Abstract
Purpose
Determine the β1/β3 integrin-mediated pathways that regulate cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells. CLANs form in glaucomatous and steroid-treated TM cells which may contribute to reducing outflow facility through the TM.
Methods
Expression of CD47 (an αvβ3 integrin co-receptor/thrombospondin-1 receptor) and integrins αvβ3 and β1 was assessed by FACS. CLANs were induced by plating cells on fibronectin (a β1 integrin ligand) in the absence or presence of the β3 integrin-activating mAb AP-5 and identified by phalloidin labeling. The role of Src kinases, PI-3 Kinase (PI-3K), Rac1 and CD47 was determined by incubating cells with the inhibitors PP2 and EPA (Src kinases), LY294002 (PI-3K), or NSC23766 (Rac1). Tiam1 and Trio siRNAs and dominant-negative Tiam1 were used to determine which Rac1-specific guanine nucleotide exchange factor was involved. The role of CD47 was determined using the thrombospondin-1-derived agonist peptide 4N1K and the CD47 function blocking antibody B6H12.2.
Results
HTM cells expressed CD47 and integrins αvβ3 and β1. β3 integrin or CD47 activation significantly increased CLAN formation over β1 integrin-induced levels while anti-CD47 mAb B6H12.2 inhibited this increase. PP2, NSC23766 and Trio siRNA decreased β3-induced CLAN formation by 72%, 45% and 67% respectively while LY294002 and dominant negative Tiam1 had no effect. LY294002 decreased β1 integrin-mediated CLAN formation by 42% and PP2 completely blocked it.
Conclusions
Distinct β1 and αvβ3 integrins signaling pathways converge to enhance CLAN formation. β1-mediated CLAN formation was PI3-K-dependent while β3-mediated CLAN formation was CD47- and Rac1/Trio-dependent and may be regulated by thrombospondin-1. Both integrin pathways were Src-dependent.
Keywords: cytoskeleton, trabecular meshwork, steroid glaucoma, CLANs, integrins, CD47, Trio
Introduction
It is well established that in both humans and animals, treatment with a glucocorticoid (GC) such as dexamethasone (DEX) can increase intraocular pressure (IOP) both in vivo and in cultured anterior segments1–8. In some cases this can cause damage to the optic nerve and result in a steroid-induced glaucoma (SIG). Studies in cultured anterior segments3 and cultured trabecular meshwork (TM) cells9–11 treated with DEX have suggested that steroid treatment can lead to a rearrangement of the actin cytoskeleton into cross-linked actin networks (CLANs) that resemble geodesic domes or polygonal actin networks12–14. CLANs have also been observed in cultured TM cells and in TM cells in isolated meshworks from glaucomatous donor eyes in the absence of any DEX treatment5, 15 which suggests these actin structures are involved in the pathogenesis of SIG as well as other forms of primary open angle glaucoma (POAG)3, 9, 11, 16. CLANs have also been found in normal TM cells in isolated meshworks albeit at a lower frequency than in glaucomatous TMs15.
The function of CLANs in the TM remains unclear at this time. CLANs can be found in both spreading12, 17 18 and non-spreading cells9, 19, 20 and were originally thought to be precursors to actin stress fibers12 or reorganized sarcomeres21. It has been suggested that CLANs are specialized structures that participate in maintaining cellular tensegrity22. Recently, it has been suggested3 that CLAN formation in TM cells may reduce the contractility of the tissue by increasing the rigidity of the cells and thus rendering them unable to change shape and “relax” under pressure. Alternatively, CLAN formation could be impacting other actin mediated biological processes of the TM that are required for normal outflow facility such as attachment to the extracellular matrix (ECM), phagocytosis, and gene expression16, 23.
CLANs are made up of interconnected F-actin bundles (spokes) radiating outward from central vertices (or hubs). The vertices appear to be composed of molecular complexes (vertisomes) composed of α-actinin, syndecan-4, phosphatidylinositol 4,5-bisphosphate (PIP2) and filamin in addition to actin17. Outside of the vertisomes, filamin, myosin and tropomyosin localize along the F-actin bundles12–14, 17. In TM cells, CLAN formation can be controlled by cooperative signaling between β1 and β3 integrins in the absence of steroid treatment17 as well as by TGF-β2 (Hoare, M.-J., IOVS, 2009, 49, ARVO E-Abstract 4876).
Integrins are transmembrane receptors that consist of a heterodimer of α and β subunits. They recognize ECM proteins by binding to the amino acid sequence Arg-Gly-Asp (RGD) or its homologues within a given protein. Signaling from integrins is dependent upon the formation of supra-molecular complexes with both integral or peripheral membrane proteins and cytoplasmic molecules. These complexes provide bidirectional signaling that allows integrins to transduce extracellular signals to the actin cytoskeleton and within the intracellular environment (outside in signaling) as well as intracellular signals to the outside environment (inside out signaling). Thus, the specific arrangement of molecules associated with integrins form an important physical link between the extracellular and intracellular environment that regulates cell function and the organization of the actin cytoskeleton24, 25.
One potential component in this supramolecular signaling complex is CD47 (Integrin-Associated Protein, IAP)26–29. CD47 was initially identified as a 50 kDa protein associated with αvβ3 integrin signaling and later shown to be a receptor for the carboxyl terminal domain of thrombospondin-1 (TSP1)26–29. It is an atypical member of both the immunoglobulin superfamily and the G-protein-coupled receptor (GPCR) family of membrane proteins. Although CD47 has only five transmembrane domains, rather than the seven that are typical of GPCRs, it has been suggested that a complex formed by CD47 and an integrin heterodimer such as αvβ3 could function as a GPCR26–29. It is unknown if CD47 expression in HTM cells is altered in response to GC treatment, however, it has been shown that expression of its ligand TSP1 is increased in DEX- or TGF-β1-treated HTM cells30. In addition to associating with αvβ3, CD47 can also interact with integrins αIIbβ3, α4β1 α5β1 and α2β1, however, CD47 appears to differentially regulate β1 and β3 integrin signaling28, 31. A CD47 antibody that blocks β3 integrin signaling causes activation of β1 integrin signaling suggesting differences in the physical interaction of this receptor with different integrin species. The CD47/integrin complex regulates such activities as platelet activation, cell motility, adhesion, migration, phagocytosis, cytokine synthesis and integrin crosstalk28, 32. The molecular basis for how CD47 affects integrin signaling is unclear, since it is not found in focal adhesions with integrins. However, αvβ3/CD47 signaling complexes can be found either in33 or out of lipid rafts34. Data suggest that CD47 may modulate integrin signaling by activating Gαi-containing heterotrimeric GTPases and stimulating phosphorylation of FAK (focal adhesion kinase) and the Src kinase Lyn28, 33, 35.
In this study we used various pharmacological agents, activating and dominant negative peptides, function blocking antibodies and siRNAs to examine the signaling components utilized by β1 and β3 integrins to promote CLAN formation in HTM cells. These data indicate that the cooperative β1 and β3 integrin signaling pathways that enhance CLAN formation in TM cells spread on a fibronectin are distinct. The β1 integrin-mediated pathway is dependent upon Src kinases and PI3-K. The β3 integrin-mediated pathway is also dependent upon Src kinases along with Rac1, the Rac1 GEF Trio, and CD47. The studies also suggest CD47 activation by a TSP1-specific peptide may play a central role.
