Abstract
The 5-HT3 receptor is rapidly potentiated by ethanol and mediates fast excitatory 5-HT transmission that modulates dopamine release in the reward circuitry. The 5-HT transporter regulates synaptic 5-HT availability. Functional polymorphisms in genes encoding the transporter and receptor may therefore influence addiction vulnerability. In this study, 360 treatment-seeking African American male patients with single and comorbid DSM-IV lifetime diagnoses of alcohol, cocaine and heroin dependence and 187 African American male controls were genotyped for the triallelic 5-HTTLPR functional polymorphism in the 5-HT transporter gene (SLC6A4) and 16 haplotype-tagging SNPs across HTR3B (including the functional rs1176744 Tyr129Ser) and HTR3A, genes encoding 5-HT3 receptors. The HTR3B rs1176744 gain of function Ser129 allele predicted alcohol dependence (p = 0.002) and low 5-HTTLPR activity predicted cocaine/heroin dependence (p = 0.01). Both the HTR3B Ser129 allele (p = 0.014, OR = 1.7 [1.1–2.6]) and low 5-HTTLPR activity (p = 0.011, OR = 2.5 [1.3–4.6]) were more common in men with alcohol + drug dependence compared with controls. Moreover, the HTR3B Ser129 allele and low 5-HTTLPR activity had an additive (but not an interactive) effect on alcohol + drug dependence (OR = 6.0 [2.1–16.6]) that accounted for 13% of the variance. One possible explanation of our findings is that increased synaptic 5-HT coupled with increased 5-HT3 receptor responsiveness may result in enhanced dopamine transmission in the reward pathway, a predictor of increased risk for addiction. Our results may have pharmacogenetic implications for 5-HT3 therapeutic antagonists such as ondansetron.
Keywords: 5-HTTLPR, HTR3B, rs1176744, alcoholism, cocaine, heroin
INTRODUCTION
Variation in serotonin (5-HT) transmission in the central nervous system (CNS) has been implicated in alcohol and drug dependence. The 5-HT transporter regulates the availability of 5-HT in the synaptic cleft through re-uptake and is the target of selective serotonin re-uptake inhibitors. A functional promoter polymorphism, 5-HTTLPR, in the 5-HT transporter gene (SLC6A4) has been associated with changes in neuronal circuitry implicated in negative affect.1 Results from studies showing associations between 5-HTTLPR and alcohol, heroin or cocaine dependence are mixed.2–6
Unlike the other twelve 5-HT receptors that are G protein coupled receptors7, 5-HT3 receptors are pentameric, ligand-gated ion channels that, when bound to 5-HT, produce fast activation of neurons, including in the mesolimbic reward circuitry.8,9 5-HT3 receptors share structural and functional homology with GABAA, nicotinic acetylcholine and glycine receptors. The members of this superfamily are targets for the acute and chronic effects of ethanol.10 Moreover, 5-HT3 receptors are the target of ondansetron which has been shown to diminish alcohol craving.8 The HTR3A and HTR3B genes both lie in a 90 Kb region on chromosome 11q23.1, and encode the 5-HT3A and 5-HT3B receptor subunits respectively.
5-HT3 receptors can be homomeric (all 5-HT3A subunits) or heteromeric (5-HT3A and 5-HT3B subunits). Although homomeric receptors are more widely distributed in the CNS than heteromeric receptors, the latter are expressed in the amygdala, the caudate and the hippocampus, locations implicated in addiction.11,12 A mis-sense polymorphism (Tyr129Ser, rs1176744) in HTR3B has been shown to alter the response of the heteromeric receptor to 5-HT. The Ser129 substitution results in an increased maximum response to 5-HT, decreased desensitization and deactivation kinetics (10–20 fold slower, respectively) and a 7-fold increase in mean channel open time.13,14 Since 5-HT3 receptors may play a role in reward by modulating DA release in the mesolimbic pathway15, we hypothesized that this gain of function receptor might have implications for alcohol dependence (AD). Moreover, we hypothesized that there might be an additive effect between the HTR3B Ser129 allele and low 5-HTTLPR activity that increases synaptic 5-HT and which has been associated with AD.2
We conducted our study in treatment seeking African American men who had both single and comorbid lifetime DSM-IV diagnoses of alcohol, heroin and cocaine dependence, and African American male controls. The primary association analyses for AD, with and without comorbid drug dependence, were conducted with the functional HTR3B SNP, rs1176744 Tyr129Ser, and the functional triallelic 5-HTTLPR polymorphism. In secondary analyses we investigated whether further information could be derived from haplotype associations by genotyping an additional 15 haplotype tagging SNPs across the HTR3B and HTR3A genes.
