Abstract
In cultured cells, palmitic acid (PA) and oleic acid (OA) confer distinct metabolic effects, yet, unclear, is whether changes in dietary fat intake impact cellular fatty acid (FA) composition. We hypothesized that short-term increases in dietary PA or OA would result in corresponding changes in the FA composition of skeletal muscle diacylglycerol (DAG) and triacylglycerol (TAG) and/or the specific FA selected for β-oxidation. Healthy males (N = 12) and females (N = 12) ingested a low-PA diet for 7 days. After fasting measurements of the serum acylcarnitine (AC) profile, subjects were randomized to either high-PA (HI PA) or low-PA/high-OA (HI OA) diets. After 7 days, the fasting AC measurement was repeated and a muscle/fat biopsy obtained. FA composition of intramyocellular DAG and TAG and serum AC was measured. HI PA increased, whereas HI OA decreased, serum concentration of 16:0 AC (P < 0.001). HI OA increased 18:1 AC (P = 0.005). HI PA was associated with a higher PA/OA ratio in muscle DAG and TAG (DAG: 1.03 ± 0.24 vs. 0.46 ± 0.08, P = 0.04; TAG: 0.63 ± 0.07 vs. 0.41 ± 0.03, P = 0.01). The PA concentration in the adipose tissue DAG (μg/mg adipose tissue) was 0.17 ± 0.02 in those receiving the HI PA diet (n = 6), compared to 0.11 ± 0.02 in the HI OA group (n = 4) (P = 0.067). The relative PA concentration in muscle DAG and TAG and the serum palmitoylcarnitine concentration was higher in those fed the high-PA diet.
INTRODUCTION
Excessive stores of fatty acids (FAs) (“obesity”) and high dietary FA intakes have been linked to dysfunction of the liver, skeletal muscle, heart, and pancreas, thereby contributing to the metabolic syndrome and type 2 diabetes (1). Individual FAs exert chemical properties according to their structure, and it is plausible that oversupply of specific FA to target tissues, not merely the accumulation of body triacylglycerol (TAG), induces cellular dysfunction. Thus, the adverse metabolic sequelae of obesity might be linked to the deposition of specific dietary FA in nonadipose tissues.
Palmitic acid (PA) (16:0) and oleic acid (OA) (18:1) are the most abundant saturated and monounsaturated FA in both the blood and skeletal muscle (2). OA and PA have distinct physical properties (3), are oxidized to a different extent (4), and have differential effects on FA oxidation (5–7), gene expression (8–11), and insulin signaling and inflammatory responses (2,12–21). Moreover, incomplete oxidation of FA might lead to the accumulation of acylcarnitines (ACs), which have been implicated as markers and perhaps mediators of insulin resistance and inflammation (9,22). Thus, to begin to translate findings in cell-based systems to humans, it is important to study how FA composition of adipose tissue and muscle lipids is affected by diet. Because of the aforementioned effects of PA in cultured cells, we wondered whether a short-term (1 week) change in dietary PA and OA composition would alter the FA composition of muscle diacylglycerol (DAG) and TAG, as it would be important to know whether such effects might be requisite for diet-induced changes in metabolic regulation. As a way to assess, in turn, whether changes in the FA composition of lipids have functional consequences, we also evaluated fasting and fed measurements of FA oxidation and resting energy expenditure, and fasting serum concentrations of lipids, glucose, and insulin. An additional, related goal was to determine whether a short-term change in dietary OA and PA would lead to a shift in the specific FAs undergoing β-oxidation, using mitochondrial-derived AC esters as a biomarker of substrate selection.
To accomplish these goals, we evaluated the FA composition of muscle and adipose tissue DAG and TAG, as well as mitochondria-derived serum ACs, using specimens from a study that examined short-term (7 days) effects of two different levels of dietary PA and OA.
METHODS AND PROCEDURES
Subjects and overall design
This study was approved by the institutional review board of the University of Vermont and the Scientific Advisory Committee of the General Clinical Research Center (GCRC) at the University of Vermont, where the clinical aspects of the study were carried out. Healthy, non-obese males (N = 12) and females (N = 12) participated in this study. There was one female and one male of Hispanic ethnicity, both whites. There was one Asian subject (female), and no African-American subjects. Subjects ingested a baseline diet for 7 days (described below). At 0625 hours, after a 12-h fast, blood was collected for measurement of concentrations of glucose, insulin, and lipids as well as the serum AC profile. Baseline measurement of respiratory quotient (RQ) and resting energy expenditure (REE) were obtained in the fasting state (0600 hours) but also in the fed state (1530 hours, after previous meals at 0630 hours and 1200 hours). The “fed state” measurement was obtained without a prior resting period because our previous studies (5,6,23) suggested that the diets altered nonresting REE but not REE. After the completion of these baseline measurements, the subjects were randomized (stratified by gender) to one of two experimental diets described in a separate section below. After 7 days of experimental diet feeding, the RQ and blood measurements were repeated and a biopsy of the right vastus lateralis muscle and the overlying adipose tissue was obtained at 1700 hours.
