Abstract
An increasing amount of data show that central inflammation contributes to many debilitating diseases and produces spontaneous pain and hyperalgesia (an increased sensitivity to painful stimuli), and these processes may be associated with the production of proinflammatory cytokines by activated microglia. In the present study, we demonstrate that neonatal intracerebral injection of lipopolysaccharide (LPS) (1 mg/kg) in postnatal day 5 (P5) rats produced hyperalgesia that lasted into adulthood as indicated by decreased latency in the tail-flick test. Neonatal LPS administration resulted in a long-lasting increase in the number of activated microglial in the P70 rat brain. The effects of interleukin-1beta (IL-1β) and IL-1 receptor antagonists on hyperalgesia were determined to examine the possible role of inflammatory cytokines in LPS-induced hyperalgesia. Our data show that neonatal intracerebral injection of IL-1β (1 µg/kg) produced a hyperalgesic tendency similar to that induced by LPS. Neonatal administration of an IL-1 receptor antagonist (0.1 mg/kg) significantly attenuated long-lasting hyperalgesia induced by LPS and reduced the number of activated microglia in the adult rat brain. These data reveal that neonatal intracerebral LPS exposure results in long-lasting hyperalgesia and an elevated number of activated microglia in later life. This effect is similar to that induced by IL-1β and can be prevented by an IL-1 receptor antagonist. The present study suggests that an IL-1 receptor antagonist effectively attenuates or blocks long-lasting hyperalgesia and microglia activation produced by LPS exposure in the neonatal period of rats.
Keywords: Lipopolysaccharide, Interleukin-1β, Hyperalgesia, Interleukin-1 receptor antagonist, Microglia
Introduction
Neonatal pain experiences and inflammation may induce a long-lasting alteration in pain sensitivity in both animal models and humans (Boisse et al., 2005; Hermann et al., 2006; Ren et al., 2004). Clinical investigations of neonatal pain suggest that preterm neonates have an increased sensitivity to pain and that acute painful stimuli or stressful stimuli, such as periventricular leukomalacia, early intraventricular hemorrhage, and peripheral tissue damage, lead to the development of prolonged periods of hyperalgesia (Anand, 1998; Bouza, 2009; Fitzgerald et al., 1989). The neuronal hypersensitivity in chronic pain states involves activation of spinal and supraspinal glial cells (De Leo et al., 2006). When stimulated, glial cells presumably increase production of inflammatory mediators such as cytokines and chemokines (De Leo et al., 2006). Interleukin-1 (IL-1), a proinflammatory cytokine, is implicated in modulation of pain sensitivity (Wolf et al., 2003). Administration of IL-1 or lipopolysaccharide (LPS) usually produces hyperalgesia (an increased sensitivity to painful stimuli), which is possibly mediated by induction of prostaglandin E2 (PEG2) (Abe et al., 2001; Boisse et al., 2005; Hori et al., 2000; Wolf et al., 2003).
Occurrence of maternal or placental infection is frequently associated with increased concentrations of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 in the infant brain (Kadhim et al., 2001, Yoon et al., 1997). In previous studies, we developed a neonatal rat model to mimic the scenario of infection/inflammation through intracerebral injection of LPS in the postnatal day 5 (P5) rat brain. LPS, an endotoxin, is a component of the cell wall of gram-negative bacteria and is responsible for most of the inflammatory effects of infection by gram-negative bacteria (Raetz and Whitfield, 2002). In this model, we found that in neonatal rats, intracerebral LPS injection resulted in brain injury and greatly increased microglial activation and brain TNF-α and IL-1β concentrations (Cai et al., 2003; Fan et al., 2005a, 2008a, 2008b; Pang et al., 2003). There are several systemic or peripheral inflammatory animal models to study hyperalgesia (Abe et al., 2001; Boisse et al., 2005; Ren et al., 2004). The present model is to study the role of central LPS in hyperalgesia and our previous data also indicated that neonatal LPS injection resulted in hyperalgesia in adult rats. However, the detailed role of microglia and inflammatory cytokines in mediating long-lasting alterations in pain sensitivity remains unclear.
IL-1β is implicated in LPS-induced modulation of pain sensitivity and mediation of hyperalgesia and allodynia (Cunha et al., 2000). Intrathecal administration of IL-1β induces mechanical allodynia and thermal hyperalgesia (Reev et al., 2000), and treatment with an IL-1 receptor antagonist can inhibit hyperalgesic responses to LPS, IL-1β, carrageenin, bradykinin, and TNF-α (Cunha et al., 2000). Impaired IL-1 signaling or chronic treatment with an IL-1 receptor antagonist resulted in lower pain sensitivity in non-inflammatory conditions in mouse models (Wolf et al., 2003). However, it is unknown whether IL-1 receptor antagonists provide long-lasting protection by attenuating or blocking the long-lasting hyperalgesia induced by neonatal LPS exposure. Therefore, the aim of this study was to examine the effect of an IL-1 receptor antagonist on long-lasting hyperalgesia induced by neonatal LPS exposure.
Materials and methods
Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO, USA). Recombinant rat IL-1β and IL-1 receptor antagonists were purchased from R&D Systems (Minneapolis, MN, USA). An OX42 monoclonal mouse antibody (CD11b) was purchased from Serotec (Raleigh, NC, USA).
Animals
Timed pregnant Sprague-Dawley rats arrived in the laboratory on day 19 of gestation. Animals were maintained in an animal room on a 12-h light/dark cycle at constant temperature (22°C ± 2°C). The day of birth was defined as postnatal day 0 (P0). After birth, the litter size was adjusted to 12 pups per litter to minimize the effect of litter size on body weight and brain size. All litters were weaned at P21 and male rats were housed in groups of 3–4 animals per cage. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center or Fu Jen Catholic University at Taiwan. Every effort was made to minimize the number of animals used and their suffering.
