Abstract
Objective
To determine if directly observed antiretroviral therapy (DOT) is more efficacious than self-administered therapy for improving adherence and reducing HIV viral load (VL) among methadone-maintained opioid users.
Design
Two-group randomized trial.
Setting
Twelve methadone maintenance clinics with on-site HIV care in the Bronx, New York.
Participants
HIV-infected adults prescribed combination antiretroviral therapy.
Intervention
24 weeks of DOT.
Main outcomes measures
Between group differences at 4 assessment points from baseline to week 24 in: (1) antiretroviral adherence measured by pill count, (2) VL, and (3) proportion with undetectable VL (< 75 copies/ml).
Results
Between June 2004 and August 2007, we enrolled 77 participants. Adherence in the DOT group was higher than in the control group at all post-baseline assessment points; by week 24 mean DOT adherence was 86% compared to 56% in the control group (p<0.0001). Group differences in mean adherence remained significant after stratifying by baseline VL (detectable versus undetectable). In addition, during the 24-week intervention, the proportion of DOT participants with undetectable VL increased from 51% to 71%.
Conclusions
Among HIV-infected opioid users, antiretroviral DOT administered in methadone clinics was efficacious for improving adherence and decreasing VL, and these improvements were maintained over a 24-week period. DOT should be more widely available to methadone patients.
Keywords: Directly observed therapy, HIV, medication adherence, methadone, randomized trial
1. INTRODUCTION
Though deaths among persons with AIDS have decreased with combination antiretroviral therapy, death rates among drug users have decreased less markedly (Tang et al.,2008). Disproportionately worse HIV treatment outcomes among drug users compared to non-drug users have been attributed partly to poor medication adherence (Arnsten et al., 2002; Malta et al.,2008). Though many adherence-improving interventions exist, few have focused specifically on drug users.
Programs providing directly observed therapy (DOT) for tuberculosis have been shown to improve medication adherence and clinical outcomes, and to reduce the incidence of drug resistance (Chaulk and Kazandjian, 1998; Weis et al., 1994). Since these same goals apply to HIV treatment, it is reasonable to extend the DOT model to antiretroviral therapy for HIV, particularly among populations at risk for non-adherence (Lanzafame et al., 2000; Mitty et al., 2002; Ford et al., 2009). Antiretroviral DOT programs have been successfully implemented in community settings using outreach workers (Altice et al., 2004; Behforouz et al., 2004; Khanlou et al., 2003; Ma et al., 2008; Mitty et al., 2005; Wohl et al., 2006), and in settings with infrastructures allowing frequent contact, such as prisons (Babudieri et al., 2000; Kirkland et al., 2002), housing facilities (Tinoco et al.,2004), and methadone clinics (Clarke et al., 2002; Conway et al., 2004; Lucas et al., 2004). While these studies have demonstrated feasibility and acceptability, few have examined efficacy using randomized designs, and none has evaluated methadone clinic-based DOT in a randomized trial.
Methadone clinics provide a promising infrastructure for DOT because federal regulations mandate that patients receive their daily methadone dose at one clinic, and the majority of doses are directly observed by nurses. However, providing co-located substance abuse treatment and HIV medical care is challenging because addiction medicine and HIV care are usually provided by specialists who may not have expertise in both fields. Though co-located medical care and methadone treatment improves outcomes among patients with tuberculosis, HIV, and Hepatitis C (Batki et al., 2002; Gourevitch et al., 2007; Litwin et al., 2009; Mauss et al., 2004), current payment policies promote segregation of substance abuse treatment and HIV care.
Support for Treatment Adherence Research through Directly Observed Therapy (STAR*DOT) was a randomized controlled trial designed to test the efficacy of directly observed antiretroviral therapy (DOT) provided on-site in methadone clinics. Our objective was to determine if DOT is more efficacious than self-administered antiretroviral therapy for improving adherence and reducing HIV viral load (VL) among methadone-maintained drug users. We hypothesized that at the end of the 24-week intervention period, participants in the DOT intervention group would have higher adherence and lower VL than participants in the treatment as usual (TAU) control group.
2. METHODS
2.1 Design and setting
Patients were randomly assigned to one of two antiretroviral treatment groups: DOT or TAU. The trial was conducted in a network of twelve methadone clinics administered by the Division of Substance Abuse (DoSA) at the Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. These affiliated clinics provide care for approximately 3,500 opioid-dependent patients, of whom 10-15% is HIV-infected. A detailed description of study procedures has been described previously (Berg et al., 2009).
2.2 Participants
Patients were eligible for inclusion if they: were HIV-infected and prescribed antiretroviral therapy; received HIV treatment at their methadone clinic or a closely affiliated site; attended the methadone clinic 5 or 6 days per week (hereafter, 5 or 6 day pick-up schedule); were on a stable dose of methadone for 2 weeks prior to baseline; and were genotypically sensitive to their prescribed antiretroviral regimen. Eligibility was not based on antiretroviral treatment experience, current drug use, or antiretroviral adherence. Participants were excluded if they were: (1) unable to provide informed consent; (2) monolingual Spanish speakers; (3) currently receiving antiretroviral DOT; or (4) if their primary HIV care provider did not agree to their trial participation.
