Abstract
Purpose
To determine the change in vision-related quality of life scores after providing eyeglasses to American Indian/Alaska Natives with under-corrected refractive error.
Study Design
Prospective, comparative (non-randomized) interventional study
Methods
We compared a group with under-corrected refractive error to a Control group who did not need a change in eyeglasses. Under-corrected refractive error was defined as distance visual acuity 20/40 or worse in the better-seeing eye that could be improved by at least 2 lines in Snellen visual acuity. Intervention was the provision of new glasses to the under-corrected refractive error group members, based on results of manifest refraction. The main outcome measures were the differences in the 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) scores from baseline (T1) to the time after providing eyeglasses (T2).
Results
The NEI-VFQ-25 median composite score at T1 was significantly lower in those with under-corrected refractive error when compared to the Control group (75 vs. 92, p=.001). The median composite score for the Under-corrected refractive error group improved to 96 (P<0.001) at T2 when compared to T1, while the Control group remained stable at 93 (p=0.417). The Under-corrected refractive error group showed significantly greater improvement than the Control group in 8 of 12 subscale scores and in the overall composite score (all p values ≤ 0.05). A multivariate linear regression analysis, which controlled for differences in age, percent self-identified American Indian/Alaskan Native and best-corrected visual acuity between the Under-corrected refractive error and Control group showed eyeglasses to be significantly associated with improvement in NEI-VFQ-25 composite score.
Conclusion
Visual impairment from under-corrected refractive error is common in American Indian/Alaskan Natives. Providing eyeglasses results in a large, significant increase in vision-related quality of life.
INTRODUCTION
The National Health Interview Survey shows that visual impairment affects more than 21 million non-institutionalized Americans over the age of 18.1 Uncorrected refractive error is a leading cause of visual impairment in adults2–7 Visual impairment is approximately two times more prevalent in American Indian/Alaskan Natives when compared to age-matched White or African Americans.3, 8–11 In American Indian/Alaskan Native populations, refractive error is believed to be common because of a high prevalence of astigmatism11–15, and studies demonstrate a higher need for eyeglasses in American Indian/Alaskan Natives when compared to other ethnic groups6, 11
Little information exists about the changes in vision-related quality of life with the provision of eyeglasses. Such studies have focused primarily on patients who are elderly16, 17 or have other co-morbidities, such as macular degeneration18, depression19, diabetes20, or cataracts21. We were interested in the amount of improvement in vision-related quality of life after providing adult American Indian/Alaskan Natives with eyeglasses.
The Indian Health Service was established to provide health care for the members of the more than 569 Federally recognized tribes in the United States. However, Indian Health Service funding is limited. Eyeglasses are not typically provided to tribal members via Federal funding.22 While eyeglasses are a relatively safe and inexpensive treatment for visual impairment, knowledge regarding the amount of change in vision-related quality of life is important information, especially when considering the relative value of eyeglasses to other competing treatments for visual impairment, such as cataract and refractive surgery.
METHODS
Selection Criteria
We enrolled American Indian/Alaskan Natives participants who were 18 years or older from two locations in the Northwest region of the United States, and one location from the Midwest. We recruited participants into one of two groups: the Under-corrected refractive error group (if presenting distance visual acuity was 20/40 or worse in the better-seeing eye and manifest refraction showed an improvement of at least 2 lines), or in the Control group (if presenting distance vision was either better than 20/40, or could not be improved by at least 2 lines on a visual acuity chart). Similar definitions for under-corrected refractive error have been used previously.23, 24 We excluded candidates who were unable to complete subjective testing.
Survey Instruments
We used the 25-Item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25)25 to assess self-reported vision-related quality of life. We calculated a Composite score for vision-related quality of life, according to published methods,25 at baseline (T1) and at follow-up after treatment (T2). We modified one item of the Near Vision Activities subscale of the NEI-VFQ-25, adding the term “bead-working”, to better reflect the cultural activities of the American Indian/Alaskan Native population.26
We interviewed participants using the NEI-VFQ-25 at T1, and repeated the questionnaire at T2, approximately 3 months later. We waited 3 months to limit test-retest bias for both groups as well as to allow the Under-corrected refractive error group sufficient time to obtain and adjust to their new eyeglasses.
