Abstract
Background
Laparoscopic surgery has been incorporated into common surgical practice. The peritoneum is an organ with various biologic functions that may be affected in different ways by laparoscopic and open techniques. Clinically, these alterations may be important in issues such as peritoneal metastasis and adhesion formation.
Methods
A literature search using the Pubmed and Cochrane databases identified articles focusing on the key issues of laparoscopy, peritoneum, inflammation, morphology, immunology, and fibrinolysis.
Results
Laparoscopic surgery induces alterations in the peritoneal integrity and causes local acidosis, probably due to peritoneal hypoxia. The local immune system and inflammation are modulated by a pneumoperitoneum. Additionally, the peritoneal plasmin system is inhibited, leading to peritoneal hypofibrinolysis.
Conclusion
Similar to open surgery, laparoscopic surgery affects both the integrity and biology of the peritoneum. These observations may have implications for various clinical conditions.
Keywords: Fibrinolysis, Growth factors, Immune system, Inflammation, Laparoscopy, Mesothelial cells, Peritoneum, Plasmin
In recent decades, laparoscopic techniques have been integrated into common surgical practices. The rationale for the acceptance of these techniques is evident. Laparoscopic procedures have been associated with a shorter hospital stay and an earlier return to normal activities and work [1, 2]. The avoidance of a median laparotomy reduces the incidence of cicatricial hernia, and by minimizing surgical trauma, likely decreases the incidence of postoperative adhesions [3]. Moreover, endoscopic surgery is associated with superior cosmesis.
The abdominal cavity is lined by the peritoneum, which comprises a single layer of mesothelial cells supported by a basement membrane and an underlying sheet of connective tissue. The peritoneal organ has multiple biologic functions including regulation of inflammation, fibrinolysis, angiogenesis, and tissue remodeling processes [4–7]. Surgical trauma results in mesothelial damage and elicits an inflammatory response. Mesothelial cells balloon and detach from the basal membrane, thereby creating denuded areas. The inflammatory reaction is accompanied by the production and release of a broad spectrum of biologically active proteins and the exudation of protein-rich fluid. The peritoneal fibrinolytic response is rapidly disturbed [8, 9].
The minimal invasive character of laparoscopic surgery may reduce surgical trauma to the peritoneum. Laparoscopic procedures, however, introduce novel entities in the abdominal cavity such as increased abdominal pressure, insufflation gases, and temperature shifts, all of which may affect peritoneal integrity and biology. The current study was performed to review the literature concerning the biologic repercussions of laparoscopic surgery to the peritoneal organ.
Methods
A search of the MEDLINE and Cochrane databases was conducted to identify reports describing peritoneal changes due to laparoscopy. The following MeSH search terms were used: “laparoscopy,” “peritoneum,” “inflammation,” “morphology,” “immunology” and “fibrinolysis.” These terms were applied in various combinations in addition to the use of the “related articles” function. A total of 113 articles were selected and assessed. Manual cross-referencing was performed, and relevant references from selected papers were reviewed. The articles were restricted to those in the English language.
Morphologic alterations
The peritoneal surface comprises a mesothelium composed of cubic, flat, or intermediate cell types delimited by a basal lamina. Cubic mesothelial cells are predominantly present in the serosa of parenchymal organs and flat mesothelial cells in the intestinal, omental, and parietal peritoneum. An intermediate mesothelial cell type is found in the gastric peritoneum [10]. The submesothelial connective tissue layer is composed of collagen fiber bundles, fibroblasts, and free cells such as macrophages, granulocytes, and mast cells and contains blood and lymphatic vessels.
During conventional surgical procedures, mesothelial cells balloon and detach from the basal membrane, thereby creating denuded areas. Various experimental studies have shown that peritoneal integrity also may be disturbed by laparoscopic surgery. Suematsu et al. [11] found in an experimental study using mice that bulging up of the mesothelial cells was present immediately after initiation of pneumoperitoneum and fully resolved after 72 h. These data were confirmed by another experimental study in which mesothelial cells were bulging up 2 h after the initiation of a pneumoperitoneum [12]. The intercellular clefts thereby increased in size, and the underlying basal lamina became visible and subsequently infiltrated by peritoneal macrophages and lymphocytes.
A disruption of the peritoneal lining is likely to be a major cause for the inclination of surfaces to adhere. Restoration of the mesothelial lining, including mesothelial repopulation of denuded areas, seems to be of importance in reducing postinflammatory and postsurgical adhesion development. Bertram et al. [13] demonstrated that adhesion formation may be reduced by intraperitoneal transplantation of mesothelial cells, most likely by nidation of “free floating” cells. Similar results were found with intraperitoneal injection of mesenchymal stem cells immediately after surgery [14].
Inflammatory cells have been found in the peritoneum already 2 h after initiation of a pneumoperitoneum, whereas after 24 h, a chronic infiltrate and reactive mesothelial cells with congestion were observed [15]. Suematsu et al. [11] found that peritoneal changes during laparoscopy may be affected by the choice of insufflation gas, the amount of pressure, and the duration of insufflation. Intercellular clefts were found after both helium and carbon dioxide (CO2) pneumoperitoneum, but not after insufflations with normal air. This was confirmed by Rosario et al. [16], who described CO2 pneumoperitoneum as more harmful to the mesothelial ultrastructure than air insufflation.
In addition, Hazebroek et al. [17] also described a retraction, bulging of mesothelial cells, and exposure of the basal lamina after insufflation with CO2. In their study, the effect was independent of the temperature and the humidifying of the CO2. In contrast to that study, Erikoglu et al. [18] found, in an electron and light microscopic study, that heated and humidified CO2 resulted in fewer peritoneal alterations than cold and dry CO2.