Surgery procedures and animal treatment
Intracerebral injection of LPS, recombinant rat IL-1β, or LPS in combination with an IL-1 receptor antagonist in 5-day old male Sprague-Dawley rat pups was performed as previously described (Cai et al., 2003, 2004; Fan et al., 2008a; 2009; Pang et al., 2003). Under light anesthesia with isoflurane (1.5%), LPS (0.1 or 1 mg/kg from Escherichia coli, serotype 055: B5), IL-1β (1 µg/kg), IL-1receptor antagonist (0.1 mg/kg), or LPS (1 mg/kg) plus IL-1 receptor antagonist (0.1 mg/kg) in sterile saline containing 0.1% BSA (total volume 2 µl) was administered to the rat brain 1.0 mm posterior and 1.0 mm left to the bregma, and 2.0 mm deep from the skull surface in a stereotaxic apparatus with a neonatal rat adapter. The dose of LPS was chosen based on previous results that produced reproducible brain injury (Cai et al., 2003; Fan et al., 2005a, 2005b, 2008a; Pang et al., 2003). The doses of IL-1β and IL-1 receptor antagonists were chosen based on the peak concentrations of IL-1β achieved in the rat pup brain following LPS administration, as reported previously (Cai et al., 2003, 2004; Fan et al., 2009; Holmin and Mathiesen, 2000; Pang et al., 2003). The injection site was located at the area just above the left cingulum. The control rats were injected with the same volume of sterile saline containing 0.1% BSA. All animals survived the intracerebral injection. Each dam had an equal litter size (12 pups) and each group contained 12 pups from different litters. Three days (P8) or sixty-five days (P70) after surgery, rats were sacrificed by transcardiac perfusion with normal saline followed by 4% paraformaldehyde for brain section preparation. Coronal frozen brain sections (10 µm thickness) were prepared in a cryostat for immunohistochemistry
Tail-flick test of pain sensitivity
The tail-flick test was performed as described by Brown et al. (1997) and Wonchanapai et al. (1998) with modifications. The test was performed for all rats daily from P5 (equals to basal data; test was done before the surgery) to P8, or weekly from P21 to P70 (adult rats). Rats were habituated to handling and to being inserted into a plastic cylindrical tube. A shallow groove cut into the Plexiglas plate supported the rat’s tail during the test trials. The center of the rat’s tail from the hip to the end of tail was placed under radiant heat (Analgesia Test Tail-Flick Type 812, Columbus Instruments, Columbus, OH, USA), and the pain sensitivity was measured by tail-flick latency defined as the time from the onset of radiant heat. The intensity of the beam was adjusted to produce mean control reaction times of 6–8 seconds to tail withdrawal. A cut-off time of 10 seconds was established to minimize skin damage (D’Amour and Smith, 1941).
Immunohistochemistry
Microglia were detected using OX42 (CD11b, Serotec, Raleigh, NC, USA) (1:100) immunostaining, which recognizes both resting and activated microglia. Sections were incubated with primary antibodies at 4°C overnight and followed by incubation with secondary antibodies conjugated with fluorescent dyes (rhodamine) for 1 h in the dark at room temperature. Sections incubated in the absence of primary antibody were used as negative controls. The resulting sections were examined under a fluorescent microscope at appropriate wavelengths.
Data analysis and statistics
The tail-flick latency was calculated as the average of 3 tail-flick latencies (sec). The data are presented as the mean ± SEM, and were analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different postnatal days, followed by Student-Newman-Keuls test. Results with p < 0.05 were considered statistically significant. Immunostaining data were quantified by counting positively stained cells. OX42 positive (OX42+) cells with a round shape and blunt processes (as indicated in Figs. 2B and C) were considered activated microglia. The number of positively stained cells in the 3 images was averaged. The brain sections at the bregma level and the middle dorsal hippocampus level were used. The cortex of 3 sections at each of the 2 section levels were examined by an observer blind to the treatment, and the mean cell counting value was used to represent 1 single brain. For the convenience of comparing results among the treated groups, results were standardized as the average number of cells/mm2. In addition to cell counting, OX42 staining was quantified by calculating the percentage area of OX42+ staining in the whole image frame using NIH image software. This method was successfully used to quantify the density of cortical serotonin transporter-immunoreactive fiber networks (Maciag et al., 2006). Quantified immunostaining data are presented as the mean ± SEM, and were analyzed by one-way ANOVA followed by Student-Newman-Keuls test. Results with p < 0.05 were considered statistically significant.
