and 40 μg/ml. However, gallic acid dramatically decreases the tube formation rate in normal brain endothelial cells at all three concentrations. Based on our data, gallic acid may be useful for targeting against angiogenesis, but further clarification of the underlying mechanisms is required.
In this study, we used the wound scratch assay to assess the motility of U87 and U251n cells and the matrigel invasion assay to assess the ability of U87 cells to penetrate the ECM. The data show that the motility of U87 and U251n cells as well as the invasion potential of U87 cells is significantly reduced by gallic acid. To investigate the underlying mechanisms of decreased invasive potential, the expression of ADAM17, Erk/p-Erk, and Akt/p-Akt was analyzed by Western blotting in U87 and U251n cells. ADAM17, p-Akt, and p-Erk expression levels are suppressed by gallic acid in both cell lines. In addition to the downregulation of its expression, the activity of ADAM17 is also significantly reduced by gallic acid at all concentrations tested.
ADAMs are best known as ectodomain sheddases, and their domains function as metalloproteases. The ADAM family belongs to one of the Zn-dependent metalloproteinases (Lu et al., 2008; Kheradmand and Werb, 2002). ADAM17 is an important member of the ADAM family and is involved in proteolysis of collagen IV of the ECM and the release from the cell surface of several integrins, suggesting that ADAM17 influences the invasive activity of different cells including glioma cells (Wildeboer et al., 2006). ADAM17 is a primary upstream component for multiple EGFR pro-ligands (Zheng et al., 2007; Zheng et al., 2008). EGFR binding with ligands subsequently activates MEK/ERK and PI3K/Akt pathways, which contribute to invasiveness and other malignant phenotypes (Tsatas et al., 2002). Gallic acid significantly downregulates the phosphorylation of members of both PI3K/Akt and Ras/MAPK signal transduction pathways, which have been implicated in cell proliferation, invasion and survival. These results suggest that suppression of ADAM17 by gallic acid may be responsible for decreased invasiveness through the downregulation of PI3K/Akt and Ras/MAPK pathways.
In summary, our data demonstrate that gallic acid significantly decreases cell viability, proliferation, invasion and tube formation. Suppressing ADAM17 may contribute to the inhibition of invasiveness through the inactivation of PI3K/Akt and Ras/MAPK signaling pathways. This study suggests that further investigations on the use of gallic acid as a treatment for glioma are warranted.
Acknowledgments
The work was in part supported by a Central Research Grant from the Hong Kong Polytechnic University and a NIH grant (Feng Jiang).
References
- Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- Chi A, Norden AD, Wen PY. Inhibition of angiogenesis and invasion in malignant gliomas. Expert Rev Anticancer Ther. 2007;7:1537–1560. doi: 10.1586/14737140.7.11.1537. [DOI] [PubMed] [Google Scholar]
- Faried A, Kurnia D, Faried LS, Usman N, Miyazaki T, Kato H, Kuwano H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int J Oncol. 2007;30:605–613. doi: 10.3892/ijo.30.3.605. [DOI] [PubMed] [Google Scholar]
- Kheradmand F, Werb Z. Shedding light on sheddases: role in growth and development. Bioessays. 2002;24:8–12. doi: 10.1002/bies.10037. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Brockmann MA, Eckerich C, Bohlen P, May C, Mangold U, Fillbrandt R, Westphal M. Inhibition of glioblastoma angiogenesis and invasion by combined treatments directed against vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and vascular endothelial-cadherin. Clin Cancer Res. 2005;11:4934–4940. doi: 10.1158/1078-0432.CCR-04-2270. [DOI] [PubMed] [Google Scholar]
- Liu Z, Schwimer J, Liu D, Lewis J, Greenway FL, York DA, Woltering EA. Gallic acid is partially responsible for the antiangiogenic activities of Rubus leaf extract. Phytother Res. 2006;20:806–813. doi: 10.1002/ptr.1966. [DOI] [PubMed] [Google Scholar]
- Lu X, Lu D, Scully M, Kakkar V. ADAM proteins - therapeutic potential in cancer. Curr Cancer Drug Targets. 2008;8:720–732. doi: 10.2174/156800908786733478. [DOI] [PubMed] [Google Scholar]
- Maggi-Capeyron MF, Ceballos P, Cristol JP, Delbosc S, Le Doucen C, Pons M, Leger CL, Descomps B. Wine phenolic antioxidants inhibit AP-1 transcriptional activity. J Agric Food Chem. 2001;49:5646–5652. doi: 10.1021/jf010595x. [DOI] [PubMed] [Google Scholar]
- Pervaiz S. Anti-cancer drugs of today and tomorrow: are we close to making the turn from treating to curing cancer? Curr Pharm Des. 2002;8:1723–1734. doi: 10.2174/1381612023394025. [DOI] [PubMed] [Google Scholar]
- Pilkington GJ. The paradox of neoplastic glial cell invasion of the brain and apparent metastatic failure. Anticancer Res. 1997;17:4103–4105. [PubMed] [Google Scholar]
- Prados MD, Levin V. Biology and treatment of malignant glioma. Semin Oncol. 2000;27:1–10. [PubMed] [Google Scholar]
- Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci. 2002;9:282–288. doi: 10.1054/jocn.2001.1063. [DOI] [PubMed] [Google Scholar]
- Voigt W. Sulforhodamine B assay and chemosensitivity. Methods Mol Med. 2005;110:39–48. doi: 10.1385/1-59259-869-2:039. [DOI] [PubMed] [Google Scholar]
- Wildeboer D, Naus S, Amy Sang QX, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol. 2006;65:516–527. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, Zhang ZG, Yang H, Chopp M. Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 2007;98:674–684. doi: 10.1111/j.1349-7006.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Jiang F, Katakowski M, Zhang X, Jiang H, Zhang ZG, Chopp M. Sensitization of cerebral tissue in nude mice with photodynamic therapy induces ADAM17/TACE and promotes glioma cell invasion. Cancer Lett. 2008;265:177–187. doi: 10.1016/j.canlet.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]