Few studies have described the peritoneal changes during laparoscopic surgery in humans. Liu et al. [19] described the peritoneal morphology in 40 patients undergoing either conventional or laparoscopic surgery. Similar to the experimental studies, pneumoperitoneum induced bulging of mesothelial cells that was evident immediately at initiation. After 30 min of surgery, intercellular spaces could be found, and at 1 h, the underlying basement membrane could be seen and had lost its continuity. After 2 h, lymphocytes and macrophages were found in the intercellular clefts. These observations are in contrast to observations during conventional surgery, in which after as long as 60 min, no marked changes in the mesothelial cells were found. After 120 min of conventional surgery, intercellular spaces became significant.
Hypoxia and acidosis in the peritoneum
In addition to morphologic alterations, laparoscopic surgery may induce metabolic changes in the peritoneum. The creation of pneumoperitoneum may affect local peritoneal oxygen levels, which may induce metabolic changes including acidosis.
In 2003, Wildbrett et al. [20] showed that a pneumoperitoneum, created with either CO2 or helium, decreased the partial oxygen pressure in the rat abdominal wall, as measured with an implanted microcatheter. In contrast, insufflation with a nonhypoxic gas mixture consisting of 80% CO2 and 20% oxygen did not affect the oxygen pressure. Based on their study and additional in vitro experiments, these authors concluded that insufflation with either CO2 or helium affects the intra- and extracellular parameters regulating essential cell functions such as oxidative phosphorylation to produce adenosine triphosphate (ATP), cell proliferation, and onset of apoptosis.
The role of local oxygen pressure was further studied by Bourdel et al. [21] in a mouse laparoscopic model with controlled respiratory support. They found that the peritoneal tissue–oxygen tension levels in noninjured peritoneum during a low-pressure CO2 pneumoperitoneum were higher than during a laparotomy in ventilated mice. After CO2 insufflation, the peritoneal tissue–oxygen tension immediately increased and remained at a higher level. A similar effect was not found when normal air was used as insufflation gas.
In two experimental studies, Molinas el al. [22, 23] showed that peritoneal hypoxia is relevant in the occurrence of postsurgical adhesions, possibly by reducing capillary flow in the superficial peritoneal layers during pneumoperitoneum. In a murine model, they found that the incidence of adhesions increased with a longer time of pneumoperitoneum and a higher insufflation pressure. Interestingly, the incidence decreased when oxygen was added to the insufflation gas. The adhesion-reducing effect of oxygen was maximal when 2 to 3% oxygen was added.
This theory was confirmed by the study of Elkelani et al. [24] using mice, in which adhesion formation decreased with the addition of 3% oxygen to the CO2 pneumoperitoneum. The addition of higher oxygen concentrations, however, was deleterious. Adhesions always increased with a longer duration of the pneumoperitoneum.
In another experiment, the role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha in CO2 pneumoperitoneum-enhanced adhesion formation was studied [25]. It was observed that CO2 pneumoperitoneum enhanced adhesion formation and that this effect was mediated, at least in part, by an upregulation of HIF-1alpha and HIF-2alpha. Additionally, Binda et al. [26] recently showed that the reactive oxygen species scavengers superoxide dismutase, catalase, melatonin, and ascorbic acid may decrease adhesion formation. Moreover, they showed that the HIF inhibitors may reduce adhesion formation.
Peritoneal hypoxia may induce metabolic changes, inducing a metabolic acidosis. Recently, it was shown that insufflation of CO2 into the peritoneal cavity results in a decreased peritoneal pH. An immediate local drop in pH to 6.6 occurred in the peritoneum after CO2 insufflation. During the pneumoperitoneum, the pH further declined, stabilizing at 6.4. The pH was completely restored during the recovery period. This peritoneal effect also appeared to affect the systemic acid–base balance, probably due to transperitoneal absorption.
Using helium as the insufflation gas, the opposite effect was found. Tissue pH increased slightly to 7.5 during insufflation, followed by a continuous decrease during pneumoperitoneum and recovery, reaching 7.2. This indicates that factors other than high intraabdominal pressure probably are involved [27].
These data confirm the study of Hanly et al. [28], who also found that abdominal insufflation with CO2 causes peritoneal acidosis independent of the systemic pH. In their study, insufflation with helium did not affect the peritoneal pH. Additionally, Wong et al. [29] also found that CO2 pneumoperitoneum results in severe peritoneal acidosis. In their study, this effect was unaltered by heating or humidification. They hypothesized that the alteration in peritoneal pH may conceivably be responsible for providing an environment favorable for tumor cell implantation during laparoscopy.
The induction of peritoneal acidosis by laparoscopy may change the peritoneal immunoprotection. Acidification of the peritoneal cavity by abdominal insufflation increased serum interleukin-10 (IL-10) and decreased serum tumor necrosis factor (TNF)-alpha levels in response to systemic lipopolysaccharide challenge. The degree of peritoneal acidification correlated with the degree of inflammatory response reduction [30]. Thus, pneumoperitoneum-mediated peritoneal cell acidification may attenuate the inflammatory response after laparoscopic surgery.
Peritoneal immunology
After conventional surgery, polymorphonuclear leucocytes infiltrate the damaged peritoneal areas, soon followed by macrophages. Degranulation of peritoneal mast cells increases vascular permeability, which results in an inflammatory response and the release of active components. These active components include complement factors and opsins. In addition, cytokines and growth factors, such as transforming growth factor-beta (TGF-β) and TNF-α, are secreted by polymorphs. Macrophages also release monokines such as interleukin-1 (IL-1), IL-6, and arachadonic acid metabolites [31]. The peritoneal macrophage function activates the immune system due to the release of acute-phase proteins, ultimately leading to the activation of repair mechanisms. In contrast to the peritoneal reaction to a laparotomy, little is known about the local immune response to endoscopic procedures.
Clinical and experimental studies have demonstrated that laparoscopic surgery may preserve the systemic immune system better than open procedures [32–35]. Both systemic C-reactive protein levels and IL-6 levels are lower in patients undergoing laparoscopy than in those undergoing laparotomy [36, 37]. An animal study comparing conventional and laparoscopically assisted colonic resection showed reduced levels of systemic IL-6 in the laparoscopic group. Laparoscopic surgery is characterized by a decreased acute-phase pro-inflammatory response of TNF-α and IL-1, with subsequent attenuation of late-phase immunosuppression seen with laparotomy [38–40]. These systemic observations, however, may not reflect the local immune response of the peritoneum to surgical trauma.