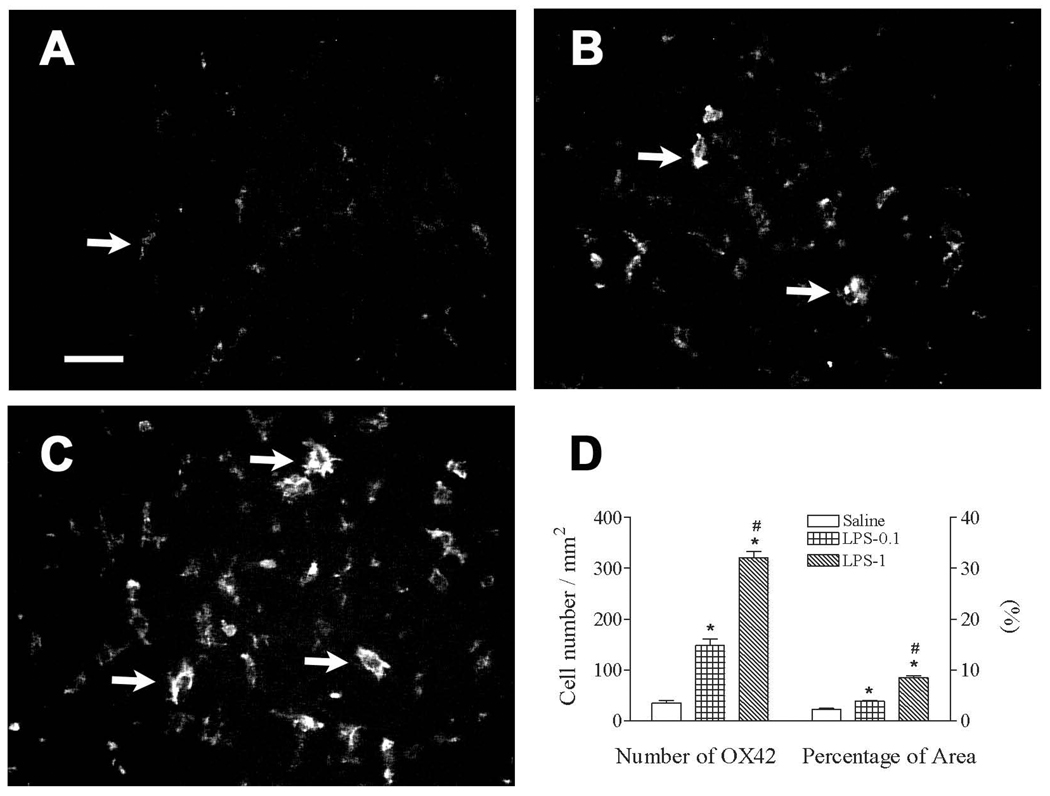
Figure 2.
Representative photomicrographs of OX42 staining in the cortex of rat brain 3 days (P8) after lipopolysaccharide (LPS) injection. LPS stimulated activation of microglia, as indicated by OX42 immunostaining (from a ramified shape to cells with an enlarged cell body and blunt processes) in the rat brain. Intracerebral injection of saline, LPS (0.1 mg/kg), or LPS (0.1 mg/kg) in P5 rats was performed as described in the methods. Most microglia were at resting status with a small rod shaped soma and ramified processes in the brain of the saline group (indicated by the arrow in A). Numerous activated microglia (indicated by arrows in B and C) with a round or elongated shaped cell body and blunt processes were observed in the rat brain 3 days after LPS (0.1 mg/kg) (B) or LPS (0.1 mg/kg) injection (C). The scale bar in A represents 50 µm for A, B, and C. Quantitation of the number of OX42+ cells and the percentage of the image area that contained OX42 staining (D) in the cortex was performed as described in the methods. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by one-way ANOVA. * P < 0.05 represents a significant difference in the LPS (0.1 mg/kg) or LPS (1 mg/kg) group compared with the saline group. # P < 0.05 represents a significant difference in the LPS (1 mg/kg) group compared with the LPS (0.1 mg/kg) group.
Results
Neonatal LPS treatment increased the pain sensitivity
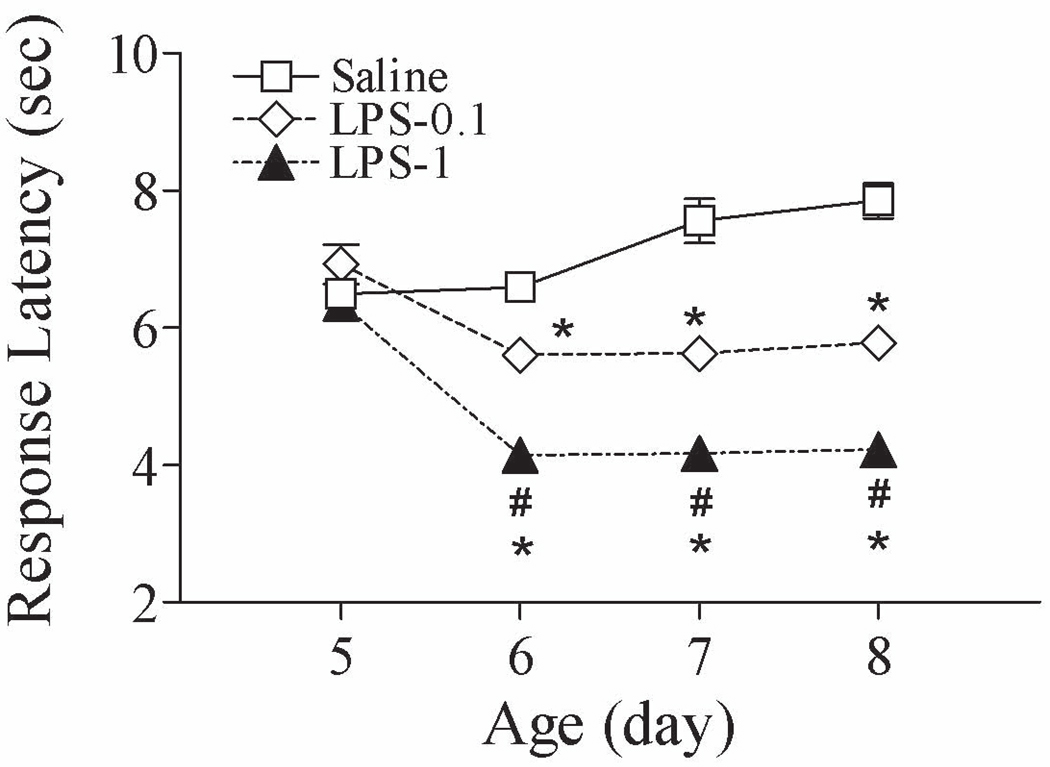
Neonatal LPS exposure (0.1 or 1 mg/kg) at P5 resulted in an enhanced response to stimuli in the neonatal rats [F(2, 143) = 128.459, p < 0.001] (P6–P8, p < 0.05), i.e., decreased latency to tail removal from a radiant heat source (Fig. 1). This decreased removal latency was characteristic of hyperalgesia in the neonatal rats. LPS-induced hyperalgesia was dose dependent, as indicated by decreased latency from approximately 8 sec (control) to 6 sec (0.1 mg/kg LPS) (p < 0.05) and 4 sec (1 mg/kg LPS) (p < 0.05) after radiant heat stimulation in the tail-flick test (Fig. 1).
