Abstract
The effects of dark chilling on the leaf-side-specific regulation of photosynthesis were characterized in the C4 grass Paspalum dilatatum. CO2- and light-response curves for photosynthesis and associated parameters were measured on whole leaves and on each leaf side independently under adaxial and abaxial illumination before and after plants were exposed to dark chilling for one or two consecutive nights. The stomata closed on the adaxial sides of the leaves under abaxial illumination and no CO2 uptake could be detected on this surface. However, high rates of whole leaf photosynthesis were still observed because CO2 assimilation rates were increased on the abaxial sides of the leaves under abaxial illumination. Under adaxial illumination both leaf surfaces contributed to the inhibition of whole leaf photosynthesis observed after one night of chilling. After two nights of chilling photosynthesis remained inhibited on the abaxial side of the leaf but the adaxial side had recovered, an effect related to increased maximal ribulose-1,5-bisphosphate carboxylation rates (Vcmax) and enhanced maximal electron transport rates (Jmax). Under abaxial illumination, whole leaf photosynthesis was decreased only after the second night of chilling. The chilling-dependent inhibition of photosynthesis was located largely on the abaxial side of the leaf and was related to decreased Vcmax and Jmax, but not to the maximal phosphoenolpyruvate carboxylase carboxylation rate (Vpmax). Each side of the leaf therefore exhibits a unique sensitivity to stress and recovery. Side-specific responses to stress are related to differences in the control of enzyme and photosynthetic electron transport activities.
Keywords: Abaxial/adaxial leaf specification, chilling responses, C4 photosynthetic models, Paspalum dilatatum, photosynthetic regulation, stomatal regulation
Introduction
The understanding of plant responses to environmental stresses is crucial for models of plant behaviour in a changing climate. Plant growth and development are strongly influenced by the perception of signals from the environment. The perception of light quality and day length, for example, influences plant growth and architecture, flowering time, germination, and other processes. Similarly, nutrient availability, temperature, and interactions with other organisms also modify plant development. Exposure to low non-freezing (chilling) temperatures induces structural, developmental, physiological, cellular, and molecular alterations in plants (Lyons, 1973; Smallwood and Bowles, 2002; Sung et al., 2003) that are largely associated with altered membrane function (Lyons, 1973). Chilling also leads to the induction of a network of cold-responsive genes, which are considered to be responsible for the biochemical and physiological changes that allow plants to acclimate to a low temperature growth environment. Cold acclimation involves the regulated expression of effecter genes that are collectively called the COLD-REGULATED (COR) genes, whose function is to protect cell membranes and thereby prevent cellular dehydration. Low-temperature induction of the COR genes is regulated by CRT/CRE BINDING FACTOR (CBF) genes, whose expression is influenced by the plant circadian clock, which regulates the magnitude of the transcriptional activation of the CBF genes by low temperatures. For example, CBF expression is lower when chilling is experienced in the dark than in the light (Maruyama et al., 2004; Fowler et al., 2005). Moreover, chilling disrupts the circadian pattern of activity of some enzymes in leaves such as sucrose phosphate synthase and nitrate reductase, whose activation is delayed following exposure to chilling (Jones et al., 1998).
Persistent inhibition of photosynthesis occurs when plants are exposed to chilling temperatures in a diverse group of plant species. The extent of chilling-induced inhibition depends on the species, the sensitivity to chilling, the duration of the stress, and the light intensity experienced in the photoperiod following the stress. Photosynthesis is decreased following exposure to chilling temperatures even when the stress is applied in the dark in some C3 (e.g. Flexas et al., 1999; van Heerden et al., 2003a; Feng and Cao, 2005) and C4 (e.g. Ludlow and Wilson, 1971; Pasternak and Wilson, 1972; Pittermann and Sage, 2001) species. Dark-chilling-induced limitations to stomatal function have been reported in C3 and C4 plants, and these contribute to the observed inhibition of photosynthesis rates at least in C3 species (e.g. Pasternak and Wilson, 1972; Martin et al., 1981; Bauer et al., 1985; van Heerden et al., 2003a; Feng and Cao, 2005). Non-stomatal limitations on whole leaf CO2 assimilation have also been reported after dark chilling in both C3 and C4 plants. For example, direct effects on electron transport (Shen et al., 1990; van Heerden et al., 2003b; Bertamini et al., 2006; Strauss et al., 2007) and the activities of photosynthetic enzymes (Jones et al., 1998; Jun et al., 2001; Pittermann and Sage, 2001; van Heerden et al., 2003a, b; Zhou et al., 2004) have been observed.
The dorsoventral regulation of photosynthesis is well characterized in dicotyledonous C3 plants and is related to the presence of two types of mesophyll cell and a continuous airspace system that provides gaseous continuity across the leaf blade (Terashima and Inoue, 1985a,b; Nishio et al., 1993; Evans, 1995; Sun et al., 1998; Sun and Nishio, 2001; Evans and Vogelmann, 2003). Unlike dicotyledonous C3 plants, the adaxial and abaxial sides of C4 monocotyledonous leaves like maize comprise essentially two separate compartments as there are physical dorsoventral restrictions to airspace continuity (Long et al., 1989). Moreover, the regulation of photosynthesis is different on each side of C4 monocotyledonous leaves that belong to the NADP-malic enzyme (NADP-ME) subtype such as Paspalum dilatatum (Soares et al., 2008; Soares-Cordeiro et al., 2009) and maize (Long et al., 1989; Driscoll et al., 2006; Soares-Cordeiro et al., 2009). Marked differences in the leaf-side-specific responses of photosynthesis and stomatal closure to light orientation are observed in such C4 monocotyledonous leaves. This led us to propose that each side of such leaves has independent signalling pathways associated with light perception, photosynthesis, and stomatal closure (Soares-Cordeiro et al., 2009).