As a first line of defense, peritoneal macrophages and polymorphonuclear neutrophil granulocytes are of primary importance in protecting the body. Experimental animal studies have shown that both air and carbon dioxide may affect the function of these cells [41]. Air exposure triggered a higher transmigration rate of polymorphonuclear neutrophils from the blood compartment into the peritoneal cavity and decreased polymorphonuclear neutrophil apoptosis compared with CO2. Another study showed that peritoneal macrophages exhibit a decreased basal TNF-α release when exposed to CO2 [42]. Interestingly, these cells showed an increased TNF-α release after a second immune stimulation using Escherichia coli, suggesting a greater competency of interaction in an immune defense reaction after CO2 exposure.
West et al. [43] reported an inhibition of IL-1 production by peritoneal macrophages in vitro after 15 min of CO2 exposure, whereas after 30 min, TNF-α production also was decreased. These findings were supported by additional in vivo experiments comparing a capno-pneumoperitoneum and a pneumoperitoneum with helium, showing less TNF-α and IL-1 secretion when CO2 was used [44]. Because the tumor-scavenging action of peritoneal macrophages is mediated by inflammatory cytokines such as TNF-α, these observations may affect tumor implantation [45]. Additionally, Neuhaus et al. [46] showed in an experimental model that the choice of insufflation gas may affect the incidence of port-site metastases, possibly in relation to local defense mechanisms.
Various factors may contribute to the changed local immune response during laparoscopic surgery. As described earlier, pneumoperitoneum causes an acidification of the peritoneal surface, which may affect the immune response. This, however, may not entirely explain why IL-1 is inhibited as early as 15 min after CO2 exposure, whereas TNF-α is inhibited after a minimum exposure of 30 min [47]. This may imply that IL-1 production is inhibited via a transcriptional control mechanism, whereas intracellular acidosis may contribute to the inhibition of TNF-α. Decreased IL-1 mRNA and normal levels of TNF-α mRNA support this assumption.
A second factor may be the abdominal temperature. Carbon dioxide insufflation has been associated with reduced body and abdominal temperature. During laparoscopy, the intraabdominal temperature has been demonstrated to decrease to as low as 27.7°C [48]. Animal studies have shown significant changes in body core temperature during laparoscopy using dry cold gas compared with the use of humidified and heated gas [49]. Hypothermia may have affected peritoneal macrophage functions. Patients undergoing a laparoscopic cholecystectomy with a pneumoperitoneum at room temperature were shown to have higher levels of cytokines in the peritoneal fluid than those undergoing the procedure with pneumoperitoneum at body temperature [50]. Both TNF-α and IL-1 were significantly increased, whereas IL-6 was marginally elevated [51].
Growth factors and adhesion molecules
Activation of peritoneal inflammation and modulation of the immune response after laparoscopic surgery eventually regulates peritoneal healing processes. Besides the previously described cytokines, growth factors and adhesion molecules also may play important roles in peritoneal healing. Peritoneal mesothelial cells produce multiple cellular mediators such as TGF-β and also may regulate the response of other cells [52]. Whereas TGF-β stimulates fibroblasts to produce many proteins including collagen, fibronectin, and integrins, it decreases the production of proteins such as collagenase and heparinase whose function is to degrade the extracellular matrix. Mesothelial cells express various adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) [53].
Other proteins present in the peritoneum are connective tissue growth factor, heparin-binding epidermal growth factor, vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet-derived growth factor. Studies focusing on laparoscopic surgery in relation to these proteins are lacking. An exception is TGF-β, which has been studied in patients undergoing a laparoscopic cholecystectomy [54]. The findings showed that short-term laparoscopy does not affect the peritoneal levels of TGF-β. The use of an ultrasonic scalpel, frequently applied in laparoscopic surgery, decreased the levels of TGF-β compared with electrocautery. The intensity of light used to illuminate the peritoneal cavity also influenced the local concentrations of TGF-β.
A range of studies support the theory that TGF-β is a major stimulator of peritoneal adhesion formation, primarily by increasing the production of plasminogen activator inhibitor (PAI-1). Furthermore, TGF-β is a stimulator of extracellular matrix deposition, resulting in a net accumulation of connective tissue [55–57]. The relation between TGF-β and adhesion formation has been established by various studies demonstrating elevated levels of TGF-β in areas of adhesion formation in humans [58, 59]. Additionally, Freeman et al. [60] showed in an experimental rat study that peritoneal adhesions have higher levels of TGF-β1 and TGF-β3 mRNA transcripts than either uninjured or normally healed peritoneum.
Sendt et al. [61] studied the expression of various indicators of the inflammatory response including IL-1, IL-6, ICAM-1, and antibodies against macrophage-inhibiting-factor-related proteins 8 and 14 in patients undergoing open-end laparoscopic cholecystectomy. They found no difference in any of the measured parameters except IL6 between the open and laparoscopic groups. The findings showed that ICAM-1 also was increased significantly in the laparoscopic group. These authors concluded that minimally invasive surgery does not necessarily mean minimal peritoneal damage.
The role of VEGF during laparoscopy was studied by Molinas et al. [62] in an experimental mouse model. After 60 min of CO2 pneumoperitoneum, an increased incidence of adhesion were observed. Antibodies against VEGF-1 receptors significantly reduced adhesion formation, suggesting that VEGF plays an important role in the complex design of peritoneal healing after laparoscopic surgery.
Peritoneal fibrinolysis
There is substantial evidence that the peritoneal plasmin system plays a crucial role in peritoneal healing and subsequent adhesion formation. Peritoneum mesothelial cells produce both activators and inhibitors of the plasmin system. Tissue-type plasminogen activator (tPA) is the major plasminogen activator in the peritoneal organ [63], followed by urokinase-type plasminogen activator (uPA). Their activity is limited by plasminogen-activating inhibitors, principally type 1 (PAI-1). Conventional abdominal surgery is accompanied by a rapid decline in the peritoneal fibrinolytic activity. The decreased peritoneal fibrinolysis may be caused by both an increase in its inhibitors [64] and a quick release of tPA from the visceral peritoneum during surgery [65].