2.3 Recruitment and randomization
Research staff recruited patients from clinic waiting areas using a brief survey that assessed HIV status and current antiretroviral therapy. If patients consented, blood was drawn for genotypic resistance testing and a baseline visit was scheduled. Prior to baseline, the study medical director (KMB) verified eligibility by reviewing medical records, conferring with the patient's HIV provider, and confirming genotypic sensitivity to prescribed medications.
Random-number tables were used to allocate subjects. Randomization was stratified by antiretroviral experience (versus naïve), and by once-daily (versus twice-daily) dosing. We randomized by blocks within each of these four strata.
2.4 DOT intervention
Participants randomized to the DOT intervention received one dose of their antiretroviral medications at the same time that they received their daily methadone. Antiretroviral medications were stored in weekly pill trays composed of seven removable single dose pillboxes. Clinic nurses dispensed and observed ingestion of both methadone and antiretroviral medications at the usual methadone dispensing locations. Non-observed antiretroviral doses included evening doses for participants on twice-daily regimens, Saturday doses for participants on 5-day pick-up schedules, and Sunday doses for all participants. In these instances, participants were given enough single-dose antiretroviral pillboxes or “take home doses” to last until their next methadone clinic visit. Participants were asked to return these pillboxes to the nurses at their next visit, whether or not they had taken the pills.
During the 24-week intervention, the study medical director phoned in prescriptions to a designated community pharmacy. HIV providers were asked to suspend writing prescriptions for DOT participants during the intervention period, and to contact the study medical director if a participant changed medications.
2.5 TAU control
Participants in the TAU group received antiretroviral prescriptions from their regular HIV provider, and self-administered their antiretroviral medications.
2.6 Resources available to both DOT and TAU participants
After completion of the baseline visit, all study participants were referred for adherence counseling, which was provided in each DoSA clinic by paraprofessional counselors (Cooperman et al., 2007). DOT and TAU participants attended a similar number of counseling visits (3.33 for DOT versus 4.92 visits for TAU; p= 0.11).
2.7 Visit schedule and data collection
The visit schedule for all participants included a baseline visit followed by 8 weekly visits and 4 monthly visits (i.e., weeks 1, 2, 3, 4, 5, 6, 7, 8, 12, 16, 20, and 24). Baseline survey data were collected using Audio Computer-Assisted Self-Interview (ACASI) technology. ACASI allows participants to read questions on a computer screen while simultaneously listening to the questions through headphones, and has been shown to improve reporting of stigmatized behaviors (DesJarlais et al., 1999; Macalino et al., 2002; Mizuno et al., 2007).
2.8 Main outcome measures
2.8.1 Adherence
For DOT participants, pills remaining in the pill trays were counted weekly. We assumed that unreturned take-home pillboxes contained pills that had not been ingested.
Pill counts for TAU participants occurred at all study visits. To increase the accuracy of pill counts, interviewers documented activities that would alter the pill count adherence rate, including: receipt of a prescription refill, ingestion of pills from another source, or loss of any pills (details available from authors).
For TAU participants, in addition to pill counts, we assessed adherence using the Medication Event Monitoring Systems (MEMS), in which a computerized cap is placed on a medication bottle and records each opening as a presumptive dose. We monitored either a protease inhibitor or non-nucleoside reverse transcriptase inhibitor. Because pill counts were not performed on the monitored medication we calculated adherence rates in two ways: using pill counts alone, and combining data from both pill counts and MEMS.
2.8.2 HIV viral load
We collected blood for quantifying HIV viral load at baseline and weeks 8, 16, and 24. Plasma viral load was quantified using the VERSANT HIV-1 bDNA 3.0 assay (Bayer, Tarrytown, NY). Virologic outcomes were: (1) median VL (log10 copies/ml), and (2) proportion of participants with undetectable VL (<75 copies/ml). We also measured CD4+ T lymphocyte count using the BD FACSCount system (BD Biosciences, San Jose, CA).
2.9 Additional variables
2.9.1 Self-reported adherence
Baseline self-reported adherence with each antiretroviral medication was assessed using standard questions (Chesney et al., 2000). We calculated adherence as the pills taken divided by pills prescribed during the prior week, and dichotomized self-reported adherence at 100% (Simoni et al., 2006).
2.9.2 Drug and alcohol use
We used the Recent Drug Abuse Survey to assess drug and alcohol use during the 30 days prior to baseline (Arnsten et al., 2002; Berg et al., 2004). In addition, urine was collected at every research visit for toxicology testing using the EMIT procedure (Siemens Healthcare Diagnostics, Deerfield, IL).
2.10 Statistical analyses
2.10.1 Assessment periods
Using an intent-to-treat approach, we examined the effect of DOT on pill count adherence during four assessment periods: baseline to week 4 (“week 4”), weeks 5 to 8 (“week 8”), weeks 9 to16 (“week 16”), and weeks 17 to 24 (“week 24”). To examine the effect of the intervention on VL, we compared the two study groups at baseline and at weeks 8, 16, and 24.