Amphistomatous leaves from dicotyledonous and monocotyledonous C3 and C4 species can exhibit differential regulation of stomatal closure on the adaxial and abaxial surfaces. Generally, the stomata on the adaxial surface are considered to be less sensitive to light intensity (Turner, 1970; Pemadasa, 1979; Travis and Mansfield, 1981; Goh et al., 1995; Soares et al., 2008), as well as to water stress conditions (Turner and Singh, 1984; Lu, 1988; Wang et al., 1998). However, stomatal conductance was shown to be more affected on the adaxial surface in the dicotyledonous C3 species Rumex obtusifolius grown under CO2 enrichment (Pearson et al., 1995). Side-specific differences in stomatal movement have been related to variations in calcium ion concentration in the guard cells (De Silva et al., 1986), which are in turn regulated by the metabolic and signalling activities of the adjacent mesophyll cells in response to changes in CO2 or light availability (Mott et al., 2008). Recent evidence suggests, however, that photosynthesis does not contribute directly to the signalling network that regulates stomatal opening (von Caemmerer et al., 2004; Baroli et al., 2008).
The presence of a two-compartment structure in C4 monocotyledonous leaves with little or no apparent airspace continuity between the upper and lower sides, would allow separate dorsoventral systemic stress signalling pathways. The hypothesis that was tested in the present study was that the leaves of C4 monocotyledonous species such as P. dilatatum (Soares et al., 2008; Soares-Cordeiro et al., 2009) might exhibit differential responses and acclimation to chilling on the adaxial and abaxial sides of the leaf. The two-airspace system may thus be useful not only in the optimization of whole leaf photosynthesis but also in the regulation of surface-specific stress defence-signalling cascades. In the following experiments therefore we have explored the possibility that each side of P. dilatatum leaves responds independently to the stress imposed by dark chilling.
To date, relatively few studies that focus on enhancing our current understanding of how the side-specific regulation of photosynthesis and stomatal movements is altered in response to environmental stress have been performed. Studies on CO2 enrichment in maize (Driscoll et al., 2006) and P. dilatatum (Soares et al., 2008) revealed that each surface of the leaf responded differently to altered CO2 availability. While photosynthesis on the abaxial surfaces was largely unchanged in the leaves of these two C4 NADP-ME species when plants were acclimated to elevated growth CO2 levels, CO2 assimilation rates were decreased on the adaxial surfaces. No information is available in the literature on the effects of chilling on the surface-specific regulation of photosynthesis and stomatal conductance values in monocotyledonous C4 species. The present study was therefore undertaken to characterize the effects of dark chilling on photosynthesis and stomatal function on each side of P. dilatatum leaves and to determine whether chilling stress results in side-specific regulation in this C4 grass species, which is partially tolerant to the stress imposed by dark chilling (Cavaco, 2000).
Materials and methods
Plant material
Paspalum dilatatum seeds were germinated and grown for 6 weeks in controlled environment cabinets (Sanyo SGC228.CFX.J; Sanyo, Osaka, Japan) as described previously (Driscoll et al., 2006; Soares et al., 2008; Soares-Cordeiro et al., 2009). The controlled environment cabinets provide a constant CO2 concentration of 350±20 μl l−1 (Eurotherm Ltd, Worthing, UK), 80% relative humidity, a temperature of 25 °C in the day and 19 °C at night, with a 16 h photoperiod at an irradiance of 600–650 μmol m−2 s−1 at pot height (400–700 nm). Irradiance was provided by Phillips Master TL5 HO 49w/830 fluorescent lamps. Plants were watered daily throughout the period of the experiments.
Dark chilling treatments and sampling
For the dark chilling treatments plants were transferred to a controlled growth chamber for one or two consecutive nights. The conditions in the controlled growth chamber were as above, except that the night temperatures were maintained at 4 °C. Leaf pigment and soluble protein determinations and gas-exchange measurements were performed on the middle (widest) part of the youngest fully expanded leaves in the subsequent light periods after one and two consecutive nights of dark chilling. Leaf harvest and measurements were performed between 1 and 5 h after the beginning of the light period.
Determination of leaf pigments and soluble protein
Leaf pigments [chlorophyll a (chla), chlb, total chlorophyll, and carotenoids] and soluble protein content were determined in the middle sections of the youngest fully expanded leaves of plants that had been either maintained at optimal growth temperatures or subjected to one or two nights of dark chilling, as above. All methods used were essentially as described previously (Soares et al., 2008; Soares-Cordeiro et al., 2009). Leaf samples were ground in liquid nitrogen and quartz sand. Pigments were determined according to the method of Lichtenhaler and Wellburn (1983) after extraction in 96% ethanol (v/v). Soluble proteins were extracted in sodium phosphate buffer as described by Soares et al. (2008). Total soluble protein content was determined according to the method of Bradford (1976).