In an in vitro model, Ziprin et al. [66] assessed the effect of a pneumoperitoneum on the fibrinolytic activity of mesothelial cells. Human peritoneal cells were incubated in CO2, helium, and standard culture conditions. These authors found enhanced plasminogen activator activity from cells exposed to both CO2 and helium in the absence of oxygen because of a reduction in PAI-1 concentrations. No changes in tPA levels were observed. Changes in insufflation pressures did not affect plasminogen activator activity.
Bergström et al. [67] found that flowing CO2 increases PAI-1 expression by mesothelial cells in vitro. In their study, one group of primarily cultured human peritoneal mesothelial cells was exposed to CO2 flowing through the box without elevated pressure and another group to CO2 at a pressure of 14 mmHg. The mesothelial cells exposed to flowing CO2 released more PAI-1 than cells exposed to pressurized CO2 and control cells. Cells exposed to flowing CO2 had an increased PAI-1 mRNA expression. Upregulation of PAI-1 has been described as a mechanism of pneumoperitoneum-enhanced adhesion formation in mice [68].
In another experimental study, Nagelschmidt et al. [69] found that a CO2 pneumoperitoneum significantly decreased peritoneal tPA activity in pigs. These experimental studies have indicated that similar to open surgery, laparoscopic surgery may lead to hypofibrinolysis by both an upregulation of inhibitors and a downregulation of activators of the plasmin system.
Tarhan et al. [70] studied peritoneal fluid after both open and laparoscopic cholecystectomy. They found higher tPA levels after the open procedure, possibly in relation to more intense tissue handling. The role of fluid concentrations in peritoneal healing and the relation to the peritoneal levels of fibrinolytic enzymes, however, remain a matter of debate. In another study with patients undergoing a laparoscopic cholecystectomy, there was no effect on the expression of peritoneal tPA, PAI-1, or uPA levels. Moreover, the biopsies taken from patients undergoing surgery with various pressures and light intensities did not differ [71]. The short operation time may have contributed to these observations.
This hypothesis was confirmed in another study assessing the effect of prolonged laparoscopic surgery [72]. Prolonged laparoscopic surgery decreased peritoneal tPA antigen expression and its activity after 90 min of surgery. It was concluded that in contrast to short-term laparoscopic surgery, prolonged laparoscopic surgery causes decreased fibrinolytic activity in the peritoneum due to decreased tPA levels.
Another factor affecting peritoneal fibrinolysis is decreased abdominal temperature. In a study comparing patients undergoing surgery with CO2 at room temperature and those who had surgery with CO2 at body temperature showed significantly increased PAI-1 levels in the first group. These observations suggest a possible adverse effect from cooling of the abdominal cavity on peritoneal fibrinolysis [73].
To date, few human studies have compared open and laparoscopic procedures. Bergström et al. [74] found an initial rise in peritoneal PAI-1 concentration during laparoscopy, which suggests an adverse effect of CO2 insufflation. At the end of the cholecystectomy, however, there was no difference between groups. Neudecker [75] found decreased tPA activity levels after both conventional and laparoscopic colonic resection. In their study, unfortunately, all the patients underwent an initial laparoscopy before randomization, which may have affected their results. A similar study [76] showed that after mobilization of the hemicolon, peritoneal levels of tPA antigen and activity were significantly higher in the laparoscopic group due to a decrease in the conventional group. At the end of the procedure, the concentrations of tPA antigen and activity significantly decreased in the laparoscopic group to levels comparable with those in the conventional group. It was concluded that both conventional and laparoscopic surgery inflict a decrease in tPA antigen and its specific activity. Peritoneal hypofibrinolysis appears to initiate more rapidly during conventional surgery than during laparoscopic surgery, which may reflect the minimal invasive character of the latter.
Discussion
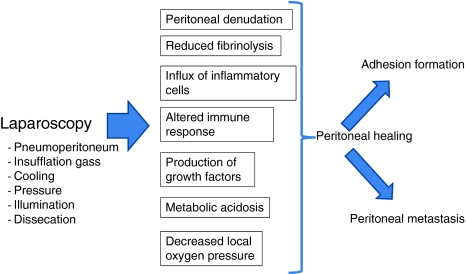
The current review shows that laparoscopic surgery has a profound effect on both peritoneal integrity and its biology. The immune system is altered and the plasmin system inhibited. Moreover, laparoscopy induces peritoneal acidosis, probably due to decreased local oxygen pressure. Many of the available data have been derived from experimental studies. It is unclear how these experimental data can be translated clinically (Fig. 1).
Fig. 1.
The described changes may have been induced by various factors of laparoscopic surgery. Which effect is induced by which component of laparoscopic surgery is not obvious. First, the abdominal temperature may be important. A decreased temperature has been shown to inhibit the plasmin system and affect local cytokine concentrations. Second, the intense illumination of the peritoneum may affect the peritoneum, either directly or indirectly, by causing local dissecation. Local TGF-β levels were affected by the intensity of light. Other components are intraabdominal pressure, duration of procedure, choice of dissection devices, dissecation, and the insufflation gas. Carbon dioxide, the most frequently used insufflation gas, has been shown to affect the peritoneal morphology and to cause local hypoxia, leading to acidosis. Moreover, CO2 affects the local inflammatory response and disturbs peritoneal fibrinolysis. Other gases, such as helium or air, do not always induce similar effects. Additional studies are needed to elucidate the individual effects of all the described components.