We used a modified Behavioral Risk Factor Surveillance Survey27 survey to determine American Indian/Alaskan Natives heritage, ocular history, and risk factors for medical and eye diseases. We also added a previously validated single question regarding self-reported depression28 to a subset of the study cohort (n=37 at T1; n=70 at T2). This question, “How often have you felt downhearted and blue in the last 4 weeks,” has 6 response options: 1= “All the time;” 2=”Most of the time;” 3=”A good bit of the time;” 4=”Some of the time;” 5=”A little of the time;” and 6=”None of the time.” All participants spoke English, and the Behavioral Risk Factor Surveillance Survey and NEI-VFQ-25 questionnaires were administered in this language.
Visual Acuity Testing
We recorded presenting Snellen distance vision using standard Early Treatment of Diabetic Retinopathy Study charts, trans-illuminated with a chart illuminator (Lighthouse Low Vision Products, Long Island City). We converted these measurements into a logMAR (logarithm of the minimum angle of resolution) scale of distance visual acuity, using published methods.29 The main outcome measure was the difference in NEI-VFQ-25 scores from T1 to T2, in the Under-corrected refractive error group compared to the Control group.
Eyeglasses
We provided distance or bifocal spectacles to the Under-corrected refractive error group (n=26); we provided bifocal spectacles to 9 participants, while the remainder received distance correction only. Those that only required reading glasses were not included in this study.
Statistical Analysis
We used non-parametric tests (Mann-Whitney U, and Kruskal-Wallis tests) when the data was not normally distributed (subscale and composite NEI-VFQ-25 scores). NEI-VFQ-25 score change from T1 to T2 was normally distributed, and we used a t-test to evaluate the change between Control and Under-corrected refractive error groups. We also used a multivariate linear regression model to control for differences in visual acuity, age, gender, and self-reported percent American Indian/Alaskan Native heritage between the Under-corrected refractive error and Control groups. All statistical analyses were performed using SPSS® (v16.0, SPSS, Inc. Chicago, Illinois) and R (R Development Core Team (2009). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org) statistical software. We checked the internal consistency of subscales by Cronbach’s alpha statistics. 30 We employed mixed-effects models with a random intercept to account for potential autocorrelation within a person within a testing location using the R “nlme” library.31
We determined whether our sample sizes were adequate based on observed group means and effect sizes. Prior to the study, we determined a sample size of 84 people per group was needed to detect a 10-point difference in composite scores, with an alpha of 0.05 (t-test), power of 0.80, and inter-temporal correlation between scores of 0.60. We further calculated that a 20-point difference would require 21 persons in each group. The amount of change in the Under-corrected refractive error group exceeded 20 points in the majority of the subscales and in the composite score; therefore, we stopped enrolling participants after recruiting 26 under-corrected refractive error participants.
RESULTS
Participants
We enrolled 114 participants: 76 into the Control group, 26 into the Under-corrected refractive error group, and 12 participants (11%) who did not complete the NEI-VFQ-25 questionnaire at T2. Therefore we report data for 102 participants. We found no statistically significant differences (p>0.05) in age, sex, percent American Indian/Alaskan Native heritage, T1 Composite scores, measures of self-reported depression or visual acuity between those who completed and those who did not complete the questionnaire at T2 (data not shown).
Table 1 shows the baseline characteristics of the Control and Under-corrected refractive error groups. Significant differences between the groups existed in age, percent self-reported American Indian/Alaskan Native heritage, level of visual impairment and median visual acuity. Control group participants showed an improvement of less than 1 line of acuity (T1 mean=0.09: median 0.00; T2 mean=0.06: median=0.00) after manifest refraction (0.30 lines), while those in the Under-corrected refractive error group improved nearly 6 lines (T1 mean=0.60: median=0.50; T2 mean=0.03: median=0.00) after manifest refraction (5.7 lines).
Table 1.
Baseline characteristics of the Control and Under-corrected Refractive Error (UCRE) groups.
| All Participants (n=102) |
Control group (n=76) |
UCRE group (n=26) |
p value | ||
|---|---|---|---|---|---|
| Age: Mean (Std. Dev) | 50.9 (15.9) | 56.2 (13.3) | 35.6 (12.5) | <0.001 | |
| Female | 56% | 61% | 42% | 0.083 | |
| AI/AN (Self-reported) | |||||
| Less than 50% | 28% | 22% | 46% | 0.012 | |
| 50% or greater | 64% | 71% | 42% | ||
| Refused a | 8% | 7% | 12% | ||
| Level of Visual Impairment | |||||
| None | 64% | 64% | 0% | <0.001 | |
| Mild | 23% | 35% | 54% | ||
| Moderate to Severe | 14% | 2% | 46% | ||
| Visual Acuity: Median logMAR (Snellen) b | 0.1 (20/25) | 0.0 (20/20) | 0.5 (20/63) | <0.001 | |
All participants were registered members of a Federally recognized tribe; some refused to reveal exact percentage.