2.10.2 Pill counts
We modified pill count adherence rates that were <0% or >100% according to the following rules. Since pill count adherence rates could be negative if participants added pills to a bottle but did not remove any pills, we considered all negative pill count adherence rates to represent 0% adherence. Pill count adherence rates >100% might occur if participants mistakenly took extra pills. We decided this scenario was plausible, but should reasonably occur no more than twice weekly, and therefore considered all pill counts between 100% and 120% to represent 100% adherence. Finally, if pill count adherence rates were >120%, we imputed new values for those data points by averaging five randomly drawn imputed values based on the mean and standard deviation from the same participant. Four percent of pill count adherence rates were <0, 77% were between 0 and 100, 9% were between 100 and 120, and 10% were >120%. Missing pill count data were not included in analyses.
Final pill count adherence rates were derived by first computing the mean pill count adherence rate for all the antiretroviral medications in the participant's regimen. We then computed the mean pill count adherence rate for all time points in the assessment period (e.g., week 8 pill count adherence represents the mean of pill count adherence rates at weeks 5, 6, 7, and 8).
In addition, we created a composite adherence rate for each participant by calculating a weighted average accounting for the number of pills in the regimen that were counted during pill counts and the number that was monitored by MEMS. Using the composite adherence rate did not change the results, we therefore present pill count adherence only.
2.10.3 Baseline comparisons
To check randomization, we used Chi-square tests and Wilcoxon Rank Sum tests as appropriate.
2.10.4 Intervention effect
We fit the repeatedly measured outcomes of pill count adherence and VL using a mixed-effects linear model, which included an intervention effect (DOT vs. TAU), a time effect, and an intervention by time effect, all of which were considered fixed. Time was entered as a discreet variable and the covariance matrix of the error term was assumed to be unstructured to account for within-subject correlations. Subsequently, based on the mixed-effects model, contrasts representing the intervention effect across the time points were constructed and tested at a two-tailed significance level of 0.05. In addition, we calculated mixed effects logistic regression to determine the odds of achieving viral suppression for DOT participants compared to TAU participants. Analyses were conducted using SAS, version 9.1 (SAS Institute; Cary, NC).
3. RESULTS
3.1 Baseline characteristics
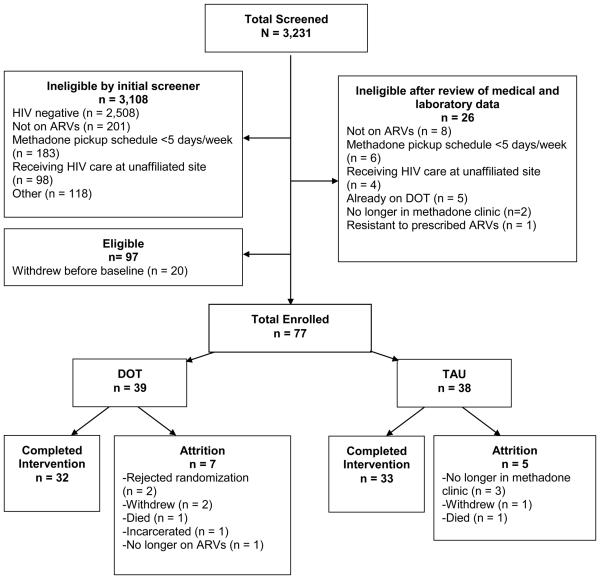
From June 2004 to August 2007, we screened 3,231 potential participants. Of these, 97 were eligible and 77 enrolled. The retention rate at 24 weeks was 84% (Figure 1).
Figure 1.
Flow chart of study recruitment, enrollment, and completion.
The sample was 53% male, 45% Hispanic and 40% Black, with a mean age of 47. All participants were antiretroviral experienced, and 45% had undetectable baseline VL. The majority (70%) was prescribed twice-daily antiretroviral regimens. The median duration of methadone treatment was 10 years [interquartile range (IQR) 5-16] and the median methadone dose was 125 mg (IQR 90-180). At baseline, 55% had urine toxicology results consistent with cocaine use, and 31% with non-methadone opioid use. All measured characteristics, including self-reported antiretroviral adherence and VL, were similar between groups (Table 1).
Table 1.