Diets
The composition of the baseline diet was as follows (% energy): total fat, 34; PA, 8; OA, 14; protein, 18; carbohydrate, 50). The PA/OA ratio of this diet was 0.57. We used two experimental diets: HI PA, patterned after an American diet (24) (actual intake as % energy: total fat, 42; PA, 15; OA, 17; protein, 15; carbohydrate, 44) or HI OA (% energy actual intake: total fat, 42; PA, 3; OA, 27; protein, 15; carbohydrate, 44). The PA/OA ratios of these respective diets were 0.88 and 0.11. There were 6 males and 6 females randomly assigned to each of the two diet groups. All food (solid and liquids except water) was provided by the GCRC, and all subjects ate breakfast and were weighed daily at the GCRC for the duration of the protocol. Each of the three diets, baseline and then either HI PA or HI OA, consisted of one menu for breakfast, lunch, and dinner, which was consumed each day. The food consisted of low fat meat plus vegetables, starchy foods, fruit, and beverages, as well as a plastic container of vegetable oil, blended to achieve the desired FA level of the respective diet. Each meal was designed to provide, approximately, the same fractional energy content of each major macronutrient as for the entire diet, and this was verified by analysis. However, the size of the meals and thus the daily energy intake was estimated to allow body weight stability during the duration of study As previously described (25), we weighed the subjects daily to assure energy balance. The subjects were instructed as to how to conveniently and efficiently combine the vegetable oil with the respective foods within a given meal, but they were also required to use a spatula and, if necessary, the mouth to be sure that all of the food and oil were consumed. Empty containers were inspected daily, and the subjects had to sign a form each day indicating whether they had consumed all the food and whether they had consumed any other food or drink. We did not identify any noncompliance using the written attestation, the inspection of the containers, or on the basis of daily conversations with the subjects by the research staff. We also did not detect noncompliance in the form of significant discrepancies between the energy intake of the food offered and any changes in daily weight.
Indirect calorimetry
The subjects remained in bed during the measurement. For the fasting measurement of RQ, the subjects rested for 30 min before the measurement. For the fed measurement in the afternoon, the subjects did not rest, before this “fed” measurement, as we wanted to capture possible effects of normal physical activity on fat oxidation and energy expenditure (5). The measurements of oxygen consumption (VO2), and CO2 production (VCO2) were obtained using a metabolic cart (Vmax SPECTRA 29; Sensor Medics, Yorba Linda, CA) (5). Because we were not able to collect urine over a stable fasting or fed period, the component of protein oxidation in the REE was estimated using the software loaded onto the instrument.
Body composition
On day 6, of the experimental diet period, fat mass and fat-free mass were assessed using dual-energy X-ray absorptiometry (GE Lunar Prodigy Densitometer, version 5.6).
Blood concentrations of cholesterol, high-density lipoprotein, triglyceride, glucose, and insulin
Serum concentrations of total cholesterol, high-density lipoprotein, and triglyceride were measured at the Clinical Chemistry Laboratory at Fletcher Allen Health Care, an affiliate of the University of Vermont, using a colorimetric method (Vitros 5.1 FS Chemistry System; Ortho-Clinical Diagnostics, Rochester, NY). Plasma glucose and insulin concentration were measured at the GCRC Core Laboratory. Glucose concentration was measured using a YSI 2300 Stat Plus glucose analyzer (YSI, Yellow Springs, OH). Serum insulin concentration was measured by radioimmunoassay (Linco Research, St. Charles, MO). For the baseline and postdiet states, we estimated insulin sensitivity using the Homeostatic Model Assessment of Insulin Resistance (HOMAIR) (26): HOMAIR = (fasting insulin, μU/ml) × (fasting glucose, mmol/l/22.5)). HOMAIR <4.65 implies <25% risk of being insulin resistant using the euglycemic/hyperinsulinemic clamp methodology (26).
Acylcarnitines
Fasting serum concentrations of AC were measured at Duke University by direct-injection electrospray tandem mass spectrometry, using a Micromass Quattro MicroTM system equipped with a model 2777 autosampler, a model 1525 HPLC solvent delivery system and a data system running 4.0 MassLynx software (Waters, Millford, MA) (8). Data were available on 22 of the 24 subjects.