Figure 1.
Effect of lipopolysaccharide (LPS) on neonatal rat pain sensitivity in the tail-flick test (P5–P8). Rats were intracerebrally injected with saline, LPS (0.1 mg/kg), or LPS (1 mg/kg) at P5 and intensity 2 was used in the tail-flick test. LPS injection resulted in enhanced responses to stimuli in the neonatal rat, i.e., decreased latency to tail removal from a radiant heat source. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different postnatal days. * P < 0.05 represents a significant difference in the LPS (0.1 mg/kg) or LPS (1 mg/kg) group compared with the saline group on the same postnatal day. # P < 0.05 represents a significant difference in the LPS (1 mg/kg) group compared with the LPS (0.1 mg/kg) group on the same postnatal day.
Neonatal LPS exposure increased the number of activated microglia
Neonatal LPS exposure resulted in increased microglial activation in the P8 rat brain as indicated by the number and morphology of OX42+ cells. Few OX42+ cells were detected in the control rat brain, and most of those cells were in a resting status with a small rod shaped soma with fine and ramified processes (Fig. 2A). A significantly increased number of OX42+ cells were observed in the cortex [F(2, 35) = 195.858, p < 0.001] of the LPS-exposed rat brain (Figs. 2B–C). Many OX42+ cells showed features typical of activated microglia, i.e., bright staining of an elongated or a round shaped cell body with blunt or no processes (indicated by arrows in Figs. 2B and C) (Godoy et al., 2008; Kreutzberg, 1996). The LPS-induced increase in microglia activation was dose dependent as indicated by an increase from 34.75 ± 6.01 cell/mm2 (control) to 148.19 ± 12.64 cell/mm2 (0.1 mg/kg LPS) (p < 0.05) and 321.64 ± 11.14 cell/mm2 (1 mg/ kg LPS) (p < 0.05) in the cortex of P8 rat brains (Fig. 2D). OX42 staining was further quantified by calculating the percentage area that contained OX42 immunostaining. A higher percentage area of OX42+ immunostaining was observed in the cortex [F(2, 35) = 139.167, p < 0.001] of the neonatal LPS-exposed rat brain (Fig. 2D). The percentage area containing OX42 immunostaining increased from 2.30 ± 0.17% (control) to 3.84 ± 0.15% (0.1 mg/kg LPS) (p < 0.05) and 8.48 ± 0.41 (1 mg/kg LPS) (p < 0.05) in the cortex of P8 rat brains (Fig. 2D).
Neonatal IL-1β treatment increased pain sensitivity
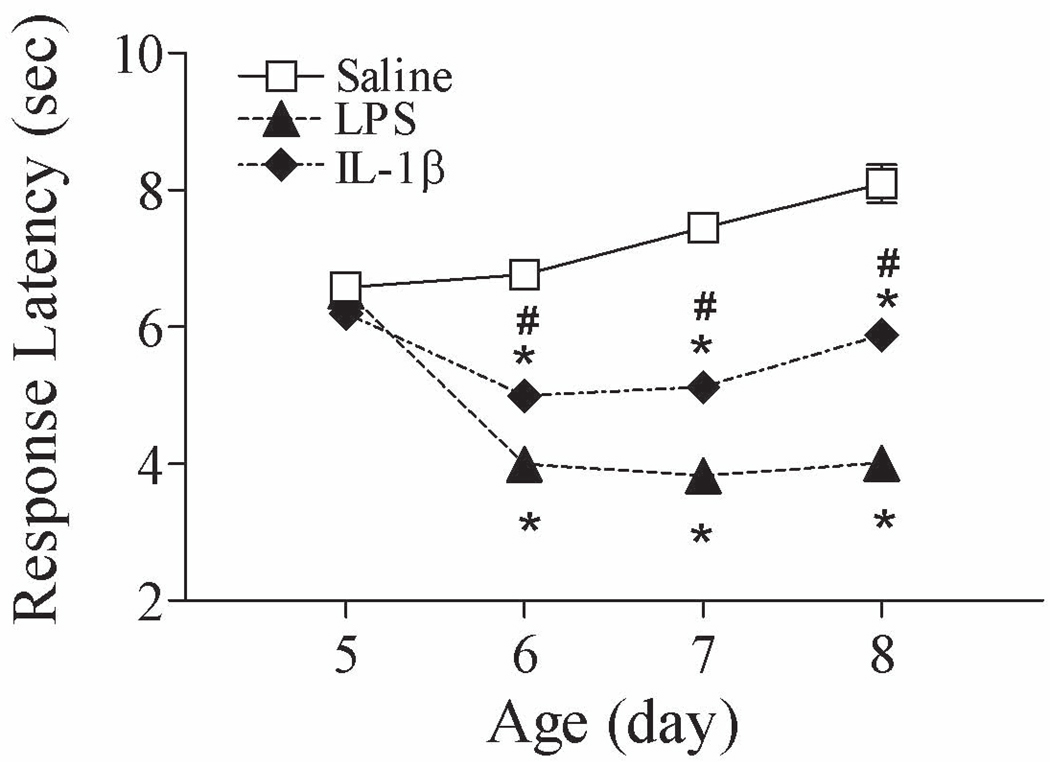
Intracerebral injection of the pro-inflammatory cytokine IL-1β (1 µg/kg) produced an enhanced response to painful stimuli in the neonatal rats [F(2, 143) = 167.371, p < 0.001] (P6–P8, p < 0.05), i.e., decreased latency to tail removal from a radiant heat source (Fig. 3), in a manner similar to LPS exposure in the neonatal rat. IL-1β-induced hyperalgesia was indicated by decreased latency from approximately 8 sec (control) to 6 sec (p < 0.05) after radiant heat stimulation in the tail-flick test (Fig. 3).