PARTICIPANTS AND METHODS
Originally, 635 African-American substance dependent men were recruited: 590 from the Substance Abuse Treatment Program (SATP) at the Department of Veteran Affairs New Jersey Healthcare System (VANJHCS), East Orange Campus and 45 men originally screened as controls (see below) who were found to have a diagnosis of lifetime substance dependence. Most of the participants recruited from the SATP were inpatients on a 21 day residential treatment ward, however some were recruited from the outpatient clinic or from the methadone clinic. Criteria for inclusion in the study were that participants were ≥ 18 years of age, met DSM-IV criteria for substance dependence, self-identified as African American and had been abstinent for at least two weeks. Exclusion criteria included mental retardation, dementia and acute psychosis. Patients were interviewed by a psychiatrist (A.R.) with the substance abuse section of the Structured Clinical Interview for DSM-IV (SCID)16 to determine lifetime substance dependence diagnoses. The mean (SD) age of the patients was 45.6 (7.8) years.
Three hundred and twenty African American male controls were recruited from churches and a blood bank in Newark, NJ, (46%) and from among insulin-dependent diabetic outpatients seen at an ophthalmology clinic (54%) at the University of Medicine and Dentistry: New Jersey Medical School (UMDNJ, Newark, NJ). All controls had a semi-structured psychiatric interview and were without a lifetime history of any substance abuse or dependence or major Axis 1 psychiatric disorder. Their mean (SD) age was 34.0 (10.1) years.
The study was approved by the Institutional Review Boards of the VANJHCS and UMDNJ. After a full description of the study was provided, all participants gave written informed consent.
Genotyping
DNA was available for 547 participants (360 patients and 187 controls). Missing DNA was random and showed no selection bias.
HTR3B and HTR3A
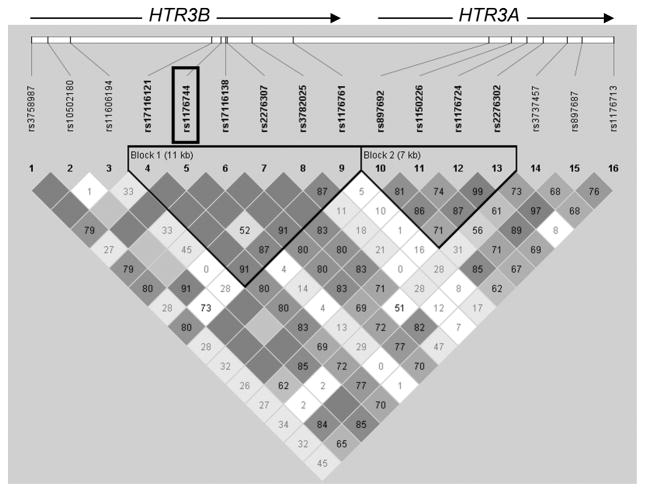
Sixteen haplotype tagging SNPs were identified using a previously described design pipeline17 to maximize haplotype capture for the region of chromosome 11 extending from 5kb upstream of HTR3B to 1kb downstream of HTR3A (NCBI Human Build 35.1) (Figure 1).17 Nine HTR3B SNPs and seven HTR3A SNPs were genotyped using the Illumina GoldenGate platform17 The SNPs listed in the direction of transcription (from left to right within Figure 1) with their minor allele frequencies in non-alcoholics are:
FIGURE 1. Haplotype Block Structure across HTR3B and HTR3A Showing the 16 Genotyped SNPs.
The numbers in the squares refer to pairwise linkage disequilibrium (LD) measured as D′. Haplotype blocks were defined using a setting of average pairwise D′ within-block of ≥ 0.80. The functional SNP rs1176744, Tyr129Ser, is outlined.
HTR3B
rs3758987 (0.39); rs10502180 (0.04); rs11606194 (0.02); rs17116121 (0.08); rs1176744 (0.39) Tyr129Ser; rs17116138 (0.08) Val183Ile; rs2276307 (0.10); rs3782025 (0.44); rs1176761 (0.17);
HTR3A
rs897692 (0.39), rs1150226 (0.32), rs1176724 (0.34), rs2276302 (0.48), rs3737457 (0.15), rs897687 (0.33) and rs1176713 (0.30).
Triallelic 5-HTTLPR polymorphism
Genotyping was performed in two stages using size discrimination for the S (103bp) and L (146bp) alleles and for the rs25531 (LA (146bp) and LG (61bp)) alleles. This assay is also capable of detecting the SG and the super long L alleles but they were not observed in these African Americans. The genotyping method is fully described in supplementary information. Genotyping accuracy was determined empirically by duplicate genotyping of 25% of the samples selected randomly. The error rate was <0.005, and the completion rate was >0.95.
Tri-allelic genotyping revealed the following 5-HTTLPR allele frequencies: S = 0.25, LA = 0.51, LG = 0.24. Genotypes were grouped as low activity (SS, S LG, LG LG) (0.23) medium activity (SLA, LALG) (0.51) and high activity (LALA) (0.26).
Assessment of population stratification using ancestry informative markers (AIMS)
A total of 186 AIMs17 were genotyped in the study sample and in the HGDP-CEPH Human Genome Diversity Cell Line Panel (1051 individuals from 51 worldwide populations) (http://www.cephb.fr/HGDP-CEPH-Panel). PHASE Structure 2.2 (http://pritch.bsd.uchicago.edu/software.html) was run simultaneously using the AIMS data from our sample and the 51 CEPH populations to identify population substructure and compute individual ethnic factor scores. This ancestry assessment showed that the European factor score was on average 0.09 (median value = 0.04). Both a Mid East factor and an Asian factor had an average score of 0.06 (median 0.04).