Methods
Cell culture
The A7-1, N27TM-1 and N27TM-2 strains of HTM cells were isolated from a 30 year old and two different 27 year old donors, respectively, with no known history of ocular disease as previously described36–38. Both N27TM cell strains were found to perform similarly to the A7-1 strain in our spreading assay although the basal level of CLAN formation were lower in these strains relative to that seen in A7-1 HTM cells. The A7-1 strain was used in all experiments except where noted in the figure legends. Cells were cultured in low glucose DMEM (Sigma, St. Louis, MO), 15% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 2 mM L-glutamine (Sigma), 1% amphoteracin B (Mediatech, Herndon, VA), 0.05% gentamicin (Mediatech) and 1 ng/mL FGF-2 (Peprotech, Rocky Hill, NJ)36, 37.
Fluorescence Activated Cell Sorting scan (FACScan) analysis
FACS analysis was performed as described previously39. Cells were incubated with anti-human CD47 (clone 2D3, eBioscience, San Diego, CA), anti-human αvβ3 (LM609, Millipore Corp., Temecula, CA) or anti-human β1 integrin (Hb1.1, Millipore Corp.) at 2 μg/mL prepared in Tris-buffered saline (20 mM Tris pH 7.6, 150 mM NaCl) with 1% bovine serum albumin (BSA) for 30 min on ice. Following incubation with FITC-conjugated anti-mouse IgG (Pierce) diluted 1:200 in TBS containing 1% BSA, the cells were analyzed by FACScan caliber flow cytometer (Becton-Dickinson, Franklin Lakes, NJ).
Spreading assays
The spreading assays were performed using duplicate determinations as described previously17. Prior to re-plating onto coverslips pre-coated with 20 nM fibronectin, suspended cells were pre-incubated for 30–60 minutes in the absence or presence of 0.25, 1, 5 or 20 μM of the Src family kinase (SFK) inhibitor PP2 (EMD Biosciences, Inc., San Diego, CA), 60 or 120 μM of the selective SFK inhibitor cis-5,8,11,14,17-eicosapentaenoic acid (EPA, Sigma), 20 μM of the phosphatidylinositol 3-kinase (PI3-K) inhibitor LY294002 (EMD Bioscience, Inc.), 20 μM of the Rac1 inhibitor NSC2376640 (kindly provided by Yi Zheng, Children's Hospital Research Foundation, Cincinnati, OH), or 100 nM of the dominant negative (DN) form of the Rac1 guanine nucleotide exchange factor (GEF) Tiam1. Cells pre-treated with DMSO were used as controls for PP2, EPA and LY294002 treatments. Treated cells were then plated in the absence or presence of 8 μg/mL mAb AP-5 which activates β3 integrins. In some experiments, cells were plated in the presence of 100–200 μg/mL CD47 agonist peptide 4N1K (KRFYVVMWKK) derived from the carboxyl-terminal domain of TSP127, 200μg/mL control peptide 4NGG (KRFYGGMWKK) or 20 μg/mL CD47 function blocking mAb B6H12.2 (Abcam, Inc., Cambridge, MA). All cells were allowed to spread for 3 hours and then fixed with 4% p-formaldehyde plus 0.18% TritonX-100 for 30 minutes. Human plasma fibronectin was prepared as described41.
Immunofluorescence microscopy and quantification of CLANs and cell area
Fixed cells were labeled with Alexa Fluor®488-conjugated phalloidin and Hoechst 33342 (both from Invitrogen, Carlsbad, CA) to visualize F-actin and nuclei respectively. DN-Tiam1 was localized in cells using Monoclonal ANTI-FLAG® M2 mouse primary antibody (Sigma) and the Alexa Fluor® 546 goat anti-mouse IgG secondary antibody (Invitrogen). Fluorescence was observed with an epifluorescence microscope (Zeiss Axioplan 2) equipped with a digital camera (Axiocam HRm) and image acquisition software (Axiovision ver. 4.5).
To quantify the number of CLAN-positive cells (CPCs), five to eight low-power (200X) fluorescence images from each treatment group were captured. The minimum requirement for an actin structure to be counted as a CLAN was the presence of three intensely fluorescent vertices connected by three actin spokes. Representative images of what we counted as CLANs are shown in Figure 1. The number of CPCs per image, along with the total number of cells, was counted to calculate the percentage of CPCs per image. Data were pooled from 3 experiments for each treatment and represent the mean percentage of CPC ± the standard deviation (s.d.) of the mean. To determine any effects on cell spreading, the cell areas from 6–8 of the images captured from the groups treated with or without PP2 were analyzed using Axiovision software (Figure 3C). All data were pooled from 3 experiments and represent the mean cell area ± s.d. of the mean. Statistical analysis comparing the different treatment groups for CLAN formation or for cell area was performed using ANOVA (figures 3, 4 and 6) or Student's t-test (figure 5). Where pairs of treatment groups had to be compared (figures 3 and 4), ANOVA analysis was used in conjunction with the Tukey HSD test.
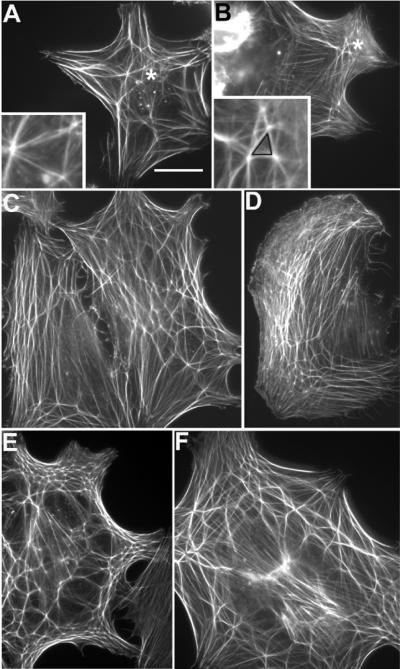
Figure 1. Representative images of CLANs.
Panels A–F show the various types of CLANs observed in all the treatment groups (A) Minimal structure for a CLAN; asterisk: region shown in inset. (B) A small CLAN; asterisk: region shown in inset. The black triangle illustrates the minimum requirement for CLAN designation. (C) Two CPCs side by side. The cell on the left contains a slightly larger CLAN than in (B) while the one on the right contains a larger, more extensive CLAN. (D) A moderately sized CLAN. (E & F) Two examples of large extensive CLANs are shown. There appeared to be a general tendency for higher order CLANs to be more frequent in cells treated with mAb AP-5. No attempt was made, however, to quantify any differences in CLAN size with respect to specific treatments. The N27TM-1 HTM strain was used to acquire these images. Bar in panel (A) = 20 μm.
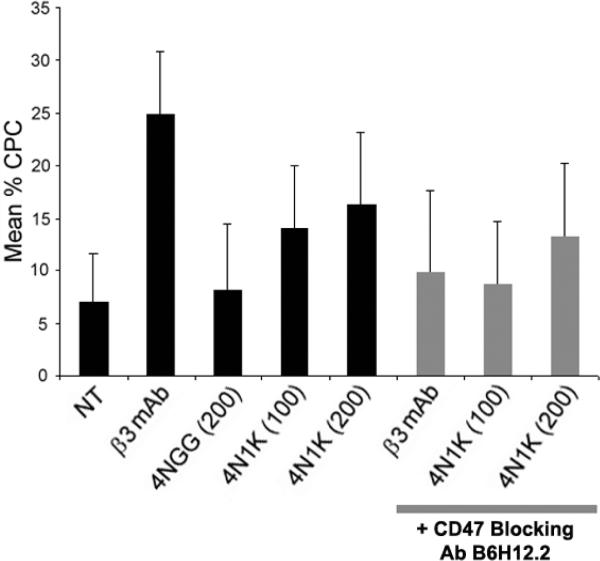
Figure 3. CD47 regulates CLAN formation through αvβ3 integrin.