Baseline characteristics of the study sample
| Total (n=77) |
Directly observed therapy (DOT) study arm (n=39) |
Treatment as usual (TAU) study arm (n=38) |
p value* |
||
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Age, mean (sd) | 47 (7) | 45 (7) | 49 (7) | 0.05 | |
| Sex, n (%) | |||||
| Male | 41 (53) | 19 (49) | 22 (58) | 0.42 | |
| Race, n (%) | |||||
| White | 10 (13) | 5 (13) | 5 (13) | 0.70 | |
| Black | 31 (40) | 14 (36) | 17 (45) | ||
| Other | 36 (47) | 20 (51) | 16 (42) | ||
| Ethnicity, n (%) | |||||
| Hispanic | 35 (45) | 20 (51) | 15 (39) | 0.30 | |
| Non-Hispanic | 42 (55) | 19 (49) | 23 (61) | ||
| Education, n (%) | |||||
| Less than high school | 38 (49) | 19 (49) | 19 (50) | 0.89 | |
| High school (partial or completed) | 20 (26) | 11 (28) | 9 (24) | ||
| College (partial or completed) | 19 (25) | 9 (23) | 10 (26) | 0.74 | |
| Marital status, n (%) | |||||
| Married or living with partner | 34 (44) | 17 (44) | 17 (45) | 0.14 | |
| Widowed, separated or divorced | 22 (29) | 8 (20) | 14 (37) | ||
| Single | 21 (27) | 14 (36) | 7 (18) | ||
| Employment status, n (%) | |||||
| Employed | 2 (3) | 1 (3) | 1 (3) | 1.00 | |
| Unemployed or unable to work, n (%) | 75 (97) | 38 (97) | 37 (97) | ||
| Insurance, n (%) † | |||||
| Medicaid | 69 (90) | 33 (85) | 36 (95) | 0.26 | |
| Medicare | 16 (21) | 10 (26) | 6 (16) | 0.40 | |
| Private insurance | 6 (8) | 3 (8) | 3 (8) | 1.00 | |
|
Antiretroviral regimen and HIV clinical characteristics |
|||||
| Self-reported seven-day antiretroviral adherence ‡ |
|||||
| 100%, n (%) | 55 (72) | 25 (66) | 30 (79) | 0.20 | |
| <100%, n (%) | 21 (27) | 13 (34) | 8 (21) | ||
| Median duration of HIV infection, years (IQR) |
13 (9-17) | 12 (8-15) | 15 (10-18) | 0.09 | |
| Duration of antiretroviral therapy, n (%) § | |||||
| <1 year | 18 (24) | 8 (21) | 10 (28) | 0.83 | |
| 1 – 5 years | 34 (44) | 18 (49) | 16 (44) | ||
| >5 years | 21 (27) | 11 (30) | 10 (28) | ||
| Number of pills in antiretroviral regimen, n (%) ¶ |
|||||
| One | 8 (10) | 4 (10) | 4 (10) | 0.12 | |
| Two | 39 (51) | 25 (64) | 14 (37) | ||
| Three | 24 (31) | 8 (21) | 16 (42) | ||
| Four | 5 (7) | 2 (5) | 3 (8) | ||
| Five | 1 (1) | 0 | 1 (3) | ||
| Frequency of antiretroviral dosing, n (%) | |||||
| Once per day | 21 (27) | 9 (23) | 12 (32) | 0.40 | |
| Two or more times per day | 56 (73) | 30 (77) | 26 (68) | ||
| Viral load (copies/ml), n (%) | |||||
| <75 | 36 (47) | 20 (51) | 16 (42) | 0.11 | |
| 75 – 400 | 5 (6) | 4 (10) | 1 (3) | ||
| 401 - 10,000 | 21 (27) | 8 (21) | 13 (34) | ||
| 10,001 - 100,000 | 12 (16) | 4 (10) | 8 (21) | ||
| > 100,000 | 3 (4) | 3 (8) | 0 | ||
| HIV viral load < 75 copies/ml, n (%) | 41 (53) | 19 (49) | 22 (58) | 0.42 | |
| Mean viral load, log10 copies/ml | 2.81 | 2.74 | 2.89 | 0.43 | |
| Median CD4+ T cell count, cells/mm3, (IQR) (n=74) |
345 (151-494) | 367 (189 - 509) | 277 (110 - 453) | 0.34 | |
| CD4+ T cell count ≥ 350, n (%) | 37 (50) | 21 (55) | 16 (44) | 0.35 | |
| Substance use characteristics | |||||
| Median duration methadone maintenance, years (IQR) (n=48) |
10 (5-16) | 9 (4-15) | 10 (6-16) | 0.93 | |
| Median methadone dose, mg (IQR) (n=74) |
125 (90-180) | 135 (90-170) | 120 (90-185) | 0.85 | |
| Reported use of illicit drug in 30 days prior to baseline, n (%) |
|||||
| Heroin | 20 (26) | 9 (24) | 11 (29) | 0.60 | |
| Cocaine | 22 (29) | 9 (24) | 13 (34) | 0.31 | |
| Crack | 27 (35) | 14 (37) | 13 (34) | 0.81 | |
| Marijuana | 11 (14) | 7 (19) | 4 (11) | 0.30 | |
| Amphetamine | 3 (4) | 1 (3) | 2 (5) | 1.00 | |
| Hazardous alcohol use (AUDIT ≥8) | 15 (20) | 6 (16) | 9 (24) | 0.36 | |
| Positive baseline urine toxicology report, n (%) |
|||||
| Opioid (non-methadone) | 24 (31) | 11 (30) | 13 (35) | 0.62 | |
| Cocaine (including crack) | 42 (55) | 19 (51) | 23 (62) | 0.35 | |
| Benzodiazepine | 7 (9) | 3 (8) | 4 (11) | 0.71 | |
| Marijuana | 13 (17) | 6 (16) | 7 (19) | 0.72 | |
| Amphetamine | 0 (0) | 0 | 0 | 1.00 |
p value for difference between DOT and TAU groups
Categories not mutually exclusive
Data missing for 1 participant
Data missing for 4 participants
Participants reporting <3 pills were prescribed combination antiretroviral medications co-formulated in one pill
3.2 Between group differences in adherence
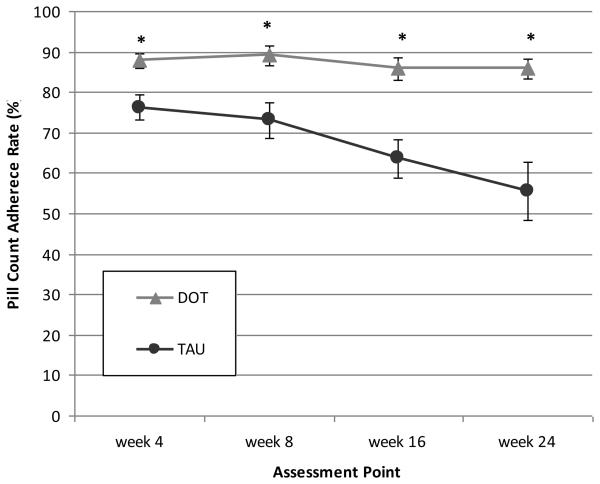
Adherence over time is presented in Figure 2a. At all assessments, adherence in the DOT group was higher than in the TAU group. During the trial, adherence in the DOT group remained high while adherence in the TAU group fell to lower levels. The difference in adherence between DOT and TAU participants increased from 12% at week 4 to 30% at week 24.