Statistical Analyses
Logistic regression analyses were undertaken using JMP 7 software. Backward stepwise regression was performed with variables (age, ethnic factor scores) being eliminated from the model in an iterative process. Ethnic factor scores were included as covariates in the final model if they had significant effects (the European factor had a significant effect on heroin addiction in most analyses but no effect on cocaine or alcohol dependence). Logistic regression models with nominal variables yielded likelihood ratio (L-R)χ2 results.
Although the mean (SD) age of the patients (45.6 (7.8) yrs) was higher than the controls (34.0 (10.1) yrs), F = 211, p< 0.0001, there were no differences in age between carriers of the three HTR3B genotypes (p = 0.7) or the three 5-HTTLPR genotype groups (p = 0.9).
Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE.18 Haploview version 2.04 Software (Whitehead Institute for Biomedical Research, USA) was used to produce linkage disequilibrium (LD) matrices (Figure 1). Since rare and uncommon haplotypes are subject to estimation errors because of increased sampling variance, all analyses were conducted with haplotypes ≥ 5% frequency.
RESULTS
Primary Analyses
HTR3B SNP rs1176744 Tyr129Ser
The rs1176744 129Ser allele was more common in the total group of alcoholics compared with controls without addiction: 0.47 vs 0.38, χ2 = 6.3, 1df, p = 0.012. As can be seen from Table 1, the strongest signal derived from the patients with only AD in whom the 129Ser allele frequency was 0.51 (χ2 = 6.4, 1df, p = 0.012). There was no association between rs1176744 and cocaine or heroin dependence (Table 1).
TABLE 1.
Influence of the Functional HTR3B Rs1176744 Tyr129Ser on Addiction Diagnoses
| Diagnosis | N | Genotype Frequency | Genotype | Tyr/Tyr vs Tyr/Ser + Ser/Ser | Ser Allele Freq | Allele | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyr/Tyr | Tyr/Ser | Ser/Ser | χ2 | P valuea | χ2 | P valueb | χ2 | P valueb | |||
| All Patients with Addiction | 357 | 0.29 | 0.53 | 0.18 | 5.1 | 0.077 | 5.0 | 0.025 | 0.45 | 4.3 | 0.037 |
| (a) Heroin/Cocaine Dependence only | 131 | 0.34 | 0.50 | 0.16 | 0.6 | 0.742 | 0.6 | 0.44 | 0.41 | 0.6 | 0.453 |
| (b) All Alcoholics | 226 | 0.26 | 0.55 | 0.19 | 7.7 | 0.021 | 7.5 | 0.006 | 0.47 | 6.3 | 0.012 |
| -- Alcohol Dependence only | 62 | 0.21 | 0.56 | 0.23 | 7.2 | 0.028 | 6.6 | 0.010 | 0.51 | 6.4 | 0.012 |
| -- Alcohol + Drug Dependence | 164 | 0.27 | 0.55 | 0.18 | 4.7 | 0.094 | 4.4 | 0.035 | 0.45 | 3.7 | 0.054 |
| No Addiction | 177 | 0.38 | 0.48 | 0.14 | 0.38 | ||||||
2df,
1df.
All groups were compared with controls without addiction. As shown in the table, the total group of patients was divided into subsets (a) and (b).
The Ser129 allele exerted a dominant effect: the frequencies of the Tyr129/Ser129 and the Ser129/Ser129 genotypes were both increased in alcoholics, and indeed as shown in Table 1 the dominant model produced the strongest results. For this reason and also for simplicity in analyses with 5-HTTLPR, two genotype groups were included in analyses: (a) Tyr129/Tyr129 homozygotes and (b) Ser129 allele carriers.
5-HTTLPR
Compared with non-addicted controls, low 5-HTTLPR activity was significantly more abundant in the total group of patients with addiction (OR = 2.49, 95% CI: 1.45 – 4.26) (Table 2). This signal derived from the patients with AD and comorbid drug dependence (OR = 2.45, 95% CI: 1.30 – 4.62) and the non-alcoholic patients with cocaine and/or heroin dependence (OR = 2.81, 95% CI: 1.43 – 5.48) but (in contrast to HTR3B Ser129) not from the patients with only AD (Table 2).
TABLE 2.
