Abstract
Excessive softening of fruits during the ripening process leads to deterioration. This is of significant global importance as softening-mediated deterioration leads to huge postharvest losses. N-glycan processing enzymes are reported to play an important role during climacteric fruit softening: however, to date these enzymes have not been characterized in non-climacteric fruit. Two ripening-specific N-glycan processing enzymes, α-mannosidase (α-Man) and β-D-N-acetylhexosaminidase (β-Hex), have been identified and targeted to enhance the shelf life in non-climacteric fruits such as capsicum (Capsicum annuum). The purification, cloning, and functional characterization of α-Man and β-Hex from capsicum, which belong to glycosyl hydrolase (GH) families 38 and 20, respectively, are described here. α-Man and β-Hex are cell wall glycoproteins that are able to cleave terminal α-mannose and β-D-N-acetylglucosamine residues of N-glycans, respectively. α-Man and β-Hex transcripts as well as enzyme activity increase with the ripening and/or softening of capsicum. The function of α-Man and β-Hex in capsicum softening is investigated through RNA interference (RNAi) in fruits. α-Man and β-Hex RNAi fruits were approximately two times firmer compared with the control and fruit deterioration was delayed by approximately 7 d. It is shown that silencing of α-Man and β-Hex enhances fruit shelf life due to the reduced degradation of N-glycoproteins which resulted in delayed softening. Altogether, the results provide evidence for the involvement of N-glycan processing in non-climacteric fruit softening. In conclusion, genetic engineering of N-glycan processing can be a common strategy in both climacteric and non-climacteric species to reduce the post-harvest crop losses.
Keywords: Capsicum, climacteric, fruit softening, N-glycans, non-climacteric, RNAi, α-mannosidase, β-D-N-acetylhexosaminidase
Introduction
One of the crucial phases of fruit development is ripening, a protracted form of senescence. When fruits attain physiological maturity, their growth ceases and the ripening process begins. The ripening process causes changes in a fruit's cellular metabolism which leads to the development of a soft edible ripe fruit with the desirable quality attributes that attract organisms to facilitate in seed release and dispersal (Giovannoni, 2001, 2004; Adams-Phillips et al., 2004). Fruit texture is the principal quality attribute for shelf life, resistance to post-harvest pathogens, transportability, and consumer acceptability (Seymour et al., 2002; Brummell, 2006; Vicente et al, 2007). The main factor that determines the texture of fruit crops is the rate of softening. Excessive softening exacerbates the damage incurred during handling, decreases the shelf life of fruits, and increases the susceptibility towards post-harvest pathogens. In developing countries, post-harvest losses of fruits and vegetables account for almost 50% of the produce. India, the world's second largest producer of fruits and vegetables, loses 35–40% of the produce due to excessive softening. Therefore, ripening-associated softening is the obvious target to extend the fruit shelf life and to control the post-harvest losses.
Extending the desirable texture during ripening is the key to increasing the shelf life of fruit (Chapple and Carpita, 1998). The loss of fruit firmness/texture during ripening is largely the consequence of disassembly of the cell wall polymers. This is due to the presence of co-ordinated and interdependent action of the numerous cell wall modifying enzymes and proteins such as polysaccharide hydrolases, transglycosylases, lyases, and other cell wall loosening proteins, such as expansin (Huber, 1983; Fischer and Bennett, 1991; Harker et al., 1997; Rose and Bennett, 1999; Wakabayashi, 2000; Brummell and Harpster, 2001; Giovannoni, 2001; Rose et al., 2003; Fry, 2004; Brummell, 2006). Successful efforts to control fruit ripening are based on either reducing the biosynthesis of the plant hormone ethylene and/or slowing down the rate of fruit softening by targeting the genes involved in cell wall modification (Causier et al., 2002). However, these approaches have limited implication to non-climacteric fruits which do not respond to ethylene during ripening and different genes need to be targeted for the different categories of fruits. Thus, identification of a common mechanism that is conserved in different types of fruits would provide new opportunities for controlling fruit softening. Previous studies revealed that the degradation of cell wall N-glycoproteins and free N-glycan levels significantly influence softening in tomato (Solanum lycopersicum), a climacteric fruit (Priem and Gross, 1992; Priem et al., 1993; Meli et al., 2010). However, to date, N-glycan processing enzymes have not been shown to be involved in softening of non-climacteric fruit. The injection of N-glycans Man3(Xyl)GlcNAc(Fuc)GlcNAc and Man3GlcNAc into mature green tomato fruits was previously noted to stimulate ripening (Priem and Gross, 1992). Free N-glycans make up a significant fraction of the soluble oligosaccharide pool in fruit pericarp and the amount increases during the ripening process due to a corresponding boost in their synthesis and/or N-glycoconjugate degradation (Priem et al., 1993). N-linked glycans are known to strongly influence the conformation, stability, and biological activity of glycoproteins, and also their secretion into the extracellular compartments (Lerouge et al., 1998; Rayon et al., 1998). Moreover, our recent studies on N-glycan processing enzymes α-mannosidase (α-Man, EC 3.2.1.24) and β-D-N-acetylhexosaminidase (β-Hex, EC 3.2.1.52) revealed their involvement in the ripening-associated softening of tomato (Meli et al., 2010).
Capsicum (Capsicum annuum) fruit is categorized as non-climacteric on the basis of the patterns of carbon dioxide and ethylene production during ripening (Saltveit, 1977; Lu et al., 1990; Biles et al., 1993). Capsicum is a commercially important vegetable crop and its texture is an important quality attribute for consumers (Priya Sethu et al., 1996). Moreover, the major post-harvest problem with this crop is the excessive softening that causes shrinkage, drying, and pathological disorders which severely reduce the quality and acceptability of the product. The enzymes, α-Man and β-Hex are present at high levels during the ripening of many fruits including capsicum (Priya Sethu and Prabha, 1997; Jagadeesh and Prabha, 2002; Jagadeesh et al., 2004). However, their molecular function remains to be elucidated in a non-climacteric fruit. Thus, these N-glycan processing enzymes were targeted for functional characterization in capsicum. α-Man and β-Hex are known to hydrolyse the glycosidic bonds between carbohydrates, as well as between carbohydrate and non-carbohydrate. α-Man, the member of glycosyl hydrolase (GH) family 38 cleaves the terminal α-mannosidic linkages from both the high mannose type and plant complex type N-glycans which are present in glycoproteins (Hossain et al., 2009). Whereas, β-Hex, the member of GH family 20 cleaves the terminal N-acetyl-D-hexosamine residues and generates the paucimannosidic N-glycans present in most of the plant glycoproteins (Gutternigg et al., 2007; Strasser et al., 2007).
In the present study, the role of α-Man and β-Hex was examined during capsicum fruit development and ripening. The active enzymes were purified and the corresponding genes involved in softening of capsicum fruit were isolated. It was observed that the RNAi-mediated suppression of α-Man and β-Hex in capsicum leads to an enhanced shelf life of fruits. The results suggest that the manipulation of N-glycan processing can be of strategic importance to reduce post-harvest losses in both climacteric and non-climacteric fruits.
