Abstract
The vaginal mucosa is commonly exposed to chemicals and therapeutic agents that may result in irritation and/or inflammation. In addition to acute effects, vaginal irritation and inflammation can make women more susceptible to infections such as HIV-1 and herpes simplex virus-2. Hence, the vaginal irritation potential of feminine care formulations and vaginally administered therapeutic agents is a significant public health concern. Traditionally, testing of such materials has been performed using the rabbit vaginal irritation (RVI) assay. In the current study, we investigated whether the organotypic, highly differentiated EpiVaginal™ tissue could be used as a non-animal alternative to the RVI test. The EpiVaginal tissue was exposed to a single application of ingredients commonly found in feminine hygiene products and the effects on tissue viability (MTT assay), barrier disruption (measured by transepithelial electrical resistance, TEER and sodium fluorescein (NaFl) leakage), and inflammatory cytokine release (interleukin (IL)-1α, IL-1β, IL-6, and IL-8) patterns were examined. When compared to untreated controls, two irritating ingredients, nonoxynol 9 and benzalkonium chloride, reduced tissue viability to <40% and TEER to <60% while increasing NaFl leakage by 11–24% and IL-1α and IL-1β release by >100%. Four other non-irritating materials had minimal effects on these parameters. Assay reproducibility was confirmed by testing the chemicals using three different tissue production lots and by using tissues reconstructed from cells obtained from three different donors. Coefficients of variation between tissue lots reconstructed with cells obtained from the same donor or lots reconstructed with cells obtained from different donors were less than 10% and 12%, respectively. In conclusion, decreases in tissue viability and barrier function and increases in IL-1α and IL-1β release appear to be useful endpoints for preclinical screening of topically applied chemicals and formulations for their vaginal irritation potential.
Keywords: EpiVaginal tissue, vaginal irritation, inflammatory cytokines, in vitro assay
Introduction
The number of alternative in vitro irritation assay models that are reproducible, less time consuming, relatively inexpensive, and more predictive of human response than animal models is increasing. For instance, reconstructed in vitro tissue culture skin models such as EpiDerm (MatTek Corporation, Ashland, MA) and EPISKIN (SkinEthic Laboratories, Nice, France) have been validated and accepted as validated tests by the European Union (EU) and Organization for Economic Cooperation and Development to identify skin irritants and corrosives (Spielmann H et al., 2007). These models address a significant market need since the 7th Amendment to the Cosmetics Directive banned the testing of cosmetic ingredients or formulations in the European Union as of March, 2009 (http://www.aavs.org/lawsProductsSeventh.html). While a lot of emphasis has been placed on developing alternatives to skin and ocular testing, in vitro alternatives to the rabbit vaginal irritation (RVI) test are limited and the RVI assay remains the predominant means of determining vaginal irritation potential.
The vaginal epithelium forms an uninterrupted mucosal barrier between the external micro-environment of the vaginal canal and the underlying tissues. Exposure of the vaginal-ectocervical tissue to chemical insult may cause damage and/or inflammation at the site of application. The public health risk caused by such reactions is signficant since increased infection rates for sexually transmitted infections (STI) such as HIV-1 can result (Fichorova RN, 2004, Weber J et al, 2005). The increased infectivity is due to: a) compromised tissue barrier which allows viral entry, b) recruitment of susceptible target cells to the site of inflammation (Catalone BJ, et al., 2005) or c) induction of inflammatory cytokines such as IL-1β and TNF-α which are known to activate HIV LTR via the NFkB pathway (Osborn L, et al. 1989).
Animal models such as the rabbit, slug, pig, and mouse have been used to screen chemicals/formulations for their irritancy and inflammation potential (Tsai CC, et al, 2003, D’Cruz OJ and Uckun FM, 2002, Achilles SL, et al, 2002, Galen BT, et al, 2007 ). Among the various models, the RVI assay is the most commonly used test and the only FDA approved animal model for vaginal irritancy testing. In the RVI assay, visual observations and subjective parameters of erythema, edema, and discharge are used to evaluate injury and inflammation. In addition, histological cross-sections from exposed tissues are scored for epithelial exfoliation, leukocyte infiltration, thickening of the lamina propria (edema), and vascular congestion. However, the rabbit cervicovaginal tissue is markedly different from the human in terms of anatomy since it lacks the cyclic reproductive human (menstrual) stages and it is unresponsive to most human genital pathogens (Belec, L., et al, 1995, Noguchi, K., et al, 2003). Due to these species related differences, the RVI test is at times not predictive of human irritancy and toxicity. For instance, the RVI test failed to predict the disruptive effects of nonoxynol-9 (N9) which led to increased HIV-1 infection rates (Stafford MK, et al. 1998; CDC 2000). In recent years, researchers have also used pig as an animal model to study inflammatory responses induced by candidate spermicides and microbicide formulations. The availability of specific probes to study inflammatory responses in pigs and the physiological and histological similarity of the genital tract of pigs with that of the human vagina were among the important factors cited to promote the use of pig as a suitable animal model for vaginal irritation studies (Squier, CA, et al, 2007). However, the pig remains an animal model and it is not suitable for high throughput screening of chemicals and formulations. Also, existing animal protocols vary substantially in relation to number of animals to be used, dose volume, and the depth of application. A comparative summary of the use of organotypic tissue and animal models for vaginal irritation testing is presented in Table 1.
Table 1.
Comparison between the in vitro EpiVaginal and animal based vaginal irritation tests
| In Vitro EpiVaginal Method | Rodents/Rabbit vaginal Irritation assay | |
|---|---|---|
| Tissue Structure | Epithelium is non-keratinized; resembles native human vaginal tissue | Epithelium is keratinized in rodents and shows structural variation in rabbits |
| Reproducibility | Highly reproducible | Variable |
| Sensitive Endpoints | Very good | Good |
| Endpoint interpretation | Quantitative (cytotoxicity, permeability, cytokine release, and TEER) | Subjective (erythema and edema), highly trained scorer/pathologist required. |
| Cytokine or molecular endpoints | Yes | Requires cross-reactive antibodies |
| Study turnaround time | 3–4 days | 15–30 days |
| High throughput screening possible | Yes | No |
| Animal alternative model | Yes | No |
| Relevance to humans | High | Moderate |
Vaginal explant cultures have also been used in many laboratories to predict in vivo irritancy of vaginally applied chemicals and feminine hygiene products. However, the scarcity of normal human tissue, short survival time (due to their rapid deterioration and loss of tissue integrity when cultured), and donor-to-donor variation makes explant tissues impractical for determining the irritancy potential of topically applied chemicals or formulations. The organotypic EpiVaginal tissue model has advantages over explants tissues in that: 1) EpiVaginal can be cultured for relatively longer time period (e.g. 2–3 weeks), 2) a large number of tissues can be produced from a single donor, 3) tissue-to-tissue variability is reduced, 4) there are fewer regulatory constraints (Cummins JE and Doncel GF 2009), and 5) the tissue can be reconstructed in the presence/absence of endocrine hormones or different cell types to mimic its in vivo counterpart. Vaginal and ectocervical monolayer cells or transformed cell lines have also been used to assess toxicity of feminine care products and spermicides. However, assays that utilize submerged monolayer epithelial cultures do not take into account the three dimensional differentiated structure of the native vaginal epithelium and as a result have a limited value. Other factors such as differences in gene and protein expression levels between monolayer cultures and vaginal epithelium and limitations of testing aqueous incompatible materials such as gels, films, and vaginal rings also make monolayer cultures poor models for vaginal irritation or toxicity studies (Ayehunie, S, et al., 2006). Further, versus animal models, the human cell-based EpiVaginal tissue is directly infectible with human pathogens such as HIV-1 (Cole, A.L., et al., 2007.) while animal models are infectible only with viruses that resemble HIV-1.