None=logMAR of −0.3 through 0.2, or Snellen visual acuity 20/10 through 20/32; Mild=logMAR of 0.3 through 0.5, or Snellen visual acuity 20/40 through 20/63; and Moderate to Severe=logMAR of 0.6 or worse, or Snellen visual acuity 20/80 or worse. Percentages may not equal 100% due to rounding.
NEI-VFQ-25 scores at Baseline (T1)
The mean subscale and Composite scores at baseline for each group at T1 are presented in Table 2. We found that the Under-corrected refractive error group scored significantly lower at T1 than the Control group in 7 of 12 subscales (General Vision, Distance Activities, Social Functioning, Mental Functioning, Role Difficulties, Dependency, Driving), as well as in the Composite score (all p values ≤ 0.05). We found ceiling effects (median value equals 100) for the subscales of color vision and peripheral vision for the Under-corrected refractive error group.
Table 2.
Baseline vision-related quality of life scores for the Control and Under-corrected Refractive Error (UCRE) groups as measured by the 25-Item National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ-25).
| Time 1 NEI-VFQ 25 Questionnaire |
|||||
|---|---|---|---|---|---|
| Control group (n=76) | UCRE group (n=26) | p value a | |||
| Mean (Std Dev) | Median | Mean (Std Dev) | Median | ||
| Composite score | 84.7 (16.9) | 91.9 | 72.7 (17.3) | 75.3 | <0.001 |
| General Health | 47.4 (24.7) | 50.0 | 51.9 (32.3) | 50.0 | 0.468 |
| General Vision | 68.7 (18.2) | 72.5 | 57.9 (23.4) | 55.0 | 0.012 |
| Ocular Pain | 82.7 (22.4) | 87.5 | 75.0 (22.9) | 75.0 | 0.073 |
| Near Vision Activities | 80.2 (22.4) | 83.3 | 77.1 (21.6) | 79.2 | 0.476 |
| Distance Vision Activities | 86.4 (21.4) | 91.7 | 65.4 (19.9) | 62.5 | <0.001 |
| Social Functioning | 93.4 (14.6) | 100 | 79.3 (21.2) | 87.5 | <0.001 |
| Mental Functioning | 78.8 (25.1) | 87.5 | 61.3 (27.8) | 68.8 | 0.003 |
| Role Difficulties | 82.1 (27.3) | 100 | 69.2 (28.3) | 75.0 | 0.010 |
| Dependency | 90.4 (21.2) | 100 | 72.1 (29.9) | 79.2 | <0.001 |
| Driving | 86.3 (14.9) | 91.7 | 64.0 (19.1) | 58.3 | <0.001 |
| Color Vision | 96.7 (14.3) | 100 | 96.2 (9.2) | 100 | 0.206 |
| Peripheral Vision | 88.8 (21.4) | 100 | 80.0 (27.0) | 100 | 0.111 |
Non-parametric Mann-Whitney U test.
NEI-VFQ-25 scores at Time 2 after providing eyeglasses
Table 3 shows a large improvement in NEI-VFQ-25 subscale and composite scores for the Under-corrected refractive error group when compared to scores at T1 (Table 2). The provision of eyeglasses resulted in subscale and composite scores in the Under-corrected refractive error group becoming more similar to Control group scores. In some cases, such as the General Vision, Driving and Composite scores, the T2 scores for the Under-corrected refractive error group were significantly higher than those found in the Control group.
Table 3.