Vastus lateralis (right) muscle biopsy and extraction and separation of muscle lipids and analysis by gas chromatography
After pretreatment for an hour of the area of the biopsy with a local anesthetic cream (2.5 g) containing 2.5% Lidocaine and 2.5% Prilocaine (EMLA; Astrazeneca Pharmaceuticals, Wilmington, DE), a percutaneous muscle biopsy was carried out as previously described by Highstead et al. (27). The muscle biopsy tissue was examined and dissected free of visible fat and then immediately frozen in liquid N2 and stored at −80 °C until further processing for determination of the concentration of FA in DAG and TAG by GC. Frozen muscle tissue was homogenized using a tissue pulverizer that was first cooled in liquid nitrogen. In order to measure the protein concentration of the muscle sample, ~3 mg of powdered muscle was weighed and homogenized in protein extraction buffer (0.6 mol/l KCl, 0.15 mol/l K phosphate, 20 mmol/l EDTA, 5 mmol/l MgCl2, 3.3 mmol/l ATP, pH 6.7), using a microfuge tube homogenizer (cat. no. 749520; Kontes Glass, Vineland, NJ), and then incubated on ice for 90 min. The homogenate was centrifuged at 6,500g for 20 s and the supernatant was analyzed for protein concentration using a Bradford assay with bovine serum albumin as a standard (Bio-Rad Laboratories, Hercules, CA) (28). Technical problems precluded accurate analysis of protein concentration on one of the 24 subjects. The remaining powdered muscle tissue (13–81 mg) was weighed, and intramyocellular lipid was extracted overnight at 4 °C in 3 ml methanol:chloroform (1:2, vol/vol) containing 0.05 mg/ml butylated hydroxytoluene after the addition of internal standards (20 μl of 0.1 mg/ml 1,3-dipentadecanoylglycerol (15:0 DAG) and 100 μl of 1 mg/ml 1,2,3-triheptadecanoylglycerol (17:0 TAG). Samples were then centrifuged at 3,500 r.p.m. for 30 min at 4 °C. The supernatant was dried under nitrogen, and lipids were resuspended in 50 μl chloroform and applied to a thin-layer chromatography (TLC) plate (cat. no. LK5D; Whatman, Maidstone, UK) (29). The TLC plate was developed in a tank containing hexane:diethyl ether:acetic acid (70:30:1, vol/vol). Subsequently, the DAG and TAG bands were visualized using a primuline spray and a 365 nm UV lamp and were scraped and placed into glass vials (29). Samples were then transmethylated at 60 °C overnight using 2 ml 5% sulfuric acid in methanol. Methyl esters were then extracted with 3 ml petroleum ether after addition of 1.5 ml of distilled water. Samples were thoroughly mixed and centrifuged at 1,500 g. The petroleum ether phase was transferred and dried under N2 (30). The methyl esters were then resuspended in dichloromethane and transferred to gas chromatography (GC) autosampler vials. The methyl esters were analyzed on an Agilent 6890N Series Gas Chromatograph with an Agilent 7683 AutoSampler and Injector (Agilent Technologies, Santa Clara, CA) equipped with a 30 m × 0.25 mm fused silica capillary column (cat. no. 24019; Supelco, Bellefonte, PA). The inlet and detector temperature were set to 230 °C. The initial oven temperature was 60 °C and was ramped to 80 °C at 10 °C/min, to 150 °C at 40 °C/min, to 200 °C at 4 °C/min, and finally to 240 °C at 60 °C/min and held for 5 min. Helium was used as the carrier gas with a flow rate of 1 ml/min. This method was used in splitless mode for DAG samples and had a split ratio of 40:1 for TAG samples. To determine the FA concentration in the GC injection vial, we used FA-specific methyl ester standards (including 15:0 and 17:0). However, 15:0 DAG and 17:0 TAG were used to estimate total recovery of FA, starting with the overnight extraction and ending with the final quantitation of the FA concentration by GC. Pentadecanoic acid (15:0) and heptadecanoic acid (17:0) are synthesized by ruminant bacteria and thus ingested in the form of dairy products and meat (beef, lamb) (31). In human skeletal muscle phospholipids, 15:0 constitutes about 0.22–0.32% of total FA, and 17:0 constitutes 0.36–0.43% (32). In human adipose tissue, 15:0 constitutes about 0.19–0.32% and 17:0 about 0.21–0.34% of FA. The amount of 15:0 and 17:0 added as internal standards greatly dwarfed the normal concentrations of these two FA, which were used only to estimate loss of FA during extraction, TLC, and derivatization, but the concentrations per se of 15:0 and 17:0 were not included in calculations of DAG or TAG content or FA composition. In muscle and adipose tissue, the concentration of 15:0 and 17:0 in DAG and TAG averaged, respectively, 37.7 and 31.5% of all measured FA. Thus, we feel that naturally abundant 15:0 and 17:0 have a trivial effect on the valid use of these two FA as internal standards.
Total DAG and TAG were estimated from the sum of the measured FA concentration (14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3). It is important to note that our values for DAG or TAG concentration given in this paper are actually DAG-derived or TAG-derived FA concentrations; therefore, in comparisons we make, later in the paper, to studies reporting DAG in molar amounts, we used a factor of 568 mg of FA/mmol, based on the molecular weight of PA.
Using this method, employing the Whatman TLC plate, we observed the following recoveries of external DAG standards, consisting of each FA at both positions on the DAG molecule, all placed in the same lane of the TLC plate (% recovered based on GC measurement):
DAG: 15:0, 65.7%; 16:0, 96.1%; 18:0, 177.0%; 18:1, 13.6%; 18:2, 6.3%;
TAG: 16:0, 65.0%; 17:0, 68.2%; 18:0, 60.0%; 18:1, 50.1%; 18:2, 50.2%;
DAG and TAG measurements of individual FA (and thus total DAG and TAG) were corrected for the respective recoveries of the internal standards, 15:0 and 17:0, and then, in addition, for the external standard recoveries. In muscle samples, both DAG and TAG coexist; so, the external standards, mentioned just above, contained both DAG and TAG for each of the major FA. However, when we employed, for comparison, DAG and TAG in separate lanes, the recovery of distearin was 75%, similar to dipalmitin, and the recovery of tristearin was 74%. The amount of DAG external standard used was 0.002 mg, compared to 0.1 mg of TAG external standard. Thus, if only 1.5% of the tristearin degraded to distearin during TLC, this would account for the increment in recovery of distearin, above 100% (i.e., 77%), when all the external standards were placed in the same lane. Of course, we have neither proof that this occurred nor whether this could in any way relate to the low oleate recovery.