Figure 2a.
Differences in mean pill count adherence rates by study arm at each assessment point during the 24-week intervention period. Precise estimates available in online supplementary materials.
DOT: directly observed therapy; TAU: treatment as usual
*p < 0.01, based on mixed effects model analysis.
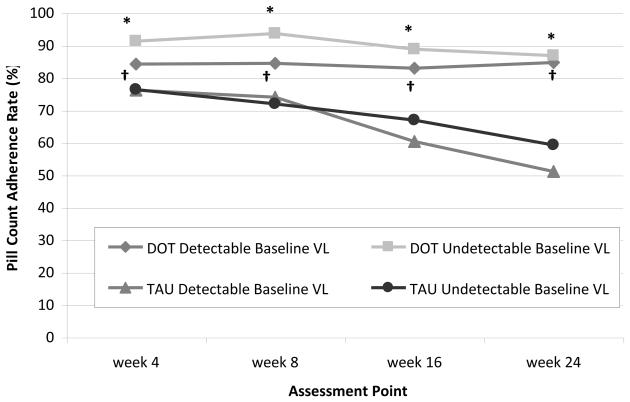
When we examined adherence over time after stratifying by baseline VL, the DOT intervention remained efficacious for improving adherence among participants with both detectable and undetectable baseline VL (Figure 2b). Among those with detectable VL, the difference in adherence between DOT and TAU increased by 26% (from 8% at week 4 to 34% at week 24), while among those with undetectable VL, the adherence difference increased by 13% (from 15% at week 4 to 28% as week 24). Further, the three-way interaction among intervention, baseline VL, and time was not significant (p=0.51), suggesting that the significance of the DOT intervention effect among the entire sample was not driven by participants with detectable baseline VL. Finally, we examined the efficacy of the DOT intervention after stratifying by baseline CD4 count (≤ 350 vs. > 350 cells/mm3), and found that rates of achieving viral suppression in each study arm were independent of baseline CD4 count (data not shown).
Figure 2b.
Differences in mean pill count adherence rates by study arm at each assessment point during the 24-week intervention period, stratified by baseline viral load (≥75 copies/ml versus <75 copes/ml). Precise estimates available in online supplementary materials.
DOT: directly observed therapy; TAU: treatment as usual; VL: viral load
*p < 0.01 for difference between DOT and TAU among participants with undetectable baseline VL.
†p < 0.01 for difference between DOT and TAU among participants with detectable baseline VL.
All p values based on mixed effects model analysis.
3.3 Between group differences in viral load
Over the 24-week period, VL in the DOT group decreased 0.52 log10 copies/ml (from 2.74 to 2.22 log10 copies/ml), while VL in the TAU group remained relatively stable (Figure 3a).
Figure 3a.
Differences in log10 viral load by study arm at each assessment point during the 24-week intervention period. Precise estimates available in online supplementary materials.
DOT: directly observed therapy; TAU: treatment as usual; VL: viral load
*p <0.01 based on mixed effects model analysis
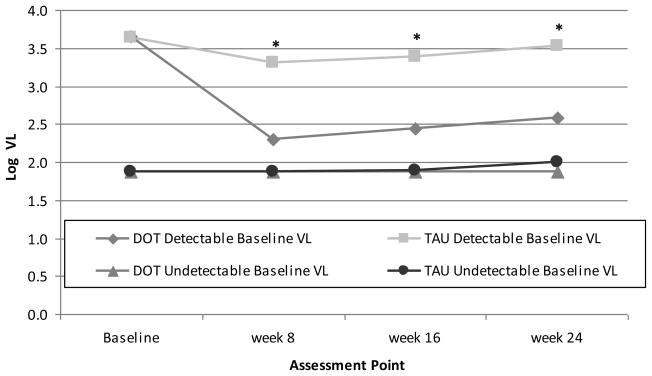
When we examined VL over time after stratifying by baseline VL, the DOT intervention was efficacious for reducing VL only among participants with detectable baseline VL (Figure 3b). Among DOT participants with detectable baseline VL, the decrease in VL was 1.07 log10 copies/ml, and 47% became undetectable by week 24.
Figure 3b.
Differences in log10 viral load by study arm at each assessment point during the 24-week intervention period, stratified by baseline viral load (≥75 copies/ml versus <75 copes/ml). Precise estimates available in online supplementary materials.