Gas-exchange measurements
Photosynthetic gas-exchange measurements were performed using an infrared gas analyser (model wa-225-mk3; ADC, Hoddesdon, UK) either on whole leaves using custom-made standard Parkinson-type whole leaf chambers designed for CO2 and water vapour analysis (Novitskaya et al., 2002) or on each leaf surface separately using custom-made dual Parkinson-type chambers designed for CO2 and water vapour analysis (Soares et al., 2008; Soares-Cordeiro et al., 2009).
Gas-exchange measurements were performed on the middle sections of the youngest fully expanded attached leaves of plants that had been either maintained at optimal growth temperatures or subjected to one or two nights of dark chilling. Light was oriented either to the adaxial surface or to the abaxial surface, by inverting the leaves in the chambers. Since P. dilatatum leaves are not large enough to fully separate the two sides of the dual chamber, the leaves were expanded with gas-tight tape to seal each half of the chamber, preventing any flux of gas between the two sides as previously described by Soares et al. (2008) and Soares-Cordeiro et al. (2009).
Three types of measurement were made on the attached leaves of plants that had been either maintained at optimal growth temperatures or subjected to one or two nights of dark chilling: (i) single measurements of gas-exchange parameters on the whole leaves and on each leaf surface separately at an ambient CO2 concentration of 350 μl l−1 and at an irradiance of between 900 and 1000 μmol m−2 s−1; (ii) CO2-response curves for photosynthesis were performed on whole leaves and on each side of the leaf separately at an irradiance between 900 and 1000 μmol m−2 s−1. In these experiments, the CO2 concentration within the chambers was increased in a stepwise manner from 50 to 1000 μl l−1, and (iii) light-response curves for photosynthesis were performed on whole leaves and on each side of the leaf separately at an ambient CO2 concentration of 350 μl l−1. In these experiments, the light intensity was increased stepwise from darkness to 1500 μmol m−2 s−1.
The whole leaf response curves for photosynthesis allow us to model some of the key parameters of C4 photosynthesis, using the C4 photosynthetic models described by von Caemmerer (2000). Such C4 photosynthetic models have only been described for whole leaf photosynthesis. It was therefore not possible to use the leaf-surface-specific data obtained from the CO2- and light-response curves for photosynthesis to determine the various C4 photosynthetic parameters on each side of the leaf. In addition, we consider that the interpretation of the maximal electron transport rate and maximal photosynthetic rate values may not be trivial using values obtained for each leaf surface (S. von Caemmerer, personal communication) because we have not determined the exact amount of light that reaches the side of the leaf that is not under direct actinic illumination.
Steady-state rates for CO2 assimilation and stomatal conductance were determined at a leaf temperature of 20 °C. This temperature was maintained constant for both whole leaf and side-specific measurements of parameters on the adaxial and abaxial surfaces, by water jackets as previously described by Soares et al. (2008). Measurements were conducted at 50% relative humidity and the irradiance was provided by overhead broad-spectrum lamps and supplied only from the top of the chamber both for the whole leaf measurements and for each leaf surface measurement (Soares et al., 2008). Data presented for whole leaf and leaf-surface-specific parameters are the mean values obtained from leaves of nine plants per measurement that were analysed on different batches of plants grown over the whole experimental period.
Measurements of the internal resistance to gas diffusion
Ambient CO2 concentration, intercellular CO2 concentration, and stomatal conductance values were determined on the adaxial and abaxial surfaces of P. dilatatum leaves as follows. The gas flow and pressure were maintained equal and constant on each side of the leaf but a differential ambient CO2 concentration was applied across the leaves such that one side was maintained at 2500 μl l−1 CO2 while the other received 0 μl l−1 CO2. Light was oriented to the adaxial leaf surface in all measurements at an intensity of 550 μmol m−2 s−1. The leaf temperature was maintained at 20 °C with a relative humidity of 50%.
C4 photosynthetic model predictions for whole leaf photosynthesis
CO2-response curves:
Whole leaf CO2-response curves for photosynthesis were used to calculate C4 photosynthetic parameters, according to the whole leaf C4 photosynthetic model of von Caemmerer and Furbank (1999) and as described in detail by von Caemmerer (2000). These models are based on the earlier models of Berry and Farquhar (1978) and Peisker (1979). This model allows the prediction of the maximal phosphoenolpyruvate carboxylase (PEPC) carboxylation rate (Vpmax) and maximal ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation rate (Vcmax) using enzyme-limited photosynthesis equations and the maximal electron transport rate (Jmax) using light- and electron-transport-limited photosynthesis equations. The model requires that a saturating light intensity is used to perform the measurements. We thus had to achieve a compromise with regard to the light intensity in order to avoid photoinhibition in the light period following the treatments when the plants were dark-chilled. While the light intensity (900–1000 μmol m−2 s−1) used in the present experiments was not fully saturating for photosynthesis according to the data shown in Fig. 4, it is nearly saturating while low enough to prevent photoinhibition in plants that had experienced dark chilling. Nevertheless, it is important to note that while the values obtained through the application of the C4 photosynthetic model in this study cannot be considered to be the absolute maximal values, they can accurately be used to compare the effects of dark chilling and light orientation on the leaf.