Figure 3.
Effect of interleukin-1beta (IL-1β on neonatal rat pain sensitivity in the tail-flick test (P5–P8). P5 rats were intracerebrally injected with saline, lipopolysaccharide (LPS) (1 mg/kg), or IL-1β (1 µg/kg). Similar to LPS exposure in the neonatal rat, IL-1β injection produced enhanced responses to painful stimuli in the neonatal rats, i.e., decreased latency to tail removal from a radiant heat source. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different postnatal days. * P < 0.05 represents a significant difference in the LPS or IL-1β group compared with the saline group on the same postnatal day. # P < 0.05 represents a significant difference in the IL-1β group compared with the LPS group on the same postnatal day.
Neonatal IL-1β exposure increased the number of activated microglia
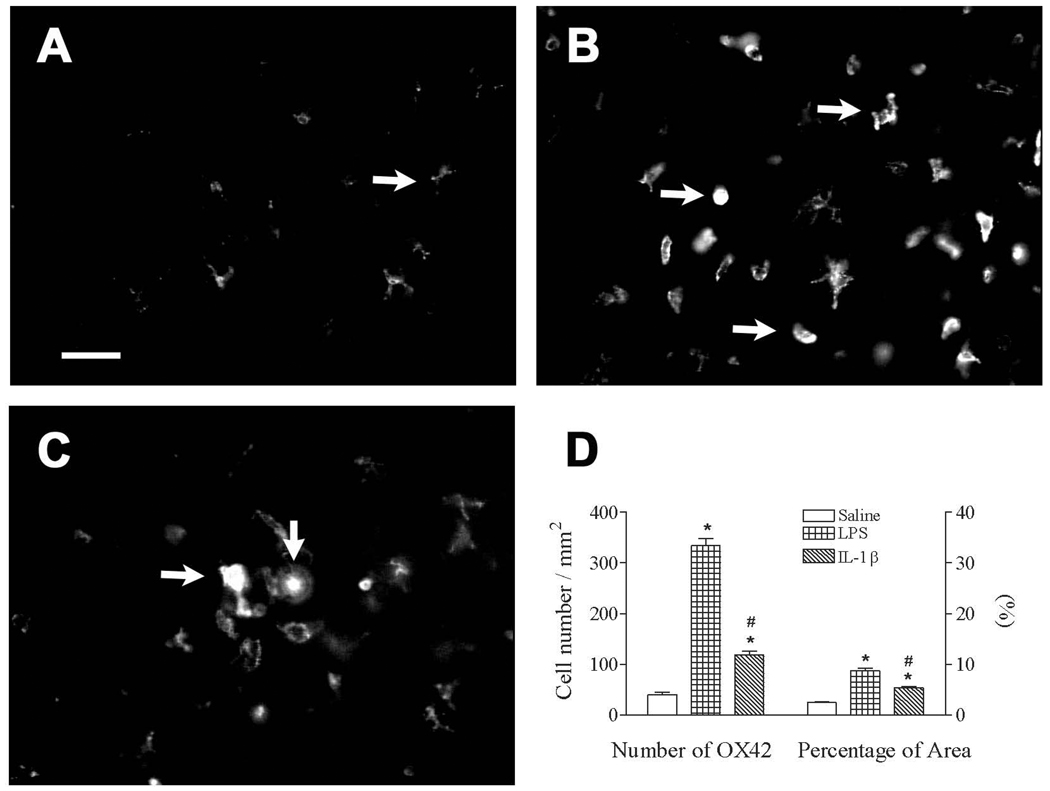
Neonatal IL-1β exposure resulted in increased microglial activation in the P8 rat brain as indicated by the number and morphology of OX42+ cells (Fig. 4). A significantly increased number of OX42+ cells were observed in the cortex [F(2, 35) = 233.536, p < 0.001] of the IL-1β-exposed rat brain (Figs. 4B–C). Neonatal IL-1β-exposure significantly increased the number of microglia (118.83 ± 8.06 cell/mm2, p < 0.05) and the percentage area that contained OX42 immunostaining (5.35 ± 0.31%, p < 0.05) compared with the control (40.01 ± 5.45 cell/mm2 and 2.44 ± 0.15%, respectively) (Fig. 4D).
Figure 4.
Representative photomicrographs of OX42 staining in the cortex of the rat brain 3 days (P8) after interleukin-1beta (IL-1β) injection. Lipopolysaccharide (LPS) or IL-1β stimulated microglia activation, as indicated by OX42 immunostaining (from a ramified shape to cells with an enlarged cell body and blunt processes), was determined in the rat brain. Intracerebral injection of saline, LPS (0.1 mg/kg), or IL-1β (1 µg/kg) in P5 rats was performed as described in the methods. Most microglia were at resting status with a small rod shaped soma and ramified processes in the brain of the saline group (indicated by the arrow in A). Numerous activated microglia (indicated by arrows in B and C) with a round or elongated shaped cell body and blunt processes were observed in the rat brain 3 days after LPS (B) or IL-1β injection (C). The scale bar in A represents 50 µm for A, B, and C. Quantitation of the number of OX42+ cells and the percentage of the image area that contained OX42 staining (D) in the cortex was performed as described in the methods. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by one-way ANOVA. * P < 0.05 represents a significant difference in the LPS or IL-1β group compared with the saline group. # P < 0.05 represents a significant difference in the IL-1β group compared with the LPS group.