Time 2 vision-related quality of life scores for the Control and Under-corrected Refractive Error (UCRE) groups as measured by the 25-Item National Eye Institute-Visual Functioning Questionnaire (NEI-VFQ-25).
| Time 2 NEI-VFQ 25 Questionnaire |
|||||
|---|---|---|---|---|---|
| Control group (n=76) | UCRE group (n=26) | p value a | |||
| Mean (Std Dev) | Median | Mean (Std Dev) | Median | ||
| Composite score | 88.8 (13.9) | 93.2 | 91.9 (14.1) | 96.4 | 0.020 |
| General Health | 53.9 (24.2) | 50.0 | 57.7 (26.2) | 50.0 | 0.602 |
| General Vision | 75.4 (18.3) | 80.0 | 91.3 (11.8) | 95.0 | <0.001 |
| Ocular Pain | 88.7 (20.5) | 100 | 89.9 (18.0) | 100 | 0.878 |
| Near Vision Activities | 86.3 (20.7) | 91.7 | 91.7 (17.5) | 100 | 0.062 |
| Distance Vision Activities | 90.4 (15.8) | 100 | 93.9 (15.9) | 100 | 0.078 |
| Social Functioning | 96.2 (12.9) | 100 | 93.8 (17.8) | 100 | 0.719 |
| Mental Functioning | 83.7 (23.2) | 93.8 | 83.2 (22.9) | 93.8 | 0.776 |
| Role Difficulties | 86.7 (25.6) | 100 | 90.8 (19.9) | 100 | 0.699 |
| Dependency | 93.5 (17.2) | 100 | 93.3 (21.5) | 100 | 0.546 |
| Driving | 85.6 (15.3) | 91.7 | 93.5 (10.6) | 100 | 0.013 |
| Color Vision | 98.0 (7.9) | 100 | 100 (0.0) | 100 | 0.182 |
| Peripheral Vision | 91.4 (18.1) | 100 | 90.4 (20.1) | 100 | 0.967 |
Non-parametric Mann-Whitney U test.
Differences in amount of score change after best-correction
Cronbach’s alpha results were as follows: General Vision=0.81; Ocular Pain=0.84; Near Activities= 0.84; Distance activities= 0.78; Vision Specific Social Functioning= 0.72; Mental Health= 0.85; Role difficulties= 0.85; Dependency= 0.90; and Driving= 0.73. Cronbach’s alpha could not be calculated for the Color Vision, Peripheral Vision, and General Health subscales because these included only one item. However, the overall Cronbach alpha statistic was 0.94. These results suggest that the internal consistency of the subscales were acceptable for the study population.30
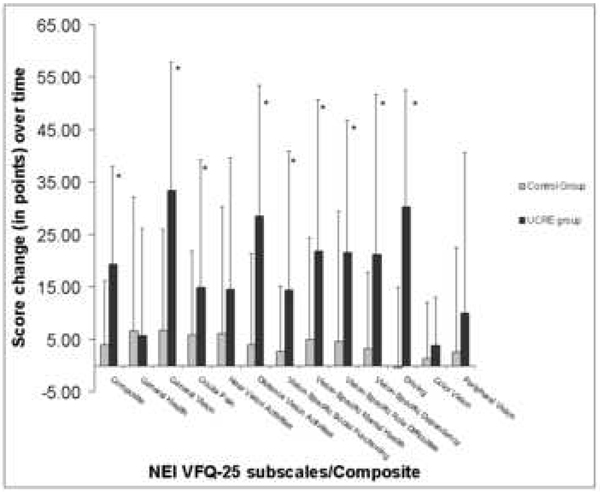
The Figure shows changes in NEI-VFQ-25 subscales and Composite scores between T1 and T2 for the Under-corrected refractive error and Control groups (calculated as Time 2 score minus Time 1 score). We found that the Under-corrected refractive error group had greater improvement in scores, when compared to the Control group, in all subscales except General Health (p=0.88), Near Vision (p=0.14), Color Vision (p=0.25), and Peripheral Vision (p=0.17).
Figure 1.
Amount of mean score change in vision-related quality of life between Time 1 and Time 2 as measured by the 25-Item National Eye Institute Visual Functioning Questionnaire, by Control and Under-corrected Refractive Error (UCRE) groups (*=p<0.05)
In those with self-reported depression scores at both T1 and T2 (n=38), depression scores were significantly improved (T1=4.12: T2=5.12, where higher scores indicate better mood) in the Under-corrected refractive error group after intervention (p=0.011), while not being significantly different (p=0.648) in the Control group (T1=4.64; T2=4.87). The mean improvement in Under-corrected refractive error group scores after eyeglasses represents a full categorical change: for example, from feeling depressed mood “Some of the time” to “A little of the time.”