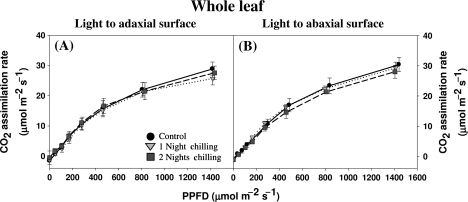
Fig. 4.
The effect of light orientation and dark chilling on the light-response curves for photosynthesis in whole P. dilatatum leaves. Plants were either maintained at optimal growth temperatures (black circles) or subjected to dark chilling for either one (light grey inverted triangles) or two (dark grey squares) nights. Light intensity was increased in a stepwise manner from 0 to 1500 μmol m−2 s−1 with orientation to either the adaxial (A) or the abaxial (B) leaf surface. Data are the mean values±SE of nine plants per treatment.
In addition, the C4 photosynthetic model requires the use of some parameters measured at 25 °C (von Caemmerer, 2000). Some parameters are dependent on temperature and thus it was important to calculate the values appropriate to the temperatures at which the present curves were performed (20 °C). This is the case for the Michaelis–Menten constants of Rubisco for CO2 (Kc) and for O2 (Ko), and the Michaelis–Menten constant of PEPC for CO2 (Kp). An Arrhenius function was used to transform the Kc, Ko, and Kp values to 20 °C, (Badger and Collatz, 1977):
where Q10 is the temperature coefficient that represents the factor by which the rate of a reaction increases for every 10 °C rise in the temperature. The tabled values of Kc and Ko at 25 °C (650 μbar and 450 mbar, respectively) and the Q10 values for Kc and Ko (2.24 and 1.63, respectively) (von Caemmerer, 2000) were used to calculate the Kc and Ko at 20 °C. The Kp value tabled at 25 °C (80 μbar) (von Caemmerer, 2000) and the Q10 value for Kp of 1.603, calculated from the PEPC activation energy value (34.8 kJ mol−1) obtained for P. dilatatum plants at temperatures >20 °C (Cavaco, 2000), were used to calculate the Kp value at 20 °C. The parameters and the values used to apply the photosynthetic model are described in Table 1.
Table 1.
Parameters used for modelling C4 photosynthesis based on the CO2-response curves for photosynthesis (von Caemmerer, 2000)
| Parameter | Value | Description |
| Kc | 0.973 mbar | Michaelis–Menten constant of Rubisco for CO2 (variable with temperature, corrected to 20 °C) |
| Ko | 575 mbar | Michaelis–Menten constant of Rubisco for O2 (variable with temperature, corrected to 20 °C) |
| Kp | 101 μmol mol−1 | Michaelis–Menten constant of PEPC for CO2 (variable with temperature, corrected to 20 °C) |
| O | 21% | O2 partial pressure in the bundle sheath and mesophyll cells |
| gbs | 3 mmol m−2 s−1 | Bundle-sheath conductance to CO2 |
| gi | 2 mol m−2 s−1 | Mesophyll conductance to CO2 |
| Rd | 0.01*Vcmax | Mitochondrial respiration |
| Rm | 0.5*Rd | Mesophyll mitochondrial respiration |
| γ* | 0.000193 | Half the reciprocal of Rubisco speficity |
| X | 0.4 | Partitioning factor of electron transport rate |
| θ | 0.7 | Empirical curvature factor |
| F | 0.15 | Factor that corrects for spectral quality of light |
| abs | 0.85 | Absorptance of leaves |
| I | 1000 μmol m−2 s−1 | Irradiance used |
| Ia | 361.2 μmol m−2 s−1 | Total absorbed irradiance |
A similar approach to that described by Massad et al. (2007) was used to estimate Vpmax, Vcmax, and Jmax. First, an asymptotic exponential model was selected as it provides the best description of the variation in CO2 assimilation rates with intercellular CO2 concentration (Ci) for each individual plant according to the least squares method using the program Statistical Package for Social Sciences (SPSS) (version 15.0, 2006; SPSS Inc., Chicago, IL, USA).
The equations that were used to determine enzyme-limited photosynthesis and light- and electron-transport-limited photosynthesis (as shown in Table 2) were applied using the Mathematica version 6.0 (2007) software (Wolfram Research Inc., Champaign, IL, USA). Data obtained from each individual plant were analysed in the range 0–500 μl l−1, in a stepwise manner (i.e. per 5 μl l−1 Ci increase) in order to determine Vpmax, Vcmax, and Jmax values at each Ci. The estimated values of Vpmax, Vcmax, and Jmax were then plotted against Ci. The mean values were then determined for each individual plant in the ranges 50–80 μl l−1 Ci for Vpmax and 300–500 μl l−1 Ci for Vcmax and Jmax. The values obtained for each plant were then used to determine the mean values for Vpmax, Vcmax, and Jmax for each treatment. Values for mitochondrial respiration and mesophyll mitochondrial respiration were then calculated from the Vpmax and Vcmax data according to von Caemmerer (2000).
Table 2.