Thus, we had no choice but to apply the above recovery corrections to our actual data in muscle. However, after completion of our muscle analyses, we discovered that the type of TLC plate affected recovery of unsaturated FA (33). Thus, we used a new TLC plate for six reanalyses of muscle, where we had remaining sample. The procedure was identical except that we spotted the samples onto a different TLC plate (cat. no. 5715-7; EMD Chemicals, Gibbstown, NJ).
Using this method, employing the EMD Chemicals TLC plate, we observed the following recoveries of external standards (% recovered based on GC measurement):
DAG: 15:0, 62.1%; 16:0, 78.6%; 18:0, 76.9%; 18:1, 76.7%; 18:2, 80.8%;
TAG: 16:0, 73.2%; 17:0, 70.8%; 18:0, 65.7%; 18:1, 76.8%; 18:2, 64.7%;
In this paper, we are mainly reporting our data using the Whatman plate and employing recovery values. However, for comparison to the work of others, we will present the repeat analyses on the six subjects using the new TLC plate, which is now in routine use in our laboratory; as the recovery of FA was fairly uniform and reasonably good, using this new plate, we have not employed recovery factors in presenting the data on these six subjects with repeat analyses. In these six subjects, there was no difference in measured TAG, but the measured total DAG value (μg/mg protein) was significantly higher using the new technique ( uncorrected for FA recovery) (3.6 ± 0.8) than using the old technique (corrected for FA recovery; 1.1 ± 0.2, P = 0.031).
Fat biopsy and preparation of tissue for GC
Approximately midway through our study, we made the decision to establish a method for studying adipose tissue proximal to the muscle biopsy site, in order to assess, indirectly, the effects of adipose tissue contamination of a muscle biopsy specimen. Thus, adipose tissue was biopsied from the subcutaneous tissue overlying the area of muscle biopsy, using gentle suction and a 14 gauge fat biopsy needle attached to a 60 cc syringe containing about 10 ml of 0.9% sodium chloride. The saline containing adipose tissue was strained through a 250 μm nylon sieve (cat. no. CMN0250-D; Small Parts, Miramar, FL). The sample was examined for contaminants and rinsed with saline. Any excess saline was removed by briefly placing the nylon sieve onto a gauze pad. The fat was then placed into liquid nitrogen and allowed to freeze completely. The adipose tissue was stored at −80 °C until time of analysis. Analysis of lipids from adipose tissue was performed as described for the muscle samples, although the EMD Chemicals TLC plate was used, and no external recovery factors were used. Results were expressed per mg adipose tissue and not mg protein in order to appreciate the fractional effects of including adipose tissue in the muscle biopsy sample.
Statistics
All data are expressed as mean ± s.e.m. Diet group effects for data for which we had baseline data (RQ, REE, blood lipids, glucose, insulin, and AC profile) were analyzed using an analysis of variance of the change in the variable with the baseline value as a covariate. The paired t-test was used to compare muscle to adipose tissue. All other data were analyzed by a two-sample t-test and simple linear regression analysis. For the latter, we assessed linearity both by inspection of scattergrams and by comparisons to rank correlation. Generally, we are reporting only the linear (Pearson) correlation coefficients, but in each case the Spearman rank correlation coefficients were similar with almost identical statistical significance.
To determine the effect of the diets on the selection of substrates for mitochondrial FA oxidation, we quantified diet-induced changes in palmitoylcarnitine and oleoylcarnitine. As noted above, PA may inhibit complete FA oxidation (8). Accumulation of specific ACs as well as increased microsomal omega FA oxidation occurs in genetic disorders of β-oxidation (34). Thus, we examined the change in the sum of medium- and short-chain ACs and computed the ratio of the sum of medium chain AC (6:0 + 8:0 + 10:0 + 12:0) to the long-chain AC (14:0 + 16:0 + 18:0 + 18:1 + 18:2). We also assessed diet-induced changes in the sum of C4, C6, and C8 dicarboxylic ACs (succinic, adipic, suberic acids).
RESULTS
Effects of diets on FA oxidation and serum concentrations of lipids, glucose, and insulin
The groups did not differ in age or body composition (Table 1). Before the experimental diet, there were no between-group differences in RQ or REE, in either the fasting or fed states, or in blood concentrations of lipids, glucose,, and insulin. The diet did not affect fed or fasting RQ or REE (with or without adjustment for fat-free mass), fasting blood glucose or insulin levels. The HOMAIR was not different between groups at the end of the baseline diet and was not affected by the dietary intervention (HI PA vs. HI OA, 1.43 ± 0.18 vs. 1.37 ± 0.16. Neither diet affected serum concentrations of high-density lipoprotein and TAG, but the HI OA diet caused a decrease in serum total cholesterol (−0.53 mmol/l or −20.5 mg/dl; P = 0.017) and low-density lipoprotein cholesterol (−0.42 mmol/l or −16.2 mg/dl; P = 0.011), compared to no change in the HI PA group (Table 1).