HTM cells were plated onto fibronectin-coated coverslips in the absence (NT) or presence of soluble β3 integrin activating mAb AP-5, 4NGG or 4N1K; other treatment groups also included soluble CD47 blocking antibody B6H12.2 together with mAb AP-5 or the peptides. Cells were fixed and labeled with phalloidin. The percentage of CLAN-positive cells (CPC) is shown as the mean ± s.d.; n ranged from 3,139–2,439 for each group. The percent CPC in β3 mAb- or 4N1K-treated (100 and 200 μg/mL) cells was significantly greater than untreated cells or cells treated with the 4NGG control peptide respectively (p < 0.0001). The percent CPC in mAb AP-5 treated cells was significantly greater than either concentration of peptide 4N1K by itself (p < 0.01). The percent CPC in cells treated with CD47 blocking antibody B6H12.2 plus mAb AP-5 was significantly less that cells treated with mAb AP-5 only (p < 0.0001). The percent CPC in cells treated with mAb B6H12.2 plus 100 μg/mL 4N1K was significantly less than cells treated with 100 μg/mL 4N1K only (p < 0.01). The percent CPC in cells treated with mAb B6H12.2 plus 200 μg/mL 4N1K was significantly greater than in untreated cells, cells treated with the control 4NGG peptide or cells treated with mAb B6H12.2 plus 100 μg/mL 4N1K (p <0.01). There was no statistical difference between untreated cells, 4NGG-, B6H12.2 plus AP-5-, and B6H12.2 plus 100 μg/mL 4N1K-treated cells.
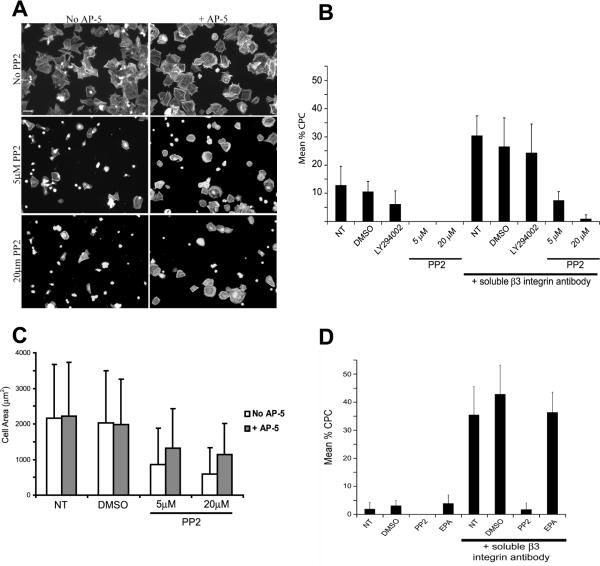
Figure 4. β1- and β3-mediated CLAN formation are dependent upon a Src family kinase while only β1-mediated CLAN formation is PI3-K dependent.
Cells were plated onto fibronectin-coated coverslips, fixed and labeled with phalloidin. (A) Representative photomicrographs of A7-1 HTM cells treated with or without 5 or 20 μM PP2 in the presence or absence of mAb AP-5. Scale bar = 50 μm. (B) CLAN formation in A7-1 HTM cells spread in the absence (NT) or presence of 0.1% DMSO, 20 μM PI3-K inhibitor LY294002 or the SFK inhibitor PP2 with or without soluble mAb AP-5. β1-mediated CLAN formation was significantly inhibited by LY294002 or PP2 (5 and 20 μM) (p < 0.01) compared to controls. β3- mediated CLAN formation (mAb AP-5 only) was significantly greater than untreated cells (p < 0.0001). Although, β3-mediated CLAN formation was statistically less than in AP-5 only-treated cells when LY294002 was added (p < 0.01), there was no statistical difference between the vehicle treated cells (β3 mAb + 0.1% DMSO) and cells treated with β3 mAb + LY294002. (C) Areas A7-1 cells spread in the absence or presence of 0.1% DMSO or PP2 (5 or 20 μM) with or without mAb AP-5. Spreading in the presence of 5 or 20 μM PP2 was statistically less than control cells (p < 0.0001) when β3 mAb AP-5 was absent. Cell spreading in the presence of AP-5 and either 5 or 20 μM PP2 was statistically greater than cells treated with PP2 alone (p < 0.0001). (D) CLAN formation in spread N27TM-1 cells in the absence or presence of 0.1% DMSO, 20 μM PP2 or 60 μM EPA with or without mAb AP-5. β1-mediated CLAN formation (no AP-5) in the presence of EPA was not statistically different from vehicle treated cells while β3-mediated CLAN formation in the presence of EPA was statistically less than vehicle treated cells (p < 0.01). The percentage of CPC is shown as the mean ± s.d.; n ranged from 2,899–1,146 for each group.
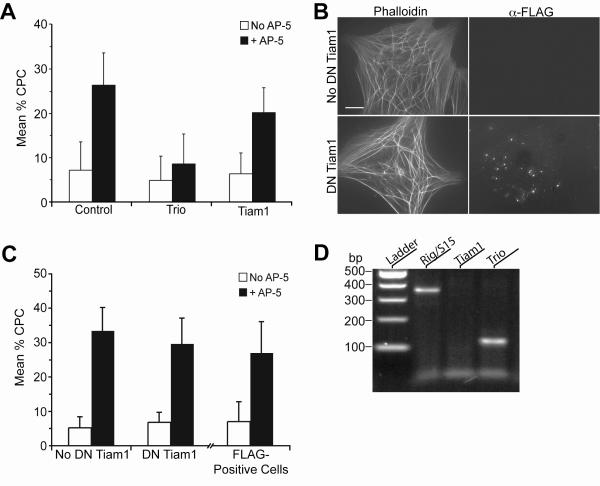
Figure 6. The Rac1 GEF Trio, not Tiam1, regulates β3-mediated CLAN formation.
N27TM-2 HTM cells were plated onto fibronectin-coated coverslips in the presence or absence of β3 integrin activating mAb AP-5. Cells were fixed, labeled with phalloidin, and stained for the FLAG-tag (panel B only), as described in Materials and Methods. (A) Quantification of CLANs in cells transfected with 125 nM Trio, Tiam1, or non-targeting control siRNA. The percentage of CPC is shown as the mean ± s.d.; n ranged from 1,273–1,794 for each group. The mean percentage of CPC in β3 antibody + Trio siRNA-treated cells is significantly less than in β3 antibody alone-treated cells (p < 0.001). (B) Photomicrograph showing CLAN formation in N27TM-1 cells transduced with DN-TAT-Tiam1 and treated with soluble mAb AP-5. Scale bar = 20 μm. (C) Quantification of CLANS in N27TM-1 cells that spread in the absence or presence of DN-TAT-Tiam1. CLAN formation was also determined in cells that were Tiam1 positive, as determined by the presence of FLAG-tag staining. The percentage of CPC is shown as the mean ± s.d.; n ranged from 2,899–1,146 for each group. There was no significant difference between untreated cells and those transduced with DN-TAT-Tiam1 that were positive for the FLAG-tag. (D) 2.5% agarose gel stained with ethidium bromide showing endogenous expression of Trio and the house-keeping gene Rig/S15 in N27TM-2 cells. Tiam1 expression was not detected. PCR primers for Trio, Tiam1, and Rig/S15 amplify 122, 97, and 361 bp fragments, respectively.