Independent Effects of 5-HTTLPR Activity and HTR3B Rs1176744 Genotypes on Addiction Diagnoses
| Diagnosis | N | 5-HTTLPR Frequencies | A. Effect of 5-HTTLPR Activity | A. Effect of HTR3B | A. Whole Model Test | B. 5-HTTLPR HTR3B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Med | High | Low/Med/High | Rs1176744 Tyr/Tyr vs Tyr/Ser+Ser/Ser | Low-Tyr/Ser+Ser/Ser Vs High-Tyr/Tyr | |||||||||||
| χ2 | P value | 2df | χ2 | P value | 1df | χ2 | P value | 3df | χ2 | P value | 1df | |||||
| All Patients with Addiction | 345 | 0.27 | 0.51 | 0.22 | 10.8 | 0.004 | 5.8 | 0.016 | 17.1 | 0.0007 | 13.0 | 0.0003 | ||||
| OR = 2.49 [1.45 – 4.26] | OR = 1.48 [1.01 – 2.16] | OR = 4.27 [1.90 – 9.57] | ||||||||||||||
| (a) Heroin/Cocaine Dependence only | 120 | 0.30 | 0.48 | 0.22 | 9.1 | 0.011 | 0.6 | 0.43 | 9.9 | 0.020 | 4.9 | 0.028 | ||||
| OR = 2.81 [1.43 – 5.48] | OR = 1.16 [0.68 – 1.86] | OR = 2.81 [1.10 – 7.16] | ||||||||||||||
| (b) All Alcoholics | 225 | 0.25 | 0.53 | 0.22 | 8.5 | 0.014 | 9.2 | 0.002 | 17.7 | 0.0005 | 15.6 | < 0.0001 | ||||
| OR = 2.24 [1.25 – 4.02] | OR = 1.73 [1.13 – 2.64] | OR = 6.1 [2.39 – 15.67] | ||||||||||||||
| -- Alcohol Dependence only | 61 | 0.25 | 0.49 | 0.26 | 2.7 | 0.26 | 7.8 | 0.005 | 10.3 | 0.0174 | 8.0 | 0.005 | ||||
| OR = 1.91 [0.81 – 4.39] | OR = 2.30 [1.16 – 4.55] | OR = 6.57 [1.57 – 25.36] | ||||||||||||||
| -- Alcohol + Drug Dependence | 164 | 0.25 | 0.54 | 0.21 | 9.1 | 0.011 | 6.0 | 0.0139 | 15.1 | 0.0017 | 12.9 | 0.0003 | ||||
| OR = 2.45 [1.30 – 4.62] | OR = 1.66 [1.05 – 2.63] | OR = 5.95 [2.12 – 16.56] | ||||||||||||||
| No Addiction | 172 | 0.17 | 0.49 | 0.34 | ||||||||||||
χ2 results are for effect likelihood ratio (L-R) tests. All groups were compared with controls without addiction. As shown in the table, the total group of patients was divided into subsets (a) and (b).
The table shows the results from logistic regression models:
A. The whole model test and the independent effects of 5-HTTLPR and HTR3B rs1176744 within the whole model. Odds ratios (OR) with [95% confidence intervals] are provided for low vs high 5-HTTLPR activity and for HTR3B Tyr/Tyr vs Tyr/Ser+Ser/Ser genotypes.
B. A model including only the following individuals: 129 Ser carriers + low 5-HTTLPR activity (N = 43); Tyr129Tyr genotype + high 5-HTTLPR activity (N = 31).
Additive Effects of 5-HTTLPR + HTR3B rs1176744 Tyr129Ser
Low, medium and high 5-HTTLPR activity and the HTR3B Tyr129/Tyr129 and Ser129 carrier genotypes were included together as independent variables in logistic regression models with diagnosis as the dependent variable. Table 2 shows that the results for the whole model tests for each diagnosis were all significant (p = 0.01 – 0.0007). When the total group of patients with addiction was divided into three component parts: (a) AD only; (b) alcohol + drug dependence; (c) heroin and/or cocaine dependence only, it became apparent that the strongest signal derived from the alcoholics with comorbid drug dependence (Table 2).
Figure 2 illustrates the results for alcohol and comorbid drug dependence. On either the HTR3B Tyr129/Tyr129 (normal activity) background or the Ser129 carrier genotypes (enhanced activity) background, low 5-HTTLPR activity was associated with an increased liability to alcohol + drug dependence compared with high 5-HTTLPR activity: OR = 2.45, 95% CI: 1.30 – 4.62. On either the low, medium or high 5-HTTLPR activity background, the enhanced activity HTR3B genotypes were associated with increased liability to alcohol + drug dependence compared with the normal activity genotype: OR = 1.66, 95% CI: 1.05 – 2.63 (Table 2).
FIGURE 2. Low 5-HTTLPR Activity and the HTR3B Rs1176744 Ser129 Gain of Function Allele predict Alcohol and Drug Dependence.
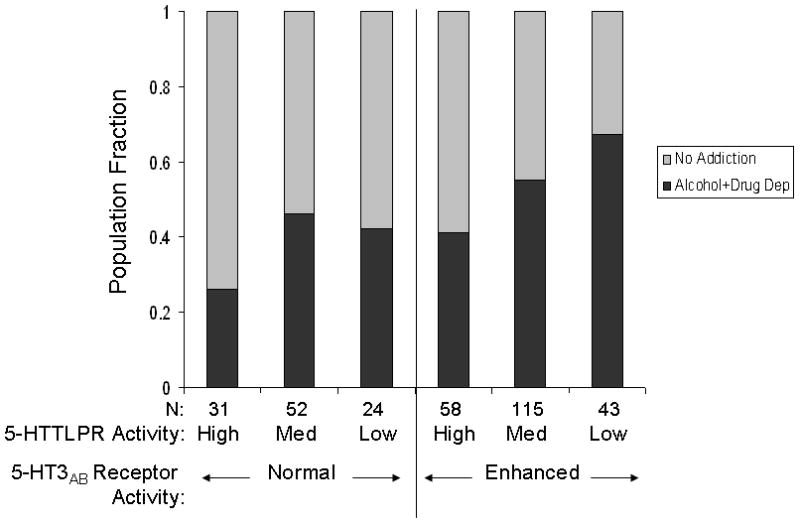
5-HT3AB: ‘normal’ activity is predicted by the rs1176744 Tyr129/Tyr129 genotype, ‘enhanced’ activity is predicted by the Ser129 allele.