Multivariate Linear Regression
We performed multivariate linear regression to control for differences between the Control and Under-corrected refractive error groups in age, best-corrected visual acuity in the better-seeing eye, percent American Indian/Alaskan Native, and gender. Using a stepwise backward selection procedure, we found decreasing age (β=−0.26, p=.03), higher best-corrected visual acuity in the better seeing eye (β=15.8, p=.09), and providing eyeglasses (β=10.5, p=.01) to be associated with change in NEI VFQ-25 composite score. When forcing age, best-corrected visual acuity, percent American Indian/Alaskan Native and gender into the model, providing eyeglasses (p=0.01) was still associated with higher NEI-VFQ-25 scores. The mixed-effects models using a random intercept to account for potential autocorrelation within a person within a test location showed similar results: age (p=0.03), best-corrected visual acuity (p=0.09), and eyeglasses (p=0.01). This suggests that eyeglasses are independently associated with higher NEI-VFQ-25 scores even when controlling for differences in age, best-corrected visual acuity, percent American Indian/Alaskan Native, gender, and autocorrelation within a person within a location between the Control and Under-corrected refractive error groups.
We also determined whether self-reported depression at T2 (n=70) was associated with change in NEI VFQ-25 composite score. Depression was not associated with T2 composite score (p=0.90). When forcing T2 depression scores into the model, higher best-corrected visual acuity in the better-seeing eye (p=0.02) and providing eyeglasses (p≤0.001) were still associated with change in NEI VFQ-25 score. The mixed-effects models showed similar results: best-corrected visual acuity (p=0.005), and eyeglasses (p=0.005). This suggests that eyeglasses are independently associated with improvement in vision-related quality of life when controlling for self-reported depression.
DISCUSSION
This study examines the impact of eyeglasses on vision-related quality of life in American Indian/Alaskan Natives. To our knowledge, little published information exists regarding the effect of eyeglasses on vision-related quality of life, and none at all in American Indian/Alaskan Native populations. We found that eyeglasses resulted in a large improvement in vision-related quality of life, even when controlling for various differences between the groups.
Impact of change in visual acuity
Overall, the Under-corrected refractive error group showed an average increase of almost 34 points in perceptions of General Vision, and 19 points in the overall Composite score. These gains in vision-related quality of life are higher than studies examining NEI-VFQ-25 score change after an intervention in eyes with diabetic retinopathy32, macular degeneration18, and macular hole33.
For example, Matza found an average gain in General Vision of 9.1 points and of nearly 4 points in the Composite score in patients with diabetic retinopathy who gained at least 2 lines of vision after treatment with ruboxistaurin32.
Another study inpatients with age-related macular degeneration found an improvement of almost 9 points in the General Vision category and nearly 6 points in overall Composite score 12 months after treatment with ranibizumab 18. Still another study in patients with macular hole found improvement of 14.4 points in the General Vision score and almost 7 points in the Composite score after cataract surgery.33
The gains in the current study were also higher than those found in studies using elderly or nursing home patients. Coleman17 found an average gain in the General Vision subscale of 10.4 points and a gain of almost 7 points in the Composite score after giving eyeglasses to elderly patients, while Owsley noted a 19% increase in General Vision scores after providing eyeglasses to nursing home residents, which is lower than the 58% increase in General Vision found in our study; however, Owsley’s study did not use the NEI-VFQ-25, and direct comparisons are difficult (see Sensitivity of the NEI-VFQ-25 section below).
The provision of eyeglasses in our study showed a somewhat similar improvement in vision-related quality of life to those who received cataract surgery with intraocular lens implants34. That work showed a 14-point (22%) increase in Composite scores and a nearly 18-point (36%) increase in General Vision scores after surgical correction, which is similar to the 26% increase in Composite score and lower than the 58% increase in General Vision found in our study. This suggests that eyeglasses may provide similar or even larger improvements in vision-related quality of life when compared to cataract surgery; however, this result needs to be confirmed in a study including American Indian/Alaskan Native participants with similar baseline characteristics such as age and pretreatment visual acuity (see Sensitivity of NEI-VFQ-25 below).