Figure 5. β3-mediated CLAN formation is dependent upon Rac1/Tiam1 or Rac1/Trio signaling while β1-mediated CLAN formation is not.
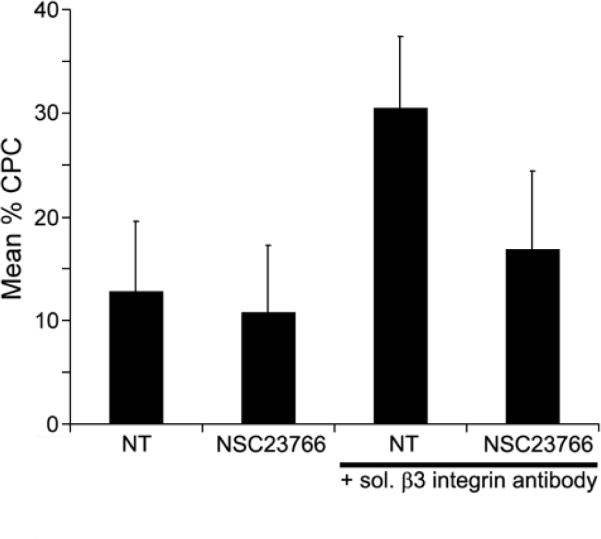
HTM cells were plated onto fibronectin-coated coverslips in the absence (NT) or presence of 20 μM Rac1 inhibitor NSC23766 with or without soluble β3 integrin activating mAb AP-5. Cells were fixed and labeled with phalloidin. The percentage of CPC is shown as the mean ± s.d.; n ranged from 2,328-1,964 for each group. The mean percentage of CPC in the presence of AP-5 and NSC23766 was significantly less than in the presence of AP-5 alone (p = 0.0001).
siRNA-mediated silencing
siRNA against human Trio (ON-TARGETplus SMARTpool L-005047-00-0005), human Tiam1 (ON-TARGETplus SMARTpool L-003932-00-0005), and a non-targeting control (ON-TARGETplus siCONTROL Non-targeting Pool D-001810-10-05) was obtained from Dharmacon (Lafayette, CO). Differentiated monolayers of N27TM-2 cells were transfected with 125 nM siRNA using Lipofectamine™2000, per manufacturer's protocol. After 48 hours, a spreading assay was performed and CPCs were quantified, as described above.
RNA extraction and RT-PCR
Total RNA was extracted from siRNA-transfected N27TM-2 cells using the Qiagen RNeasy™ Plus Mini Kit (Valencia, CA), according to the manufacturer's instructions. The RNA was then reverse transcribed with the RETROscript™ Kit (Applied Biosystems, Foster City, CA). Each reaction was performed using 2 μg RNA, 5 μM random decamers, 500 μM of each dNTP, 10 U RNase inhibitor, and 100 U MMLV reverse transcriptase. Reactions were incubated at 44°C for 1 hour and then 92°C for 10 minutes. After first strand synthesis, the cDNA was amplified by PCR using 1 U Platinum™ Taq DNA Polymerase (Invitrogen, Carlsbad, CA), 1.5 mM MgCl2, 125 μM of each dNTP, and 5 μM gene specific primer pairs. The sequences for the Trio and Tiam1 primers that were used have been published previously18. Amplification conditions were: 94°C for 1 minute, 30 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, followed by a final extension at 72°C for 5 minutes. The PCR products were separated on a 2.5% agarose gel and stained with ethidium bromide.
Cloning, Expression, and Purification of DN-Tiam1
N-terminally truncated Tiam1 cDNA (C1199) was used as a template for polymerase chain reaction amplification of a DNA sequence encoding amino acids 393–854 of Tiam1. The Tiam1 cDNA was a gift of Dr. John G. Collard (The Netherlands Cancer Institute, Amsterdam, The Netherlands). The sense primer, 5'-AATAACCGGTATGAGTACCACCAACAG-3', generated an AgeI restriction site (in boldface), and the antisense primer, 5'-AATAGCATGCTCACGCGTCTGACTTCTCAA-3', introduced a SphI restriction site (in boldface) and stop codon (underlined). The amplified DNA was ligated into the pGEX-PTD4-FLAG vector provided by Dr. Jennifer Faralli (University of Wisconsin, Madison, WI) and contains a PTD4 transduction sequence (YARAAARQARA)42 and a FLAG tag (DYKDDDDK) upstream of the multiple cloning site. The vector was introduced into E. coli BL21-CodonPlus™ competent cells (Stratagene, La Jolla, CA) according to the manufacturers' instructions.
Expression of the FLAG tagged TAT-DN-Tiam1 (DN-Tiam1) fusion protein was induced with 0.4 mM isopropyl β-D-thiogalactopyranoside at 30°C, for 3 hours. Following induction, the bacteria were resuspended in buffer consisting of 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 4 mM KCl, and 0.1 mM DTT and then lysed with 0.5 mg/mL lysozyme, and 25 μg/mL DNaseI for 20 minutes. This was followed by sonication at 20% for 5 minutes in pulse mode. The lysate was incubated with 1% Triton X-100 for 20 minutes and the insoluble material was sedimented by centrifugation at 10,000 × g for 30 minutes at 4°C. The DN-Tiam1 was isolated from the clarified lysate by affinity chromatography on glutathione Sepharose 4B, followed by on-column cleavage by thrombin and incubation with p-aminobenzamidine Sepharose 6B, as described previously43.
The biological activity of the recombinant DN-Tiam1 was confirmed by examining its effect on cell morphology and cell contacts in monolayers of immortalized human TM-1 cells44. DN-Tiam1 caused extensive cell rounding of TM-1 cells when they were transduced with DN-Tiam1 (not shown). This activity is consistent with the role that Tiam1 plays in maintaining cell-cell adhesions45.
Results
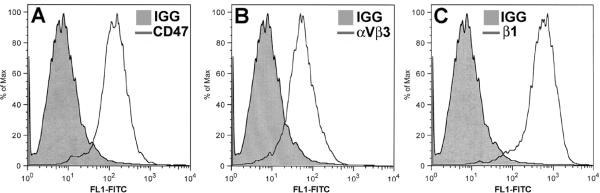
CD47 participates in β3 integrin-mediated CLAN formation
Previous studies demonstrated that the basal level of CLANs formed by plating cells onto a β1 integrin binding substrate is enhanced by activation of β3 integrin using the activating β3 mAb AP-517. Since CD47 is a known co-receptor for β3 integrin, FACs analysis was performed in order to determine if CD47 is present on the surface of TM cells and thus could also be involved. Figure 2 shows that HTM cells express CD47 in addition to αvβ3 andβ1 integrins. To see if CD47 participated in CLAN formation, HTM cells were plated in the presence or absence of the CD47 agonist peptide 4NIK27 which contains a peptide sequence from the cell-binding domain of TSP1. The peptide 4NGG was used as a control. Figure 3 shows that both 100 and 200 μg/mL of 4N1K caused an approximately 2-fold increase (p < 0.0001) in CLAN-positive cells (CPCs) relative to untreated cells or cells treated with the 4NGG control peptide while the β3 integrin-activating mAb AP-5 caused a 3.5-fold increase in CPCs relative to untreated cells (p < 0.0001). The increase in CPCs in the presence of mAb AP-5 was significantly greater than that seen in the presence of either 4N1K concentration (p < 0.01). In contrast, treatment with the control peptide 4NGG showed only 7–8% of the cells as CLAN positive which is comparable to the level of CLAN formation previously observed in untreated cells17. Treatment of HTM cells with either 4N1K or 4NGG did not have any obvious effect on cell spreading compared to untreated cells or cells treated with mAb AP-5 (not shown).