Materials and methods
Plant material and growth conditions
Capsicum (cv. California Wonder) seeds, obtained from the National Seeds Corporation Ltd., New Delhi, were germinated in presterilized soil and 15-d-old seedlings were transplanted into pots containing agropeat and vermiculite (2:1 v/v). Plants were grown in a growth chamber with ∼25 °C day temperature, ∼ 22 °C night temperature, ∼65% relative humidity, and a 16/8 h light/dark regimen. Four developmental stages, S1 (7 d after fruit set, DAF), S2 (14 DAF), S3 (21 DAF), and S4 (28 DAF) and three ripening stages, mature green, breaker, and red were selected for the analysis.
Enzyme assay and purification of α-Man and β-Hex from capsicum fruit
Enzyme reactions were performed using 0.4 mM substrates, pNP-Man (for α-Man) and pNP-GlcNAc (for β-Hex) in 10 mM TRIS-Cl, pH 6.0 at 37 °C for 15 min and the reaction was terminated by adding 20 mM Na2CO3. The reaction was monitored colormetrically by measuring the absorbance at 405 nm and the amount of pNP released was determined using a standard curve.
Frozen fruit pericarp was powdered in liquid nitrogen and resuspended in a one-quarter volume of extraction buffer (100 mM TRIS -Cl, pH 7.0 with 0.25 M NaCl and 4 mM PMSF) and extracted overnight at 4 °C. After passing through four layers of cheesecloth, the extract was centrifuged at 10 000 g to precipitate the debris. The supernatant was subjected to 40–60% (w/v) ammonium sulphate precipitation (proteins precipitated by 40% saturation were discarded and proteins precipitated by 60% saturation were taken for further purification). The pellet obtained after the precipitation was reconstituted and dialysed overnight against 25 mM TRIS-Cl, pH 7.0 with one change. The sample was then chromatographed on Q-Sepharose, pH 7.0 (DEAE Sepharose for β-Hex) and eluted with increasing gradient of NaCl (up to 1 M NaCl). The samples (unbound and eluted fractions at 120 mM NaCl for α-Man and β-Hex, respectively) containing the activity were pooled and concentrated by 0–90% (w/v) ammonium sulphate saturation. The pellet was dissolved in 25 mM TRIS-Cl, pH 7.0 and directly loaded onto the Sephadex G100 gel filtration column. Fractions were collected after the void volume, assayed for enzyme activity and resolved on 12.5% SDS-PAGE. For β-Hex purification, ion exchange purified samples were subjected to affinity chromatography on a ConA Sepharose column (eluted with 50 mM α-D-methylmannopyranoside), followed by gel filtration on Sephadex G100. The purified fractions were concentrated using CentriconYM30 (Millipore) and stored at 4 °C.
SDS-PAGE, mass spectrometry and immunoblotting
Proteins were resolved on 12.5% SDS-PAGE. The electrophoresed proteins were stained with Coomassie Brilliant Blue and gel images were digitized with a FluorS imaging system (Bio-Rad). The experimental molecular mass was calculated using standard molecular mass marker proteins. The spots were cut from the gel and analysed by electrospray ion trap time-of-flight mass spectrometry (LC-MS/MS) (Q-Star Pulsar i, Applied Biosystems). The spectra were analysed by Mascot sequence matching software (Matrix Science) against the Viridiplantae (green plants) database.
For immunoblot analysis, 50 μg of protein, quantified by the Bio-Rad protein assay kit, was resolved on 12.5% SDS-PAGE and electrotransferred to Hybond-C Extra membrane (Amersham Biosciences) at a constant current of 150 mA for 2–3 h. Non-specific sites on the membrane were blocked by Blotto in TBS for 1 h and incubated with the primary antibody (1:2500) overnight at 4 °C. Primary antibodies were raised in rabbit using purified α-Man and β-Hex proteins and antibodies were further purified through a protein A sepharose matrix (Amersham Biosciences). Immunodetection was carried out with horseradish peroxidase conjugated anti-rabbit antiserum as the secondary antibody for 1 h and exposing the blot to chemiluminescence substrate (Thermo Scientific).
Glycoprotein staining
Glycoprotein staining was performed according to the instruction manual of the GelCode® Glycoprotein staining kit (Pierce biotechnology). EndoH digestion was performed according to the manufacturer's instruction (New England Biolabs). After the reaction, the samples were resolved on 12.5% SDS PAGE and the gel was stained with Coomassie Brilliant Blue.
HPAE chromatography
HPAE chromatography was used to identify the sugars released by the purified α-Man and β-Hex following incubation with N-glycans. The standard sugar (mannose or GlcNAc) was prepared in deionized water and injected at a concentration of 100 nmoles before and after the analysis of sample. The HPAE-PAD system (Dionex DX 500 BioLC) equipped with a gradient pump (GP 40), an anion exchange column (Carbopac PA-1, 4×250 mm), and an eluant degas module (EDM-2) for pressurizing the eluants with argon was used for the analysis of monosaccharides. The separated monosaccharide was detected by an ED 40 detector equipped with a gold electrode and an Ag/AgCl reference electrode. The resulting chromatographic data were integrated and plotted using a PC based oracle 2 data acquisition system (Indtech Analytical, Bombay). Ten micrograms of N-glycan substrates (Dextra) were incubated at 37 °C for 12 h with 0.5 μg of purified protein in a 100 μl reaction mixture. Then, the sample was filtered through a PVDF membrane to remove the protein and 10 μl of the filtered sample was loaded onto the column.
Subcellular localization
Immunolocalization was carried out as described previously with a few modifications (Sauer et al., 2006). In brief, fruit pericarp sections (5×5 mm) were fixed in 4% (w/v) paraformaldehyde for 14–16 h. Sections were then washed with 1× PBS and sectioned (10–15 μm) with the help of a cryostat microtome (Leica CM1510S). After blocking the non-specific sites with 3% (w/v) BSA for 1 h, the sections were incubated with primary antibody (described in the immunobloting section) at 4 °C for ∼12 h, followed by incubation with FITC-labelled secondary antibody for ∼3 h. After washing with 1× PBS, the sections were viewed with the help of a Nikon 80i-epi-fluorescent/phase contrast/bright field microscope.
Cloning and sequence analysis
Gene-specific degenerate oligonucleotides were designed based on the peptide sequence tags obtained from mass spectrometry (LC-MS/MS) analysis and motifs identified from multiple sequence alignments (see Supplementary Figs S5–S7 at JXB online). Total RNA was isolated from the pericarp of capsicum fruit and reverse transcribed to generate cDNA using a polyA tail specific oligonucleotide (3′ RACE adapter primer; Invitrogen). The left primer corresponding to the peptide QHVADDYAK (5′-CAACATGTKGCTRATGATTATGCMA-3′) and the right primer corresponding to the peptide SGAYVFRP (5′-TGGRCGAAAMACATATGCTCCAGA-3′), were used to amplify a fragment of α-Man that was later cloned into pGEM-T Easy vector (Promega) and sequenced. Then, the remaining 5′ and 3′ regions were amplified using a RACE kit (Invitrogen/Clontech). A β-Hex gene-specific degenerate primer corresponding to the peptide KLNVLHWH (5′-AARYTIAATGTTYTICAYTGGCA-3′) and a nested primer derived from the 3′ RACE adapter primer (Invitrogen) were used to amplify a DNA fragment which was cloned into the pGEM-T Easy vector and sequenced. Further, RACE-PCR was performed to determine the 5′ end sequence of the β-Hex (Clontech). Related protein sequences from other species were taken and phylogenic analysis (MEGA4) was performed (Tamura et al., 2007).