Equations for enzyme-limited photosynthesis and for light- and electron-transport-limited photosynthesis used to predict the maximal PEPC carboxylation rate (Vpmax), maximal Rubisco carboxylation rate (Vcmax), and the maximal electron transport rate (Jmax) from the CO2-response curves of whole leaf photosynthesis according to von Caemmerer (2000)
| Parameter | Equations |
| Vpmax and Vcmax | Equations for enzyme-limited photosynthesis: 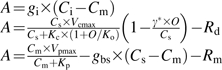
|
| Jmax | Equations for light- and electron-transported-limited photosynthesis: 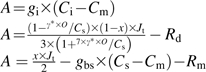
|
Equation characterizing the relationship between electron transport rates and absorbed irradiance: 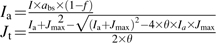
|
See Table 1 for all abbreviations except A (CO2 assimilation rate), Ci (intercellular CO2 concentration), Cm (mesophyll cells CO2 concentration), Cs (bundle sheath cells CO2 concentration), and Jt (predicted electron transport rate).
Light-response curves:
Light-response curves for photosynthesis in whole leaves were analysed (Statistica 8.0, 2007; StatSoft, Inc., OK, USA) to determine the apparent quantum yield (ϕ), curvature degree (θ), maximal rate of photosynthesis (Amax), and mitochondrial respiration (Rd), using the equations below as described by von Caemmerer (2000) and Lambers et al. (1998):
where, A is the CO2 assimilation rate, and I the irradiance used.
Each parameter was calculated for each individual curve and then the mean values were obtained for each dark chilling and light orientation treatment. The C4 photosynthetic light-response curve model requires that analysis is performed under conditions of saturating CO2 concentrations. In the present study, the light-response curves for photosynthesis were performed at 350 μl l−1 CO2 (corresponding to a Ci value of ∼120–200 μl l−1), which is a saturating CO2 concentration (according to Fig. 3). The predicted ϕ, θ, Amax, and Rd values determined from the model are therefore considered to be accurate.
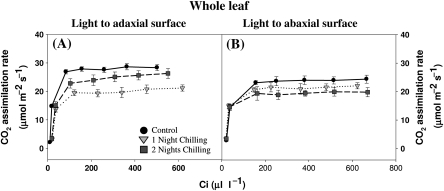
Fig. 3.
The effect of light orientation and dark chilling on the CO2-response curves for photosynthesis in whole P. dilatatum leaves. The light-response curves for photosynthesis were performed at 350 μl l−1 CO2 (corresponding to a Ci value of ∼120–200 μl l−1), which is a saturating CO2 concentration. Plants were either maintained at optimal growth temperatures (black circles), or subjected to dark chilling for either one (light grey inverted triangles) or two (dark grey squares) nights. The light intensity was orientated either to the adaxial (A) or the abaxial (B) leaf surface. Data are the mean±SE of nine plants per treatment.
Statistical analysis
Statistical analysis of the data was performed using parametric tests at a stringency of P<0.05. The significance of variation for mean values regarding pigment, protein, single gas-exchange measurements obtained for whole leaves and for each leaf side separately and for whole leaf photosynthetic model determinations was analysed using an ANOVA followed by a Tukey HSD test. All the analyses were performed separately for each parameter.
Results
Effects of dark chilling stress on leaf pigments and protein
The leaves of plants that had been subjected to a single night of dark chilling showed a decrease in the content of both chla and chlb. However, the chla/chlb ratio was increased because the chilling-induced decrease (19%) in chlb was higher than that (9%) in chla (Table 3). After a second night of chilling the decrease in chla and chlb was of a similar magnitude (13% and 14%, respectively). Thus, the ratio chla/chlb was not significantly different from that of the control plants that had been maintained under optimal temperatures. Leaf carotenoid content decreased significantly after the second consecutive night of chilling (Table 3) but the ratio of chla plus chlb to carotenoids was unaffected by dark chilling. The amount of soluble protein in the leaves was not affected by the chilling treatments (Table 3).
Table 3.
Effects of dark chilling on leaf pigment and protein contents. Plants were either maintained at optimal growth temperatures (controls) or were subjected to dark chilling for either one or two consecutive nights
Data represent the mean values ± SE of seven leaves per treatment. Statistical analysis was performed separately for each parameter. The different letters represent statistical differences at P<0.05. Chl, chlorophyll.