When looking at the joint effects of 5-HTTLPR and HTR3B Tyr129Ser (Table 2, Figure 2) it can be seen that, comparing the two extremes, individuals with low 5-HTTLPR activity coupled with the gain of function HTR3B Ser129 allele (N = 43) were significantly more likely to have alcohol + drug dependence than individuals with high 5-HTTLPR activity and the normal function HTR3B Tyr129/Tyr129 genotype (N = 31): OR = 5.95, 95% CI: 2.12 – 16.56. Within these 74 individuals, the additive effect of variation in these two genes accounted for 13% of the variance in alcohol and drug dependence. There was no statistical gene-gene interaction (p = 0.49). Thus 5-HTTLPR and HTR3B Tyr129Ser appear to have additive effects on the risk for alcohol + drug dependence.
Secondary Analyses
Secondary analyses were undertaken to determine whether HTR3B and HTR3A haplotype analyses could confer further information about genetic risk for alcohol and drug dependence.
HTR3B and HTR3A haplotype blocks
HTR3B and HTR3A are closely adjacent genes but are in different haplotype blocks although there is evidence of modest long distance linkage disequilibrium (LD) across the two genes (Figure 1).
HTR3B haplotype analyses
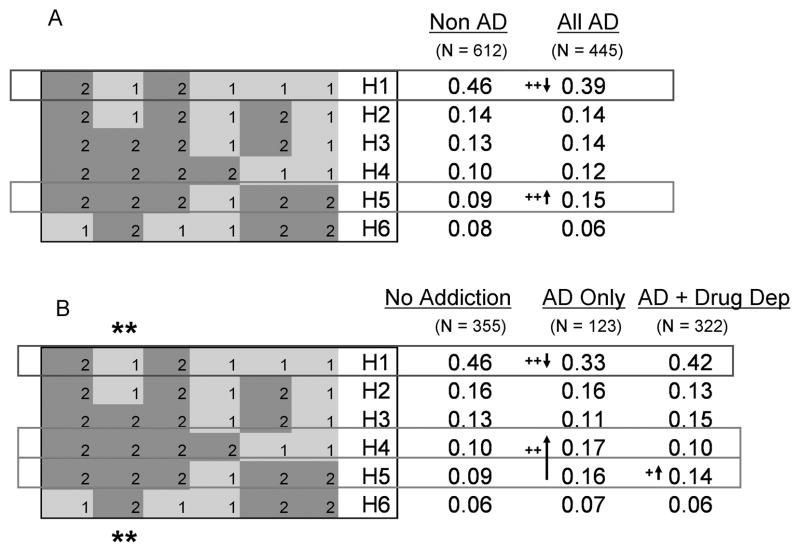
Six SNPs, listed from left to right in the direction of transcription: rs17116121, rs1176744, rs17116138, rs2276307, rs3782025 and rs1176761 were included in one haplotype block (Figure 1). Within this block there were 6 haplotypes with ≥ 0.05 frequency that accounted for 98% of the haplotype diversity. These haplotypes (H1 – H6), together with their frequencies, are shown in Figure 3A.
FIGURE 3. HTR3B Haplotype Association with Alcohol Dependenc.
++P < 0.05, +P < 0.1.
A: in the total group of alcoholics (All AD), the frequency of haplotype H5 is increased and H1 is decreased. B: the signal derives from alcoholics without drug dependence: the frequency of haplotypes H4 and H5 are increased and H1 is decreased compared with controls without addiction.
The numbers 1, 2 within the blocks represent allele 1 and allele 2. For each SNP, alleles 1 and 2 are located on opposite DNA strands. The haplotypes within the HTR3B haplotype block derive from the following 6 SNPs [listed from left to right in the direction of transcription with allele1, allele 2 bases in parentheses]: rs17116121 (A,G); rs1176744 (T,G); rs17116138 (A,G); rs2276307 (A,G); rs3782025 (T,C); rs1176761 (A,T). Since these are haplotype analyses the N’s are the number of chromosomes. The 6 haplotypes, H1 – H6, account for 0.98 of the haplotype diversity in the total sample. The analyses were conducted on just these 6 haplotypes and therefore the total frequencies are 1.00 for each group.
** identifies the functional SNP rs1176744: Tyr129 (allele1), Ser129 (allele 2).
In order to increase the power of analyses with the lower frequency haplotypes we designated non-alcoholics (‘non-AD’) to include controls without addiction and non-alcoholic patients with cocaine/heroin addiction. The justification for this grouping was that the frequencies of the H1, H3, H4 and H5 haplotypes in non-AD were identical to the frequencies in controls without addiction, and the frequencies of haplotypes H2 and H6 were very similar in both groups (Figure 3A, 3B).