RNA isolation and quantitative RT-PCR
RNA was isolated according to the LiCl precipitation method (Menke et al., 1999) and purified using the RNeasy Mini Kit (Qiagen). Five micrograms of total RNA, quantified using a nanodrop (ND 100) was reverse transcribed using Superscript II (Invitrogen) and diluted five times before using in the quantitative real-time PCR reaction. Quantitative RT-PCR was performed using One Step Real Time RT-PCR (Applied Biosystems) with SYBR Green dye. The oligonucleotide primers used in the amplification are RTMANF 5′-GTTGCTGCTTCAATACCACA-3′ and RTMANR 5′-CTCCAGAGAGCTTCTAACCTG-3′ for α-Man; RTCAPH1F 5′-CGGCGTCAACGAATCCTACT-3′ and RTCAPH1R 5′-TGTAGGCGGCGGAAGAAC-3′ for β-Hex, CAPGAPDHL 5′-AGGCTGCTATCAAGGAGGAGTCT-3′ and CAPGAPDHR 5′-CCTTGGCGTCAAAGATGCTT-3′ for endogenous control GAPDH. Relative gene expression was analysed using the 2–ΔΔCt method (Bovy et al., 2002).
Vectors and Agrobacterium-based transient transformation
RNAi constructs were developed as described previously by Meli et al. (2010). In brief, ∼500 bp 5′ or 3′ coding region of the gene including UTR was cloned into pHANNIBAL (Wesley et al., 2001) in sense and antisense directions at either site of the PDK intron to express hairpin RNAs under the control of CaMV 35S promoter. Finally, a NotI restricted fragment obtained from pHANNIBAL was sub-cloned into a binary vector, pART27. Agroinjection was performed into the pericarp of capsicum fruits at the mature green stage, as described previously with a few modifications (Orzaez et al., 2006). Five millilitres of YEP medium containing rifampicin (50 μg ml−1) and spectinomycin (100 μg ml−1) was inoculated with a single isolated colony of A. tumefaciens (EHA 105) transformed with the appropriate binary vector and incubated at 28 °C for ∼24 h. After that, 200 μl of the grown culture was used to inoculate 50 ml induction medium (0.5% beef extract, 0.1% yeast extract, 0.5% peptone, 0.5% sucrose, 2 mM MgSO4, 20 mM acetosyringone, 10 mM MES, pH 5.6) with antibiotics (rifampicin and spectinomycin) and grown at 28 °C until the OD600 of the culture reached 0.8–1.0. Cells were then recovered by centrifugation (5000 g for 10 min), resuspended in 50 ml of infiltration medium (10 mM MgCl2, 10 mM MES, 200 mM acetosyringone, pH 5.6) and again incubated at room temperature with gentle agitation (20 rpm) for 2 h. Culture was then injected into the fruits at 4–5 spots with the help of a syringe (1 ml; needle size, 0.33×13 mm). The needle was introduced up to 3–4 mm in depth into the fruit tissue and the infiltration solution was gently injected. The total volume of solution injected varied with the size of the fruit, with a maximum of 2 ml in mature green fruits. The progress of the injection process could be observed by a slight change in colour in the infiltrated areas. Blank binary vector pART27 (containing the NotI restricted fragment from pHANNIBAL) was used as a control for the agroinjection experiments.
Textural analysis
Fruit firmness was determined using TA-XT Plus texture analyser (Stable Microsystems). Pericarp discs (10 mm in diameter) were dissected from agroinjected regions in the capsicum fruits and compressed (1 mm of vertical displacement) with a cylindrical probe (2 mm diameter). The test speed was set to 1 mm s−1. The firmness was defined as the response force to a 5 g applied force. The values were subjected to Student's t test to find the significant difference. Gene silencing in the agroinjected areas was confirmed by checking transcript levels of the targeted gene through quantitative RT-PCR. For the determination of texture, compression data were taken for fruits which showed ∼70% suppression of the gene.
Lectin blotting
Lectin blotting was performed as described previously (Meli et al., 2010) to find out the status of the N-glycoproteins in RNAi fruits. To do this, proteins (50 μg) were resolved on 12.5% SDS-PAGE and electrotransferred to the Hybond-C Extra membrane (Amersham Biosciences) for 2–3 h at a constant current of 150 mA. After blocking of the non-specific sites with TBS containing 0.075% (v/v) Tween 20, the membrane was incubated with 0.5 μg ml−1of peroxidase-conjugated wheat germ agglutinin (binds to GlcNAc, Sigma) or 1 μg ml−1 of digoxigenin conjugated Galanthus nivalis agglutinin (binds to mannose, Roche) in blocking buffer for 1 h. The membrane was then washed and developed with chemiluminescent substrate (Thermo Scientific) or incubated for 1 h with anti-digoxigenin-alkaline phosphatase in blocking buffer, further washed and developed with nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP).
The nucleotide sequences for α-mannosidase and β-D-N-acetylhexosaminidase have been deposited in the GenBank database under GenBank Accession numbers GU356593 and GU356594, respectively.
Results
Activity of α-Man and β-Hex commences with ripening of capsicum
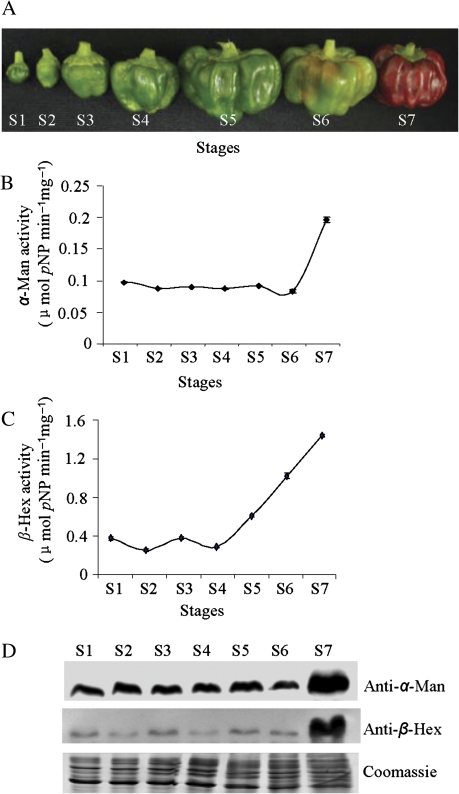
Fleshy fruits like capsicum, after physiological maturity undergo the process of ripening which is accompanied by extensive changes in nutrient composition, texture, pigmentation, aroma, and flavour (Fischer and Bennett, 1991; Priya Sethu et al., 1996; Giovannoni, 2001; Brummell, 2006; Vicente et al., 2007). The texture index of capsicum fruit declines with the advancement of ripening due to excessive softening (Priya Sethu et al., 1996). The development and ripening of this non-climacteric fruit is categorized into seven stages (Fig. 1A). α-Man and β-Hex enzyme assays were performed at all stages of capsicum fruit development and ripening, using p-nitrophenyl-α-D-mannopyranoside (pNP-Man) and p-nitrophenyl-β-D-N-acetylglucosaminide (pNP-GlcNAc) as the substrates. The maximum activity of α-Man and β-Hex was found at the red stage (S7) of capsicum fruit ripening (Fig. 1B, C). However, α-Man and β-Hex activities were not detected in other plant parts such as the stem, leaves, and roots (data not shown). To analyse the expression specificities of the α-Man and β-Hex proteins, various samples extracted from different developmental and ripening stages of fruits were examined by immunoblotting with anti-α-Man and anti-β-Hex antibodies. It was noticed that α-Man and β-Hex proteins accumulate more in ripe fruit compared with unripe fruit (Fig. 1D). This onset of α-Man and β-Hex activity at the critical stages of capsicum fruit ripening suggests their possible role in the process, which prompted us to target these enzymes for further characterization.