The effects of laparoscopy on peritoneal integrity and biology may have repercussions for the occurrence of peritoneal metastasis. Although dissemination of tumor cells to the peritoneum is not exceptional, mechanisms of action have not been fully elucidated. Only when the mesothelial cell layer has been breached may tumor cells infiltrate and proliferate within the submesothelial connective tissue matrix. The early bulging of mesothelial cells during laparoscopic procedures may facilitate this phenomenon. Some experimental studies have suggested a prominent role of neutrophils in the occurrence of peritoneal metastasis. Others have proposed that tumor-conditioned media or exogenous inflammatory cytokines may induce mesothelial retraction and disaggregation to gain access to the submesothelial connective tissue.
The results from the current review show a remarkable effect of laparoscopic surgery on peritoneal immunology and inflammation. Adhesion molecules may play an important role as well. Tumor cells have been shown to adhere rapidly to the mesothelial cell layer and play a role for the cell adhesion molecule CD44 and the integrins β1, α2, α3, and α5 in mesothelial invasion [77]. Studies focusing on the effects that laparoscopic surgery has on the expression of these adhesion molecules are therefore indicated.
Postsurgical adhesion formation remains a major concern because it may cause severe and life-threatening complications such as small bowel obstruction. The economic burden of adhesions is high [78]. Despite the observations that the incidence of adhesions after laparoscopic surgery may be lower than after open surgery, efforts to reduce their occurrence still appear to be indicated.
There is considerable evidence that the plasmin system plays a key role in the pathophysiology of intraabdominal adhesions. Clinical studies on the peritoneal fibrinolytic response to conventional surgery using sequential biopsies taken during the procedure have shown a progressive peritoneal hypofibrinolysis. During laparoscopic procedures, a prolonged period of surgery was needed before tPA levels decreased, a finding in contrast to the situation during open surgery, in which a rapid decline in tPA levels has been shown in several studies. This might be due to a less intensive or a different peritoneal trauma during laparoscopic surgery, compared with open surgery. These observations warrant further research focused on various components of laparoscopic surgery and the peritoneal healing process.
Laparoscopic procedures are regularly indicated for various infectious diseases such as perforated appendicitis and diverticulitis. Laparoscopic peritoneal lavage is frequently performed under these circumstances. Animal studies have suggested that microbial adherence to the peritoneum is high, which may negatively affect the results of the lavage. Whether the effects of laparoscopic surgery on peritoneal biology affect this microbial adherence either positively or negatively compared with open lavage remains to be investigated. This may be clinically important because lavage-resistant bacteria may be a source of persistent infection and may cause, through translocation, septic complications.
In conclusion, the results of the current review show that laparoscopic surgery affects the integrity of the peritoneum and its biologic activity. Information about separate components of laparoscopic surgery and their specific effects remains to be elucidated.
Acknowledgments
Disclosures
W. J. A. Brokelman, M. Lensvelt, I. H. M. Borel Rinkes, J. H. G. Klinkenbijl, and M. M. P. J. Reijnen have no conflicts of interest or financial ties to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Berggren U, Gordt T, Grama D, Haglund U, Rastad J, Arvidsson D. Laparoscopic versus open cholecystectomy: hospitalization, sick leave, analgesia, and trauma responses. Br J Surg. 1994;81:1362–1365. doi: 10.1002/bjs.1800810936. [DOI] [PubMed] [Google Scholar]
- 2.Berrevoet E, Biglari M, Sinove Y, De Baardemaeker L, Troise R, de Hemptinne B. Outpatient laparoscopic cholecystectomy in Belgium: what are we waiting for? Acta Chir Belg. 2006;106:537–540. doi: 10.1080/00015458.2006.11679947. [DOI] [PubMed] [Google Scholar]
- 3.Gutt CN, Oniu T, Schemmer P, Mehrabi A, Buchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18:898–906. doi: 10.1007/s00464-004-0056-7. [DOI] [PubMed] [Google Scholar]
- 4.Yung S, Chan TM. Mesothelial cells. Perit Dial Int. 2007;27(Suppl 2):S110–S115. [PubMed] [Google Scholar]
- 5.Nachtsheim R, Dudley B, McNeil PL, Howdieshell TR. The peritoneal cavity is a distinct compartment of angiogenic molecular mediators. J Surg Res. 2006;134:28–35. doi: 10.1016/j.jss.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Chegini N. Peritoneal molecular environment, adhesion formation, and clinical implication. Front Biosci. 2002;7:e91–e115. doi: 10.2741/chegini. [DOI] [PubMed] [Google Scholar]
- 7.diZerega GS. Biochemical events in peritoneal tissue repair. Eur J Surg Suppl. 1997;577:10–16. [PubMed] [Google Scholar]
- 8.Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012–1019. doi: 10.1080/110241599750007810. [DOI] [PubMed] [Google Scholar]