There was a haplotype association with AD: global p value = 0.023, 5 df, L-R χ2 = 13.1. As can be seen from Figure 3A, the most abundant haplotype H1 was more common in non-AD (0.46) than in alcoholics (0.39) p = 0.044, 1 df, L-R χ2 = 4.1. In contrast, haplotype H5 was more common in alcoholics (0.15) compared with non-AD (0.09): p = 0.006, 1 df, L-R χ2 = 7.7; H1 vs H5: p = 0.001, 1 df, L-R χ2 = 10.7.
Secondary analyses showed that the signal for the haplotype association largely derived from the patients with AD only (Figure 3B). Haplotypes H4 and H5 were more abundant in patients with AD only compared with controls without addiction (0.17 vs 0.10; 0.16 vs 0.09, respectively; H4+H5 vs rest: p = 0.003, 1 df, L-R χ2 = 8.8). In contrast, there was only a weak signal from the alcoholics with drug dependence when compared with controls without addiction (H5 vs rest: p = 0.086, 1df, L-R χ2 = 2.9).
It can be deduced from Figure 3B that haplotypes H4 and H5 together differ from haplotype H1 by only SNP rs 1176744. Our results therefore suggest that the haplotype association was driven by rs176744 Tyr129Ser.
HTR3A haplotype blocks
There was one haplotype block extending from rs897692 to rs2276302 that included 8 haplotypes with ≥ 0.01 frequency that accounted for 0.97 of the haplotype diversity. Of these, four haplotypes occurred with ≥ 0.05 frequency: 2211 (0.41), 1122 (0.26), 2212 (0.14) and 1222 (0.06). There was no HTR3A haplotype association with alcohol or drug dependence.
HTR3B and HTR3A individual SNP analyses
Other than HTR3B rs1176744, none of the SNPs showed a significant association with AD (p = 0.11 to 0.99).
Comparison with earlier HTR3B haplotype analyses
In an earlier study19 we genotyped exactly the same HTR3B SNPs as in our current study and reported a 4-SNP haplotype association with AD plus comorbid antisocial personality disorder (ASPD) in Finnish Caucasian men. The original 4-SNP haplotype analyses did not include rs1176744. We re-did the haplotype analyses and found that the 6-SNP haplotype block (including rs1176744) that was present in the African Americans was also present in the Finns (Supplementary Figure S1). Five of the 6 African American haplotypes (not H5) were also present in the Finns but at different frequencies: H1: 0.29, H2: 0.41, H3: 0.03, H4: 0.17, H6: 0.10. In the Finns there was a haplotype association with AD comorbid with ASPD: global p value = 0.0278, 4 df, L-R χ2 = 10.9. Haplotype H4 was more abundant in individuals with AD + ASPD compared with non-alcoholics: p = 0.019, 1 df, L-R χ2 = 5.5 (Supplementary Figure S2).
DISCUSSION
Our study has shown that the predominant effect of the HTR3B rs1176744 Ser129 allele was on risk for AD and there was no effect of this allele on heroin and cocaine dependence. In contrast, low 5-HTTLPR activity had a significant effect in the group of non-alcoholic patients with heroin and/or cocaine dependence but not in the patients with AD only. 5-HT3 receptors are rapidly potentiated by ethanol at concentrations that are within the intoxicating range in humans.20 Ethanol increases the rate of channel activation while decreasing the rates of deactivation and desensitization.21,22 The effects of ethanol on 5-HT3 receptors are similar to the effects of the HTR3B Ser129 allele on heteromeric 5-HT3AB receptor function.13,14 Although the effect of ethanol is greater in homomeric than heteromeric receptors23,24 the potentiating effects of ethanol on the gain of function 5-HT3AB variant receptor may have relevance to addiction since heteromeric receptors are found in the reward circuitry.11,12
Furthermore, this study has shown that low 5-HTTLPR activity and the HTR3B Ser129 allele that respectively increase synaptic 5-HT and 5-HT3AB receptor responsiveness to 5-HT, have significant, independent effects on the risk for alcohol and drug dependence and moreover have an additive effect on risk. Individuals with low 5-HTTLPR activity coupled with the gain of function HTR3B Ser129 allele were over 2 ½ times more likely to have a diagnosis of alcohol and drug dependence compared with individuals with both high 5-HTTLPR activity and the HTR3B Tyr129/Tyr129 genotype. Finally, in-depth HTR3B and HTR3A haplotype and SNP analyses showed that only the HTR3B Tyr129Ser polymorphism influenced alcohol and drug dependence. This study illustrates the logic of studying genetic variation that might be expected to result in additive or interactive biological effects.