In this study, we used an organotypic 3D vaginal tissue model cultured from reconstructed from normal human cells to predict irritation of ingredients commonly found in vaginally administered products. Tissue viability, structure, barrier function, and cytokine release patterns were monitored to correlate in vitro results to in vivo irritation. Using these biomarkers, nonoxynol-9 (N9), which induced mild irritation in the RVI assay, and benzalkonium chloride, known to be a vaginal irritant (Fichorova RN, 2004), were correctly identified as irritants.
Material and Methods
Source of epithelial cells
Human vaginal-ectocervical (VEC) tissues were obtained from healthy women (age 34–44) undergoing hysterectomies for benign indications following Internal Review Board (IRB) approval. The ectocervix was used as a source of epithelial cells and fibroblasts. Pieces of ectocervical tissue were placed in Dulbecco’s Modified Eagle’s Medium (DMEM; Cambrex, MD) containing penicillin, streptomycin, and gentamicin (10 mg/ml, Cambrex) and processed within 24 h of surgical removal. The underlying connective tissue was dissected away from epithelial layers and cultured in DMEM plus 10% fetal bovine serum (FBS) at 37°C and 5% CO2 to isolate vaginal fibroblasts. The epithelial tissue was placed in 0.025% trypsin/0.025% EDTA (Cambrex) and incubated for 1 h at 37°C and 5% CO2. After 1 h with gentle stirring, individual cells separated from the tissue and were centrifuged for 5 min. Cells were re-suspended in phosphate buffered saline (PBS)/soybean trypsin inhibitor (STI), centrifuged, re-suspended, and plated in DMEM into tissue culture treated petri dishes at a density of 4500 cells/cm2. After the cells had reached 60–70% confluence, the cells were harvested using trypsin/EDTA. The resulting cell suspension was centrifuged, the medium was drawn off and the cells were re-suspended in fresh medium. The cells were seeded as before into petri dishes, grown to 60–70% confluence, harvested by trypsinization, and cryopreserved. Cell aliquots of 1×106cells/ml were cryopreserved using a rate-controlled freezer (CryoMed).
Tissue stratification
Partial thickness EpiVaginal tissue (VEC-100)
Cryopreserved cells from a single donor were thawed and plated into 150-mm petri dishes. When the cell density reached 60–70% confluence, cells were trypsinized, counted, and seeded onto polycarbonate tissue culture treated microporous membrane cell culture inserts (NalgeneNunc International, Naperville, IL). Inserts were cultured at 37°C, 5% CO2, 98% rH for 4 days submerged and 7 days at the air liquid interface using a serum free differentiation medium (VEC-100-MM, MatTek Corporation, Ashland, MA) to produce the VEC tissue.
Full-thickness EpiVaginal tissue (VEC-100-FT)
To mimic the in vivo situation, a full-thickness VEC tissue model (VEC-FT) was also developed. Initial preparation of VEC-FT tissue was similar to methods previously described (Delvenne P, et al, 2001; Ayehunie S, et al, 2006, Klausner M, et al, 2007). Briefly, normal fibroblasts were obtained from the human vaginal-ectocervical tissues by standard collagenase treatment and expanded in DMEM medium containing 10% serum (DMEM-10). 1.0 ml of serum containing 1×106 fibroblasts was seeded with a collagen solution (3.2 mg/ml) to form a fibroblast-collagen gel matrix. The mixture was allowed to gel by incubating at 37°C for 1 h. The gel was equilibrated with 1.0 ml of DMEM-10 and cultured for 24 h. Thereafter, autologous VEC epithelial cells were seeded atop the matrix and the epithelial cell/fibroblast matrix was cultured for 4 days at submerged condition and for an additional 7 days at air-liquid interface (ALI) (total culture time 11 days).
Test chemicals
Six test articles (TA) at 3 concentrations each (Table 2) were tested using the EpiVaginal tissue and in RVI assay. The concentrations were chosen based on preliminary experiments in which a broad range of concentrations were tested. An approximate concentration which reduced tissue viability to 50% following a 24 hour exposure was determined (data not shown) and two additional concentrations were chosen which were 4X above and below this concentration (Table 2). Note: For N9, a series of 10 fold dilutions were used. Water (H2O) was used as vehicle control.
Table 2.
Test articles and concentrations used for in vitro and in vivo testing.
| Test Articles | Company | Catalog # | Concentrations (%) |
|---|---|---|---|
| Nonoxynol-9 (N9) | JEEN | CHEM NP-9 | 0.20, 0.02, 0.002 |
| Benzocaine (BEN) | Sigma | E1501-100G | 0.5, 2.0, 8.0 |
| Benzalalkonium chloride (BZK) | Sigma | 234427-5G | 0.008, 0.031, 0.125 |
| Povidone Iodide (PI) | Sigma | PVP1-100G | 1.25, 5.0, 20 |
| Miconazole Nitrate (MIC) | Sigma | M3512-25G | 0.008, 0.031, 0.125 |
| Polydimethylsiloxane (SIL) | Sigma | DMPSV-500g | 1.25, 5.0, 20 |
| Ultrapure water ( H2O ) | MatTek | ---- | 15–18 MOhm---- |
Dose volume
EpiVaginal tissues were dosed with 50 μl of each test article by topical application onto the apical surface of the tissue. The 50 μl volume was chosen to match the in vivo dose used in the RVI test, based on the following calculation. The rabbit vagina has an approximate surface area of 15 cm2. In standard RVI tests, 1.5 ml of test article are applied resulting in a dose = 0.1 ml/cm2. Thus, a 50 μl dose onto the in vitro tissues (surface area = 0.5 cm2 and 0.6 cm2 for partial and full-thickness tissues, respectively) approximates the in vivo dose of 0.1 ml/cm2. The dosed tissues were incubated for 24 hours (37°C/5% CO2). After 24 hours, the tissues were rinsed with PBS and analyzed using the MTT, TEER, and sodium fluorescein leakage assays, as described below. In addition, the medium contacting the basal surface of the tissue was saved for cytokine release analysis.
Histology
To examine the morphology of the in vitro reconstructed VEC tissues, the tissues were fixed by submerging the tissue-containing inserts in 10% formalin (overnight, at room temperature). Standard histology procedures were utilized and tissue cross-sections were cut (5–7 μm thick), mounted on microscopic slides, and stained with hematoxylin and eosin (H & E) (Figure 1). The stained cross-sections were observed and photographed using a Nikon Diaphot microscope. To evaluate structural changes to the tissues following chemical exposure, tissues were processed as above and compared with untreated control tissues.
Figure 1.

H & E stained cross-sections of: A) Partial thickness, epithelial VEC-100 tissue, B) Full-thickness VEC-100-FT tissue with epithelial and lamina propria layers, and C) Native human explant vaginal tissue. The epithelium in all tissues contains nucleated basal and suprabasal cell layers. As the apical surface is approached, the cells lose their nuclei and become filled with glycogen. In the full thickness and explant tissues, a lamina propria layer consisting of collagen gel matrix containing vaginal fibroblasts is observed. Conclusion: Good morphological correspondence between the EpiVaginal and native explant tissues was observed.
Tissue viability (MTT)
To asses the tissue viability following exposure to the various TA, the tissues were rinsed with PBS and loaded with (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma). Tissues were placed into a 24-well plate containing 300 μl of MTT (1 mg/ml) and incubated for 3 hrs at 37°C, 5% CO2. After incubation with MTT, the culture inserts were transferred into 24-well plates containing 2.0 ml of extractant (isopropyl alcohol); extraction of the MTT from the tissues was allowed to continue overnight (in the dark, at room temperature). The resulting extract was quantified by measuring the optical density (OD) at 570 nm using an E-MAX 96-well plate reader (Molecular Devices, Menlo Park, CA). The tissue viability was determined by normalizing to the OD for untreated tissues using the equation: % viability = OD (treated tissue)/OD (untreated tissue) X 100.