- 9.van der Wal JB, Jeekel J. Biology of the peritoneum in normal homeostasis and after surgical trauma. Colorectal Dis. 2007;9(Suppl 2):9–13. doi: 10.1111/j.1463-1318.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 10.Michailova K, Wassilev W, Wedel T. Scanning and transmission electron microscopic study of visceral and parietal peritoneal regions in the rat. Ann Anat. 1999;181:253–260. doi: 10.1016/S0940-9602(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 11.Suematsu T, Hirabayashi Y, Shiraishi N, Adachi Y, Kitamura H, Kitano S. Morphology of the murine peritoneum after pneumoperitoneum vs. laparotomy. Surg Endosc. 2001;15:954–958. doi: 10.1007/s004640090100. [DOI] [PubMed] [Google Scholar]
- 12.Volz J, Koster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13:611–614. doi: 10.1007/s004649901052. [DOI] [PubMed] [Google Scholar]
- 13.Bertram P, Tietze L, Hoopmann M, Treutner KH, Mittermayer C, Schumpelick V. Intraperitoneal transplantation of isologous mesothelial cells for prevention of adhesions. Eur J Surg. 1999;165:705–709. doi: 10.1080/11024159950189780. [DOI] [PubMed] [Google Scholar]
- 14.Lucas PA, Warejcka DJ, Zhang LM, Newman WH, Young HE. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 1996;62:229–232. doi: 10.1006/jsre.1996.0200. [DOI] [PubMed] [Google Scholar]
- 15.Papparella A, Noviello C, Romano M, Parmeggiani P, Paciello O, Papparella S. Local and systemic impact of pneumoperitoneum on prepuberal rats. Pediatr Surg Int. 2007;23:453–457. doi: 10.1007/s00383-006-1860-z. [DOI] [PubMed] [Google Scholar]
- 16.Rosario MT, Ribeiro U, Jr, Corbett CE, Ozaki AC, Bresciani CC, Zilberstein B, Gama-Rodriques JJ. Does CO2 pneumoperitoneum alter the ultra-structure of the mesothelium? J Surg Res. 2006;133:84–88. doi: 10.1016/j.jss.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Hazebroek EJ, Schreve MA, Visser P, De Bruin RW, Marquet RL, Bonjer HJ. Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A. 2002;12:355–364. doi: 10.1089/109264202320884108. [DOI] [PubMed] [Google Scholar]
- 18.Erikoglu M, Yol S, Avunduk MC, Erdemli E, Can A. Electron-microscopic alterations of the peritoneum after both cold and heated carbon dioxide pneumoperitoneum. J Surg Res. 2005;125:73–77. doi: 10.1016/j.jss.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Hou QX. Effect of carbon dioxide pneumoperitoneum during laparoscopic surgery on morphology of peritoneum. Zhonghua Yi Xue Za Zhi. 2006;86:164–166. [PubMed] [Google Scholar]
- 20.Wildbrett P, Oh A, Naundorf D, Volk T, Jacobi CA. Impact of laparoscopic gases on peritoneal microenvironment and essential parameters of cell function. Surg Endosc. 2003;17:78–82. doi: 10.1007/s00464-002-9015-3. [DOI] [PubMed] [Google Scholar]
- 21.Bourdel N, Matsuzaki S, Bazin JE, Pouly JL, Mage G, Canis M. Peritoneal tissue–oxygen tension during a carbon dioxide pneumoperitoneum in a mouse laparoscopic model with controlled respiratory support. Hum Reprod. 2007;22:1149–1155. doi: 10.1093/humrep/del482. [DOI] [PubMed] [Google Scholar]
- 22.Molinas CR, Koninckx PR. Hypoxaemia induced by CO2 or helium pneumoperitoneum is a cofactor in adhesion formation in rabbits. Hum Reprod. 2000;15:1758–1763. doi: 10.1093/humrep/15.8.1758. [DOI] [PubMed] [Google Scholar]
- 23.Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560–567. doi: 10.1016/S0015-0282(01)01964-1. [DOI] [PubMed] [Google Scholar]
- 24.Elkelani OA, Binda MM, Molinas CR, Koninckx PR. Effect of adding more than 3% oxygen to carbon dioxide pneumoperitoneum on adhesion formation in a laparoscopic mouse model. Fertil Steril. 2004;82:1616–1622. doi: 10.1016/j.fertnstert.2004.07.933. [DOI] [PubMed] [Google Scholar]
- 25.Molinas CR, Campo R, Elkelani OA, Binda MM, Carmeliet P, Koninckx PR. Role of hypoxia-inducible factors 1alpha and 2alpha in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80(Suppl 2):795–802. doi: 10.1016/S0015-0282(03)00779-9. [DOI] [PubMed] [Google Scholar]
- 26.Binda MM, Molinas CR, Bastidas A, Koninckx PR. Effect of reactive oxygen species scavengers, antiinflammatory drugs, and calcium-channel blockers on carbon dioxide pneumoperitoneum-enhanced adhesions in a laparoscopic mouse model. Surg Endosc. 2007;21:1826–1834. doi: 10.1007/s00464-007-9296-7. [DOI] [PubMed] [Google Scholar]
- 27.Bergstrom M, Falk P, Park PO, Holmdahl L. Peritoneal and systemic pH during pneumoperitoneum with CO2 and helium in a pig model. Surg Endosc. 2008;22:359–364. doi: 10.1007/s00464-007-9409-3. [DOI] [PubMed] [Google Scholar]
- 28.Hanly EJ, Aurora AR, Fuentes JM, Shih SP, Marohn MR, De Maio A, Talamini MA. Abdominal insufflation with CO2 causes peritoneal acidosis independent of systemic pH. J Gastrointest Surg. 2005;9:1245–1251. doi: 10.1016/j.gassur.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Wong YT, Shah PC, Birkett DH, Brams DM. Carbon dioxide pneumoperitoneum causes severe peritoneal acidosis, unaltered by heating, humidification, or bicarbonate in a porcine model. Surg Endosc. 2004;18:1498–1503. doi: 10.1007/s00464-003-9290-7. [DOI] [PubMed] [Google Scholar]
- 30.Hanly EJ, Aurora AA, Shih SP, Fuentes JM, Marohn MR, De Maio A, Talamini MA. Peritoneal acidosis mediates immunoprotection in laparoscopic surgery. Surgery. 2007;142:357–364. doi: 10.1016/j.surg.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Heel KA, Hall JC. Peritoneal defences and peritoneum-associated lymphoid tissue. Br J Surg. 1996;83:1031–1036. doi: 10.1002/bjs.1800830804. [DOI] [PubMed] [Google Scholar]
- 32.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs. laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13:233–235. doi: 10.1007/s004649900952. [DOI] [PubMed] [Google Scholar]