Co-administration of LPS and an IL-1 receptor antagonist attenuated long-lasting LPS-induced hyperalgesia in adult rats
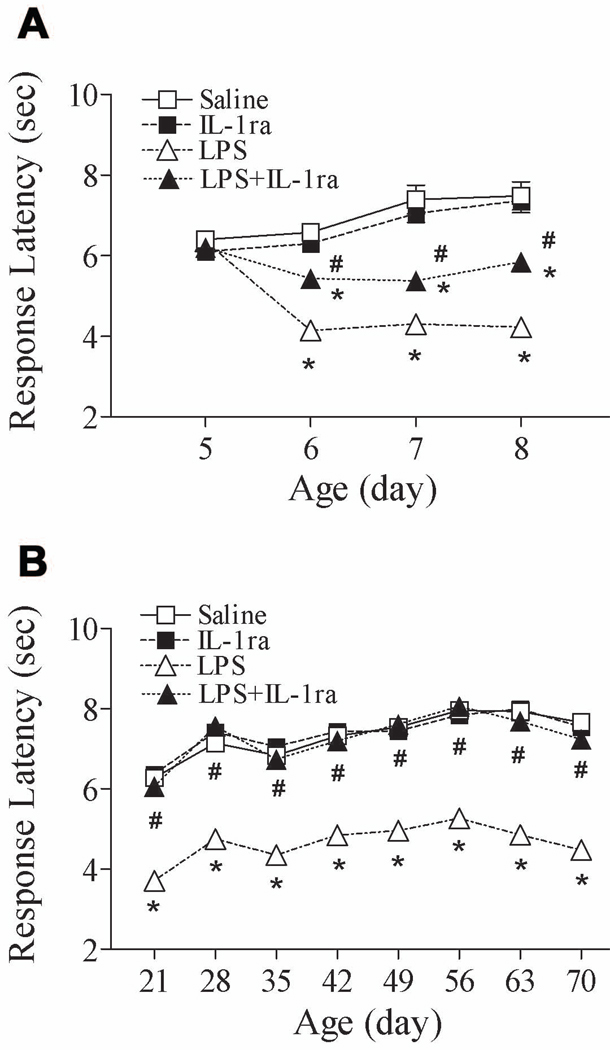
P5 rats displayed shorter tail-flick latency after LPS injection during the neonatal period [F(3, 191) = 58.564, p < 0.001] (P6–P8, p < 0.05) (Fig. 5A) and during the adult period [F(3, 383) = 343.547, p < 0.001] (P21–P70, p < 0.05) (Fig. 5B). The long-lasting hyperalgesia induced by neonatal LPS was prevented by co-treatment with an IL-1 receptor antagonist (P < 0.05) (Figs. 5A and B).
Figure 5.
Co-administration of lipopolysaccharide (LPS) and an interleukin-1 (IL-1) receptor antagonist attenuated LPS-induced pain sensitivity in neonatal rats (A, P5–P8) and adult rats (B, P21–P70) in the tail-flick test. Rats were intracerebrally injected with saline or LPS (1 mg/kg), and/or co-administration with an IL-1 receptor antagonist (0.1 mg/kg) at P5. LPS caused a long-lasting decrease in latency to tail removal from a radiant heat source in neonatal rats. Co-administration of LPS and an IL-1 receptor antagonist significantly reduced the LPS-induced pain sensitivity observed in rats treated with LPS alone. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different postnatal days. * P < 0.05 represents a significant difference in the LPS or LPS+IL-1 receptor antagonist group compared with the saline group on the same postnatal day. # P < 0.05 represents a significant difference in the LPS+IL-1 receptor antagonist group compared with the LPS group on the same postnatal day.
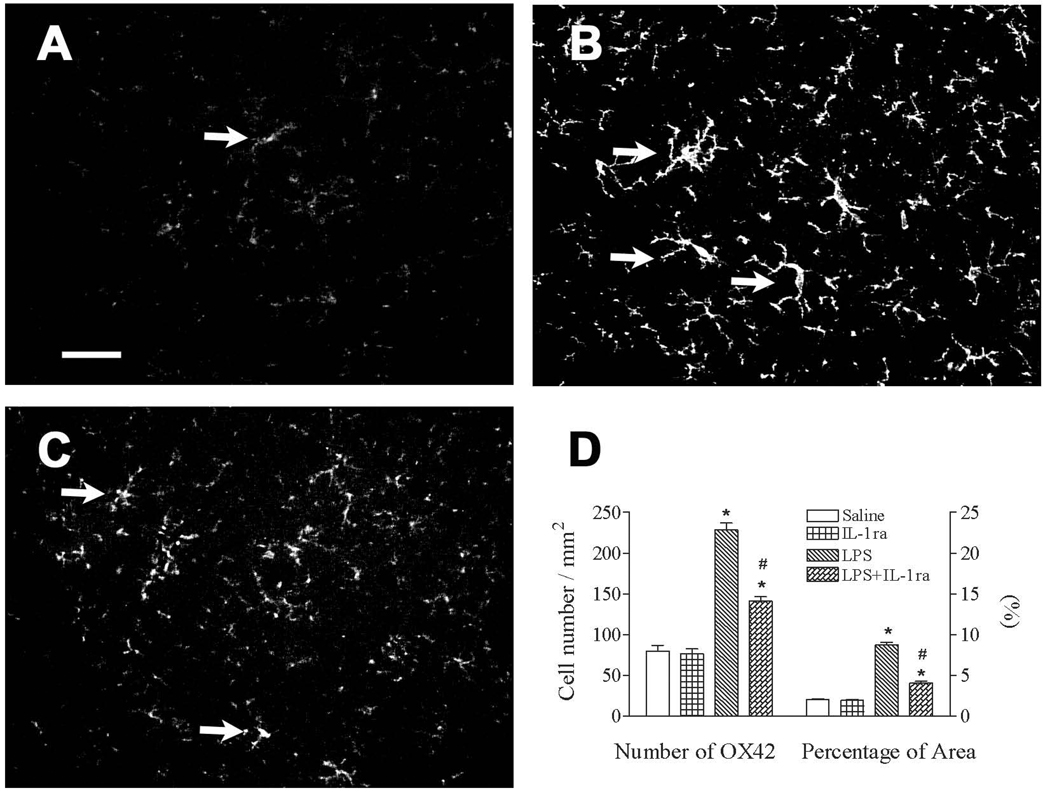
The protective effect of the IL-1 receptor antagonist was associated with a reduced number of LPS-activated microglia
LPS stimulated long-lasting activation of microglia as indicated by the increased number of OX42+ cells [F(3, 47) = 114.227, p < 0.001] and the percentage area that contained OX42 immunostaining [F(3, 47) = 239.462, p < 0.001] in the rat brain. Most OX42+ cells observed in the brain 65 days after injection were in resting status with a ramified shape (activated microglia: 80.01 6.59 cells/mm2, percentage area: 2.05 0.13%) in the control rats (Figs. 6A and D), while those in the LPS group were activated with an enlarged cell body with blunt processes 65 days after the injection (228.92 ± 8.29 cells/mm2, p < 0.05; 8.74 ± 0.33%, p < 0.05) (Figs. 6B and D). Co-treatment with LPS and an IL-1 receptor antagonist significantly reduced the number of activated microglia in the neonatal rat brain (141.28 ± 5.17 cells/mm2, P < 0.05; 4.08 ± 0.21%, p < 0.05) (Figs. 6C and D).