Transepithelial electrical resistance (TEER)
Changes in barrier function were quantitifed using transepithelial electrical resistance (TEER) measurements. TEER monitors the presence of functional tight junctions, which are responsible for the barrier function and which limit paracellular permeation of water and solutes. Similar methods have been used by others to evaluate epithelial toxicity of candidate microbicides (Gali Y, et al., 2010). TEER measurements were made using an EVOM volt-ohmmeter equipped with an EndOhm electrode chamber (World Precision Instruments, Sarasota, FL). Calculations of ohm * cm2 were made by multiplying the readings of each tissue by the surface area of the tissue (0.6 or 0.5 cm2 depending on the insert type). TEER measurements were normalized as a percentage of the untreated control tissues: % TEER = TEER (Ohms*cm2) of treated tissues (TTT) divided by the TEER of untreated tissues (TUT) times 100 (% TEER = (TTT/TUT*100).
Sodium fluorescein (NaFl) leakage – barrier disruption test
Changes induced to the barrier function of the tissues were also monitored by measuring the permeability of sodium fluorescein (NaFl) through the TA-treated tissues. After exposure to the test articles was complete (24 hr), the tissues were rinsed with PBS to remove the test article, the TEER for each tissue was measured, and 300 μL of 0.01% NaFl were applied to the apical tissue surface. Tissues were placed in a 24-well plate containing 0.5 ml PBS and incubated for 1 hr at 37°C/5% CO2. Untreated tissues were used as the negative controls. The NaFl concentration in the receiver solution (PBS) was determined using a fluorescence plate reader (Synergy HT, Multi-Detection Microplate reader, Winooski, VT) at 480/530 nm (excitation/emission). 100% NaFl permeability was determined for the naked tissue culture inserts (i.e. inserts without any tissues). Percent (%) permeability for the treated tissues was equal to: % NaFl (TA treated) = NaFl (TA treated)/(NaFl (naked inserts) *100. Methods similar to those previously reported were utilized (Ward, RK et al, 1997, Clothier R, et al 1999).
Cytokine analysis
The use of cytokine release following exposure to the test articles for hazard identification was also examined. The medium in contact with the basolateral tissue surface was collected and analyzed for four inflammatory cytokines IL-1α, IL-1β, IL-6, and IL-8 using commercially available ELISA assay kits. Fold increases were calculated by dividing the values obtained from treated tissues by the values obtained from the negative control (H2O treated) tissues.
In vivo studies
In vivo responses were monitored using a RVI test for the same set of model chemicals (Table 2). Histological cross-sections of the rabbit vaginal tissues were examined for 4 types of changes: a) epithelial exfoliation, b) vascular congestion, c) lamina propria thickness (edema), and d) leukocyte infiltration. Each category was graded on a scale of 0–4 for the intensity of the change: 0 = no change, 1 = minimal, 2 = mild, 3 = moderate and 4 = severe. The irritation scores were assigned based on the semi-quantitative scoring system for inflammation of Eckstein (Eckstein P et al, 1969). Based on the cumulative score for these 4 categories, the level of irritation was ranked as: a) 1–4 minimal, b) 5–8 mild, c) 9–11 moderate, and d) 11–16 severe irritation. In vivo studies were performed at MB Research Laboratories (Spinnerstown, PA).
Evaluation of in vitro tissue model reproducibility
Tissue-to-tissue variability within a given tissue lot (intra-lot variability) and long term variability over time need to be determined if EpiVaginal is to serve as an in vitro replacement for the RVI assay. Long term variability of the tissue model was assessed by testing 3 independent lots of EpiVaginal tissues that were reconstructed using cells obtained from a single donor and by testing 3 tissues lots reconstructed with cells from different donors (N=3). Inter-lot variability in assay performance was determined for tissue viability, barrier function, and cytokine release in these tissues.
Results
Tissue viability (MTT assay)
Representative tissue viability results following exposure to the 6 test articles are shown in Figure 2. There was no significant difference in tissue viability between the partial-thickness and full-thickness tissues for the materials tested. In all tissue lots tested (n=3), the highest concentrations of BZK (0.125%), PI (20%), and N9 (0.02 and 0.2%) reduced tissue viability below 50% (Figure 2). None of the Benzocaine (BEN), Miconazole (MIC), or polydimethylsiloxane (SIL) concentrations significantly reduced tissue viability.
Figure 2.
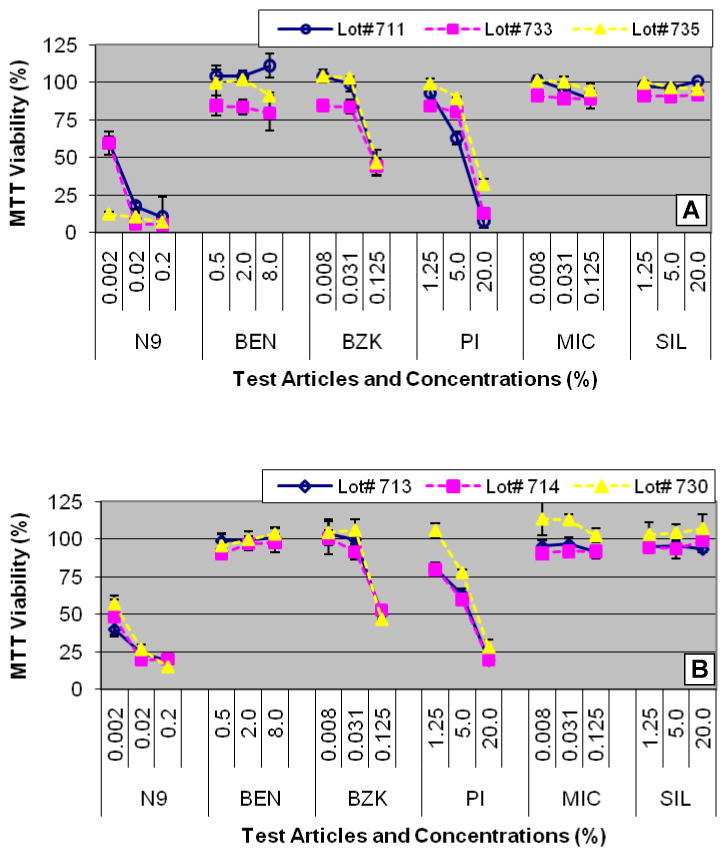
Tissue viability results for: A) Partial-thickness VEC-100 tissues cultured with cells from 3 different donors (lots # 711, 733, and 735) and B) 3 independent lots (lots # 713, 714, and 730) of full-thickness VEC-100-FT tissues obtained from cells from a single donor. Tissues were topically dosed with the test articles (Table 2) for 24 hr.
Histology
Although structural damage to the tissue often parallels decreases in tissue viability, histological evaluation provides a second, independent assessment of the effect of test articles on the tissue. Representative micrographs of EpiVaginal tissue histology following exposure to the various test articles are presented in Figure 3. Treatment with polydimethylsiloxane (SIL), benzocaine (BEN), and Miconazole (MIC) showed no or minimal effects to tissue morphology; there were slight effects to the apical and glycogen filled tissue layers but no effect on the viable basal and parabasal tissue layers. Likewise, these test materials had little effect on tissue viability which did not decrease below 85% (Table 3). In contrast, benzalkonium chloride (BZK), povidine iodide (PI), and Nonoxynol-9 (N9) all show disruption or loss of apical tissue layers and hypochromic staining of the viable tissue layers at higher concentrations, indicative of cytotoxicity (Figure 3); MTT results also show that tissue viability was reduced (to < 50%) for these test materials (Table 3).
Figure 3.