Figure 2. FACS analysis of HTM cells for expression of CD47, αvβ3 and β1 integrins.
HTM cells were labeled with either non-specific mouse IgG or antibodies against (A) CD47, (B) αvβ3 integrin or (C) β1 integrins prior to FACS analysis. Primary antibodies were detected with FITC-conjugated anti-mouse IgG.
To further confirm that CD47 plays a role in β3 integrin-mediated CLAN formation, HTM cells were incubated with the CD47 function blocking mAb B6H12.227. As shown in Figure 3, this mAb not only blocked 100 μg/mL 4N1K-induced CLAN formation as expected (p < 0.01), but also completely blocked AP-5 induced CLAN formation (p < 0.01). The affect of mAb B6H12.2 could be overcome by increasing the concentration of the 4N1K peptide to 200μg/ml. These data suggest that modulation of β3 integrin activity by activating CD47 with the TSP1 peptide promotes CLAN formation in HTM cells.
β1 integrin, but not β3 integrin, utilizes a PI-3 Kinase dependent pathway to mediate CLAN formation
To determine if β1 and β3 integrins differentially regulated CLAN formation, we examined the role of several signaling molecules known to be involved in integrin signaling. The first molecules examined were members of the Src family kinases (SFK). SFK members are important regulators of integrin signaling and are one of the first signaling molecules activated upon integrin engagement46. To assess the potential role that SFK members played in regulating CLAN formation we used the inhibitor PP2 which inhibits all SFKs47–50. As shown in Figure 4A and 4C, cell spreading in the presence of 5 or 20 μM PP2 was strikingly impaired compared to untreated cells or cells treated with DMSO alone. Both 5 μM and 20 μM PP2 decreased the average area of cell spreading by 58% and 71% (p < 0.0001), respectively, and not surprisingly, CLAN formation was completely absent (Figure 4B) even in cells that exhibited partial spreading in the presence of PP2.
To determine if β1 integrin-mediated CLAN formation could be separated from cell spreading, experiments were repeated using lower concentrations of PP2 (0.25 μM and 1 μM PP2). These concentrations of PP2 effectively blocked CLAN formation by greater than 10-fold (p < 0.01) compared to control cells. In untreated cells or 0.1% DMSO-treated cells, the number of CLAN positive cells were 2.95 ± 1.2% CPC and 2.6 ± 1.2% CPC, respectively. In contrast, the number of CLAN positive cells in cultures treated with 0.25 μM or 1 μM PP2 was 0.3 ± 0.6% CPC and 0% CPC. Despite the fact that CLAN formation was nearly completely blocked by these lower concentrations of PP2, cell spreading was only partially affected. Compared to the mean area of spread cells in the 0.1% DMSO control, cell spreading was decreased by 48% in 1 μM PP2-treated cultures(1690 ±1432 μm2 vs. 874 ± 1025 μm2, p < 0.0001) and by 14% in 0.25 μM PP2-treated cultures(1690 ±1432 μm2 vs. 1450 ± 1249 μm2, p < 0.0001). Thus, β1-mediated CLAN formation could be decoupled from cell spreading on fibronectin suggesting that SFK signaling directly regulates β1 integrin-mediated CLAN formation.
When β3 integrin signaling was activated with the AP-5 antibody in HTM cells pretreated with 5 μM or 20 μM PP2, cell spreading significantly recovered. As shown in Figures 4A and 4C, the average cell area increased by 66.5% and 58%, respectively, in cells pretreated with 5 μM (p < 0.0001) or 20 μM (p < 0.0001) PP2 in the presence of soluble mAb AP-5 compared to cells treated with PP2 alone. However, despite the recovery of cell spreading, 5 μM PP2 reduced CLAN formation by 72% (p < 0.0001) relative to cells treated with DMSO only (Figure 4B) and 20 μm PP2 essentially blocked all CLAN formation. Thus, SFK signaling also plays a direct role in β3- integrin-mediated CLAN formation.
In order to narrow down the possible SFK members that participated in cell spreading and subsequent CLAN formation in TM cells, the studies were repeated with the selective SFK inhibitor EPA which inhibits the activity of Fyn and Lck but not Src51, 52. As shown in Figure 4D, 60μM EPA, a concentration shown to be highly effective in other cell types51, 52, had little or no effect on spreading (not shown) or β1-mediated CLAN formation (Figure 4D). β3-mediated CLAN formation was only slightly affected by 60 μM EPA which decreased CLAN formation by 15% relative to the 0.1% DMSO control (p < 0.01).
The next molecule examined was PI-3 Kinase (PI3-K) which is known to be downstream of Src in integrin signaling pathways and is also a key regulator of the actin cytoskeleton53, 54. Unlike the Src inhibitor PP2, treatment of cells with the PI3-K inhibitor LY294002 did not appear to dramatically alter overall cell spreading either in the absence or presence of mAb AP-5 compared to untreated cells or cells treated with DMSO only (not shown). LY294002, however, did affect CLAN formation. In the absence of mAb AP-5, basal levels of CLAN formation mediated by β1 integrins were decreased by 42% in cells treated with LY294002 (Figure 4B) compared to cells treated with DMSO only (p < 0.01) and 52.5% compared to untreated cells (p < 0.01). This suggests that β1 integrin-mediated CLAN formation is dependent on PI3-K activity. In contrast, β3 integrin-mediated CLAN formation was not significantly altered after treatment with LY294002. The 20% decrease observed in these cells may be largely attributable to the DMSO solvent used to dissolve the inhibitor, since DMSO alone decreased β3-mediated CLAN formation by 13% and there was no difference between the decrease observed in the presence of DMSO alone compared to that in the presence of LY294002. Together these data suggest that β1 integrin-mediated CLAN formation is dependent upon Src/PI3-K signaling while β3 integrin-mediated CLAN formation involves a Src dependent, PI3-K independent pathway.
β3 integrin-mediated CLAN formation, but not β3 integrin- mediated CLAN formation is regulated by Rac1/Trio signaling
We then examined the role that Rac1 might play in regulating CLAN formation in HTM cells. Rac1 is a small GTPase known to regulate the polymerization of branched actin filaments during migration and lamellipodia formation during cell spreading55. For this study we utilized the Rac1-specific inhibitor NSC23766 which blocks a subset of Rac1 signaling mediated by the GEFs Tiam1 and Trio40. 20 μM NSC23766, like LY294002, also differentially affected β1 and β3 integrin-mediated CLAN formation (Figure 5). NSC23766 inhibited β3 integrin-mediated CLAN formation by nearly 45% (p = 0.0001) demonstrating a role for Rac1 signaling mediated by either Tiam1 or Trio in CLAN formation. In contrast, CLAN formation mediated by β1 integrins was not affected by NSC23766 (p = 0.23) indicating that basal CLAN formation is not regulated by Rac1/Tiam1 or Rac1/Trio signaling. As observed with cells spread in the presence of LY294002, NSC23766 did not visibly alter overall cell spreading compared to untreated cells spread under the same conditions (not shown).
Because the Rac1-specific inhibitor NSC23766 blocks both Tiam1 and Trio, we performed additional experiments in order to determine which Rac1 GEF was involved in regulating β3 integrin-mediated CLAN formation. In the first set of experiments, quiescent HTM cells were transfected with siRNA targeting either Tiam1 or Trio. As expected, cultures transfected with Trio or Tiam1 siRNA did not show a change in β1-mediated CLAN formation relative to control cells (Figure 6A). In the presence of mAb AP-5, however, cultures transfected with Trio, but not Tiam1, siRNA demonstrated a 67% decrease in CLAN formation relative to control cells (p < 0.001). Tiam1-transfected cultures did not show any significant change in β3 integrin-mediated CLAN formation.