When we re-analyzed data from an earlier study19 we showed that in both African American and Finnish men, haplotype H4 was more abundant in individuals with AD. However in the Finns HTR3B variation was associated with AD comorbid with ASPD. Moreover, the earlier study showed that in the Finns, rs3782025 and not rs1176744 was associated with AD comorbid with ASPD, nevertheless these two SNPs are in strong LD with each other. Finally, the earlier study found no HTR3B SNP or haplotype association with AD in a community sample of Plains American Indian men and women and we found no association with the 6-SNP haplotypes (data not shown). Clearly the association of HRT3B genetic variation with AD is complex and may vary with ethnicity and alcoholism phenotype.
In a study in Japanese participants, Yamada et al showed that two HTR3B Tyr129 haplotypes and Tyr129/Tyr129 homozygotes were significantly associated with major depression (MD) in women only.25 Therefore our and the Yamada et al study together imply that the HTR3B Ser129 allele that increases the effectiveness of 5-HT transmission appears to be both protective against depression and also a risk factor for AD. This result might seem surprising because of the known co-occurrence of MD and alcoholism in epidemiological samples. The explanation may lie in sex differences: the Yamada et al study found a significant association only in women, however our study was conducted only in men and a population based twin study has shown that factors underlying MD in women and alcoholism in men are different. 26
Presynaptic 5-HT3 receptors modulate the synaptic release of various neurotransmitters including GABA27 whereas postsynaptic receptors are responsible for the fast excitatory response to 5-HT. 5-HT3receptors play a significant role in regulating the activity of VTA dopamine neurons.8 5-HT3B subunits can only assemble as 5-HT3AB receptors11 and these heteromeric receptors are expressed in several regions of the human brain including the hippocampus, the amygdala and the caudate11,12,28 and have been detected in the CNS of rodents.29 One possible explanation for our finding is that increased synaptic 5-HT coupled with increased 5-HT3AB receptor responsiveness to 5-HT might result in enhanced dopamine transmission in the reward pathway that is associated with a greater risk for addiction. Moreover, 5-HT is a less potent activator of heteromeric receptors than homomeric receptors.11,30 Therefore genetic variants that increase the responsiveness of heteromeric receptors in the reward pathway may make an impact on addiction vulnerability.
Studies in humans have shown that the 5-HT3 receptor antagonist ondansetron decreases alcohol cue-induced activation of the ventral striatum31 and influences drinking behavior.32–34 The results of our study may have pharmacogenetic implications for ondansetron. It is conceivable that alcoholics with and without the gain of function heteromeric receptor may respond differently to this therapeutic agent.
The rs1176744 Ser129 variant is common in all ethnic groups. The frequencies in non-addicted controls in the current and earlier study19 are: African Americans: 0.38, Plains American Indians: 0.38, Finnish Caucasians: 0.29. Curiously, the more abundant Tyr129 allele is only found in humans, ranging in frequency in HapMap populations from 0.57 in Africans to 0.83 in Chinese. From Figure 3 it can be seen that the Ser129 allele is found on four different haplotype backgrounds (H3-H6) whereas the Tyr129 allele is found on two almost identical haplotypes (H1 and H2) suggesting that the Tyr129 allele is a relatively recent development. The fact that the ancestral Ser129 allele is at a lower frequency suggests that selective pressure unique to humans maintains the Tyr129 allele in all ethnic groups. 5-HT3AB receptors are abundant in the gut and are implicated in the neurocircuitry associated with emesis.12 It is conceivable that the Tyr129 allele has a selective advantage in this context, perhaps by inducing protective vomiting in response to toxic substances.35
There are some limitations to the present study. Data on other Axis 1 diagnoses were not available for the patients. Since it is known that there is high comorbidity between substance dependence and other psychiatric disorders, particularly ASPD and major depression, and the controls were free of all Axis 1 diagnoses, it is possible that the signals for association found in our study derived from hidden comorbidity. The controls were recruited from two sources and although their mean age was appreciably lower than that of patients they had largely passed through the peak risk age for onset of addictive disorders.
In conclusion, our study has shown that functional variants in the genes encoding the 5-HT3AB receptor and the 5-HT transporter respectively had independent effects on risk for AD and for heroin/cocaine dependence, and furthermore had additive effects on the risk for alcohol + drug dependence. It is possible that increased synaptic 5-HT plus increased 5-HT3 receptor responsiveness to 5-HT might result in enhanced dopamine transmission in the reward pathway that is associated with a greater risk for addiction. The results of our study may have pharmacogenetic implications for therapeutic 5-HT3 antagonists such as ondansetron.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH and in part by grant RO1 DA 10336-02 to AR from the National Institute of Drug Abuse, NIH.