Fig. 1.
α-Man and β-Hex activity during capsicum fruit development and ripening. (A) Capsicum fruits used in this study. Flowers were tagged at anthesis and fruits were harvested at different stages of development and ripening at 7 (S1), 14 (S2), 21 (S3), 28 (S4) d after fruit set (DAF) and mature green (S5), breaker (S6), and red (S7) stages. (B) Specific activity of the α-Man enzyme was determined in different stages of fruits. Data are mean ±sem, n=3. (C) Specific activity of the β-Hex enzyme in different stages of fruits. Data are mean ±sem, n=3. (D) α-Man and β-Hex protein levels during the fruit development and ripening stages of capsicum were determined by immunoblotting.
Purification and characterization of capsicum α-Man and β-hex
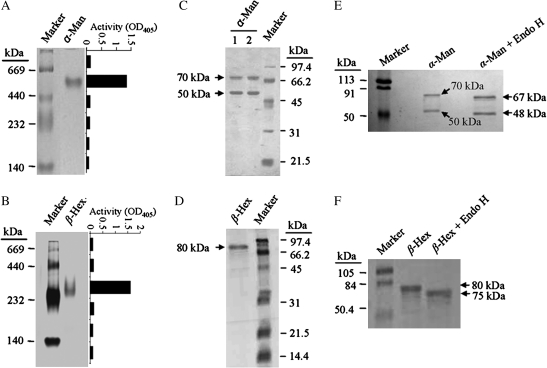
The enzymes α-Man and β-Hex were purified to electrophoretic homogeneity from capsicum fruit pericarp by subjecting them to various column chromatography techniques. The purification procedure consisted of ammonium sulphate precipitation, anion exchange, ConA lectin affinity and gel filtration chromatography (see Supplementary Figs S1 and S2 at JXB online). The specific activity of the α-Man and β-Hex increased upto ∼37-fold and ∼175-fold from the crude extract with the recovery of ∼7% and ∼21%, respectively (see Supplementary Table S1 at JXB online). The optimum pH was found to be pH 6.0 for the pNP-Man (Km 1.6 mM) and pNP-GlcNAc (Km 0.141 mM) hydrolysis by α-Man and β-Hex, respectively, and both the enzymes displayed a wide range of pH stability (see Supplementary Fig. S3A, B at JXB online). However, they preferred different temperatures (55 °C for α-Man and 45 °C for β-Hex) for optimum activity (see Supplementary Fig. S3C, D at JXB online). α-Man and β-Hex were found to be stable at 60 °C and 42 °C, respectively, for 1 h without considerable loss of activity (see Supplementary Fig. S3E, F at JXB online). The molecular masses of α-Man and β-Hex were ∼500 kDa and ∼300 kDa, respectively, on non-denaturing PAGE (Fig. 2A, B). However, the molecular masses of α-Man and β-Hex on the Superdex 200 analytical gel filtration column were found to be ∼290 kDa and ∼219 kDa, respectively. This appeared to be different from the molecular mass determined by the non-denaturing PAGE. In order to confirm that the protein band observed in non-denaturing PAGE corresponds to α-Man or β-Hex, an enzyme assay was performed on excised gel slices from the unstained non-denaturing PAGE, which corresponds to the band position in the stained gel. This was achieved by extracting proteins from mashed gel slices in the extraction buffer and analysing enzyme activity in the extracted protein. The assay conferred α-Man or β-Hex activity, confirming the proteins (Fig. 2A, B). Therefore, the discrepancy in molecular mass determined by the gel filtration and non-denaturing PAGE analysis could be due to glycoproteic nature of α-Man and β-Hex. This was confirmed by periodic acid–Schiff (PAS) staining (see Supplementary Fig. S4A, B at JXB online) and deglycosylation with endoglycosidase H (Fig. 2E, F). Further, when resolved on SDS-PAGE, α-Man and β-Hex represented two (70 kDa and 50 kDa) and one (80 kDa) polypeptide(s), respectively, suggesting that they function as the oligomeric proteins in capsicum (Fig. 2C, D).
Fig. 2.
Purification and characterization of α-Man and β-Hex from capsicum fruit pericarp. (A) Purified α-Man resolved on 6% non-denaturing PAGE showing single protein band of ∼500 kDa. The graph represents the activity of α-Man determined in the corresponding gel area. (B) Purified β-Hex resolved on 6% non-denaturing PAGE showing a single protein of ∼300 kDa. The graph depicts the enzyme activity measured in the corresponding gel area. (C) Purified α-Man resolved on 12.5% SDS-PAGE showing two subunits (70 kDa and 50 kDa). Lane 1, α-Man treated with β-mercaptoethanol, lane 2, α-Man without β-mercaptoethanol treatment. (D) Purified β-Hex denatured and resolved on 12.5% SDS-PAGE, indicating a single polypeptide of 80 kDa. (E) Deglycosylation of the purified α-Man by EndoH revealed a shift as compared to the undigested. (F) EndoH digestion of the purified β-Hex confirmed the presence of glycan moiety.
Capsicum α-Man and β-Hex are N-glycan processing enzymes localized in the cell wall
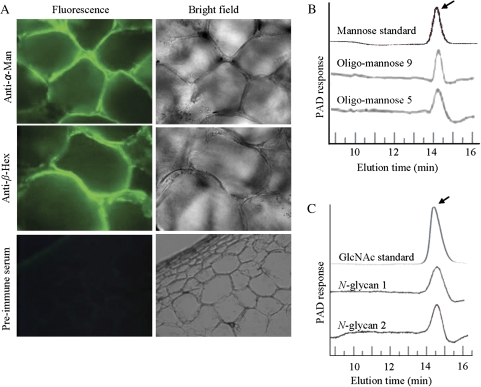
In the pericarp of fleshy fruits like tomato, free N-glycans account for more than 1 μg g−1 of the fresh weight, which further increases during the ripening process (Priem et al., 1993). Moreover, the blocking of N-glycosylation with tunicamycin delays fruit ripening. Further, when injected into the fruits, N-glycans are known to stimulate ripening (Priem and Gross, 1992). It is also known that tomato α-Man and β-Hex are involved in N-glycan processing (Meli et al., 2010). Therefore, to verify the N-glycans processing functions of the capsicum α-Man and β-Hex, the purified enzymes were incubated with different N-glycans commonly found in fruits and the release of mannose or GlcNAc was determined by high performance anion exchange chromatography (HPAE; Fig. 3B, C). During the ripening of fleshy fruits like capsicum, cell wall disassembly contributes to changes in the cell wall rheological properties and softening of the ripe fruit (Fry, 2004; Brummell, 2006). To investigate the subcellular localization of α-Man and β-Hex, immunolocalization experiments were performed using anti-α-Man and anti-β-Hex antibodies. Immunolocalization assays revealed that α-Man and β-Hex were cell wall proteins (Fig. 3A), which was further supported by the reports of α-Man and β-Hex homologues in tomato and Arabidopsis (Jamet et al., 2006; Meli et al., 2010).