DOT: directly observed therapy; TAU: treatment as usual; VL: viral load
*p<0.02 for difference between DOT and TAU among participants with detectable baseline VL, based on mixed effects model analysis.
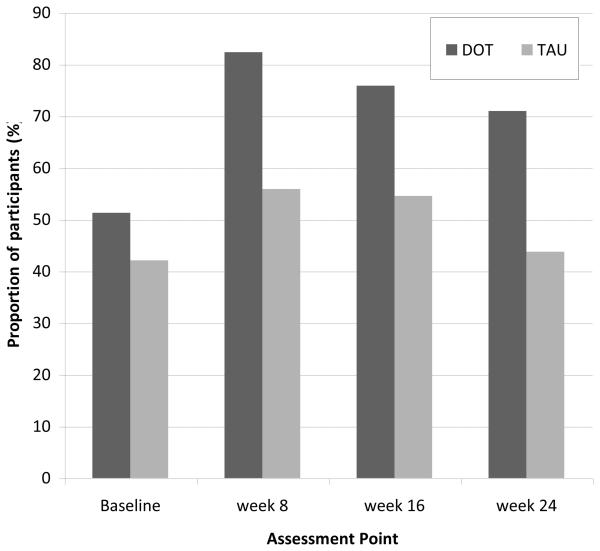
A similar proportion of participants in both groups had undetectable VL at baseline (51% for DOT and 42% for TAU; p=0.42). After baseline, the proportion of participants with undetectable VL was significantly higher in the DOT group than the TAU group at all assessment points (Figure 4). At week 24, the odds of having undetectable VL were 3-fold greater for DOT than TAU participants [ORunadj = 3.1 (95% confidence interval 1.1-5.4)]. Further, among TAU participants with undetectable baseline VL, the proportion that remained undetectable decreased to 92% at week 16 and 79% at week 24. Therefore, among TAU participants with undetectable baseline VL, 21% became detectable during the course of the 24-week trial.
Figure 4.
Proportions of participants with undetectable viral load (<75 copies/ml) by study arm at each assessment point during the 24-week intervention period. Precise estimates available in online supplementary materials.
DOT: directly observed therapy; TAU: treatment as usual
4. DISCUSSION
Among antiretroviral-experienced adults taking methadone for opioid dependence, 24-weeks of DOT was more efficacious for improving adherence and lowering VL than self-administered antiretroviral medications. Using an intent-to-treat approach, adherence in the DOT group was significantly higher at all assessment points than adherence in the TAU group. During the 24-week trial, participants in the DOT group maintained high adherence, while adherence among participants in the TAU group dropped. Further, while there was no change in VL in the TAU group, VL decreased by 0.5 log10 copies/ml in the entire DOT group, and by 1.07 log10 copies/ml among DOT participants with detectable baseline VL. Despite similar proportions of participants in both groups with undetectable VL at baseline (51% DOT, 42% TAU), at week 24 this proportion increased to 71% in the DOT group and did not change in the TAU group. In fact, among TAU participants with undetectable baseline VL, 21% experienced viral rebound. While several DOT studies have reported decreases in VL (Altice et al., 2007; Conway et al., 2004; Lucas et al., 2006; Macalino et al., 2007) and modest improvements in self-reported adherence (Altice et al., 2007; MA et al., 2008; Pearson et al., 2007), ours is one of the few DOT trials to report improvements in objectively measured adherence at multiple time points.
Unlike most DOT programs, we enrolled participants with both detectable and undetectable baseline VL (Goggin et al., 2007). To examine the effect of DOT among those with undetectable baseline VL, who are often not considered DOT candidates, we stratified analyses by baseline VL (detectable versus undetectable). We found that the DOT intervention significantly improved adherence even among participants with undetectable baseline VL.
The decrease of 0.52 log10 copies/ml we found among the entire DOT group is less than that found in other DOT studies. In addition to our trial participants having a lower baseline log VL (2.81 copies/ml) than those in other DOT studies (Lucas et al., 2006; Macalino et al., 2007; Wohl et al., 2006), another explanation for this modest VL decrease is the inclusion of participants with undetectable baseline VL. While the proportion of DOT participants with viral suppression increased from 51% to 71% during the trial, almost half (47%) the DOT participants with detectable baseline VL achieved viral suppression. Our finding of a three-fold increase in the odds of viral suppression at trial end among DOT participants compared to TAU participants is consistent with the work of both Altice et al and Lucas et al, who reported similar results among samples of drug users (Altice et al., 2007; Lucas et al., 2004). Taken together, these data strongly suggest that DOT is an effective intervention for achieving viral suppression, the ultimate goal of HIV treatment, among drug using populations.
Enrolling patients with undetectable baseline VL into DOT trials is relatively uncommon, and our ability to examine the effect of the intervention in this group is unique. Though VL did not change significantly in the TAU group, 21% of TAU participants with undetectable baseline VL experienced viral rebound. The effect of DOT on viral suppression among participants with undetectable baseline VL has been examined by Altice et al who also found that among those with undetectable baseline VL, more DOT than standard care participants maintained viral suppression (Altice et al., 2007). These results suggest that DOT is efficacious in preventing virologic failure among participants who are already meeting clinical goals.