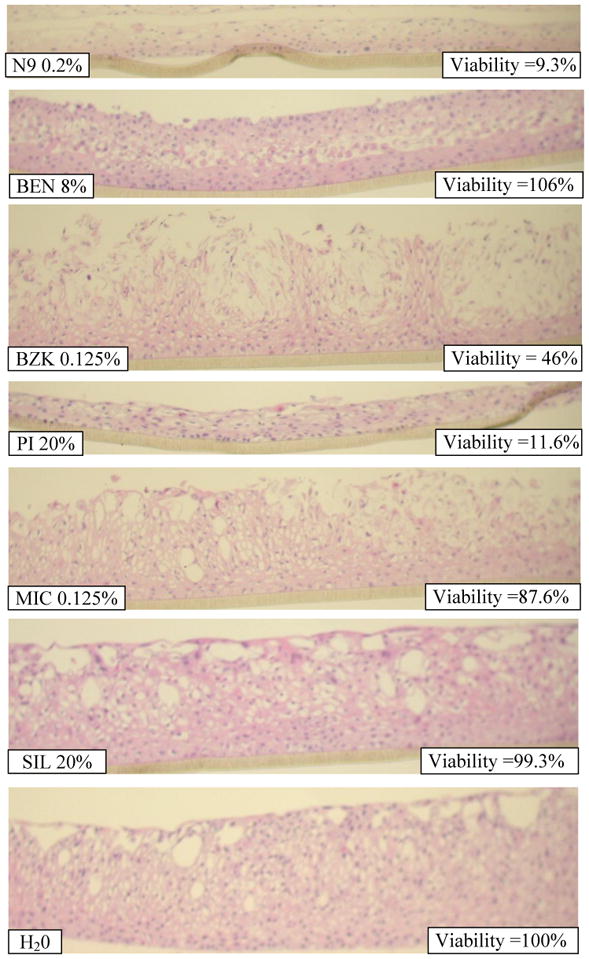
H&E stained cross-sections of EpiVaginal tissues following 24 hour exposure to the highest concentration of the test articles (Table 2).
Table 3.
Summary of tissue viability, barrier function (TEER), and histological results for the EpiVaginal tissue following 24 hour topical exposure to the test articles (Table 2). Data for the highest concentrations of each test article are presented.
| Test Article | % Conc. | Tissue Viability (%) | % CV | TEER (Ohm*cm2) | Remarks |
|---|---|---|---|---|---|
| Nonoxynol-9 (N9) | 0.2 | 10.5 | 3.4 | 24 | Complete loss of apical tissues layers. Hypochromic staining of basal cell layers. |
| Benzocaine (BEN) | 8.0 | 111.1 | 12.3 | 87 | Removal of outermost, glycogen filled apical tissue layers. No effect on viable basal/parabasal cell layers. |
| Benzalkonium chloride (BZK) | 0.125 | 46.1 | 17.1 | 51 | Disruption of apical tissue layers. Hypochromic staining in basal cell layers. |
| Povidine Iodide (PI) | 20.0 | 7.8 | 4.5 | 38 | Complete loss of apical tissues layers. Hypochromic staining of basal cell layers. |
| Miconazole Nitrate (MIC) | 0.125 | 89.2 | 5.5 | 242 | No effect |
| Polydimethylsiloxane (SIL) | 20.0 | 100.8 | 4 | 329 | No effect |
| Ultrapure Water | 100.0 | 100.0 | 8.6 | 218 | No effect |
Transepithelial resistance (TEER) measurements
The effects of the 6 test articles on partial and full-thickness EpiVaginal tissue barrier and viability are shown in Figure 4. The effects to the TEER values parallel the MTT results, i.e. decreases in viability are accompanied by decreases in TEER values. However, for benzocaine (BEN) and benzalkonium chloride (BZK), the TEER declines at lower concentrations where no changes in tissue viability are observed. This is an important observation which shows that decreases in TEER values may indicate early stages of cytotoxicity. A summary of histology, MTT tissue viability, and TEER values is presented in Table 3.
Figure 4.
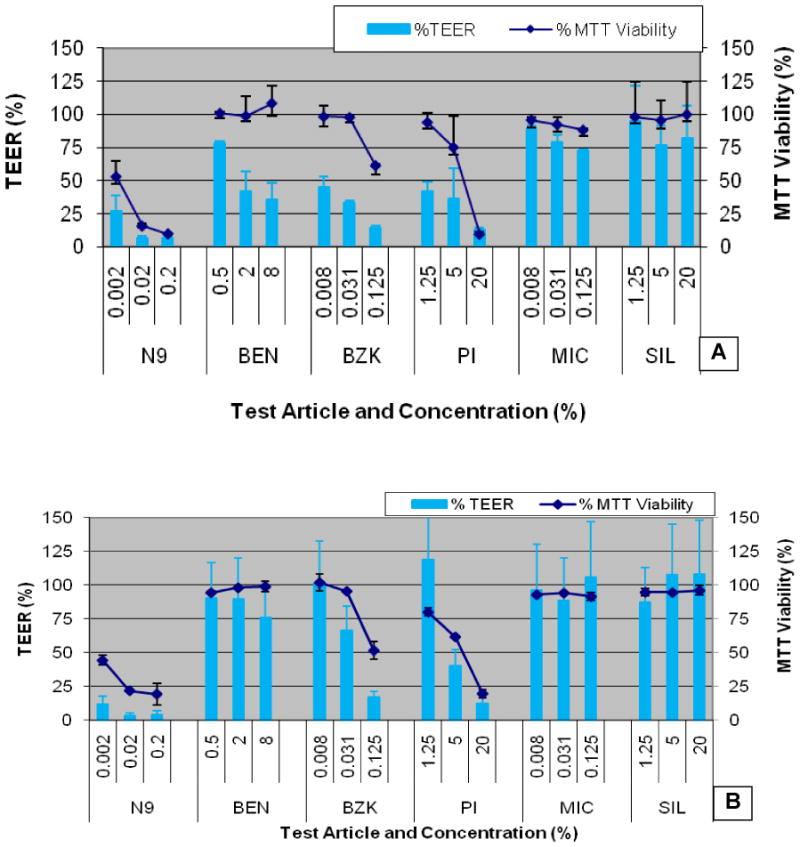
Transepithelial electrical resistance (TEER) and tissue viability (MTT) measurements for EpiVaginal tissues following 24 hr exposure to the test articles (Table 2). Averages (n=2 lots) are presented for: A) Partial-thickness VEC-100 and B) Full-thickness VEC-100-FT tissues. Exposure to ultrapure H2O was used the negative control. Conclusion: TEER values are parallel to, or are slightly more sensitive than, the tissue viability measurements.
Sodium fluorescein (NaFl)
To examine tissue permeability a sodium fluorecein leakage test was performed using a different tissue lot of full-thickness EpiVaginal tissues. The results from the fluorescein leakage test are presented in Figure 5A. Significant increases in NaFl permeability were observed for all dilutions of N9 (19–24% leakage rate) and for the highest concentrations of BZK (11% leakage rate) and PI (6% leakage rate). These concentrations also resulted in significant reductions in TEER and tissue viability (Figure 5B). TEER and NaFl permeability appear to be slightly more sensitive than the viability assay as evidenced by the results for 0.125% BZK, 0.002% N9, and 5% PI (Figure 5).
Figure 5.
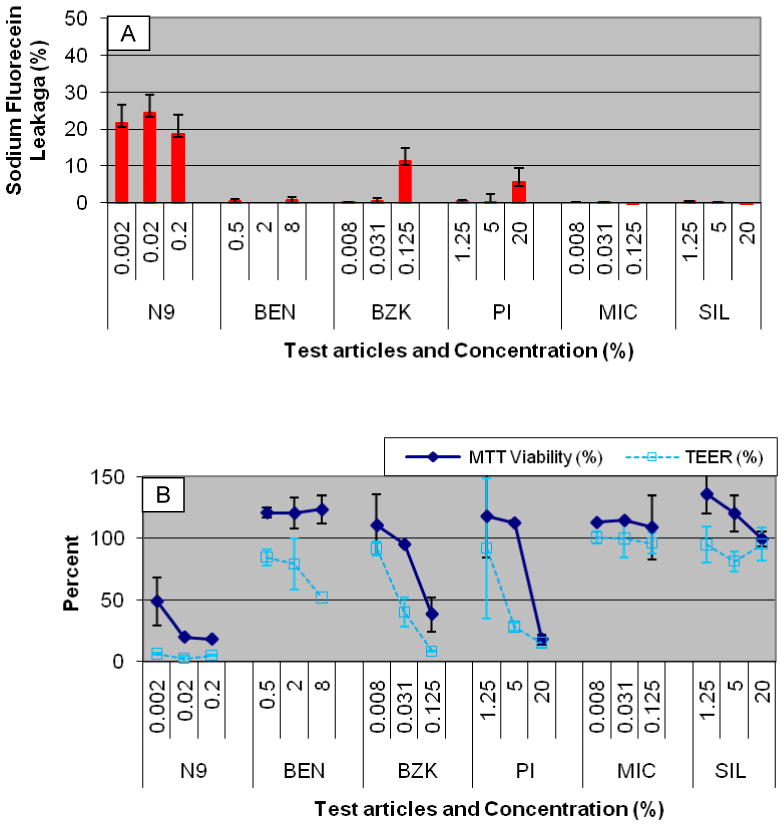
Results for the full thickness VEC-100-FT tissue following 24 hour exposure to the test articles (Table 2). A) Sodium fluorescein (NaFl) leakage and B) Tissue viability (MTT) and TEER assays. Conclusion: NaFl leakage increases when tissue viability and TEER decrease.