Footnotes
Supplementary information is available at the Molecular Psychiatry website.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 2.Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- 3.Gerra G, Garofano L, Santoro G, Bosari S, Pellegrini C, Zaimovic A, et al. Association between low-activity serotonin transporter genotype and heroin dependence: behavioral and personality correlates. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:37–42. doi: 10.1002/ajmg.b.20111. [DOI] [PubMed] [Google Scholar]
- 4.Mannelli P, Patkar AA, Murray HW, Certa K, Peindl K, Mattila-Evenden M, et al. Polymorphism in the serotonin transporter gene and response to treatment in African American cocaine and alcohol-abusing individuals. Addict Biol. 2005;10:261–268. doi: 10.1080/13556210500235540. [DOI] [PubMed] [Google Scholar]
- 5.Patkar AA, Berrettini WH, Hoehe M, Hill KP, Sterling RC, Gottheil E, et al. Serotonin transporter (5-HTT) gene polymorphisms and susceptibility to cocaine dependence among African-American individuals. Addict Biol. 2001;6:337–345. doi: 10.1080/13556210020077064. [DOI] [PubMed] [Google Scholar]
- 6.Saiz PA, Garcia-Portilla MP, Florez G, Arango C, Corcoran P, Morales B, et al. Differential role of serotonergic polymorphisms in alcohol and heroin dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:695–700. doi: 10.1016/j.pnpbp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Cravchik A, Goldman D. Neurochemical individuality: genetic diversity among human dopamine and serotonin receptors and transporters. Arch Gen Psychiatry. 2000;57:1105–1114. doi: 10.1001/archpsyc.57.12.1105. [DOI] [PubMed] [Google Scholar]
- 8.McBride WJ, Lovinger DM, Machu T, Thielen RJ, Rodd ZA, Murphy JM, et al. Serotonin-3 receptors in the actions of alcohol, alcohol reinforcement, and alcoholism. Alcohol Clin Exp Res. 2004;28:257–267. doi: 10.1097/01.alc.0000113419.99915.da. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11:527–540. doi: 10.1517/14728222.11.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 11.Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- 12.Jensen AA, Davies PA, Bräuner-Osborne H, Krzywkowski K. 3B but which 3B? And that’s just one of the questions: the heterogeneity of human 5-HT(3) receptors. Trends Pharmacol Sci. 2008;29:437–444. doi: 10.1016/j.tips.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krzywkowski K, Davies PA, Feinberg-Zadek PL, Bräuner-Osborne H, Jensen AA. High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc Natl Acad Sci U S A. 2008;105:722–727. doi: 10.1073/pnas.0708454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walstab J, Hammer C, Bönisch H, Rappold G, Niesler B. Naturally occurring variants in the HTR3B gene significantly alter properties of human heteromeric 5 hydroxytryptamine-3A/B receptors. Pharmacogenet Genomics. 2008;18:793–802. doi: 10.1097/FPC.0b013e3283050117. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute, Biometrics Research; New York, U.S.A: 1995. [Google Scholar]
- 17.Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducci F, Enoch MA, Yuan Q, Shen PH, White KV, Hodgkinson C, et al. HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power--an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol. 2009;43:73–84. doi: 10.1016/j.alcohol.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung KW, Engel SR, Allan AM, Lovinger DM. 5-HT(3) receptor function and potentiation by alcohols in frontal cortex neurons from transgenic mice overexpressing the receptor. Neuropharmacology. 2000;39:2346–2351. doi: 10.1016/s0028-3908(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 21.Lovinger DM, Sung KW, Zhou Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology. 2000;39:561–570. doi: 10.1016/s0028-3908(99)00164-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Hosoi M, Fukuzawa M, Sun H, Rawlings RR, Weight FF. Distinct molecular basis for differential sensitivity of the serotonin type 3A receptor to ethanol in the absence and presence of agonist. J Biol Chem. 2002;277:46256–46264. doi: 10.1074/jbc.M207683200. [DOI] [PubMed] [Google Scholar]
- 23.Rüsch D, Musset B, Wulf H, Schuster A, Raines DE. Subunit-dependent modulation of the 5-hydroxytryptamine type 3 receptor open-close equilibrium by n-alcohols. J Pharmacol Exp Ther. 2007;321:1069–1074. doi: 10.1124/jpet.106.118752. [DOI] [PubMed] [Google Scholar]
- 24.Stevens R, Rüsch D, Solt K, Raines DE, Davies PA. Modulation of human 5-hydroxytryptamine type 3AB receptors by volatile anesthetics and n-alcohols. J Pharmacol Exp Ther. 2005;314:338–345. doi: 10.1124/jpet.105.085076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, et al. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol Psychiatry. 2006;60:192–201. doi: 10.1016/j.biopsych.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57:803–811. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- 27.Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Brady CA, Dover TJ, Massoura AN, Princivalle AP, Hope AG, Barnes NM. Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharmacology. 2007;52:1284–1290. doi: 10.1016/j.neuropharm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Doucet E, Latrémolière A, Darmon M, Hamon M, Emerit MB. Immunolabelling of the 5-HT 3B receptor subunit in the central and peripheral nervous systems in rodents. Eur J Neurosci. 2007;26:355–366. doi: 10.1111/j.1460-9568.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- 30.Dubin AE, Huvar R, D’Andrea MR, Pyati J, Zhu JY, Joy KC, et al. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J Biol Chem. 1999;274:30799–30810. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- 31.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18:8879–8885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 34.Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27:1150–1155. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- 35.Sugai T, Suzuki Y, Sawamura K, Fukui N, Inoue Y, Someya T. The effect of 5-hydroxytryptamine 3A and 3B receptor genes on nausea induced by paroxetine. Pharmacogenomics J. 2006;6:351–356. doi: 10.1038/sj.tpj.6500382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.