Table 1.
Age, body composition, RQ, REE, and change in serum lipid concentrations
| HI OA | HI PA | |
|---|---|---|
| Age (years) | 28.3 ± 1.8a | 29.8 ± 1.9 |
| BMI | 22.0 ± 0.8 | 23.4 ± 0.7 |
| % Body fat | 25.5 ± 3.1 | 28.7 ± 2.6 |
| Fat mass (kg) | 15.9 ± 2.1 | 18.4 ± 1.5 |
| FFM (kg) | 49.9 ± 3.7 | 50.5 ± 3.7 |
| RQpre fed | 0.88 ± 0.02 | 0.91 ± 0.01 |
| RQpre fast | 0.85 ± 0.01 | 0.87 ± 0.01 |
| RQpost fed | 0.88 ± 0.01 | 0.90 ± 0.02 |
| RQpost fast | 0.86 ± 0.01 | 0.85 ± 0.01 |
| REEpre fed (kcal/kg FFM/min) | 0.024 ± 0.001 | 0.025 ± 0.001 |
| REEpre fast (kcal/kg FFM/min) | 0.021 ± 0.001 | 0.020 ± 0.001 |
| REEpost fed (kcal/kg FFM/min) | 0.022 ± 0.002 | 0.024 ±.0.001 |
| REEpost fast (kcal/kg FFM/min) | 0.020 ± 0.001 | 0.020 ± 0.001 |
| Δ total cholesterol (mmol/l) | −0.53 ± 0.14b | −0.04 ± 0.13 |
| Δ LDL cholesterol (mmol/l) | −0.42 ± 0.11c | −0.04 ± 0.08 |
FFM, fat-free mass; HI OA, high oleic acid; HI PA, high palmitic acid; LDL, low-density lipoprotein; REE, resting energy expenditure; RQ, respiratory quotient.
Mean ± s.e.m. Subscripts “pre” and “post” refer to baseline (pre) and postexperimental diet values. No differences between groups for any variable, except where indicated. Δ total cholesterol, serum cholesterol concentration (postexperimental diet minus pre-experimental diet); Δ LDL cholesterol, serum LDL cholesterol concentration (postexperimental diet minus pre-experimental diet).
HI OA vs. HI PA, P = 0.017 (analysis of covariance).
HI OA vs. HI PA, P = 0.011 (analysis of covariance).
FA composition of ACs
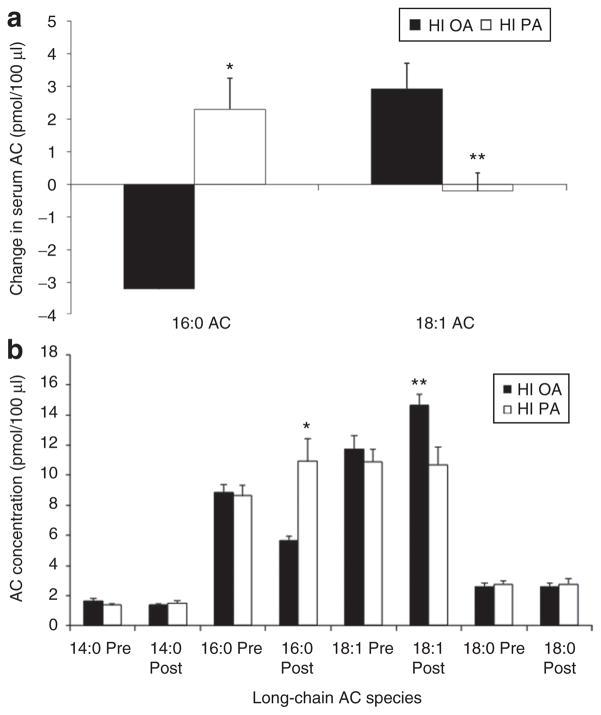
The HI PA diet caused an increased concentration (pmol/100 μl) of the 16:0 AC (palmitoylcarnitine), whereas this species decreased on the HI OA diet (P < 0.001) (Figure 1a). Conversely, the HI OA diet, caused a marked increase in the 18:1 AC (oleoylcarnitine), whereas this species slightly decreased in subjects on the HI PA diet (which contained approximately the same OA concentration as the baseline diet, P = 0.005) (Figure 1a).
Figure 1.
Serum acylcarnitine (AC) concentrations (pmol/100 μl) in subjects fed either a high-palmitic acid diet (HI PA) or a high-oleic acid/low-palmitic acid diet (HI OA). (a) Changes in the fatty acid composition of AC. HI PA vs. HI OA: *P < 0.001; **P = 0.005. (b) Absolute concentrations of selected long-chain AC, pre-experimental diet (Pre) and after the experimental diets (Post). HI PA vs. HI OA: *P < 0.001; **P = 0.005.