Rabbit vaginal irritation (RVI) results
To check the validity of the EpiVaginal model, experiments were also performed using the RVI test for the same 6 materials (Table 2). In the RVI study, only the highest concentration of N9 (0.2%) showed mild irritation (RVI score = 5.6) (Table 4). All the other test chemicals at the concentrations tested were scored as minimal/non-irritating.
Table 4.
Average RVI scores for the test articles at highest concentration used for each test article. The average score for each category was derived from the scores for 6 rabbits/test material.
| RVI Category | N9 (0.2%) | BEN (8 %) | BZK (0.125%) | PI (20%) | MIC (0.125%) | SIL (20%) | Control (H2O) | Untreated Control |
|---|---|---|---|---|---|---|---|---|
| Exfoliation | 2.6 | 0.1 | 0.3 | 0.4 | 0.2 | 0.2 | 0.0 | 0.0 |
| Congestion | 0.7 | 0.0 | 0.2 | 0.2 | 0.2 | 0.0 | 0.6 | 0.0 |
| Edema | 1.6 | 0.6 | 0.2 | 0.4 | 0.4 | 0.3 | 0.9 | 0.0 |
| Infiltrate | 0.7 | 0.4 | 0.1 | 0.3 | 0.1 | 0.1 | 0.7 | 0.0 |
| Total RVI | 5.6 | 1.0 | 0.8 | 1.3 | 0.9 | 0.6 | 2.2 | 0.0 |
Cytokine Release - IL-1α and IL-1β
Analysis of culture supernatants revealed increased levels of IL-1α and IL-1β following exposure to the 6 test materials (Figures 6 – 7). In most cases, both IL-1α and IL-1β release corresponded to tissue viability, i.e. increases in cytokine release were noted following treatments that decreased the tissue viability. Only PI (20%) significantly decreased tissue viability but did not result in increased IL-1β release; likewise, IL-1α did not increase following exposure of 20% PI, despite a significant decrease in tissue viability.
Figure 6.
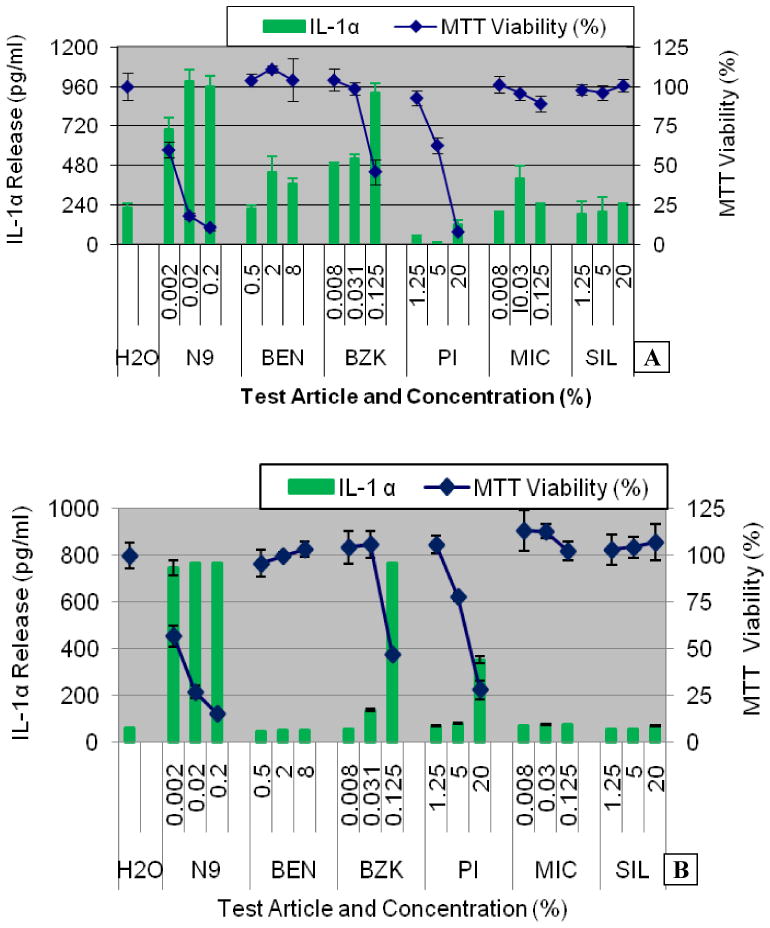
IL-1α release into culture supernatants of: A) Partial thickness VEC-100 and B) Full-thickness VEC-100-FT EpiVaginal tissues following topical exposure to the test articles (Table 2) for 24 hours. Conclusion: IL-1α release increases when tissue viability decreases.
Figure 7.
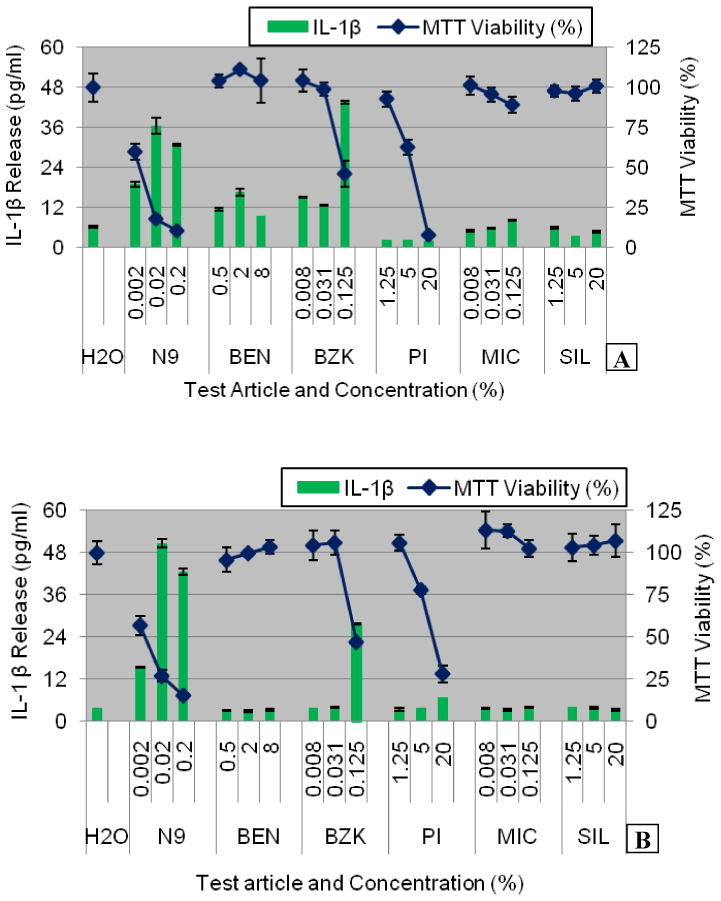
IL-1β release into culture supernatants of: A) Partial thickness VEC-100 and B) Full-thickness VEC-100-FT EpiVaginal tissues following topical exposure to the test articles (Table 2) for 24 hours. Conclusion: IL-1β release increases when tissue viability decreases except for test article PI.