| Control | 1 Night Chilling | 2 Nights Chilling | |
| Chla (mg m-2) | 572 ± 15.7 a | 518 ± 14.7 b | 499 ± 4.9 b |
| Chlb (mg m-2) | 156 ± 4.9 a | 127 ± 4.3 b | 135 ± 2.0 b |
| Chla+b (mg m-2) | 727 ± 2.0 a | 645 ± 18.9 b | 634 ± 5.7 b |
| Carotenoids (mg m-2) | 114 ± 3.3 a | 108 ± 2.6 b,a | 100 ± 1.4 b |
| Chla/Chl b ratio | 3.7 ± 0.06 a | 4.1 ± 0.04 b | 3.7 ± 0.05 a,b |
| Chla+b/Carotenoid ratio | 6.4 ± 0.07 a,b | 6.0 ± 0.06 a | 6.3 ± 0.10 a,b |
| Soluble proteins (mg m-2) | 3084 ± 148.9 a | 2773 ± 65.3 a | 2797 ± 78.8 a |
Effects of dark chilling on whole leaf photosynthesis and stomatal conductance
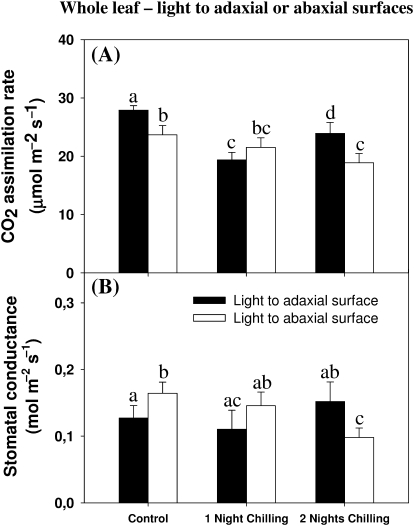
Whole leaf photosynthesis rates at ambient CO2 and a light intensity of 900–1000 μmol m−2 s−1 were 14% lower when CO2 assimilation was measured with light oriented to the abaxial surface (Fig. 1A). Whole leaf CO2 assimilation rates measured under adaxial illumination were 32% and 14% lower after, respectively, one and two nights exposure to dark chilling compared with controls that had been maintained under optimal temperatures. Photosynthesis was only decreased under abaxial illumination after two nights of chilling and then only by 20%. Stomatal conductance values were higher in control plants under abaxial illumination than under adaxial illumination (Fig. 1B). No effects of dark chilling on the stomatal conductance were detected either under adaxial illumination after one or two nights of dark chilling or under abaxial illumination after one night of chilling. However, a 40% decrease in stomatal conductance was observed under abaxial illumination after two nights of stress (Fig. 1B).
Fig. 1.
The effect of light orientation and dark chilling on whole leaf photosynthesis (A) and stomatal conductance (B) in P. dilatatum leaves. Plants were either maintained at optimal growth temperature (controls), or subjected to dark chilling for either one or two nights. Light was oriented to either the adaxial (closed bars) or the abaxial (open bars) leaf surface. Data are the mean±SE of nine plants per treatment with each gas-exchange parameter measured separately. The different letters represent significant differences (P<0.05).
Effects of dark chilling on photosynthesis and stomatal conductance on each leaf surface
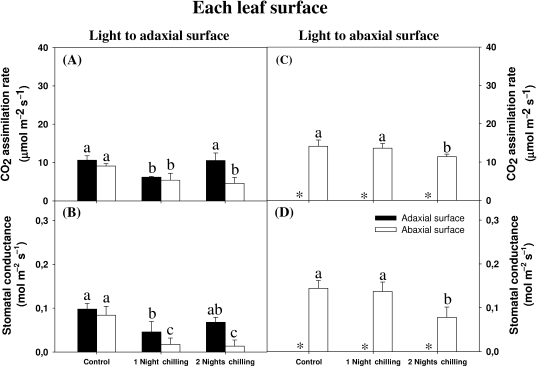
The dark chilling treatments affected CO2 assimilation differently on each leaf surface under adaxial illumination. The CO2 assimilation rates on each leaf surface were similar under adaxial illumination in control plants (Fig. 2A). Under the same light condition, a single night of exposure to the chilling stress was sufficient to decrease the photosynthetic rates by half. However, photosynthesis rates recovered on the adaxial surface, but not on the abaxial surface after two nights of chilling in these conditions.
Fig. 2.
The effect of light orientation and dark chilling on leaf-side-specific rates of photosynthesis (A) and stomatal conductance (B) in P. dilatatum leaves. Plants were either maintained at optimal growth temperatures (controls), or subjected to dark chilling for either one or two nights. CO2-assimilation rates (A, C) and stomatal conductance (B, D) were determined on the adaxial (closed bars) and the abaxial (open bars) leaf surfaces. Light was oriented to either the adaxial (A, B) or the abaxial (C, D) leaf surface. Data are the mean values±SE of nine plants per treatment with each gas-exchange parameter measured separately. The different letters represent significant differences (P<0.05) and * indicates values below the levels of detection.
Photosynthesis on the adaxial surface was below the level of detection under abaxial illumination. However, the abaxial surface had increased CO2 assimilation rates compared with the values obtained under adaxial illumination (Fig. 2A, C). In contrast to photosynthesis under adaxial illumination, CO2 assimilation rates on the abaxial surface were unaffected by one night of chilling, but they were decreased (21%) after two consecutive nights of chilling (Fig. 2C). Dark chilling had similar effects on stomatal conductance under adaxial (Fig. 2B) and abaxial illumination (Fig. 2D).
Effects of dark chilling on the whole leaf CO2-response curves for photosynthesis
Whole leaf CO2 assimilation rates increased with Ci under all conditions irrespective of the light orientation (Fig. 3). The steady-state rates of photosynthesis were similar after one night of chilling when light was oriented either to the adaxial or the abaxial surface of the leaves. However, steady-state rates of photosynthesis in the control leaves maintained under optimal growth temperatures and those of plants that had experienced two nights of dark chilling were highest when light was oriented to the adaxial surface (Fig. 3A). The initial slopes of the CO2-response curves for photosynthesis were similar in control and chilled leaves regardless of light orientation. However, chilling had different effects on the steady-state rates of photosynthesis measured under adaxial or abaxial illumination. Photosynthesis rates were decreased under adaxial illumination after the plants had been chilled for one night (Fig. 3A). However, a partial recovery was observed after the second chilling treatment (Fig. 3A). In contrast, a second night of chilling led to a greater inhibition of photosynthesis when measured under abaxial illumination (Fig. 3B). These data indicate that the adaxial sides of the leaves are better able to recover from chilling-induced inhibition of photosynthesis than the abaxial sides.