To confirm that Tiam1 is not involved in CLAN formation, HTM cells were transduced with a FLAG tagged TAT-DN-Tiam1 fusion protein (DN-Tiam1)56. The TAT sequence was added to allow for the rapid transduction of recombinant DN-Tiam1 into cells, while the FLAG tag provided a means by which to detect the Tiam1 construct in the cells (Figure 6B)42. Using fluorescence microscopy, the transduction rate of DN-Tiam1 into the HTM cultures was found to be 50–54% (data not shown). As shown in Figure 6B, overall cell spreading in cultures transduced with DN-Tiam1 did not differ relative to untransduced cultures. Cultures transduced with DN-Tiam1 also did not show a significant change in either β1 integrin- or β3-integrin mediated CLAN formation compared to control cells (Figure 6C).
RT-PCR analysis of Tiam1 and Trio RNA levels also indicated that Trio, rather than Tiam1, was the GEF involved in CLAN formation. The expression of Trio, but not Tiam1, RNA could be detected indicating that HTM cells express Trio but not Tiam1 (Figure 6D). The absence of Tiam1 in these cells was also confirmed by Western blot (data not shown).
Discussion
In this study, we found that distinct signaling pathways activated by β1 and β3 integrins converge and cooperate to promote CLAN formation (Figure 7). The basal level of CLAN formation in HTM cells mediated by β1 integrins involved a SFK/PI3-K-dependent signaling pathway. Although it is likely that the SFK is Src, we have not yet ruled out that two other SFK members, Yes, and Lyn57, 58, are involved given that all SFK members can be inhibited by PP247–50 and EPA only inhibits Fyn and Lck51, 52. This basal level of CLAN formation induced via β1-integrin could be further enhanced by co-activating a β3 integrin signaling pathway that utilized distinct and separate signaling components. β3 integrins enhanced CLAN formation via a Src dependent, PI3-K independent pathway that also involved Rac1-Trio. Furthermore this β3 integrin pathway could be activated by the G-protein coupled receptor CD47 via the TSP1-derived peptide 4N1K. These data suggest β1-and β3-mediated signaling pathways converge downstream of PI3-K and Rac1-Trio to promote CLAN formation (Figure 7).
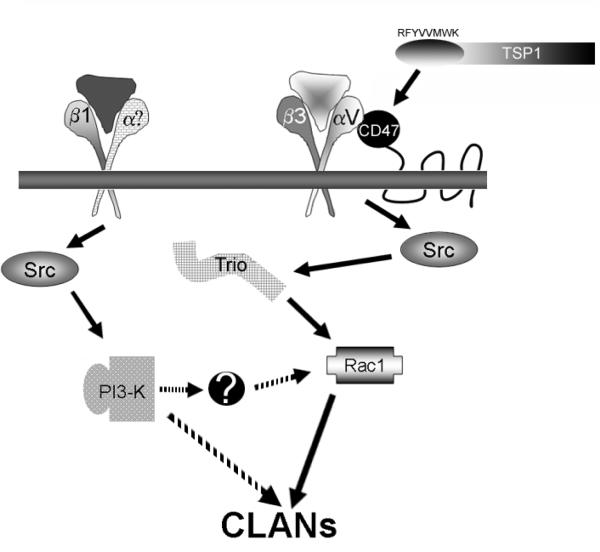
Figure 7. Schematic diagram showing that β1- and β3-mediated CLAN formation is the result of distinct signaling pathways.
Both pathways are dependent upon a SFK, possibly Src. The β1 integrin-activated pathway involves PI3-K while the αvβ3-activated pathway involves CD47 and Rac1 signaling mediated by the GEF Trio. CD47 activation by TSP1 containing the carboxy-terminal sequence found in peptide 4N1K (RFYVVMWK) may be an important regulator of CLAN formation. Convergence of the two pathways could potentially occur at two points. The β1 integrin pathway might also signal through Rac1, but via activation of a Rac1 GEF distinct from Trio. Alternatively, PI-3K could bypass Rac1 completely and the two pathways would converge at a different downstream effector to promote CLAN formation.
CD47 induced CLAN formation was most likely mediated through interactions with αvβ3 integrins rather than αIIbβ3 or α2β1 integrins59, 60. The expression of αIIbβ3 integrins is restricted to megakaryocyte-derived cells and previous studies showed that direct activation of α2β1 integrin did not induce significant CLAN formation17. Thus, the contribution of CD47-mediated activation of αIIbβ3 and/or α2β1 integrins to TM cell's CLAN formation is minimal. Furthermore, the CD47 function blocking antibody B6H12.2 blocked CLAN formation induced by the β3 integrin activating antibody mAb AP-5 further suggesting an interaction between a β3 integrin and CD47.
The involvement of CD47 in β3-induced CLAN formation raises some very interesting questions about how this might possibly relate to the induction of CLAN formation by DEX in cultured anterior segments and cells3, 9–11. CD47 is a receptor for TSP1, the expression of which is up-regulated by both TGF-β1 and DEX treatment in human and mouse TM cells, and increased TSP1 deposition has been found in the ECM of the meshwork in cases of POAG and SIG30. Additionally, Liu et al23 showed that DEX caused a decrease in TSP1 released into culture medium, consistent with the idea that DEX treatment results in an increase in matrix- or cell-associated TSP1, similar to what has been demonstrated for other ECM components44, 61–63. Increased TSP1 within the ECM could lead to increased CD47 activation thereby contributing to αvβ3-integrin-mediated CLAN formation in DEX-treated HTM cells. The suppression of TSP1 production and subsequent decreased CLAN formation in HTM cells in response to the actin-disrupting drug LAT-A would further support the notion that TSP1 and CLAN formation are connected23.
The point where these two pathways converge to enhance CLAN formation could not be determined from these studies. Clearly, these signaling pathways must converge since activation β3 integrin signaling alone is not sufficient to induce CLAN formation in TM cells17. One point may be Src, since PP2 blocked both β1- and β3-mediated CLAN formation. However, Src is generally thought of as an early upstream signaling event before Rac1, so this would not explain the differential inhibition of β1- and β3-mediated CLAN formation by LY94002 and NSC23766, respectively. The most likely explanation is that the pathways converge at Rac1 or downstream of Rac1 activation at one of the steps involved in the formation of a branched actin network since Rac1 is known to regulate the formation of these structures55. However, only αvβ3 integrin-mediated CLAN formation was blocked by the Rac1 inhibitor, NSC23766 which is somewhat puzzling because β1-mediated CLAN formation is regulated by PI3-K and there are numerous studies that show Rac1 activation being dependent upon PI3-K64, 65. The NSC23766 inhibitor that was used, however, specifically blocks Rac1 signaling mediated by the GEFs Tiam1 or Trio. Hence. it is possible that β1-mediated CLAN formation involves activation of Rac1 by another Rac1 GEF such as Vav166. The fact that gene-silencing experiments indicated that β3-mediated CLAN formation involved Trio rather than Tiam1 supports the idea that CLAN formation in HTM cells is regulated by specific GEFs.