Though evidence in support of antiretroviral DOT programs is mounting, important questions still remain. In particular, the minimally efficacious dose of DOT is unclear. Because of differences in antiretroviral dosing and methadone pick-up schedules the proportion of observed antiretroviral doses in our trial varied from 86% among participants on once-daily regimens with a 6 day/week methadone pick-up schedule to 36% among participants on twice-daily regimens with a 5 day pick-up schedule. Among drug users in a community-based DOT program, Altice et al found that adherence was significantly higher with observed than unobserved medication doses, suggesting that a greater number of observed doses may improve outcomes (Altice et al., 2004). In contrast, Lucas et al found that in a methadone clinic based DOT program, the percent of observed doses was not associated with virologic failure, suggesting that even minimal participation in a DOT program may improve outcomes (Lucas et al., 2007). The effect of DOT programs on adherence with unobserved, or “take-home”, doses merits further research.
Despite ongoing research, little is known about the optimal target population for antiretroviral DOT programs, and this has important cost implications. Our intervention was efficacious among participants who had been HIV-infected for a median of 13 years and were all antiretroviral experienced. Despite long-term opioid replacement therapy with high-doses of methadone, over half was using cocaine at baseline, and approximately one third was using non-methadone opioids. These findings support the recommendation that DOT should be offered to all methadone maintained patients, regardless of active drug use. To better understand the potential benefit of DOT to drug users, future research should examine the effect of DOT on active versus former drug users, and the efficacy of DOT for reducing substance use.
Our study has several important strengths. First, HIV providers made all clinical decisions and we did not restrict antiretroviral dosing regimens. Second, frequency and duration of research visits were the same in each group, minimizing the risk of attention bias. Lastly, the DOT intervention utilized existing methadone resources. The main difference between our trial and a program without additional funding was the specially prepared pill trays for observed and take-home antiretroviral doses. Ad hoc DOT occurs in our clinics for non-HIV medications (e.g., benzodiazepines), and in these cases nurses dispense daily doses directly from patients' medication bottles, which are stored in the clinic. Nonetheless, the cost effectiveness of DOT programs remains an open question (Goldie et al., 2003).
Limitations to generalizability should be noted. First, because our trial was conducted in a network of methadone clinics with fully integrated onsite HIV care, the feasibility and efficacy of DOT in methadone clinics with off-site medical care is unknown. In addition, we only enrolled patients who attended the methadone clinic at least 5 days per week limiting our ability to extend our results to patients who attend clinic less frequently.
These findings have important policy implications. HIV care and substance abuse treatment must be further integrated before DOT can be widely implemented in methadone clinics. Though this model would be most effective if methadone treatment programs had on-site HIV care, antiretroviral DOT could be achieved through close partnerships between HIV clinics and substance abuse treatment programs. For example, with coordination among off-site HIV specialists, community pharmacies, and methadone clinic staff, antiretroviral mediations could be delivered directly to methadone clinics and then dispensed by methadone clinic nurses as observed doses. Lastly, to increase the feasibility of methadone based DOT programs, efforts to provide on-site HIV care in methadone clinics should be aggressively pursued. Allowing higher reimbursement rates for treatment of HIV is one step towards this goal, and could increase the number of existing methadone clinics capable of providing DOT.
In conclusion, this study adds to the literature on antiretroviral DOT programs by demonstrating efficacy of DOT in a randomized trial of antiretroviral experienced, methadone maintained patients, many with ongoing drug use. Future DOT trials should determine the optimal dose and duration of antiretroviral DOT for drug users. In addition, to inform allocation of resources, research efforts should focus on identifying the most appropriate target population from both a clinical and cost-effectiveness perspective. Our finding that DOT was efficacious in a sample that was heterogeneous regarding both baseline VL and drug use suggests that antiretroviral DOT programs have the potential to provide substantial clinical benefit to drug users on methadone for opioid dependence.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A set of tables corresponding to figures 2-5 provide detailed information on data presented (e.g., sample sizes, means, standard deviations, proportions), and can be found in the supplementary materials by accessing the online version of this paper at doi:xxx/j.drugalcdep.xxx . . .