Effects of dark chilling on the whole leaf light-response curves for photosynthesis
Under saturating light intensities (900–1000 μmol m−2 s−1) photosynthesis rates were significantly lower when light was oriented to the abaxial surface (Fig. 1). However, this inhibition was not observed under lower light intensities (Fig. 4). Thus, the kinetics of the light-response curves for photosynthesis were largely unaffected by dark chilling under either adaxial or abaxial illumination (Fig. 4). Only the light-saturated rate was decreased (Figs 1, 4).
Effects of dark chilling on calculated photosynthetic response curve parameters
The orientation of the leaf to light has effects on whole leaf Vpmax, Vcmax, and Jmax values. In plants maintained at optimal growth temperatures, these parameters were 48%, 19%, and 22% lower, respectively, under abaxial relative to adaxial illumination (Table 4). However, the estimated values for the whole leaf Amax, ϕ, θ, and Rd were not affected by light orientation at optimal growth temperatures (Table 5).
Table 4.
The effects of light orientation and dark chilling on whole leaf maximal phosphoenolpyruvate carboxylase (PEPC) carboxylation rates (Vpmax), maximal ribulose-1,5-bisphosphate (Rubisco) carboxylation rates (Vcmax) and maximal electron transport rates (Jmax). Plants were either maintained at optimal growth temperatures (controls) or subjected to dark chilling for either one or two consecutive nights
Data represent the mean values ± SE of nine plants per treatment and were calculated following the application of the C4 photosynthetic model of von Caemmerer (2000) to the whole leaf CO2-response curves (See Fig. 3). Statistical analysis was performed separately for each parameter. The different letters represent statistical differences at P<0.05.
| Vpmax(μmol m-2 s-1) | Vcmax(μmol m-2 s-1) | Jmax(μmol m-2 s-1) | |
| Light Adaxial | |||
| Control | 111 ± 4.3 a | 32 ± 1.0 a | 214 ± 15.6 a |
| 1 Night Chilling | 52 ± 1.4 c | 22 ± 0.7 c | 144 ± 11.3 b |
| 2 Nights Chilling | 63 ± 1.0 b,c | 28 ± 1.1 a,b | 183 ± 10.2 a,b |
| Light Abaxial | |||
| Control | 58 ± 2.9 b,c | 26 ± 0.5 b | 167 ± 1.6 b |
| 1 Night Chilling | 67 ± 4.8 b | 24 ± 0.8 b,c | 152 ± 6.4 b,c |
| 2 Nights Chilling | 66 ± 2.5 b,c | 22 ± 1.1 c | 148 ± 3.5 c |
Table 5.
The effect of light orientation and dark chilling on whole leaf maximal rate of photosynthesis (Amax), apparent quantum yield (φ), curvature degree (θ) and mitochondrial respiration (Rd) in plants that had been maintained at optimal growth temperatures (controls) or subjected to dark chilling for either one or two consecutive nights
Data represent the mean values ± SE of nine plants per treatment and was calculated accordingly to von Caemmerer (2000) and Lambers et al. (1998) from the whole leaf light-response curves (See Fig. 4). Statistical analysis was performed separately for each parameter. The different letters represent statistical differences at P<0.05.
| Amax(μmol m-2 s-1) | ϕ(*102 μmol μmol-1) | θ(relative units) | Rd(μmol m-2 s-1) | |
| Light Adaxial | ||||
| Control | 41 ± 2.4 a | 5.7 ± 0.58 a | 0.64 ± 0.045 a | 1.9 ± 0.06 a |
| 1 Night Chilling | 26 ± 0.6 b | 4.6 ± 0.66 a | 0.82 ± 0.044 a | 1.8 ± 0.08 a |
| 2 Nights Chilling | 39 ± 2.7 a | 6.5 ± 0.23 a | 0.69 ± 0.090 a | 1.9 ± 0.13 a |
| Light Abaxial | ||||
| Control | 43 ± 1.7 a | 4.2 ± 0.40 a | 0.64 ± 0.073 a | 1.6 ± 0.05 a |
| 1 Night Chilling | 37 ± 1.0 a | 5.4 ± 0.12 a | 0.61 ± 0.035 a | 1.6 ± 0.03 a |
| 2 Nights Chilling | 37 ± 1.0 a | 4.6 ± 0.50 a | 0.64 ± 0.073 a | 1.7 ± 0.07 a |
One night of chilling decreased the Vpmax, Vcmax, Jmax, and Amax values by between 30 and 50% when light was oriented to the adaxial leaf surface (Tables 4, 5). These parameters largely recovered to control values after the second night of chilling, except for Vpmax, which showed values similar to those measured after one night of dark chilling (∼45% decrease) (Table 4). The Vpmax and Amax values were similar in control and dark-chilled plants when the light was oriented to the abaxial surface (Tables 4, 5). Under abaxial illumination the estimated values for Vcmax and Jmax decreased by 15% and 11%, respectively, but only after two nights of chilling (Table 4). Dark chilling and light orientation had no effects on ϕ, θ, and Rd (Table 5).