At the end of the baseline diet, the ratio of the 16:0 AC to the 18:1 AC was 0.80 ± 0.03 on those subsequently ingesting the HI PA diet and 0.77 ± 0.03 in those subsequently ingesting the HI OA diet (NS). After 1 week on the respective diets, the ratio of the 16:0 AC to the 18:1 AC increased 28% in the subjects on the HI PA diet (postvalue: 1.02 ± 0.06; change: 0.22 ± 0.05) and decreased 49 % in those on the HI OA diet (postvalue: 0.39 ± 0.02; change: −0.38 ± 0.03, P < 0.001). Figure 1b shows the absolute serum concentrations of 16:0 and 18:1. For the entire data set, there was no correlation of 18:1 AC with 16:0 AC, but this correlation was positive within the HI PA group (r = 0.82, P = 0.003) and within the HI OA group (r = 0.66, P = 0.027).
We observed no statistically significant difference in the change in the concentration of medium chain ACs between groups, although we observed an increase in the HI PA group and a decrease in the HI OA group (HI PA vs. HI OA groups: +32.1 ± 58.7 vs. −26.7 ± 81.4). Also, there was no statistically significant difference in the ratio of medium chain/long chain AC between the HI PA and HI OA groups (0.069 ± 0.084 and 0.034 ± 0.061, respectively). The sum of C4, C6, and C8 dicarboxylic ACs also was not statistically different in the HI PA and HI OA groups (0.928 ± 0.608 vs. 0.087 ± 0.389).
FA composition of DAG and TAG in muscle
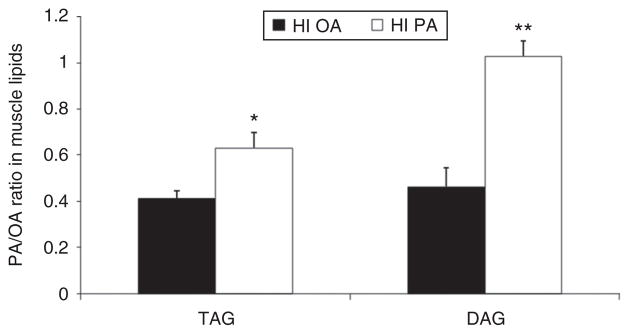
Figure 2 depicts the 122% higher PA/OA ratio in DAG in subjects receiving the HI PA diet (P = 0.043) and as well as the 54% higher PA/OA ratio in TAG (P = 0.011). There was no group effect on the individual OA or PA concentrations in either DAG or TAG (in contrast to the AC data, compare Figure 1). However, in one of our subjects on the HI PA diet, a woman with the highest BMI (28.5) the concentrations of PA and OA in DAG were 1.30 and 3.21 μg/mg protein, respectively, compared to the next highest values among the remaining subjects of 0.76 and 1.24, respectively. When this subject was excluded, we not only detected significant differences between the groups in the ratio of PA/OA in DAG and TAG but also a significantly higher value for the OA content of DAG in the HI OA group (0.61 ± 0.09 vs. 0.31 ± 0.04, P = 0.01).
Figure 2.
FA composition of diacylglycerol (DAG) and triacylglycerol (TAG) in muscle in subjects fed either a high-palmitic acid diet (HI PA) or a high-oleic acid/low-palmitic acid diet (HI OA). HI PA vs. HI OA: *P = 0.011; **P = 0.043.
In the subset of 10 subjects who underwent adipose tissue biopsy, the PA/OA ratio in muscle DAG was, respectively, 1.21 ± 0.42 and 0.59 ± 0.14 in the HI PA and HI OA groups. The PA/OA ratio in TAG was 0.66 ± 0.11 and 0.42 ± 0.05 in the two groups, respectively.
There was no significant diet group effect (HI PA vs. HI OA) on DAG concentration (1.49 ± 0.50 vs. 1.45 ± 0.20 μg/mg protein) or TAG concentration (175.9 ± 57.6 vs. 199.5 ± 96.5 μg/mg protein). There also was no gender effect. Mean DAG concentration, expressed as μg/mg muscle wet weight, was 0.17 ± 0.04 for the entire group of 24 subjects. In the six subjects reassessed using the now preferred EMD TLC plate, the muscle DAG concentration was 0.284 ± 0.05 μg/mg muscle wet weight.
FA composition of adipose tissue
Ten subjects (N = 6, HI PA; N = 4, HI OA) participated in this substudy. The PA/OA ratio in the adipose tissue DAG was 1.10 ± 0.21 and 0.91 ± 0.05 in the HI PA and HI OA groups, respectively (NS). There was a borderline significant (P = 0.067), 54% higher concentration of PA in the DAG isolated from adipose tissue (μg/mg adipose tissue) in those receiving the HI PA diet (0.17 ± 0.02), compared to the HI OA group (0.11 ± 0.02). The PA/OA ratio in the adipose tissue TAG was, respectively, 0.48 ± 0.03 and 0.42 ± 0.02 in the HI PA and HI OA groups (NS). We did observe a significant correlation between the PA/OA ratio in TAG in adipose tissue and muscle (Pearson r = 0.70, P = 0.024; Spearman r = 0.76, P = 0.011).