IL-6 and IL-8
No significant increases in IL-6 or IL-8 release were observed in the partial thickness EpiVaginal tissue (Table 5). However, for the full thickness tissue, increases in IL-6 were observed which were not linked to tissue viability. For instance, PI, BZK, and MIC all showed significant increases in IL-6 even at high tissue viability (Table 5). In addition, no increase in IL-6 was observed for N9 even though it induced high levels of cytotoxicity. On the other hand, IL-8 only showed significant increases for PI at doses which caused moderate or significant loss in tissue viability. Thus, a combination of IL-1α, IL-1β, and IL-8 release patterns may be useful biomarkers for hazard identification of topically applied. vaginal chemicals/formulations.
Table 5.
Summary of tissue viability and cytokine responses in partial thickness and full-thickness EpiVaginal tissues exposed to the test articles for 24 hr. Cytokine values are reported as the fold increase (FI) above the negative control (H2O). Significant decreases in tissue viability and significant fold increases in cytokine levels are bolded.
| Partial thickness EpiVaginal tissue | Full-Thickness EpiVaginal tissue | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Lot #711) | (Lot #730) | ||||||||||||
| MTT | IL-1α | IL-1β | IL-6 | IL-8 | MTT | IL-1α | IL-1β | IL-6 | IL-8 | ||||
| TA | % Conc. | % Viability | % CV | Fold Inc. | Fold Inc. | Fold Inc. | Fold Inc. | % Viability | % CV | Fold Inc. | Fold Inc. | Fold Inc. | Fold Inc. |
| N9 | 0.002 | 59.9 | 10.9 | 3.1 | 3.0 | 0.5 | 0.9 | 57.0 | 9.9 | 12 | 4.1 | 0.9 | 1.0 |
| 0.02 | 17.9 | 13.1 | 4.5 | 5.8 | 0.5 | 1.0 | 26.8 | 13 | 12.3 | 13.4 | 0.9 | 1.2 | |
| 0.2 | 10.5 | 3.4 | 4.3 | 4.9 | 0.6 | 0.9 | 15.2 | 11 | 12.3 | 11.3 | 1.3 | 1.1 | |
| BEN | 0.5 | 104 | 3.7 | 1.0 | 1.8 | 1.0 | 0.8 | 95.7 | 7.6 | 0.8 | 0.8 | 1.3 | 1.0 |
| 2.0 | 104 | 2.0 | 2.0 | 2.7 | 0.6 | 0.8 | 99.9 | 2.0 | 0.8 | 0.8 | 1.3 | 0.8 | |
| 8.0 | 111 | 12.3 | 1.7 | 1.5 | 0.6 | 0.8 | 104 | 4.0 | 0.8 | 0.9 | 2.1 | 0.8 | |
| BZK | 0.008 | 104 | 6.6 | 2.2 | 2.4 | 0.9 | 1.0 | 104 | 8.5 | 0.9 | 0.9 | 4.0 | 1.3 |
| 0.031 | 98.8 | 4.1 | 2.3 | 2.0 | 1.0 | 1.0 | 106 | 6.7 | 2.2 | 1.0 | 2.7 | 1.9 | |
| 0.125 | 46.1 | 17.1 | 4.2 | 7.0 | 1.3 | 1.2 | 47.0 | 2.9 | 12.3 | 7.3 | 3.2 | 2.1 | |
| PI | 1.25 | 92.9 | 4.9 | 0.2 | 0.4 | 1.8 | 1.2 | 106 | 4.4 | 1.2 | 0.9 | 6.7 | 1.5 |
| 5.0 | 62.7 | 7.8 | 0.0 | 0.3 | 1.4 | 1.0 | 78.0 | 2.8 | 1.3 | 1.0 | 6.5 | 3.1 | |
| 20.0 | 7.8 | 4.5 | 0.5 | 0.3 | 0.3 | 0.1 | 28.2 | 18 | 5.6 | 1.7 | 3.0 | 3.5 | |
| MIC | 0.008 | 101 | 5.3 | 0.9 | 0.8 | 0.8 | 1.2 | 114 | 9.5 | 1.1 | 1.0 | 1.1 | 1.2 |
| 0.03 | 95.9 | 4.7 | 1.8 | 0.9 | 0.7 | 1.2 | 113 | 3.2 | 1.2 | 0.9 | 2.4 | 1.2 | |
| 0.125 | 89.2 | 5.5 | 1.1 | 1.3 | 1.1 | 1.6 | 102 | 4.8 | 1.2 | 1.0 | 3.9 | 1.8 | |
| SIL | 1.25 | 97.9 | 3.4 | 0.9 | 1.0 | 1.4 | 1.2 | 103 | 7.8 | 0.9 | 1.1 | 1.2 | 1.4 |
| 5.0 | 96.2 | 4.6 | 0.9 | 0.5 | 1.2 | 1.1 | 104 | 5.3 | 0.9 | 1.0 | 1.7 | 1.2 | |
| 20.0 | 101 | 4.0 | 1.1 | 0.7 | 0.5 | 1.1 | 107 | 9.0 | 1.1 | 0.9 | 2.1 | 1.5 | |
| H2O | 50 ul | 100 | 8.6 | 1.0 | 1.0 | 1.0 | 1.0 | 100 | 6.7 | 1.0 | 1.0 | 1.0 | 1.0 |
| Avg CV | 6.7 | Avg CV | 7.2 | ||||||||||
Key: TA = Test article; N9 =Nonoxynol-9; BEN=Benzocaine; BZK = Benzalkonium chloride; PI=Povidine Iodide; MIC = Miconazole Nitrate; SIL= Polydimethylsiloxane; H2O = Water control; and Fold Inc. = Fold Increase (normalized to H2O negative control).
Note: Significant decreases in tissue viability (>50%) and fold increases in cytokine release (> 2.0) are bolded.
Reproducibility of in vitro results
A high level of tissue-to-tissue reproducibility is evident based on the modest error bars in Figures 2, 4, 5, 6, 7, 8, 9, & 10 and the coefficients of variation listed in Tables 3 & 5. The average coefficients of variation (CV) for the partial thickness (lot #711) and full thickness EpiVaginal tissues (lot #730) tissues were 6.7% and 7.2%, respectively (Table 5). TEER reproducibility in the partial and full-thickness EpiVaginal tissues is shown in Figures 4 and 10. Likewise, inter-lot reproducibility of cytokine release is high (Figures 8 & 9). Finally, as shown in Figures 2, 8, and 9, 3 lots of EpiVaginal tissues cultured from cells derived from 3 different donors gave highly reproducible tissue viability and cytokine release results.
Figure 8.
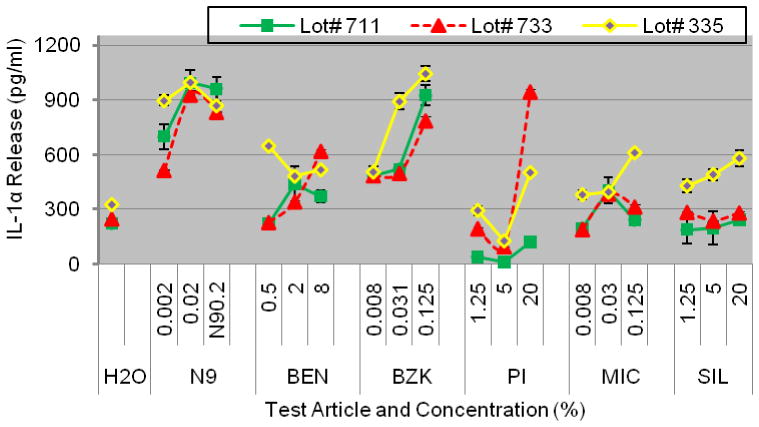
Reproducibility of IL-1α release into culture supernatants of partial thickness VEC-100 EpiVaginal tissue cultured with cells obtained from 3 different donors (Lots # 711, 733, and 735). Conclusion: IL-1α release was highly reproducible for all 3 cell donors.