Reference List
- Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin.Infect.Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, Springer SA, Friedland GH. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin.Infect.Dis. 2004;38(Suppl 5):S376–S387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J.Gen.Intern.Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babudieri S, Aceti A, D'Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA: The Journal of the American Medical Association. 2000;284:179–180. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug Alcohol Depend. 2002;66:283–293. doi: 10.1016/s0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J.Acquir.Immune.Defic.Syndr. 2004;36:642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J.Gen.Intern.Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Mouriz J, Li X, Duggan E, Goldberg U, Arnsten JH. Rationale, design, and sample characteristics of a randomized controlled trial of directly observed antiretroviral therapy delivered in methadone clinics. Contemp.Clin.Trials. 2009;30:481–489. doi: 10.1016/j.cct.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus Statement of the Public Health Tuberculosis Guidelines Panel. JAMA: The Journal of the American Medical Association. 1998;279:943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Clarke S, Keenan E, Ryan M, Barry M, Mulcahy F. Directly observed antiretroviral therapy for injection drug users with HIV infection. AIDS Read. 2002;12:305–306. [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, Smith N, Mead A, DeVlaming S. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin.Infect.Dis. 2004;38(Suppl 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Parsons JT, Chabon B, Berg KM, Arnsten JH. The Development and Feasibility of an Intervention to Improve HAART Adherence Among HIV-Positive Patients Receiving Primary Care in Methadone Clinics. Journal of HIV./AIDS & Social.Services. 2007;6:101–120. [Google Scholar]
- DesJarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, Shi Q, Hagan H, Friedman SR. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet. 1999;353:1657–1661. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- Ford N, Nachega JB, Engel ME, Mills EH. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374(9707):2064–2071. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- Goggin K, Liston RJ, Mitty JA. Modified directly observed therapy for antiretroviral therapy: a primer from the field. Public Health Rep. 2007;122:472–481. doi: 10.1177/003335490712200408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, III, Kimmel AD, Walensky RP, Sax PE, Freedberg KA. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am.J.Med. 2003;115:632–641. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Gourevitch MN, Chatterji P, Deb N, Schoenbaum EE, Turner BJ. On-site medical care in methadone maintenance: associations with health care use and expenditures. J.Subst.Abuse Treat. 2007;32:143–151. doi: 10.1016/j.jsat.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Khanlou H, Kandula VR, Yeh V, Stein TG, Sanchez S, Ricaurte JC, Bhatti L, Farthing CF. Pilot study of directly observed therapy in highly nonadherent HIV-infected patients in an urban community-based institution. J.Acquir.Immune.Defic.Syndr. 2003;33:651–653. doi: 10.1097/00126334-200308150-00017. [DOI] [PubMed] [Google Scholar]
- Kirkland LR, Fischl MA, Tashima KT, Paar D, Gensler T, Graham NM, Gao H, Rosenzweig JR, McClernon DR, Pittman G, Hessenthaler SM, Hernandez JE. Response to lamivudine-zidovudine plus abacavir twice daily in antiretroviral-naive, incarcerated patients with HIV infection taking directly observed treatment. Clin.Infect.Dis. 2002;34:511–518. doi: 10.1086/338400. [DOI] [PubMed] [Google Scholar]
- Lanzafame M, Trevenzoli M, Cattelan AM, Rovere P, Parrinello A. Directly observed therapy in HIV therapy: A realistic perspective? J.Acquir.Immune.Defic.Syndr. 2000;25:200–201. doi: 10.1097/00042560-200010010-00018. [DOI] [PubMed] [Google Scholar]
- Litwin AH, Harris KA, Jr., Nahvi S, Zamor PJ, Soloway IJ, Tenore PL, Kaswan D, Gourevitch MN, Arnsten JH. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J.Subst.Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care STDS. 2007;21:564–574. doi: 10.1089/apc.2006.0192. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin.Infect.Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin.Infect.Dis. 2004;38(Suppl 5):S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- Ma M, Brown BR, Coleman M, Kibler JL, Loewenthal H, Mitty JA. The feasibility of modified directly observed therapy for HIV-seropositive African American substance users. AIDS Patient.Care STDS. 2008;22:139–146. doi: 10.1089/apc.2007.0063. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Celentano DD, Latkin C, Strathdee SA, Vlahov D. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ.Prev. 2002;14:367–378. doi: 10.1521/aeap.14.6.367.24075. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Hogan JW, Mitty JA, Bazerman LB, Delong AK, Loewenthal H, Caliendo AM, Flanigan TP. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to Antiretroviral Therapy for Human Immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- Mitty JA, Macalino GE, Bazerman LB, Loewenthal HG, Hogan JW, MacLeod CJ, Flanigan TP. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J.Acquir.Immune.Defic.Syndr. 2005;39:545–550. [PubMed] [Google Scholar]
- Mitty JA, Stone VE, Sands M, Macalino G, Flanigan T. Directly observed therapy for the treatment of people with human immunodeficiency virus infection: a work in progress. Clin.Infect.Dis. 2002;34:984–990. doi: 10.1086/339447. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Purcell DW, Mackenzie S, Tobin KE, Wunch T, Arnsten JH, Metsch LR. Acceptability of A-CASI by HIV-positive IDUs in a multisite, randomized, controlled trial of behavioral intervention (INSPIRE) J.Acquir.Immune.Defic.Syndr. 2007;46(Suppl 2):S48–S54. doi: 10.1097/QAI.0b013e3181576795. [DOI] [PubMed] [Google Scholar]
- Pearson CR, Micek MA, Simoni JM, Hoff PD, Matediana E, Martin DP, Gloyd SS. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J.Acquir.Immune.Defic.Syndr. 2007;46:238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-Report Measures of Antiretroviral Therapy Adherence: A Review with Recommendations for HIV Research and Clinical Management. AIDS and Behavior. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YH, Pang SM, Chan MF, Yeung GS, Yeung VT. Health literacy, complication awareness, and diabetic control in patients with type 2 diabetes mellitus. J.Adv.Nurs. 2008;62:74–83. doi: 10.1111/j.1365-2648.2007.04526.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, Vergara de CA, Rodriguez-Felix L, Serrano A, Bascunana A. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. European Journal of Clinical Microbiology & Infectious Diseases. 2004;23(4):331–5. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, Gomez E, Foresman BH. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N.Engl.J.Med. 1994;330:1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Garland WH, Valencia R, Squires K, Witt MD, Kovacs A, Larsen R, Hader S, Anthony MN, Weidle PJ. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin.Infect.Dis. 2006;42:1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.