It is not surprising that Trio was found to be the active GEF in HTM cultures. Trio is highly expressed in neuronal tissues67, and HTM cells have a neural crest origin68while Tiam1 expression appears to be more widespread69. It is interesting though that the β3 integrin signaling was linked to Trio-Rac1. Integrin signaling has previously been associated with Tiam170, 71 while Trio has been associated with heterotrimeric G proteins72. To our knowledge, this is the first time that any integrin signaling has been linked to Trio-Rac1. However, the fact that CD47 is considered an atypical GPCR that can activate the Gαi subfamily of heterotrimeric G proteins35 and is also a co-receptor for β3 integrin, supports the idea that the β3 integrin/CD47 complex activates Trio-Rac1 signaling.
Additional data suggest that CLAN formation may involve Gαi signaling. A recent study in Schwann cells73 showed that CLANs (“geodesic actin networks”) were formed in response to lysophosphatidic acid (LPA) or sphingosine 1-phosphate (S1P). Both LPA and S1P signal via GPCRs and activate the Gαi signaling pathway74, 75. Interestingly, LPA- and S1P- induced CLAN formation in Schwann cells was also found to be dependent upon Rac1, rather than RhoA, activation. Clearly, future studies will be needed to explore possible links among CD47, Rac1 and Trio in CLAN formation.
Although the function of CLAN formation in HTM cells is still unknown, CLANs do not appear to be necessary for cell spreading, since the assembly of actin filaments into a CLAN appears to involve a distinct process that is not involved in cell spreading. Under conditions when β1-mediated CLAN formation was inhibited by the Src kinase inhibitor, PP2, cell spreading was only partially inhibited. Also, activation of β3-mediated CLAN formation in the presence of PP2 induced a partial recovery of cell spreading, but not CLAN formation. Thus, we were able to de-couple cell spreading from CLAN formation and show that CLAN formation is not needed for cell spreading. We can only speculate as to why CLAN formation, but not cell spreading was dependent on Src. However, Src-independent cell spreading has previously been observed in osteoclasts76 and it is possible that this can occur in TM cells.
Interestingly, it took a higher concentration of PP2 to inhibit β3 integrin-mediated CLAN formation than β1-mediated CLAN formation. It is possible that αvβ3-dependent activation of Src is a later event in the formation of CLANs than in β1-mediated CLAN formation and that activation of αvβ3 could lead to the additional recruitment of activated Src to the cell membrane. It has been shown in platelets77 and osteoclasts78 that there is a pool of inactive Src that is bound to β3 integrins in the absence of any ligand binding. This Src pool becomes activated afterβ3 integrins are activated. Thus, the recruitment of this pool of Src upon activation of αvβ3 via mAb AP-5 could have been sufficient to partially overcome the effects of PP2 at relatively low concentrations and lead to a partial recovery of CLAN formation.
In summary, these studies suggest that increases in CLAN formation might be due to a significant up-regulation in one or the other integrin signaling pathways. Clearly additional studies examining the physiological role of these signaling molecules in CLAN formation are warranted and should be useful in furthering our understanding of the organization of the actin cytoskeleton in the TM and its role in outflow facility and the pathophysiology of glaucoma.
Acknowledgments
Grant Support: This work was supported by NEI grants EY06665-01, EY017006, EY012515 (D.M.P.), EY02698 (P.L.K.), EY16995 (N.S.), EY018274 (M.K.S.), a Core grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665), a Shaffer Grant from the Glaucoma Research Foundation (M.S.F.) and a Career Development Award from the Research to Prevent Blindness Foundation (N.S.).
References
- 1.Knepper PA, Collins JA, Frederick R. Effects of dexamethasone, progesterone, and testosterone on iop and gags in the rabbit eye. Invest OphthalmolVis Sci. 1985;26:1093–100. [PubMed] [Google Scholar]
- 2.Kass M, Cheetham J, Duzman E, et al. The ocular hypertensive effect of 0.25% fluorometholone in corticosteroid responders. Am J Ophthalmol. 1986;102:159–63. doi: 10.1016/0002-9394(86)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskel. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 4.Gelatt KN, Mackay EO. The ocular hypertensive effects of topical 0.1% dexamethasone in beagles with inherited glaucoma. J Ocul Pharmacol Therapeut. 1998;14:57–66. doi: 10.1089/jop.1998.14.57. [DOI] [PubMed] [Google Scholar]
- 5.Clark AF, Miggans ST, Wilson K, et al. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- 6.Gerometta R, Podos SM, Candia OA, et al. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004;122:1492–97. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- 7.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: A review of the literature. Eye. 2006;20:407–16. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 8.Sihota R, Konkal VL, Dada T, et al. Prospective, long-term evaluation of steroid-induced glaucoma. Eye. 2008;22:26–30. doi: 10.1038/sj.eye.6702474. [DOI] [PubMed] [Google Scholar]
- 9.Clark AF, Wilson K, McCartney MD, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–94. [PubMed] [Google Scholar]
- 10.Clark AF, Lane D, Wilson K, et al. Inhibition of dexamethasone-induced cytoskeletal changes in cultured human trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci. 1996;37:805–13. [PubMed] [Google Scholar]
- 11.Wilson K, McCartney MD, Miggans ST, et al. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993;12:783–93. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- 12.Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976;68:202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon WE, 3rd, Bushnell A. Immunofluorescent and ultrastructural studies of polygonal microfilament networks in respreading non-muscle cells. Exp Cell Res. 1979;120:335–48. doi: 10.1016/0014-4827(79)90393-8. [DOI] [PubMed] [Google Scholar]
- 14.Sanger JM, Mittal B, Pochapin M, et al. Observations of microfilament bundles in living cells microinjected with fluorescently labelled contractile proteins. J Cell Sci Supp. 1986;5:17–44. doi: 10.1242/jcs.1986.supplement_5.2. [DOI] [PubMed] [Google Scholar]
- 15.Hoare M-J, Grierson I, Brotchie D, et al. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2706. [DOI] [PubMed] [Google Scholar]
- 16.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: Towards a better understanding of glaucoma. Prog Ret Eye Res. 1999;18:629–67. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 17.Filla MS, Woods A, Kaufman PL, et al. β1 and β3 integrins cooperate to induce syndecan-4 containing cross-linked actin networks (CLANs) in human trabecular meshwork (HTM) cells. Invest Ophthalmol Vis Sci. 2006;47:1956–67. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane D, Martin TA, Mansel RE, et al. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and Tiam-1 in human breast cancer. Int Semin Surg Oncol. 2008;16:23. doi: 10.1186/1477-7800-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochizuki Y, Furukawa K, Mitaka T, et al. Polygonal networks, “Geodomes”, of adult rat hepatocytes in primary culture. Cell Biol Int Rep. 1988;12:1–7. doi: 10.1016/0309-1651(88)90105-1. [DOI] [PubMed] [Google Scholar]
- 20.Ireland GW, Voon FC. Polygonal networks in living chick embryonic cells. J Cell Sci. 1981;52:55–69. doi: 10.1242/jcs.52.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Lin ZX, Holtzer S, Schultheiss T, et al. Polygons and adhesion plaques and the disassembly and assembly of myofibrils in cardiac myocytes. J Cell Biol. 1989;108:2355–2367. doi: 10.1083/jcb.108.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Wu Z, Sheibani N, et al. Low dose Latrunculin-a inhibits dexamethasone-induced changes in the actin cytoskeleton and alters extracellular matrix protein expression in cultured human trabecular meshwork cells. Exp Eye Res. 2003;77:181–188. doi: 10.1016/s0014-4835(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 24.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 25.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao A-G, Lindberg FP, Dimitry JM, et al. Thrombospondin modulates αvβ3 function through Integrin-Associated Protein. J Cell Biol. 1996;135:533–44. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao A-G, Lindberg FP, Finn MB, et al. Integrin-Associated Protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–4. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Brown EJ, Frazier WA. Integrin-Associated Protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–35. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 29.Isenberg JS, Roberts DD, Frazier WA. Cd47: A new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:615–21. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flugel-Koch C, Ohlmann A, Fuchshofer R, et al. Thrombospondin-1 in the trabecular meshwork: Localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79:649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Barazi HO, Li Z, Cashel JA, et al. Regulation of integrin function by CD47 ligands: Differential effects on αvβ3 and α4β1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–66. doi: 10.1074/jbc.M206849200. [DOI] [PubMed] [Google Scholar]