Figure 6.
Representative photomicrographs of OX42 staining in the cortex of the rat brain 65 days (P70) after lipopolysaccharide (LPS) injection. LPS stimulated activation of microglia, as indicated by OX42 immunostaining (from a ramified shape to cells with an enlarged cell body and blunt processes), was determined in the rat brain. Intracerebral injection of LPS (1 mg/kg), and/or co-administration of interleukin-1 (IL-1) receptor antagonist (0.1 mg/kg) in P5 rats was performed as described in the methods. Most microglia were at resting status with a ramified shape in the brain of the saline and the LPS+IL-1 receptor antagonist groups (A and C, respectively). Numerous activated microglia with an enlarged cell body and blunt processes were observed in the rat brain 65 days after LPS injection (indicated by arrows in B). The scale bar in A represents 50 µm for A, B, and C. Quantitation of the number of OX42+ cells and the percentage of the image area that contained OX42 staining (D) in the cortex was performed as described in the methods. The results are expressed as the mean ± SEM of 12 animals in each group, and were analyzed by one-way ANOVA. * P < 0.05 represents a significant difference in the LPS or LPS+IL-1 receptor antagonist group compared with the saline group. # P < 0.05 represents a significant difference in the LPS+IL-1 receptor antagonist group compared with the LPS group.
Discussion
Early abnormal experiences can produce permanent alterations in the sensory system and the stimulation of nociceptive information during windows of vulnerability may produce long-lasting alterations in pain sensitivity (Lidow, 2002). The long-term effects of neonatal pain stimulation on the development of nociceptive systems have been examined by treatment with repeated noxious insults, inflammatory insults, colonic irritation, and physical nerve damage (Lidow, 2002). Neonatal inflammatory insults, colonic irritation, and physical nerve damage caused chronic hyperalgesia (Lidow, 2002). Our data show that central inflammation induced by neonatal LPS in rats (5 days of age, which is developmentally comparable to human preterm infants) produced hyperalgesia that extended to adulthood (70 days of age). In our preliminary study, vocalization was observed in all adult animals (12/12) from the neonatal LPS exposure group when their hairs were tenderly touched, but this phenomenon was not observed in control rats (0/12). Vocalization induced by hair-touching may indicate long-lasting tactile allodynia (a pain reaction to normally innocuous stimuli) in rats following neonatal LPS administration, and suggests that the rat’s first postnatal week is a significant window for inducing an aberrant pain response. Similar results were reported from peripheral LPS injection in neonatal rats (P14) (Boisse et al., 2005). However, a different type of neonatal local inflammatory insult (carrageenin) produced different results (Ren et al., 2004), and rats that received 4 needle pricks daily for 7 days exhibited decreased pain sensitivity (Lidow, 2002). These effects were not observed in the present study. Both control and LPS-exposed rats were repeatedly used for testing (from P5 to P70), and each group was compared to the control group at the same age to minimize potential errors from repeated testing during development.