- 33.Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, Doorman J, Balli JE, Glass J, Gonzalez JJ, Bessler M, Wie H, Treat M. Postoperative cell-mediated immune response is better preserved after laparoscopic vs. open colorectal resection in humans. Surg Endosc. 2003;17:972–978. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- 34.Gitzelmann CA, Mendoza-Sagaon M, Talamini MA, Ahmad SA, Pegoli W, Jr, Paidas CN. Cell-mediated immune response is better preserved by laparoscopy than laparotomy. Surgery. 2000;127:65–71. doi: 10.1067/msy.2000.101152. [DOI] [PubMed] [Google Scholar]
- 35.Wichmann MW, Huttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM, Jauch KW. Immunological effects of laparoscopic vs. open colorectal surgery: a prospective clinical study. Arch Surg. 2005;140:692–697. doi: 10.1001/archsurg.140.7.692. [DOI] [PubMed] [Google Scholar]
- 36.Glaser F, Sannwald GA, Buhr HJ, Kuntz C, Mayer H, Klee F, Herfarth C. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995;221:372–380. doi: 10.1097/00000658-199504000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho JM, LaPorta AJ, Clark JR, Schofield MJ, Hammond SL, Mallory PL. Response of serum cytokines in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 1994;8:1380–1383. doi: 10.1007/BF00187340. [DOI] [PubMed] [Google Scholar]
- 38.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 39.Kuntz C, Wunsch A, Bay F, Windeler J, Glaser F, Herfarth C. Prospective randomized study of stress and immune response after laparoscopic vs. conventional colonic resection. Surg Endosc. 1998;12:963–967. doi: 10.1007/s004649900757. [DOI] [PubMed] [Google Scholar]
- 40.Redmond HP, Watson RW, Houghton T, Condron C, Watson RG, Bouchier-Hayes D. Immune function in patients undergoing open vs. laparoscopic cholecystectomy. Arch Surg. 1994;129:1240–1246. doi: 10.1001/archsurg.1994.01420360030003. [DOI] [PubMed] [Google Scholar]
- 41.Moehrlen U, Ziegler U, Boneberg E, Reichmann E, Gitzelmann CA, Meuli M, Hamacher J. Impact of carbon dioxide versus air pneumoperitoneum on peritoneal cell migration and cell fate. Surg Endosc. 2006;20:1607–1613. doi: 10.1007/s00464-005-0775-4. [DOI] [PubMed] [Google Scholar]
- 42.Moehrlen U, Schwoebel F, Reichmann E, Stauffer U, Gitzelmann CA, Hamacher J. Early peritoneal macrophage function after laparoscopic surgery compared with laparotomy in a mouse mode. Surg Endosc. 2005;19:958–963. doi: 10.1007/s00464-004-2118-2. [DOI] [PubMed] [Google Scholar]
- 43.West MA, Hackam DJ, Baker J, Rodriquez JL, Bellingham J, Rotstein OD. Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide: relevance to laparoscopic surgery. Ann Surg. 1997;226:179–190. doi: 10.1097/00000658-199708000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathew G, Watson DI, Ellis TS, Jamieson GG, Rofe AM. The role of peritoneal immunity and the tumour-bearing state on the development of wound and peritoneal metastases after laparoscopy. Aust N Z J Surg. 1999;69:14–18. doi: 10.1046/j.1440-1622.1999.01484.x. [DOI] [PubMed] [Google Scholar]
- 45.Volz J, Koster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. 1999;86:770–774. doi: 10.1002/(SICI)1097-0142(19990901)86:5<770::AID-CNCR11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Neuhaus SJ, Watson DI, Ellies T, Rowland R, Rofe AM, Pike GK, Mathew G, Jamieson GG. Wound metastasis after laparoscopy with different insufflation gases. Surgery. 1998;123:579–583. doi: 10.1067/msy.1998.88089. [DOI] [PubMed] [Google Scholar]
- 47.West MA, Baker J, Bellingham J. Kinetics of decreased LPS-stimulated cytokine release by macrophages exposed to CO2. J Surg Res. 1996;63:269–274. doi: 10.1006/jsre.1996.0259. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs VR, Morrison JE, Jr, Mundhenke C, Golombeck K, Jonat W. Intraoperative evaluation of laparoscopic insufflation technique for quality control in the OR. JSLS. 2000;4:189–195. [PMC free article] [PubMed] [Google Scholar]
- 49.Bessell JR, Ludbrook G, Miljard SH, Baxter PS, Ubhi SS, Maddern GJ. Humidified gas prevents hypothermia induced by laparoscopic insufflation: a randomized controlled study in a pig model. Surg Endosc. 1999;13:101–105. doi: 10.1007/s004649900914. [DOI] [PubMed] [Google Scholar]
- 50.Puttick MI, Scott-Coombes DM, Dye J, Nduka CC, Menzies-Gow NM, Mansfield AO, Darzi A. Comparison of immunologic and physiologic effects of CO2 pneumoperitoneum at room and body temperatures. Surg Endosc. 1999;13:572–575. doi: 10.1007/s004649901043. [DOI] [PubMed] [Google Scholar]
- 51.Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, Gotoh M, Kishimoto T, Mori T. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992;111:201–209. [PubMed] [Google Scholar]
- 52.Yao V, Platell C, Hall JC. Peritoneal mesothelial cells produce inflammatory related cytokines. ANZ J Surg. 2004;74:997–1002. doi: 10.1111/j.1445-1433.2004.03220.x. [DOI] [PubMed] [Google Scholar]
- 53.Liberek T, Topley N, Luttmann W, Williams JD. Adherence of neutrophils to human peritoneal mesothelial cells: role of intercellular adhesion molecule-1. J Am Soc Nephrol. 1996;7:208–217. doi: 10.1681/ASN.V72208. [DOI] [PubMed] [Google Scholar]
- 54.Brokelman WJ, Holmdahl L, Berstrom M, Falk P, Klinkenbijl JH, Reijnen MM. Peritoneal transforming growth factor beta-1 expression during laparoscopic surgery: a clinical trial. Surg Endosc. 2007;21:1537–1541. doi: 10.1007/s00464-006-9164-x. [DOI] [PubMed] [Google Scholar]