Fig. 3.
α-Man and β-Hex are the cell wall localized N-glycan processing enzymes. (A) The cell wall localization of the α-Man and β-Hex proteins was revealed using affinity purified antibodies. Preimmune serum was used as the negative control. (B) HPAE chromatograms show the N-glycan processing ability of α-Man. Arrow indicates the release of mannose residues. (C) N-glycan processing function of the β-Hex was determined by HPAE chromatography. N-glycan 1 and N-glycan 2 are biantennary N-linked core pentasaccharide and asialo, agalacto, biantennary, respectively. The arrow indicates the release of GlcNAc residues.
Cloning and expression analysis of capsicum α-Man and β-Hex
In order to clone the polynucleotide sequences encoding the capsicum α-Man and β-Hex enzymes, gene-specific degenerate oligonucleotides were designed. This was based on the peptide sequence information obtained from mass spectrometry analysis (see Supplementary Fig. S7A, C at JXB online) and the conserved motifs identified from multiple sequence alignment of the plant α-Man and β-Hex sequences (see Supplementary Figs S5 and S6 at JXB online). cDNAs specific to the α-Man (3093 bp) and β-Hex (1725 bp) were obtained from the total RNAs of fruit pericarp by a combination of reverse transcription (RT)-PCR amplifications with degenerate primers, and the rapid amplification of cDNA ends (5′ and 3′ RACE). The capsicum α-Man encodes a polypeptide of 1030 amino acids (∼117 kDa) which shares sequence homology with the GH 38 family proteins (see Supplementary Fig. S5 at JXB online). Three domains, related to the GH 38 family were identified in α-Man protein using a conserved domain architecture retrieval tool (CDART, www.ncbi.nlm.nih.gov). They are an N-terminal polysaccharide deacetylase, a C-terminal GH 38, and an internal α-Man middle domain (see Supplementary Figs S7B, S8A at JXB online). This type of polysaccharide deacetylase domain is also found in nodulation protein B of Rhizobium which is a chitooligosaccharide deacetylase (Freiberg et al., 1997), yeast chitin deacetylase (Mishra et al., 1997) and endoxylanases which hydrolyses glucosidic bonds in xylan (Millward-Sadler et al., 1994). The α-Man domain which is predominantly found in the enzyme α-mannosidase, adopts a structure that consists of three alpha helices, in an immunoglobulin/albumin-binding domain-like fold. The coding region of β-Hex is 1.725 kb which encodes a polypeptide of 574 amino acids (64 kDa) that showed sequence homology with the GH 20 family proteins (see Supplementary Fig. S6 at JXB online). Capsicum β-Hex has two domains: an N-terminal GH 20b domain which contains a zincin-like fold and a C-terminal GH 20 domain which contains a TIM barrel fold (see Supplementary Figs S7D and S8B at JXB online). The TIM barrel is a conserved protein fold consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone (Tews et al., 1996). This structure is named after triosephosphate isomerase, a conserved glycolytic enzyme. However, the zincin-like fold is named after metalloproteases (zincins) consisting of mixed β sheets with connections over the free side of the sheet. In silico analysis revealed the presence of eight probable N-glycosylation sites in both α-Man and β-Hex proteins. Moreover, PSORT and TargetP programs predicted N-terminal signal peptide sequence in α-Man and β-Hex with probable cleavage site between amino acids 23 to 24 (see Supplementary Fig. S7A, C at JXB online). Analysis of the protein sequences and comparative homology modelling of α-Man and β-Hex orthologues in capsicum and tomato revealed a similar organization of the GH domains, suggesting that their function is similar in these Solanaceae family plants (see Supplementary Fig. S8A, B at JXB online).
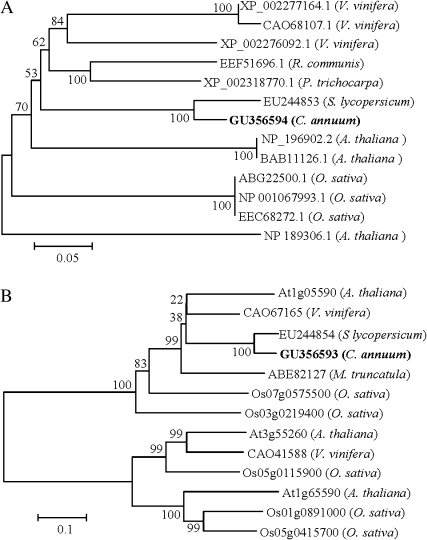
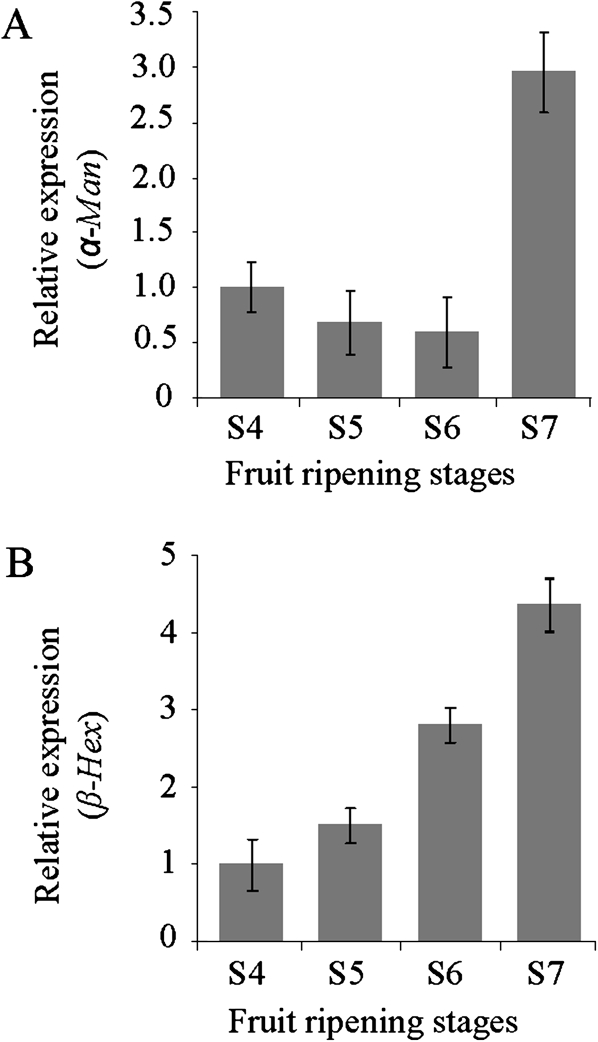
Phylogenetic analysis using full-length sequences revealed that capsicum α-Man and β-Hex proteins are most similar to tomato orthologues with 91% and 88% identity, respectively (Fig. 4A, B). The enzyme activity and the level of α-Man and β-Hex proteins increase during capsicum fruit ripening at the red stage (Fig. 1B–D). Hence, the relative transcript level of the α-Man and β-Hex was determined in the capsicum fruits, to correlate it with enzyme activity. Quantitative RT-PCR analysis revealed a ∼3-fold and a ∼4-fold increase in the transcript level of α-Man and β-Hex, respectively, at the red stage fruits compared with the unripe fruit (Fig. 5A, B). However, no detectable level of transcript was found in other parts of the plant, for example, stem, leaves, and roots (data not shown).