Figure 9.
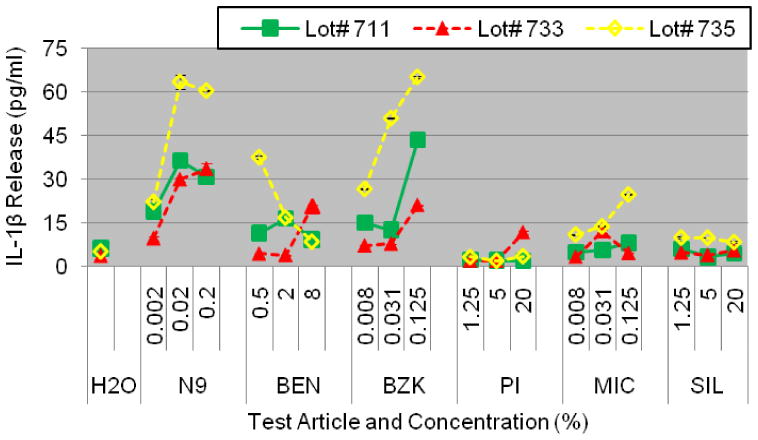
Reproducibility of IL-1β release into culture supernatants of partial thickness VEC-100 EpiVaignal tissue cultured with cells obtained from 3 different donors (Lots # 711, 733, and 735). Conclusion: IL-1β release was highly reproducible for all 3 cell donors.
Figure 10.
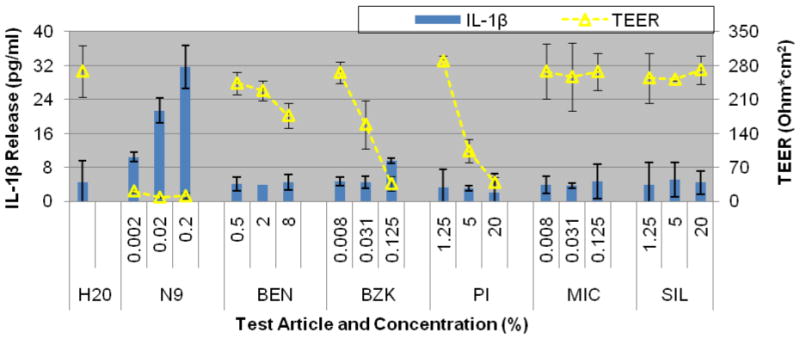
Reproducibility of transepithelial electrical resistance (TEER) measurements and IL-1β release by the full-thickness EpiVaginal tissue model following 24 hour exposure to the test articles (Table 2). Averages for N=2 independent tissue lots are shown. Exposure to ultrapure H2O was used the negative control. Conclusions: a) IL-1β release appears independent of changes in TEER values and b) IL-1β and TEER values show high levels of reproducibility.
Discussion
The EpiVaginal tissue model is very similar to native in vivo tissue in many important aspects. It is reconstructed using low passage (≤ 3rd passage) normal human Vaginal/ectocervical cells. Histologically, EpiVaginal has a 3-dimensional, stratified structure that contains basal, suprabasal, intermediate, and superficial cell layers (Figure 1). As the apical surface of the tissue is approached, cells become enucleated and the amount of glycogen in the cells increases, similar to native vaginal-ectocervical tissue (Figure 1C). Tight junctions between cells, that are important in determining the barrier function of the tissue, are intact (Figure 2) and the tissue expresses the differentiation markers cytokeratins 13 and 14, similar to native tissue (Ayehunie et al, 2006). These aspects of differentiated structure have important implications for how exogenous materials interact with the tissue.
Unlike explant tissues, the EpiVaginal tissues can be cultured for extended time periods (2–3 weeks), and up to 50,000 highly reproducible tissues can be reconstructed from cells isolated and expanded from a single ectocervical tissue explant (unpublished results). The ability to expand the cell population results in production of more EpiVaginal tissues which in turn allows researchers and toxicologists to perform multiple experiments utilizing cells obtained from the same donor, thereby improving the reproducibility of results. Also, since EpiVaginal is comprised of normal human cells, inter-species extrapolation is not necessary and findings have direct relevance to women’s health. Our current results indicate that the EpiVaginal tissue model is a promising candidate for pre-clinical screening of topically applied chemicals, formulations, and other therapeutic agents for their safety. In addition, others have shown that EpiVaginal is useful for testing the efficacy microbicides designed to inactivate sexually transmitted pathogens such as HIV (Cole, AL., 2007).
Disruption of the epithelial barrier can lead to a higher likelihood of pathogen exposure or to exudation as observed in vivo (e.g. in RVI tests or in human clinic trials). Transmission electron microscopic observations showed that EpiVaginal epithelial layers produce tight junctions and desmosomes between cells (Ayehunie et al, 2006), both of which impart barrier function to the vaginal tissue. These structures are known to inhibit the permeation of low molecular weight solutes and ions across the tissue, thereby conferring electrical resistance to the tissue. Thus, transepithelial electrical resistance (TEER) measurements were used to monitor the barrier function of the EpiVaginal tissue. Results presented herein showed that TEER measurements gave an early signal regarding the hazard potential of a test article.
Increases in the number of people infected with HIV-1 and other STI has intensified the need for the development of safe and effective female-controlled topical microbicides. However, many of the new vaginal antimicrobial agents are cytotoxic and cause vaginal irritation, particularly, when used at higher doses (Zaneveld LJ et al 2002, Stafford MK, et al, 1998, Roddy RE, et al, 1993). In mice, intravaginal and intrauterine administration of the commonly used spermicide, nonoxynol-9 (N9), has resulted in uterine epithelial sloughing or complete epithelial loss (Dayal MB et al, 2003; Phillips DM, et al 2000). Such irritation of the vaginal mucosa can increase the risk of acquiring STI pathogens such as the human herpes simplex virus (HSV) and HIV-1 in sexually active women (Mansergh G, et al, 2002). The use of an in vitro 3D-tissue model for such studies will be advantageous in that it will: 1) allow researchers to understand the discrete steps in a specific sequence of events which is difficult to do in whole animals or humans (Kniewald J, et al, 2005), 2) provide a high throughput format for large scale screening of the growing number of topically applied formulations, and 3) reduce the number of animals needed for experimentation,.
In this study, vaginal irritants such as nonoxynol 9 and benzalkonium chloride induced > 2 fold increases in IL-1β, a cytokine that activates a number of proinflammatory pathways and that is involved in the recruitment of leukocytes (Bamforth SD, et al, 1997). Although not thoroughly examined in humans, such responses may have implications on women’s health, because attracted leukocytes can be targets of infection by STI organisms such as HIV-1 and herpes simplex virus (HSV)-2. Previous studies of vaginal microbicides also suggest induction of IL-1β correlates with vaginal irritation (Passmore JS, et al, 2007, Fichorova, RN, et al, 2004). In RVI models, high levels of IL-1b were associated with increased CD3+T cells and significant influx of CD4+ T cells (Gustavo DF and Fichorova NR, 2004). Since the cells are of human origin, inter-species extrapolation of results is not needed, and animal welfare concerns are avoided. In fact, the partial thickness EpiVaginal tissue model (VEC-100) has recently been utilized to predict vaginal irritation (Ayehunie et al, 2006, Trifonova, R.T, et al, 2005, Dover, S.E, et al 2007, Fletcher, P.S, et al, 2008) and to model bacterial vaginosis (Valore, E.V, et al, 2006). The full thickness EpiVaginal containing dendritic cells (VLC-100-FT) has also been used for HIV-1 infection/microbicide efficacy studies (Venkataraman N, et al, 2005, Cole, A.L, et al, 2007).