- 55.Kagami S, Kuhara T, Yasutomo K, Okada K, Loster K, Reutte W, Kuroda Y. Transforming growth factor-beta (TGF-beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Exp Cell Res. 1996;229:1–6. doi: 10.1006/excr.1996.0336. [DOI] [PubMed] [Google Scholar]
- 56.Ignotz RA, Massague J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987;51:189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- 57.Duron JJ. Postoperative intraperitoneal adhesion pathophysiology. Colorectal Dis. 2007;9(Suppl 2):14–24. doi: 10.1111/j.1463-1318.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 58.Hobson KG, DeWing M, Ho HS, Wolfe BM, Cho K, Greenhalgh DG. Expression of transforming growth factor beta1 in patients with and without previous abdominal surgery. Arch Surg. 2003;138:1249–1252. doi: 10.1001/archsurg.138.11.1249. [DOI] [PubMed] [Google Scholar]
- 59.Holmdahl L, Kotseos K, Bergström M, Falk P, Ivarsson ML, Chegini N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 2001;129:626–632. doi: 10.1067/msy.2001.113039. [DOI] [PubMed] [Google Scholar]
- 60.Freeman ML, Saed GM, Elhammady EF, Diamond MP. Expression of transforming growth factor beta isoform mRNA in injured peritoneum that healed with adhesions and without adhesions and in uninjured peritoneum. Fertil Steril. 2003;80(Suppl 2):708–713. doi: 10.1016/S0015-0282(03)00770-2. [DOI] [PubMed] [Google Scholar]
- 61.Sendt W, Amberg R, Schöffel U, Hassan A, von Specht BU, Farthmann EH. Local inflammatory peritoneal response to operative trauma: studies on cell activity, cytokine expression, and adhesion molecules. Eur J Surg. 1999;165:1024–1030. doi: 10.1080/110241599750007838. [DOI] [PubMed] [Google Scholar]
- 62.Molinas CR, Binda MM, Carmeliet P, Koninckx PR. Role of vascular endothelial growth factor receptor 1 in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in mice. Fertil Steril. 2004;82(Suppl 3):1149–1153. doi: 10.1016/j.fertnstert.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Holmdahl L, Eriksson E, al Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119:701–705. doi: 10.1016/S0039-6060(96)80196-6. [DOI] [PubMed] [Google Scholar]
- 64.Scott-Coombes DM, Whawell SA, Thompson JN. The operative peritoneal fibrinolytic response to abdominal operation. Eur J Surg. 1995;161:395–399. [PubMed] [Google Scholar]
- 65.Ivarsson ML, Falk P, Holmdahl L. Response of visceral peritoneum to abdominal surgery. Br J Surg. 2001;88:148–151. doi: 10.1046/j.1365-2168.2001.01630.x. [DOI] [PubMed] [Google Scholar]
- 66.Ziprin P, Ridgway PF, Peck DH, Darzi AW. Laparoscopic-type environment enhances mesothelial cell fibrinolytic activity in vitro via a downregulation of plasminogen activator inhibitor-1 activity. Surgery. 2003;134:758–765. doi: 10.1016/S0039-6060(03)00293-9. [DOI] [PubMed] [Google Scholar]
- 67.Bergström M, Falk P, Holmdahl L. CO2 promotes plasminogen activator inhibitor type 1 expression in human mesothelial cells. Surg Endosc. 2003;17:1818–1822. doi: 10.1007/s00464-002-9113-2. [DOI] [PubMed] [Google Scholar]
- 68.Molinas CR, Elkelani O, Campo R, Luttun A, Carmeliet P, Koninckx PR. Role of the plasminogen system in basal adhesion formation and carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80:184–192. doi: 10.1016/S0015-0282(03)00496-5. [DOI] [PubMed] [Google Scholar]
- 69.Nagelschmidt M, Gerbecks D, Minor T. The impact of gas laparoscopy on abdominal plasminogen activator activity. Surg Endosc. 2001;15:585–588. doi: 10.1007/s004640010282. [DOI] [PubMed] [Google Scholar]
- 70.Tarhan OR, Barut I, Akdeniz Y, Sutcu R, Cerci C, Bulbul M. Fibrinolytic responses of human peritoneal fluid in laparoscopic cholecystectomy: a prospective clinical study. Surg Endosc. 2008;22:1008–1013. doi: 10.1007/s00464-007-9566-4. [DOI] [PubMed] [Google Scholar]
- 71.Brokelman WJ, Holmdahl L, Bergström M, Falk P, Klinkenbijl JH, Reijnen MM. Peritoneal fibrinolytic response to various aspects of laparoscopic surgery: a randomized trial. J Surg Res. 2006;136:309–313. doi: 10.1016/j.jss.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 72.Brokelman WJ, Holmdahl L, Janssen IM, Falk P, Bergström M, Klinkenbijl JH, Reijnen MM. Decreased peritoneal tissue plasminogen activator during prolonged laparoscopic surgery. J Surg Res. 2008;151:89–93. doi: 10.1016/j.jss.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Brokelman WJ, Holmdahl L, Bergström M, Falk P, Klinkenbijl JH, Reijnen MM. Heating of carbon dioxide during insufflation alters the peritoneal fibrinolytic response to laparoscopic surgery: a clinical trial. Surg Endosc. 2007;5:1232–1236. doi: 10.1007/s00464-007-9597-x. [DOI] [PubMed] [Google Scholar]
- 74.Bergström M, Ivarsson ML, Holmdahl L. Peritoneal response to pneumoperitoneum and laparoscopic surgery. Br J Surg. 2002;89:1465–1469. doi: 10.1046/j.1365-2168.2002.02228.x. [DOI] [PubMed] [Google Scholar]
- 75.Neudecker J, Junghans T, Ziemer S, Raue W, Schwenk W. Effect of laparoscopic and conventional colorectal resection on peritoneal fibrinolytic capacity: a prospective randomized clinical trial. Int J Colorectal Dis. 2002;17:426–429. doi: 10.1007/s00384-002-0391-x. [DOI] [PubMed] [Google Scholar]
- 76.Brokelman W, Holmdahl L, Falk P, Klinkenbijl J, Reijnen M (2009) The peritoneal fibrinolytic response to conventional and laparoscopic colonic surgery. J Laparoendosc Adv Surg Tech A 19:489–93 [DOI] [PubMed]
- 77.Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- 78.Wilson MS. Practicalities and costs of adhesions. Colorectal Dis. 2007;9(Suppl 2):60–65. doi: 10.1111/j.1463-1318.2007.01360.x. [DOI] [PubMed] [Google Scholar]