In addition to LPS- and IL-1β-induced hyperalgesia, we observed that the number of activated microglia were increased in the P8 rat brain in a manner similar to our previous studies (Cai et al., 2003; Fan et al., 2005a, 2008a, 2008b, 2009). Microglia, which are the only non-neuronal cell type that expresses the Toll-like receptor 4 (TLR4), has been identified as the major LPS-responsive cell in the CNS (Lehnardt et al., 2002). These data show that neonatal LPS-induced brain injury and behavioral deficits are not transient and may be related to the activation of microglia. It is possible that microglia activated by LPS increase production of inflammatory mediators such as IL-1β, a proinflammatory cytokine that has been implicated in modulation of pain sensitivity (De Leo et al., 2006; Wolf et al., 2003). Administration of IL-1β or LPS typically produces hyperalgesia, which is presumably mediated through the induction of PEG2 (Abe et al., 2001; Boisse et al., 2005; Cunha et al., 2000; Hori et al., 2000; Wolf et al., 2003). Our previous studies showed that intracerebral injection of LPS in the neonatal rat resulted in brain injury and increased IL-1β concentration in the brain of rats (Cai et al., 2003; Fan et al., 2005a, 2008a, 2008b; Pang et al., 2003). We have also observed that neonatal LPS injection resulted in increased IL-1β concentration in the P70 rat brain (data not shown), prompting us to design an experiment to investigate the interactions between LPS-induced hyperalgesia and IL-1β in rats. We found that hyperalgesia induced by intracerebral injection of IL-1β is similar to hyperalgesia resulting from intracerebral injection of LPS in neonatal rats. In addition, an IL-1 receptor antagonist provided long-term protection by attenuating or blocking long-lasting hyperalgesia and microglia activation produced by LPS exposure during the neonatal period (Figs. 5 and 6). These data suggest that LPS-induced hyperalgesia is associated with microglia activation and production of inflammatory mediators such as IL-1β. Intrathecal administration of IL-1β induces mechanical allodynia and thermal hyperalgesia (Reev et al., 2000) and treatment with an IL-1 receptor antagonist can inhibit the hyperalgesic response induced by LPS, IL-1β, carrageenin, bradykinin, and TNF-α (Cunha et al., 2000). Impaired IL-1 signaling or chronic treatment with an IL-1 receptor antagonist resulted in lower pain sensitivity under non-inflammatory conditions in a mouse model (Wolf et al., 2003). Terrando et al. (2010) also indicated that blocking IL-1 signaling prevented the behavioral abnormality, and attenuated the inflammatory cascade to LPS by reducing microglial activation. In addition to microglia, IL-1β may possible come from other cell type, such as astrocyte which is also a source of inflammatory cytokines upon stimulation by endotoxin (Kopnisky et al., 1997). However, the detailed mechanism of LPS-induced hyperalgesia should be further investigated.
The neuronal hypersensitivity in chronic pain states may involve the activation of spinal and supraspinal glial cells (microglia and astrocytes)(De Leo et al., 2006). It was suggested that the immaturity of sensory processing within the newborn spinal cord leads to lower excitation and sensitization thresholds, potentially maximizing the central responses of these tissue-damaging inputs (Fitzgerald and Beggs, 2001). The abnormal activity related to pain and injury in early life may alter synaptic development and change somatosensory processing (Walker et al., 2003). The long-term plasticity of both peripheral and central sensory connections during the neonatal period suggests that damage in infancy can cause structural and functional alterations in pain pathways that last into adulthood (Fitzgerald and Beggs, 2001). Neonatal LPS exposure can increase microglia activity and the concentration of IL-1β in the rat brain. It was shown that the pro-inflammatory cytokine, IL-1β, potentiates the excitability of nociceptive trigeminal ganglion neurons via membrane depolarization and up-regulation of IL-1β receptors in the neuronal soma, causing trigeminal inflammatory hyperalgesia (Takeda et al., 2008).
In conclusion, this study provides evidence that a single neonatal central inflammatory challenge could induce long-lasting hyperalgesia. This hyperalgesia is related to the activation of microglia, which is possibly mediated by IL-1β.
Acknowledgements
This work was supported by a grant SKH-FJU-97-04 (to KC Wang and LT Tien) from Shin Kong Wu Ho-Su Memorial Hospital, Taiwan. NIH grants HD 35496 (to Z Cai), NS 54278 (Z Cai), Newborn Medicine Funds (to PG. Rhodes) and a research grant from the Department of Pediatrics (to LW Fan), University of Mississippi Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Abe M, Oka T, Hori T, Takahashi S. Prostanoids in the preoptic hypothalamus mediate systemic lipopolysaccharide-induced hyperalgesia in rats. Brain Res. 2001;916:41–49. doi: 10.1016/s0006-8993(01)02861-x. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Boissé L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–141. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med. 2009;22:722–732. doi: 10.3109/14767050902926962. [DOI] [PubMed] [Google Scholar]
- Brown RF, Jackson GD, Martin T, Westbrook RF. Bacterial lipopolysaccharides induce peripheral nerve disturbances in rats that mimic human immune-mediated polyneuropathies. Lab Anim Sci. 1997;47:354–361. [PubMed] [Google Scholar]
- Cai Z, Lin S, Pang Y, Rhodes PG. Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr Res. 2004;56:377–384. doi: 10.1203/01.PDR.0000134249.92944.14. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced neuronal injury in the neonatal rat brain. Neuroscience. 2008a;151:737–744. doi: 10.1016/j.neuroscience.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Tien LT, Rhodes PG, Cai Z. Interleukin-1β-induced brain injury in the neonatal rat can be ameliorated by α-phenyl-n-tert-butyl-nitrone. Exp Neurol. 2009;220:143–153. doi: 10.1016/j.expneurol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neurol. 2008b;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005a;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien L-T, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005b;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakça S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Intracerebral administration of interleukin-1α and induction of inflammation, apoptosis, and vasogenic edama. J Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- Hori T, Oka T, Hosoi M, Abe M, Oka K. Hypothalamic mechanisms of pain modulatory actions of cytokines and prostaglandin E2. Ann NY Acad Sci. 2000;917:106–120. doi: 10.1111/j.1749-6632.2000.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Sumners C, Chandler LJ. Cytokine and endotoxin induced nitric oxide synthase in rat astroglial cultures: differential and modulation by angiotensin II. J Neurochem. 1997;68:935–944. doi: 10.1046/j.1471-4159.1997.68030935.x. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett P, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The Toll-like receptor TLP4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99:377–383. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kitagawa J, Takahashi M, Matsumoto S. Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain. 2008;139:594–602. doi: 10.1016/j.pain.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Wongchanapai W, Tsang BK, He Z, Ho IK. Differential involvement of opioid receptors in intrathecal butorphanol-induced analgesia: compared to morphine. Pharmacol Biochem Behav. 1998;59:723–727. doi: 10.1016/s0091-3057(97)00558-3. [DOI] [PubMed] [Google Scholar]
- Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K, Shavit Y. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain. 2003;104:471–480. doi: 10.1016/S0304-3959(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-α and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]