Fig. 4.
The phylogenetic relationships among the α-Man and β-Hex homologues. Values at the branch nodes indicate the numbers of trials out of 100 that produced each node. The 0.05 and 0.1 scale represents 5% and 10% change, respectively. (A) Phylogenetic analysis of the α-Man polypeptide sequences taken from selected plants. (B) Phylogram showing the relationship of selected β-Hex from plants.
Fig. 5.
α-Man and β-Hex transcripts accumulate specifically during the ripening of capsicum. The relative transcript level of the α-Man and β-Hex was determined during capsicum fruit ripening by quantitative RT-PCR analysis. GAPDH was used as the endogenous control. Data are mean ±sem, n=3. (A) Relative expression of the α-Man during the different stages of capsicum ripening. (B) Relative expression of the β-Hex during capsicum fruit ripening stages.
Silencing of α-Man or β-Hex leads to reduced softening and enhanced shelf life of capsicum fruits
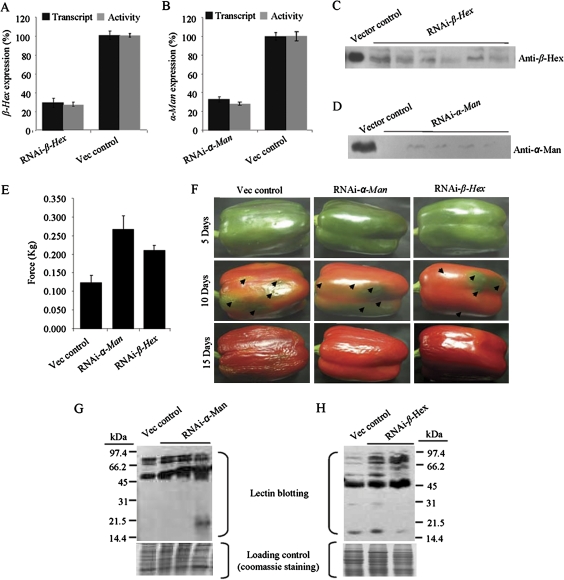
Backed by many observations, the functional characterizations of α-Man and β-Hex became imperative to demonstrate their role in ripening and/or softening of capsicum. Suppression of the genes by agroinjection has become a handy tool for analysing the gene function (Orzaez et al., 2006). In order to verify the effectiveness of the agroinjection method for analysing fruit ripening traits, tomato orthologues of α-Man and β-Hex, whose function is known in fruit ripening, were targeted (Meli et al., 2010). After 1 week, the RNAi agroinjected tomatoes for both the genes developed green sectors around the agroinjected spots, indicating delayed ripening (see Supplementary Fig. S9A, B at JXB online). As the stable transformation in capsicum is not well documented, the endogenous expression of α-Man or β-Hex gene was also silenced in capsicum fruits by Agrobacterium-mediated transient expression of gene-specific hairpin RNAs, under the control of the CaMV 35S promoter. The expression cassette consisted of a ∼500 bp fragment of the gene, sub-cloned in the sense and antisense orientations at either site of an intron that are designed to assemble into dsRNA upon expression (Wesley et al., 2001; Meli et al., 2010). Each mature green fruit was agroinjected at 4–5 spots on the surface depending upon its size. Fruits were harvested 5–7 d after agroinjection and analysed for the silencing of α-Man or β-Hex gene. To confirm and quantitate suppression at the molecular level, quantitative RT-PCR and immunoblot analyses were performed, which revealed up to 70% suppression (Fig. 6A, B) and reduced accumulation of the α-Man and β-Hex proteins (Fig. 6C, D). The reduction in transcript and protein level was found to be correlated with the loss of α-Man and β-Hex enzyme activity in the suppressed fruits (Fig. 6A, B).
Fig. 6.
RNAi mediated silencing of the α-Man or β-Hex enhances capsicum fruit shelf life and reduces the rate of fruit softening and N-glycoprotein degradation. (A, B) Capsicum fruits were agroinjected at the mature green stage. The transcript level and enzyme activity was determined in the RNAi fruits 7 d after agroinjection by quantitative RT-PCR and enzyme assay, respectively, which revealed ∼70% suppression of the α-Man and β-Hex expression. Data are mean ±sem, n=3. (C, D) Immunoblot analysis of agroinjected fruits using antibodies specific to β-Hex or α-Man (70 kDa subunit). Lanes represent proteins isolated from individual RNAi fruits. (E) Firmness of fruit pericarp was quantitatively measured 15 d after agroinjection using a texture analyser (1 mm compression). Data are mean ±sem, n=15. P is 0.0021 and 0.0008 for RNAi-α-Man and β-hex, respectively. (F) The progression of fruit deterioration was recorded by time lapse photography. Time after agroinjection is specified by days. Arrows indicate injection marks on fruit. (G, H) Lectin blots, using G. nivalis agglutinin (G) and wheat germ agglutinin (H) show enhanced level of the α-mannose- and GlcNAc-containing glycoproteins in the α-Man and β-Hex RNAi fruits, respectively. Total proteins isolated from individual RNAi fruits were loaded onto separate lanes of SDS-PAGE. The analysis was done with fruits 10 d after agroinjection.
In order to determine the effect of silencing of α-Man and β-Hex on fruit texture, the rate of softening of RNAi fruits was determined by quantitative measurement of the fruit firmness using a Texture Analyser (TA-XT plus, Stable Microsystem). For this, the pericarp discs of agroinjected fruits were subjected to texture analysis (1 mm compression) using a cylindrical probe which revealed significant difference in firmness between RNAi- and vector only- (control) agroinjected fruits. The pericarps of α-Man and β-Hex RNAi fruits were ∼2.2 and ∼1.7 times firmer than the control, respectively, (Fig. 6E). In addition to compression analysis, the loss of fruit firmness during ripening and the consequent spoilage of fruit could be determined qualitatively by the degree of wrinkling of the fruit skin. For this, RNAi- and vector only-agroinjected fruits were harvested 5–7 d after agroinjection and stored at 22–24 °C temperatures in 55–60% relative humidity. Time-lapse photography was performed at 5 d intervals to see the visible signs of fruit softening. Although ripening was normal under these post-harvest storage conditions, only the vector-agroinjected fruits started deteriorating rapidly after 8–10 d of harvest compared with RNAi fruits which showed no signs of softening upto 15–17 d after harvest (Fig. 6F). In order to determine the status of the mannose- and GlcNAc-containing glycoproteins in the RNAi fruits, lectin blotting was performed using G. nivalis agglutinin and wheat germ agglutinin. The analysis revealed an enhanced level of the mannose- and GlcNAc-containing glycoproteins in the fruits of α-Man and β-Hex RNAi agroinjection, respectively (Fig. 6G, H).
Discussion
The identification and functional characterization of α-Man and β-Hex from capsicum, a non-climacteric fruit crop, in contrast to tomato which is climacteric (requires ethylene for fruit ripening) are reported here. Our results demonstrate that α-Man and β-Hex are involved in the ripening-associated softening of capsicum fruit. It was observed that α-Man and β-Hex transcripts and proteins show ripening-specific accumulation that can be correlated with the increase in enzyme activity (Fig. 1B–D). The temporal relationship between the transcript level and enzyme activity suggests that the expression of both α-Man and β-Hex is regulated at the level of transcription. The ripening-specific expression of these genes indicates their function in ripening and/or softening of capsicum. The roles of α-Man and β-Hex in the ripening and/or softening of capsicum were examined by cloning the genes and then systematically testing their functions.