Differences in vision-related quality of life scores after treatment
Differences in scores at baseline between the Under-corrected refractive error group and the Control group were largely negated at T2 after the Under-corrected refractive error group received their eyeglasses. Interestingly, the Under-corrected refractive error participants had higher scores in some measures of vision-related quality of life than the Control group at T2. This is likely due to the large improvement in visual acuity after receiving eyeglasses in the Under-corrected refractive error group.
Self-reported Depression and the NEI-VFQ-25
We controlled for depression, since it was associated with vision-related quality of life scores in our study as well as other studies. 16, 35–40 When controlling for depression, the increase in vision-related quality of life was still associated with obtaining eyeglasses. We also found that depression scores improved significantly in T2 in the Under-corrected refractive error group after receiving eyeglasses. It is possible that improvement in visual acuity could have a positive effect on self-perceptions of mood16. Further research is needed to determine the effects of depression on visual impairment and NEI-VFQ-25 scores in American Indian/Alaskan Native populations.
Sensitivity of the NEI-VFQ-25
One should remember that change in vision-related quality of life is dependent on both the sensitivity of the instrument used to detect change in vision-related quality of life as well as the level of improvement in visual acuity in the study. We recently found that the NEI-VFQ-25 was sensitive to changes in visual acuity in American Indian/Alaskan Natives26 with results similar to studies in other ethnic groups25, 41–43 Other studies with differences in age or comorbidities such as macular hole, macular degeneration, etc., differences in the change in visual acuity with eyeglasses, or in the sensitivity of the instrument to detect change in vision-related quality of life may have different results from our study.
The NEI-VFQ-25 scoring weights subscales equally when calculating the overall composite score. The Composite score should be interpreted with caution, and researchers should pay close attention to which subscales/domains are generating the significant changes in overall Composite score. Finally, we found a ceiling effect in the Under-corrected refractive error group for color vision and peripheral vision. This ceiling effect would make it difficult to identify an increase in color vision and peripheral vision QOL with the procurement of glasses. However, this ceiling effect is common in many studies using the NEI-VFQ-25, suggesting that these domains are either uncommon or unrelated to overall vision-related QOL.
Limitations
One should remember that the high proportion of visual impairment in this sample does not reflect an accurate proportion of visual impairment in American Indian/Alaskan Natives as a whole. In a previous paper with population-based random selection, our group found 7% of American Indian/Alaskan Natives had visual impairment for distance vision because of uncorrected refractive error.11
Lowered socioeconomic status may be associated with higher rates of uncorrected refractive error 7, 22, 41, 44 because of difficulty affording eyeglasses, and possibly a lack of access to eye care providers. We did not ask participants to report their socio-economic status for this work, but this information may be important when examining the effect of eyeglasses on vision-related quality of life.
We had a difference in demographic characteristics (such as age) between the Under-corrected refractive error and Control groups because of the criteria for recruiting those into the Under-corrected refractive error and Control groups. When controlling for these factors, eyeglasses were still a significant predictor of improvement in vision-related quality of life score.
Acknowledgements
Funding/Support: This research was supported by grant funding from the National Eye Institute (NEI 3 K23 EY0155501-01), the Centers for Disease Control (CDC U48 DP000024-01), the American Glaucoma Society, and the Good Samaritan Foundation at Legacy Health.
Biographies

Steven L. Mansberger, M.D., M.P.H. is an Associate Scientist and Director of Glaucoma Services for Devers Eye Institute in Portland, Oregon. He completed a medical degree from Indiana University, an ophthalmology residency at the University of California, San Diego, a glaucoma fellowship at Devers Eye Institute, and a Masters in Public Health (MPH Biostatistics/Epidemiology) from Oregon Health Science University. He is an Editorial Board member of Journal of Glaucoma and American Journal of Ophthalmology.

Tina McClure is a Senior Research Assistant and Clinical Trials Coordinator at Devers Eye Institute. She completed a Bachelor of Science degree in Psychology from Portland State University. She has a special interest in psychometrics, questionnaire design, and quality of life outcomes, with an emphasis on the effect of depression on vision-related outcomes. She has previously published on quality of life outcomes in American Indian/Alaskan Natives.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None
Author Contributions:
Design of the study: SLM, TMB, TMM
Conduct of the study: SLM, TMB, TMM, KW, CN
Data collection: KW, CN, TMM, SLM, TMB
Data management: TMM, KW, CN, SLM
Data analysis: TMM, SLM, DC
Data interpretation: TMM, SLM, TMB, KW, CN, DC
Manuscript preparation: TMM, SLM
Manuscript review and approval: TMM, KW, CN, TMB, SLM, DC
Conformity with Author Information: This research protocol was approved by the Internal Review Boards (IRB) of Legacy Health System (Portland, Oregon) and the Northwest Portland Area Indian Health Board (Portland, Oregon). Informed consent was obtained from all participants, and the study was conducted in accordance with the tenets of the Declaration of Helsinki for human subjects research.