- 32.Blystone SD, Lindberg FP, LaFlamme SE, et al. Integrin beta 3 cytoplasmic tail is necessary and sufficient for regulation of alpha 5 beta 1 phagocytosis by alpha v beta 3 and Integrin-Associated Protein. J Cell Biol. 1995;130:745–54. doi: 10.1083/jcb.130.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green JM, Zhelesnyak A, Chung J, et al. Role of cholesterol in formation and function of a signaling complex involving αvβ3, Integrin-Associated Protein (CD47) and heterotrimeric G proteins. J Cell Biol. 1999;146:673–82. doi: 10.1083/jcb.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maile LA, Imai Y, Clarke JB, et al. Insulin-like growth factor I increases αvβ3 affinity by increasing the amount of Integrin-Associated Protein that is associated with non-raft domains of the cellular membrane. J Biol Chem. 2002;277:1800–05. doi: 10.1074/jbc.M108380200. [DOI] [PubMed] [Google Scholar]
- 35.Frazier WA, Gao A-G, Dimitry J, et al. The thrombospondin receptor Integrin-Associated Protein (CD47) functionally couples to heterotrimeric Gi. J Biol Chem. 1999;274:8554–60. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- 36.Polansky JR, Weinreb RN, Baxter JD, et al. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–9. [PubMed] [Google Scholar]
- 37.Polansky JR, Weinreb R, Alvarado JA. Studies on human trabecular cells propagated in vitro. Vis Res. 1981;21:155–60. doi: 10.1016/0042-6989(81)90151-6. [DOI] [PubMed] [Google Scholar]
- 38.Filla MS, David G, Weinreb RN, et al. Distribution of syndecans 1–4 within the anterior segment of the human eye: Expression of a variant syndecan-3 and matrix associated syndecan-2. Exp Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Peterson JA, Sheibani N, David G, et al. Heparin II domain of fibronectin uses α4β1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J Biol Chem. 2005;280:6915–22. doi: 10.1074/jbc.M406625200. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Dickerson JB, Guo F, et al. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosher DF, Johnson RB. In vitro formation of disulfide-bonded fibronectin multimers. J Biol Chem. 1983;258:6595–6560. [PubMed] [Google Scholar]
- 42.Ho A, Schwarze SR, Mermelstein SJ, et al. Synthetic protein transduction domains: Enhanced transduction potential in vitro and in vivo. Canc Res. 2001;61:474–77. [PubMed] [Google Scholar]
- 43.Bultmann H, Santas AJ, Peters DM. Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J Biol Chem. 1998;273:2601–9. doi: 10.1074/jbc.273.5.2601. [DOI] [PubMed] [Google Scholar]
- 44.Filla MS, Lui X, Nguyen TD, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- 45.Malliri A, van Es S, Huveneers S, et al. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279:30092–98. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- 46.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 47.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kannan S, Audet A, Huang H, et al. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during pseudomonas aeruginosa infection. JImmunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 49.Osterhout DJ, Wolven A, Wolf RM, et al. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–18. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai W-B, Zhang X, Sharma D, et al. Role of Yes kinase during early zebrafish development. Develop Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 51.Xua D, Kishia H, Kawamichi H, et al. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Letters. 2007;581:5227–5233. doi: 10.1016/j.febslet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Nakao F, Kobayashi S, Mogami K, et al. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-Kinase pathway. Circ Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- 53.Westhoff MA, Serrels B, Fincham VJ, et al. Src-mediated phosphorylation of Focal Adhesion Kinase couples actin and adhesion dynamics to survival signaling. Molec Cell Biol. 2004;24:8113–33. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez L, Agullo-Ortuno MT, Garcia-Martinez JM, et al. Role of c-Src in human MCF7 breast cancer cell tumorigenesis. J Biol Chem. 2006;281:20851–64. doi: 10.1074/jbc.M601570200. [DOI] [PubMed] [Google Scholar]
- 55.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 56.Stam JC, Sander EE, Michiels F, et al. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–54. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- 57.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–09. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 59.Chung J, Gao A-G, Frazier WA. Thrombspondin acts via Integrin-Associated Protein to activate the platelet integrin αIIbβ3. J Biol Chem. 1997;272:14740–46. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- 60.Wang X-Q, Lindberg FP, Frazier WA. Integrin-Associated Protein stimulates α2βl-dependent chemotaxis via gi-mediated inhibition of adenylate cyclase and Extracellular-Regulated Kinases. J Cell Biol. 1999;147:389–99. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickerson JE, Jr., Steely HT, Jr., English-Wright SL, et al. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp Eye Res. 1998;66:731–8. doi: 10.1006/exer.1997.0470. [DOI] [PubMed] [Google Scholar]
- 62.Steely HT, Browder SL, Julian MB, et al. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- 63.Zhou L, Li Y, Yue BY. Glucocorticords effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–346. [PubMed] [Google Scholar]
- 64.Keely PJ, Westwick JK, Whitehead IP, et al. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–36. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 65.Bäumer AT, ten Freyhaus H, Sauer H, et al. Phosphatidylinositol 3-Kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283:7864–76. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- 66.Villalba M, Bi K, Rodriguez F, et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–38. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma XM, Huang JP, Eipper BA, et al. Expression of Trio, a member of the Dbl family of Rho GEFs in the developing rat brain. J Comp Neurol. 2005;482:333–48. doi: 10.1002/cne.20404. [DOI] [PubMed] [Google Scholar]
- 68.Tripathi BJ, Tripathi RC. Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am J Ophthalmol. 1989;107:583–90. doi: 10.1016/0002-9394(89)90253-5. [DOI] [PubMed] [Google Scholar]
- 69.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on Rho GTPases with guanine nucleotide-exchange factors. Nat Rev Molec Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 70.Hamelers IHL, Olivo C, Mertens AEE, et al. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2006;171:871–81. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Leeuwen FN, Kain HET, van der Kammen RA, et al. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rojas RJ, Yohe ME, Gershburg S, et al. Gαq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–10. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barber SC, Mellor H, Gampel A, et al. S1P and LPA trigger schwann cell actin changes and migration. Eur J Neurosci. 2004;19:3142–3150. doi: 10.1111/j.0953-816X.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- 74.Moolenaar W. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–52. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 75.Tahaa TA, Argravesb KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura I, Lipfert L, Rodan GA, et al. Convergence of αvβ3 integrin– and macrophage colony stimulating factor–mediated signals on Phospholipase Cυ in prefusion osteoclasts. J Cell Biol. 2001;152:361–73. doi: 10.1083/jcb.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberegfell A, Eto K, Mocsai A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αiibβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hruska KA, Rolnick F, Huskey M, et al. Engagement of the osteoclast integrin αvβ3 osteopontin stimulates Phosphatidylinositol 3-hydroxyl Kinase activity. Endocrinol. 1995;136:2984–92. doi: 10.1210/endo.136.7.7540546. [DOI] [PubMed] [Google Scholar]