The calculated molecular mass of the α-Man and β-Hex polypeptides was found to be less than the molecular mass determined by SDS-PAGE analysis (Fig. 2C, D), suggesting that both the proteins undergo modification(s) after their translation. The post-translational modifications could possibly be glycosylation, as both the proteins have N-glycosylation sites (see Supplementary Fig. S7A, C at JXB online) and the glycoproteic nature of the proteins was confirmed by the PAS staining (see Supplementary Fig. S4A, B at JXB online). However, deglycosylation of the purified protein with EndoH revealed a ∼5 kDa glycans moiety (Fig. 2E, F). This is less than the molecular mass difference in β-Hex, determined by SDS-PAGE (80 kDa) and calculated based on the protein sequences (64 kDa), suggesting the involvement of another kind of post-translational modification(s) in β-Hex, in addition to glycosylation. A previous report suggests that the N-glycans can protect the protein from proteolytic degradation and they are responsible for the stability and biological activity of the glycoproteins (Rayon et al., 1998). The presence of N-glycans also affects both the co- and post-translational folding of the proteins. Therefore, the stability of α-Man and β-Hex at a wide pH range can be attributed to their glycosylation. This is also likely to be true for their thermostability.
It was observed that the α-Man consists of two separable subunits, indicating an association between two subunits and the involvement of hydrophobic interaction, rather than the inter-disulphide bonds, as subunits were dissociated under reducing as well as non-reducing conditions (Fig. 2C). These two subunits are probably derived from the processing of the 117 kDa precursor polypeptide (see Supplementary Fig. S7A at JXB online), which is encoded by the α-Man mRNA (3093 nt). This assumption was made on the basis of the fact that the amino acid sequences of 70 kDa and 50 kDa polypeptides actually matched the N-terminal and C-terminal, respectively, of the encoded polypeptide (mass spectrometry analysis). Further, the single mRNA for α-Man suggests that the subunits are the consequence of post-translational protease cleavage rather than post-transcriptional modification.
Our immunolocalization assay revealed that both α-Man and β-Hex are localized in the cell wall (Fig. 3A), which was further reinforced by the reports in tomato and Arabidopsis (Jamet et al., 2006; Meli et al., 2010) and the presence of the signal peptide in the protein sequences for their entry into the secretory pathway (see Supplementary Fig. S7A, C at JXB online). It has been shown that the capsicum α-Man and β-Hex are able to cleave mannose and GlcNAc residues from N-glycans (Fig. 3B, C). There is also evidence in tomato and Arabidopsis that α-Man and β-Hex are involved in N-glycan processing (Strasser et al., 2007; Gutternigg et al., 2007; Meli et al., 2010). The cell wall localization and N-glycan processing abilities of α-Man and β-Hex suggest their participation in the degradation of cell wall N-glycoproteins and the generation of free N-glycans, which further stimulates ripening. This is possibly by interaction with other protein(s) to transduce the potential ripening signal.
The silencing of α-Man and β-Hex in capsicum results in the enhancement of the fruit shelf life (Fig. 6F). This may be due to a reduced rate of softening, as the pericarp of RNAi fruits were found to be firmer than the control (Fig. 6E). The enzymes, α-Man and β-Hex target glycoproteins and cleave the terminal mannose and GlcNAc residues, respectively, present in the N-linked glycans (Fig. 6G, H). These results suggest that the intact cell wall polysaccharides are broken down to a lesser extent in α-Man and β-Hex silenced fruits as compared with control. Furthermore, the reduced degradation of N-glycoproteins in α-Man and β-Hex silenced fruits suggests a link between N-glycoprotein degradation and, consequently, the increase in the free level of N-glycans and fruit softening in capsicum. A similar function was proposed for the α-Man and β-Hex in climacteric fruit softening using tomato as a model plant (Meli et al., 2010). The tomato and capsicum orthologues of these two proteins show a high degree of sequence similarity (∼90% identity). Moreover, the predicted 3D structures of α-Man and β-Hex are found to be similar in tomato and capsicum (see Supplementary Fig. S8A, B at JXB online). All these observations indicate that the α-Man and β-Hex genes have a similar function during the ripening of tomato and capsicum. The data also suggest that N-glycan processing is a conserved event operating during ripening of both climacteric and non-climacteric fruits.
This work identified two N-glycan processing enzymes and their novel cognate genes, contributing to the ripening-associated softening in capsicum fruit. Overall, the data demonstrate that the N-glycan processing enzymes like α-Man and β-Hex can be targeted for the improvement of shelf life in non-climacteric fruits. The silencing of α-Man and β-Hex will not only block the degradation of the cell wall N-glycoproteins, but also inhibit the possible ripening-inducing events activated by the free N-glycans. Moreover, the possibility cannot be ruled out of α-Man and β-Hex modulating the function of cell wall-modifying enzymes during their maturation. The high level of α-Man and β-Hex activities in fruits like tomato, capsicum, papaya, banana, mango, etc. and the near identities with the sequences of V. vinifera orthologues suggest their potential involvement in both climacteric and non-climacteric fruit softening (Priya Sethu and Prabha, 1997; Jagadeesh and Prabha, 2002; Jagadeesh et al., 2004; Meli et al., 2010). In conclusion, genetic engineering of N-glycan processing is an important strategy for the improvement of fruit crops, which can be applied to both climacteric and non-climacteric species.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Purification of capsicum α-Man.
Supplementary Fig. S2. Purification of capsicum β-Hex.
Supplementary Fig. S3. Biochemical characterization of capsicum α-Man and β-Hex.
Supplementary Fig. S4. Capsicum α-Man and β-Hex are glycoproteins.
Supplementary Fig. S5. Multiple sequence alignments of α-Man proteins from different plant species.
Supplementary Fig. S6. Multiple sequence alignments of β-Hex proteins from different plant species.
Supplementary Fig. S7. The protein sequences of capsicum α-Man and β-Hex deduced from the cloned cDNA sequences.
Supplementary Fig. S8. Comparative homology modelling of tomato and capsicum α-Man and β-Hex.
Supplementary Fig. S9. Agrobacterium-mediated transient silencing of α-Man and β-Hex in tomato.
Supplementary Table S1. Summary of capsicum α-Man and β-Hex protein purification.
Supplementary Material
Acknowledgments
We thank CSIRO Plant Industry Australia for the pHANNIBAL and pART 27 vectors. SG and VSM have received fellowships from the Council of Scientific and Industrial Research and AK has received a fellowship from the National Institute of Plant Genome Research. This work was supported by the Department of Biotechnology, the Ministry of Science and Technology, Government of India.