Emerging technologies offer ample opportunities to update approaches in toxicological studies. New models are replacing the traditional animal based experimentations with alternative in vitro assays for hazard identification. Our preliminary comparison of the in vitro assay method with that of the established RVI assay identified N9 as an irritant to mucosal tissues both in vitro and in vivo. In addition, 2% BZK is a known vaginal irritant (Fitchrova et al. 2004). In this study, BZK was correctly identified as an irritant by the in vitro assay but not by the RVI assay. The differences between the in vitro and in vivo results are likely due to the 0.125% BZK concentration used in the current studies. Since the organotypic tissue based assay identified BZK as an irritant at a much lower concentration, the in vitro assay is likely more sensitive than the RVI assay in identifying vaginal irritants. This would be beneficial to product formulators interested in producing ultra-mild feminine hygiene products. These data warrant further investigation to validate the EpiVaginal system as an in vitro alternative assay method for preclinical safety studies of chemicals and formulations such as microbicides. Whether the in vitro system will be used as a stand alone assay or in conjunction with the RVI assay needs to be addressed in future studies using: 1) larger number of test articles, 2) standardized working protocols, and 3) data obtained from multi-testing laboratories.
Conclusion
The development of the EpiVaginal test system offers industrial companies and academic institutions the prospect of a new tool which is rapid, easy to use, economical, and reproducible to screen new chemicals, formulations, and therapeutic agents that can affect women’s health. The in vitro endpoints, tissue viability, histology, TEER, NaFl leakage measurements, and cytokine release, all give important information on how a material interacts with vaginal tissue. Additional studies are underway further investigating the use of these endpoints to develop a validated in vitro, human tissue based system to predict vaginal irritation and inflammation caused by topically applied feminine hygiene and other therapeutic products.
Acknowledgments
This work was supported by the National Institutes of Health Grants of 2 R43 HD050023 and Grant # 5U01AI070914 to SA..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achilles SL, et al. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sex Transm Dis. 2002;29:655–664. doi: 10.1097/00007435-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Ayehunie S, et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicology in Vitro. 2006;20:689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bamforth SD, et al. Ultrastructural analysis of interleukin-1beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol & Visual Science. 1997;38:25–35. [PubMed] [Google Scholar]
- Belec L, et al. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine. 1995;7:568–74. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- Catalone BJ, et al. Comparative safety evaluation of candidate vaginal microbicide C31G. Antimicrobial Agents and Chemothrapy. 2005;49:1509–1520. doi: 10.1128/AAC.49.4.1509-1520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Letter from the director. National Center for HIV, STD, and TB Prevention; Aug 4, 2000. posting date. http:/www.cdc.gov/hiv/pubs/mmwr/mmwr11aug00.htm. [Google Scholar]
- Clothier R, et al. Assessment of initial damage and recovery following exposure of MDCK cells to an Irritant. Toxicology in Vitro. 1999;13:713–717. doi: 10.1016/s0887-2333(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Cole AL, et al. Herasimtschuk. The retrocyclin analogue RC-101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology. 2007;121:140–145. doi: 10.1111/j.1365-2567.2006.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JE, Doncel G. Biomarkers of cervicovaginal inflammation for the assessment of microbicide safety. Sexually Transmitted Diseases. 2009;36:S84–S91. doi: 10.1097/OLQ.0b013e3181994191. [DOI] [PubMed] [Google Scholar]
- Dayal MB, et al. Disruption of upper female reproductive tract epithelium by nonoxynol-9. Contraception. 2003;68:273–279. doi: 10.1016/s0010-7824(03)00178-1. [DOI] [PubMed] [Google Scholar]
- D’Cruz OJ, Uckun FM. Pre-clinical safety evaluation of novel nucleoside analogue-based dual-function microbicides (WHI-05 and WHI-07) J Antimicrob Chemother. 2002;50:793–803. doi: 10.1093/jac/dkg001. [DOI] [PubMed] [Google Scholar]
- Delvenne P, et al. The organotypic culture of HPV-transformed keratinocytes: an effective in vitro model for the development of new immunotherapeutic approaches for mucosal (pre) neoplastic lesions. Vaccine. 2001;19:2557–2564. doi: 10.1016/s0264-410x(00)00489-8. [DOI] [PubMed] [Google Scholar]
- Doncel G, et al. Preclinical assessment of the proinflammatory potential of microbicide candidates. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;37:S174–S180. [PubMed] [Google Scholar]
- Dover SE, et al. Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal lactobacillus rhamnosus. Infect Diseases in Obstetrics and Gynecology. 2007 doi: 10.1155/2007/78248. Article ID#78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein P, et al. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. Journal of Reproduction and Fertility. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, et al. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of Vaginal microbicidal contraceptives. Biology of Reproduction. 2004;71:761–769. doi: 10.1095/biolreprod.104.029603. [DOI] [PubMed] [Google Scholar]
- Fletcher PS, et al. Preclinical evaluation of lime juice as a topical microbicide candidate. Retrovirology. 2008;5:3. doi: 10.1186/1742-4690-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen BT, et al. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. 2007;195:1332–1339. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- Gali Y, et al. Development of an in vitro dual-chamber model of the female genital tract as a screening tool for epithelial toxicity. Journal of Virological Methods. 2010;165:186–197. doi: 10.1016/j.jviromet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Klausner M, et al. Organotypic human oral tissue models for toxicological studies. Toxicology in Vitro. 2007;21:938–949. doi: 10.1016/j.tiv.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Kniewald J, et al. Alternative Models for toxicity testing of xenobiotics. Alternative for Toxicological Testing. 2005;56:195–204. [PubMed] [Google Scholar]
- Mansergh G, et al. Continued use of Nonoxynol-9 (N9) in a diverse sample of men who have sex with men (MSM) and a potentially effective prevention message to reduce N9 use. Int Conf AIDS. 2002 Jul 7–12;14 abstract no. MoPeD3547. [Google Scholar]
- Noguchi K, et al. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp Med. 2003;53:404–12. [PubMed] [Google Scholar]
- Osborn L, et al. Tumor necrosis factor-α and interleukin -1 stimulate the human immunodeficiency virus enhancer by activation of nuclear factor kappa B. Proc Natl Acad Sci. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore JS, et al. Papanicolaou smears and cervical inflammatory cytokine responses. J Inflamm (London) 2007;4:8. doi: 10.1186/1476-9255-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM, et al. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception. 2000;62:149–54. doi: 10.1016/s0010-7824(00)00156-6. [DOI] [PubMed] [Google Scholar]
- Roddy RE, et al. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS. 1993;4:165–7. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- Squier CA, et al. Procine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J pharmaceutical Sci. 2007 doi: 10.1002/jps.21077. [DOI] [PubMed] [Google Scholar]
- Spielmann H, et al. The ECVAM international validation study on in vitro tests for acute skin irritation: report on the validity of the EPISKIN and EpiDerm assays and on the Skin Integrity Function Test. Altern Lab Anim. 2007;35:559–601. doi: 10.1177/026119290703500614. [DOI] [PubMed] [Google Scholar]
- Stafford MK, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- Tsai CC, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV- 189.6P in macaques. AIDS Res Hum Retroviruses. 2003;19:535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- Trifonova RT, Pasicznyk J-M, Fichorova RN, et al. Vaginal Formulation- How Innocuous Are They To The Vaginal Epithelial Immune Function? American Journal of Reproductive Immunology. 2005;54:121. [Google Scholar]
- Valore EV, et al. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infection and Immunity. 2006;74:5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman N, et al. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- Ward RK, et al. Evaluation of tissue culture insert membrane compatibility in the fluorescein leakage assay. Toxicology in Vitro. 1997;11:761–768. doi: 10.1016/s0887-2333(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Weber J, et al. The Development of Vaginal Microbicides for the Prevention of HIV Transmission. PLoS Med. 2005;2:e142, 392–395. doi: 10.1371/journal.pmed.0020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneveld LJ, et al. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly (sodium 4-styrenesulfonate) Biol Reprod. 2002;66:886–94. doi: 10.1095/biolreprod66.4.886. [DOI] [PubMed] [Google Scholar]