The TAG concentration of adipose tissue (μg/mg adipose tissue; 316 ± 42) was about 12.3 times that of muscle (26 ± 11, P < 0.001). The amount of DAG in adipose tissue (μg/mg adipose tissue; 0.45 ± 0.05) was higher that of muscle tissue (μg/mg; 0.24 ± 0.08, P = 0.051). This implies that a 10% contamination of a muscle biopsy with adjacent adipose tissue would inflate the DAG value by about 9% (i.e., 0.26 μg/mg muscle).
DISCUSSION
We have demonstrated, for the first time in humans, that a 1-week alteration in dietary FA composition produced corresponding changes in the FA composition of skeletal muscle TAG and DAG and in the FA profile of serum ACs. These results offer evidence that the intracellular FA milieu as a whole, and at least some intramuscular glycerolipid pools, are acutely responsive to dietary manipulations. In contrast, short-term dietary changes did not influence the PA/OA ratio in adipose tissue, but in the HI PA group, there was a borderline higher concentration of PA in DAG isolated from adipose tissue. The observed effects on muscle suggest that the FA composition of intracellular lipid pools might be used as biomarkers for change in dietary FA intake. In addition, this study highlights the importance of controlling dietary FA composition when designing human trials, as it appears that even a 1-week diet intervention alters the FA composition of cellular lipid pools (ACs, DAG, TAG).
We acknowledge that the design of this study was not ideal in that we were unable to obtain baseline (prediet) muscle biopsies. However, the PA/OA ratio of the serum AC was nearly identical between groups at the start of the study. Moreover, the postdiet PA/OA ratio of serum AC (1.02 and 0.39, for the HI PA and HI OA diets, respectively) was very similar to that measured in muscle DAG (1.03 and 0.46). These findings suggest that the dietary intervention, rather than individual differences between subjects, accounted for the group differences in the PA/OA ratio of muscle lipids.
The muscle concentration of DAG averaged 0.17 μg/mg muscle wet weight for the 24 subjects, but in the six subjects reassessed using the EMD TLC plate, the muscle DAG concentration was higher, 0.284 μg/mg muscle wet weight. Using calculations described in the Methods and Procedures, our estimates of muscle DAG concentration were comparable to those in the literature (35,36). Muscle DAG averaged ~0.233 μg/mg muscle wet weight in nine obese subjects studied by Bruce et al. (35), who also used the TLC/GC methodology (Kieselgel 60 TLC plate) (feeding state not specified). A study by Itani et al. (36), who used TLC (plate not specified) and a 32P-ATP-dependent enzymatic method, reported 0.091 μg of DAG-derived FA/mg muscle, when measured after a 6-h insulin-plus-lipid infusion. The disparity in these results might be related to methodology, protocol differences in metabolic state, or between subject variability.
Andersson et al. (32) examined the FA composition of TAG from muscle biopsied after high- and low-PA diets, prescribed over a 3-month test period but without rigid, “GCRC-type” dietary control. Despite a smaller difference in the PA/OA ratio of the two diets in our study compared to theirs (32), we observed a 450% greater arithmetic difference in the PA/OA ratio of the muscle TAG between the two diets. Thus, in our shorter duration study (1 week vs. 3 months), intense dietary control magnified the response (FA composition of muscle TAG) in proportion to the diet intervention.
Over the short intervention period, we observed no effects of the diets on RQ or REE. In a previous longer term (4 weeks) dietary intervention study, we found that the HI OA and HI PA diets resulted in distinct changes in whole body FA oxidation, modified by the gender of the subjects (5,6). This effect was not observed during the first 14 h of diet administration (37). Notably, however, in this study, our estimates of RQ in both the fed or fasted state were based on a single 20-min measurement.
We found that the HI OA diet decreased serum concentrations of total cholesterol and low-density lipoprotein cholesterol, whereas these outcome measures were unchanged in subjects assigned to the HI PA diet. Our previous study showed similar effects of the HI OA diet after 4 weeks of feeding (6). In patients with hyperlipidemia, the effects of changes in dietary FA composition on serum cholesterol concentration require 1–2 weeks to stabilize (38,39). Our finding that a 1-week, low-PA diet, caused a lowering of total and low-density lipoprotein concentration could have implications to both routine patient care (annual evaluations of serum lipids) as well as epidemiological studies, because it means that very short-term alterations in dietary FA intake will affect serum lipid concentrations.
In conclusion, we found that feeding a high-PA diet for 7 days caused a corresponding change in the PA and OA composition of intramyocellular DAG and TAG and in the serum AC profile, as compared to a low-PA/high-OA diet. Our results imply that the FA composition of muscle lipids and AC metabolites both can be used as biomarkers of dietary FA composition. Also, although our data are preliminary, the measured DAG concentration of adipose tissue suggests that even a 10% contamination of a muscle biopsy with adjacent fat would result in a very small difference in measured DAG concentration of muscle, and perhaps similar conclusions with regard to the effects of a diet on PA composition of DAG.