References
- 1.Pleis JR, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2007. Vital Health Stat 10. 2009 May;(240):1–159. [PubMed] [Google Scholar]
- 2.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999 Jun;106(6):1066–1072. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrov PN, Mukesh BN, McCarty CA, Taylor HR. Five-year incidence of bilateral cause-specific visual impairment in the Melbourne Visual Impairment Project. Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5075–5081. doi: 10.1167/iovs.02-0457. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991 Aug;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 5.Munoz B, West SK, Rodriguez J, et al. Blindness, visual impairment and the problem of uncorrected refractive error in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002 Mar;43(3):608–614. [PubMed] [Google Scholar]
- 6.Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108(2):286–290. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 7.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. Jama. 2006 May 10;295(18):2158–2163. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 8.Chia EM, Mitchell P, Ojaimi E, Rochtchina E, Wang JJ. Assessment of vision-related quality of life in an older population subsample: The Blue Mountains Eye Study. Ophthalmic Epidemiol. 2006 Dec;13(6):371–377. doi: 10.1080/09286580600864794. [DOI] [PubMed] [Google Scholar]
- 9.Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002 Nov;120(11):1544–1550. doi: 10.1001/archopht.120.11.1544. [DOI] [PubMed] [Google Scholar]
- 10.Lee ET, Russell D, Morris T, Warn A, Kingsley R, Ogola G. Visual impairment and eye abnormalities in Oklahoma Indians. Arch Ophthalmol. 2005 Dec;123(12):1699–1704. doi: 10.1001/archopht.123.12.1699. [DOI] [PubMed] [Google Scholar]
- 11.Mansberger SL, Romero FC, Smith NH, et al. Causes of visual impairment and common eye problems in Northwest American Indians and Alaska Natives. Am J Public Health. 2005 May;95(5):881–886. doi: 10.2105/AJPH.2004.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosvenor T. What causes astigmatism? J Am Optom Assoc. 1976 Jul;47(7):926–932. [PubMed] [Google Scholar]
- 13.Luneburg R. Practice among American Indians; study of clinical findings. Opt J. Rev Optom. 1975;112(8):40–46. [Google Scholar]
- 14.Pensyl CD, Harrison RA, Simpson P, Waterbor JW. Distribution of astigmatism among Sioux Indians in South Dakota. J Am Optom Assoc. 1997 Jul;68(7):425–431. [PubMed] [Google Scholar]
- 15.van Rens GH, Arkell SM. Refractive errors and axial length among Alaskan Eskimos. Acta Ophthalmol (Copenh) 1991 Feb;69(1):27–32. doi: 10.1111/j.1755-3768.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 16.Owsley C, McGwin G, Jr, Scilley K, Meek GC, Seker D, Dyer A. Effect of refractive error correction on health-related quality of life and depression in older nursing home residents. Arch Ophthalmol. 2007 Nov;125(11):1471–1477. doi: 10.1001/archopht.125.11.1471. [DOI] [PubMed] [Google Scholar]
- 17.Coleman AL, Yu F, Keeler E, Mangione CM. Treatment of uncorrected refractive error improves vision-specific quality of life. J Am Geriatr Soc. 2006 Jun;54(6):883–890. doi: 10.1111/j.1532-5415.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007 Nov;125(11):1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 19.Owsley C, McGwin G., Jr Depression and the 25-item National Eye Institute Visual Function Questionnaire in older adults. Ophthalmology. 2004 Dec;111(12):2259–2264. doi: 10.1016/j.ophtha.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Correctable visual impairment among persons with diabetes--United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2006 Nov 3;55(43):1169–1172. [PubMed] [Google Scholar]
- 21.Maki J, Kusakul S, Morley K, et al. The effect of glasses on visual function following cataract surgery in a cataract camp. Br J Ophthalmol. 2008 Jul;92(7):883–887. doi: 10.1136/bjo.2007.132423. [DOI] [PubMed] [Google Scholar]
- 22.Shepard S. On the Rez and beyond. The New Physician. 2004 April;53(3):1–4. [Google Scholar]