References
- Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends in Plant Science. 2004;9:331–338. doi: 10.1016/j.tplants.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Biles CL, Wall MM, Blackstone K. Morphological and physiological changes during maturation of New Mexican type peppers. Journal of the American Society for Horticultural Science. 1993;118:476–480. [Google Scholar]
- Bovy A, de Vos R, Kemper M, et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor gene LC and C1. The Plant Cell. 2002;14:2509–2526. doi: 10.1105/tpc.004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology. 2001;47:311–340. [PubMed] [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Functional Plant Biology. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Causier B, Kieffer M, Davies B. MADS-Box genes reach maturity. Science. 2002;296:275–276. doi: 10.1126/science.1071401. [DOI] [PubMed] [Google Scholar]
- Chapple C, Carpita N. Plant cell walls as targets for biotechnology. Current Opinion in Plant Biology. 1998;1:179–185. doi: 10.1016/s1369-5266(98)80022-8. [DOI] [PubMed] [Google Scholar]
- Fischer RL, Bennett AB. Role of cell wall hydrolases in fruit ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:675–703. [Google Scholar]
- Freiberg C, Fellay R, Bairoch A, Broughton WJ, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ. Genetic regulation of fruit development and ripening. The Plant Cell. 2004;16:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutternigg M, Kretschmer-Lubich D, Paschinger K, Rendic D, Hader J, Geier P, Ranftl R, Jantsch V, Lochnit G, Wilson IBH. Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated mechanisms in nematodes, plants, and insects. Journal of Biological Chemistry. 2007;282:27825–27840. doi: 10.1074/jbc.M704235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker FR, Redgwell RJ, Hallett IC, Murray SH, Carter G. Texture of fresh fruit. Horticultural Reviews. 1997;20:121–224. [Google Scholar]
- Hossain MA, Nakamura K, Kimura Y. α-Mannosidase involved in turnover of plant complex type N-glycans in tomato (Lycopersicum esculentum) fruits. Bioscience, Biotechnology, and Biochemistry. 2009;73:140–146. doi: 10.1271/bbb.80561. [DOI] [PubMed] [Google Scholar]
- Huber DJ. The role of cell wall hydrolases in fruit softening. Horticultural Reviews. 1983;5:169–219. [Google Scholar]
- Jagadeesh BH, Prabha TN, Srinivasan K. Activities of β-hexosaminidase and α-mannosidase during development and ripening of bell capsicum (Capsicum annuum var. variata) Plant Science. 2004;167:1263–1271. [Google Scholar]
- Jagadeesh BH, Prabha TN. β-Hexosaminidase, an enzyme from ripening bell capsicum (Capsicum annuum var. variata) Phytochemistry. 2002;61:295–300. doi: 10.1016/s0031-9422(02)00181-4. [DOI] [PubMed] [Google Scholar]
- Jamet E, Canut H, Boudart G, Pont-Lezica RF. Cell wall proteins: a new insight through proteomics. Trends in Plant Science. 2006;11:33–39. doi: 10.1016/j.tplants.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L. N-Glycoprotein biosynthesis in plants: recent developments and future trends. Plant Molecular Biology. 1998;38:31–48. [PubMed] [Google Scholar]
- Lu G, Yang C, Liang H, Lu Z. Changjiao hot peppers are nonclimacteric. HortScience. 1990;25:807. [Google Scholar]
- Meli VS, Ghosh S, Prabha TN, Chakraborty N, Chakraborty S, Datta A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proceedings of the National Academy of Sciences, USA. 2010;107:2413–2418. doi: 10.1073/pnas.0909329107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke FLH, Parchmann S, Mueller MJ, Kijne JW, Memelink J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthesis genes in Catharanthus roseus. Plant Physiology. 1999;119:1289–1296. doi: 10.1104/pp.119.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Poole DM, Henrissat B, Hazlewood GP, Clarke JH, Gilbert HJ. Evidence for a general role for high-affinity non-catalytic cellulose binding domains in microbial plant cell wall hydrolases. Molecular Microbiology. 1994;11:375–382. doi: 10.1111/j.1365-2958.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Mishra C, Semino CE, McCreath KJ, de la Vega H, Jones BJ, Specht CA, Robbins PW. Cloning and expression of two chitin deacetylase genes of Saccharomyces cerevisiae. Yeast. 1997;13:327–336. doi: 10.1002/(SICI)1097-0061(19970330)13:4<327::AID-YEA96>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Orzaez D, Mirabel S, Wieland WH, Granell A. Agroinjection of tomato fruits. a tool for rapid functional analysis of transgenes directly in fruit. Plant Physiology. 2006;140:3–11. doi: 10.1104/pp.105.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priem B, Gitti R, Bush CA, Gross KC. Structure of ten free N-glycans in ripening tomato fruit (arabinose is a constituent of a plant N-glycan) Plant Physiology. 1993;102:445–458. doi: 10.1104/pp.102.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priem B, Gross KC. Mannosyl and xylosyl-containing glycans promote tomato (Lycopersicon esculentum Mill.) fruit ripening. Plant Physiology. 1992;98:399–401. doi: 10.1104/pp.98.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya Sethu KM, Prabha TN, Tharanathan RN. Post-harvest biochemical changes associated with the softening phenomenon in Capsicum annuum fruits. Phytochemistry. 1996;42:961–966. [Google Scholar]
- Priya Sethu KM, Prabha TN. α-d-Mannosidase from Capsicum annuum. Phytochemistry. 1997;44:383–387. [Google Scholar]
- Rayon C, Lerouge P, Faye L. The protein N-glycosylation in plants. Journal of Experimental Botany. 1998;49:1463–1472. [Google Scholar]
- Rose JKC, Bennett AB. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends in Plant Science. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- Rose JKC, Catala C, Gonzalez-Carranza CZH, Roberts JA. Plant cell wall disassembly. In: Rose JKC, editor. The plant cell wall. Oxford, UK: Blackwell Publishing Ltd; 2003. pp. 264–324. [Google Scholar]
- Saltveit ME. Carbon dioxide, ethylene and color development in ripening mature green bell peppers. Journal of the American Society for Horticultural Sciences. 1977;102:523–525. [Google Scholar]
- Sauer M, Paciorek T, Benkova E, Friml J. Immunocytochemical techniques for whole-mount in situ protein localization in plants. Nature Protocols. 2006;1:98–103. doi: 10.1038/nprot.2006.15. [DOI] [PubMed] [Google Scholar]
- Seymour GB, Manning K, Eriksson EM, Popovich AH, King GJ. Genetic identification and genomic organization of factors affecting fruit texture. Journal of Experimental Botany. 2002;53:2065–2071. doi: 10.1093/jxb/erf087. [DOI] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Schoberer J, Svoboda B, Liebminger E, Glossl J, Altmann F, Steinkellner H, Mach L. Enzymatic properties and subcellular localization of Arabidopsis β- N-acetylhexosaminidases. Plant Physiology. 2007;145:5–16. doi: 10.1104/pp.107.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, Vorgias CE. Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nature Structural Biology. 1996;3:638–648. doi: 10.1038/nsb0796-638. [DOI] [PubMed] [Google Scholar]
- Vicente AR, Saladie M, Rose JKC, Labavitch JM. The linkage between cell wall metabolism and fruit softening: looking to the future. Journal of the Science of Food and Agriculture. 2007;87:1435–1448. [Google Scholar]
- Wakabayashi K. Changes in cell wall polysaccharides during fruit ripening. Journal of Plant Research. 2000;113:231–237. [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, et al. Construct design for efficient, effective and high throughput gene silencing in plants. The Plant Journal. 2001;27:581–590. doi: 10.1046/j.1365-313x.2001.01105.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.