Acknowledgments
This study was supported by NIH project Grants R01 DK55384 (C.L.K.) and R01 DK073284 (C.L.K.) and by M01 RR00109 (General Clinical Research Center at the University of Vermont) and by R01 AG028930 (D.M.M.) (Duke University). None of the authors had a financial conflict of interest pertaining to this study. We thank all of the staff at the University of Vermont GCRC for help with various aspects of the study, and Mary Schmitz Brown and Dayong Sun, formerly at UTMB, Galveston, TX and Bruce O’Rourke and Rhonda Maple, University of Vermont, with initial technical assistance. We thank Catherine M. Champagne, Pennington Biomedical Research Center, Baton Rouge, LA for assistance with developing the defined diets. Finally, we thank William T. Donahoo, who performed a muscle biopsy in Dr Kien’s absence.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep. 2009;9:43–50. doi: 10.1007/s11892-009-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Moffitt JH, Fielding BA, Evershed R, et al. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res. 1999;40:2322–2332. [PubMed] [Google Scholar]
- 5.Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr. 2005;82:320–326. doi: 10.1093/ajcn.82.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity (Silver Spring) 2008;16:29–33. doi: 10.1038/oby.2007.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piers LS, Walker KZ, Stoney RM, Soares MJ, O’Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717–727. doi: 10.1079/bjn2003948. [DOI] [PubMed] [Google Scholar]
- 8.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 9.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 10.Coll T, Jové M, Rodríguez-Calvo R, et al. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes. 2006;55:2779–2787. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 11.Crunkhorn S, Dearie F, Mantzoros C, et al. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:15439–15450. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 12.Coll T, Eyre E, Rodríguez-Calvo R, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 13.Thrush AB, Heigenhauser GJ, Mullen KL, Wright DC, Dyck DJ. Palmitate acutely induces insulin resistance in isolated muscle from obese but not lean humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1205–R1212. doi: 10.1152/ajpregu.00909.2007. [DOI] [PubMed] [Google Scholar]
- 14.Jové M, Planavila A, Sánchez RM, et al. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology. 2006;147:552–561. doi: 10.1210/en.2005-0440. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Perdomo G, Brown NF, O’Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 18.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Pinnamaneni SK, Eo SJ, et al. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 20.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E1341–E1350. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- 22.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Børsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metab Clin Exp. 2006;55:1215–1221. doi: 10.1016/j.metabol.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustad VA, Jonnalagadda SS, Smutko SA, et al. Comparative lipid and lipoprotein responses to solid-food diets and defined liquid-formula diets. Am J Clin Nutr. 1999;70:839–846. doi: 10.1093/ajcn/70.5.839. [DOI] [PubMed] [Google Scholar]
- 25.Kien CL, Ugrasbul F. Prediction of daily energy expenditure during a feeding trial using measurements of resting energy expenditure, fat-free mass, or Harris-Benedict equations. Am J Clin Nutr. 2004;80:876–880. doi: 10.1093/ajcn/80.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern SE, Williams K, Ferrannini E, et al. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54:333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 27.Highstead RG, Tipton KD, Creson DL, Wolfe RR, Ferrando AA. Incidence of associated events during the performance of invasive procedures in healthy human volunteers. J Appl Physiol. 2005;98:1202–1206. doi: 10.1152/japplphysiol.01076.2004. [DOI] [PubMed] [Google Scholar]
- 28.Toth MJ, Palmer BM, LeWinter MM. Effect of heart failure on skeletal muscle myofibrillar protein content, isoform expression and calcium sensitivity. Int J Cardiol. 2006;107:211–219. doi: 10.1016/j.ijcard.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Thyfault JP, Cree MG, Zheng D, et al. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol, Cell Physiol. 2007;292:C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 30.Boberg M, Croon LB, Gustafsson IB, Vessby B. Platelet fatty acid composition in relation to fatty acid composition in plasma and to serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin Sci. 1985;68:581–587. doi: 10.1042/cs0680581. [DOI] [PubMed] [Google Scholar]
- 31.Brevik A, Veierød MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr. 2005;59:1417–1422. doi: 10.1038/sj.ejcn.1602256. [DOI] [PubMed] [Google Scholar]
- 32.Andersson A, Nälsén C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- 33.Sowa JM, Subbaiah PV. Variable recoveries of fatty acids following the separation of lipids on commercial silica gel TLC plates Selective loss of unsaturated fatty acids on certain brands of plates. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;813:159–166. doi: 10.1016/j.jchromb.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Roe CR, Coates PM. Mitochondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 1995. pp. 1501–1533. [Google Scholar]
- 35.Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 36.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 37.Kien CL, Bunn JY. Effects of palmitate and oleate on the respiratory quotient during acute feeding. Obesity (Silver Spring) 2007;15:1640–1642. doi: 10.1038/oby.2007.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grundy SM, Ahrens EH., Jr The effects of unsaturated dietary fats on absorption, excretion, synthesis, and distribution of cholesterol in man. J Clin Invest. 1970;49:1135–1152. doi: 10.1172/JCI106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spritz N, Ahrens EH, Jr, Grundy S. Sterol balance in man as plasma cholesterol concentrations are altered by exchanges of dietary fats. J Clin Invest. 1965;44:1482–1493. doi: 10.1172/JCI105255. [DOI] [PMC free article] [PubMed] [Google Scholar]