- 23.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003 Oct;136(4):655–661. doi: 10.1016/s0002-9394(03)00388-x. [DOI] [PubMed] [Google Scholar]
- 24.Davis LJ, Schechtman KB, Wilson BS, et al. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci. 2006 Feb;47(2):489–500. doi: 10.1167/iovs.05-0381. [DOI] [PubMed] [Google Scholar]
- 25.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001 Jul;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 26.McClure TM, Choi D, Becker T, Cioffi GA, Mansberger SL. The effect of visual impairment on vision-related quality of life in American Indian/Alaska Natives. Ophthalmic Epidemiol. 2009 Mar–Apr;16(2):128–135. doi: 10.1080/09286580902745428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: Centers for Disease Control and Prevention (CDC), U. Department of Health and Human Services; 2001. 2001. [Google Scholar]
- 28.Paz SH, Globe DR, Wu J, Azen SP, Varma R. Relationship between self-reported depression and self-reported visual function in Latinos. Arch Ophthalmol. 2003 Jul;121(7):1021–1027. doi: 10.1001/archopht.121.7.1021. [DOI] [PubMed] [Google Scholar]
- 29.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982 Jul;94(1):91–96. [PubMed] [Google Scholar]
- 30.Hays R, Anderson R, Revicki D. Quality of Life Assessment in Clinical Trials: Methods and Practice. Oxford University Press; 1998. Assessing reliability and validity of measurement in clinical trials; pp. 169–182. [Google Scholar]
- 31.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 32.Matza LS, Rousculp MD, Malley K, Boye KS, Oglesby A. The longitudinal link between visual acuity and health-related quality of life in patients with diabetic retinopathy. Health Qual Life Outcomes. 2008;6:95. doi: 10.1186/1477-7525-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda S, Okamoto F, Yuasa M, et al. Vision-related quality of life and visual function in patients undergoing vitrectomy, gas tamponade and cataract surgery for macular hole. Br J Ophthalmol. 2009 Dec;93(12):1595–1599. doi: 10.1136/bjo.2008.155440. [DOI] [PubMed] [Google Scholar]
- 34.Lin IC, Wang IJ, Lei MS, Lin LL, Hu FR. Improvements in vision-related quality of life with AcrySof IQ SN60WF aspherical intraocular lenses. J Cataract Refract Surg. 2008 Aug;34(8):1312–1317. doi: 10.1016/j.jcrs.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993 Apr;41(4):401–407. doi: 10.1111/j.1532-5415.1993.tb06948.x. [DOI] [PubMed] [Google Scholar]
- 36.Hayman KJ, Kerse NM, La Grow SJ, Wouldes T, Robertson MC, Campbell AJ. Depression in older people: visual impairment and subjective ratings of health. Optom Vis Sci. 2007 Nov;84(11):1024–1030. doi: 10.1097/OPX.0b013e318157a6b1. [DOI] [PubMed] [Google Scholar]
- 37.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol. 2008 Sep;146(3):404–409. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Jampel HD, Frick KD, Janz NK, et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007 Aug;144(2):238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006 Dec 21;4(1):97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettit T, Livingston G, Manela M, Kitchen G, Katona C, Bowling A. Validation and normative data of health status measures in older people: the Islington study. Int J Geriatr Psychiatry. 2001 Nov;16(11):1061–1070. doi: 10.1002/gps.479. [DOI] [PubMed] [Google Scholar]
- 41.Broman AT, Munoz B, Rodriguez J, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(11):3393–3398. [PubMed] [Google Scholar]
- 42.Globe DR, Wu J, Azen SP, Varma R. The impact of visual impairment on self-reported visual functioning in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004 Jun;111(6):1141–1149. doi: 10.1016/j.ophtha.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998 Feb;116(2):227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 44.Lamoureux EL, Hooper CY, Lim L, et al. Impact of cataract surgery on quality of life in patients with early age-related macular degeneration. Optom Vis Sci. 2007 Aug;84(8):683–688. doi: 10.1097/OPX.0b013e31812f755f. [DOI] [PubMed] [Google Scholar]