CO2 diffusion between the adaxial and abaxial sides of the leaf
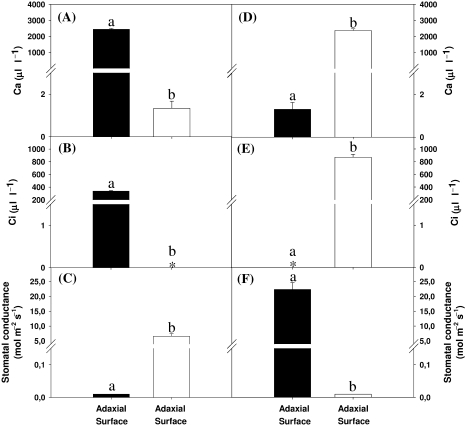
The diffusion of CO2 between the adaxial and abaxial sides of the P. dilatatum leaves was measured in plants maintained at optimal growth temperatures (Fig. 5). When the adaxial side of the leaf was exposed to high atmospheric CO2 levels with zero amounts on the abaxial side of the leaf (Fig. 5A), Ci values were below the level of detection on the abaxial surface (Fig. 5B) despite the stomata being fully open on that surface (Fig. 5C). Conversely, when the abaxial side of the leaf was exposed to high atmospheric CO2 levels with zero amounts on the adaxial side of the leaf (Fig. 5D), Ci values were below the level of detection on the adaxial side (Fig. 5E) despite the stoma being fully open on that surface (Fig. 5F).
Fig. 5.
Internal resistance to CO2 diffusion across P. dilatatum leaves. Ambient CO2 concentrations (Ca; A, D), intercellular CO2 concentrations (Ci; B, E), and stomatal conductance values (C, F) were determined on the adaxial (dark bars) and abaxial (white bars) surfaces of attached P. dilatatum leaves, on plants that were maintained at optimal growth temperatures. The ambient CO2 concentration was maintained either at 2500 μl l−1 on the adaxial surface and 0 μl l−1 on the abaxial surface (A–C) or 2500 μl l−1 on the abaxial surface and 0 μl l−1 on the adaxial surface (D–F). Light was oriented to the adaxial leaf surface. Data are the mean values±SE of four plants per treatment. The different letters represent significant differences (P<0.05) and * indicates values below the level of detection.
Discussion
Plant responses to environmental stresses are important in determining overall productivity and crop yields. The decreases in leaf protein and chlorophyll following short-term exposure to low temperatures observed in the leaves of P. dilatatum, which is nominally regarded as semi-tolerant to dark chilling (Cavaco et al., 2003), are consistent with previous observations in chilling-sensitive plants (Lyons, 1973; Slack et al., 1974; Forde et al., 1975; Haldimann et al., 1995; Nie et al., 1995; Haldimann, 1996; Cavaco et al., 2003; Naidu et al., 2003; Strauss et al., 2007). More cold-tolerant C4 species such as Miscanthus show a greater degree of cold acclimation capacity (Naidu et al., 2003). The information provided here on the chilling-dependent inhibition of photosynthesis in P. dilatatum leaves, together with observations concerning the differential sensitivities of each leaf surface to chilling-induced inhibition, advances our current understanding of how exposure to stress influences the dorsoventral regulation of photosynthesis and allows us to draw the following conclusions.
(i) Light orientation modifies whole leaf responses to dark chilling:
Under optimal growth conditions whole leaf photosynthesis was decreased under abaxial illumination compared with adaxial illumination (Fig. 1A). Moreover, differences in the calculated Vpmax, Vcmax, and Jmax values (Table 4) are related to the lower photosynthesis rates on the adaxial surface under abaxial illumination (Fig. 2C), in agreement with our earlier observations on this and other monocotyledonous C4 NADP-ME species (Soares et al., 2008; Soares-Cordeiro et al., 2009). Under abaxial illumination, the stomata on the adaxial surface of the leaf were tightly closed and thus it is not possible to determine CO2 exchange for the adaxial surface. Under these conditions the abaxial surface alone contributes to detectable CO2 assimilation rates. One possible reason for these differences is that the abaxial surface is more limited by light absorption under adaxial illumination. Under most circumstances the lower side of the leaf would experience much lower light levels than the upper surface (Soares et al., 2008). While side-specific differences in the capacity for light absorption have not been detected to date (Soares et al., 2008), there may be subtle variations that cannot be determined by simple whole leaf measurements. Moreover, the sensitivities of enzymes such as PEPC might be different on the two leaf surfaces.
When the abaxial surface was directly illuminated, the limitation by light availability was diminished and thus there was a marked increase in CO2 assimilation rates. The rates of photosynthesis on the abaxial surface under abaxial illumination were much higher than those obtained on the adaxial surface under adaxial illumination. This is presumably because the abaxial surface has a higher capacity to absorb light and to assimilate CO2.
The results presented here show that the responses of whole leaf photosynthesis to dark chilling were modified by light orientation (Fig. 1A, B). This result is similar to that obtained previously with P. dilatatum plants, in response to another environmental stress, i.e. growth with CO2 enrichment (Soares et al., 2008). It has long been known that dark chilling decreases photosynthesis under adaxial illumination in chilling-sensitive species (e.g. Ludlow and Wilson, 1971; Ivory and Whiteman, 1978; van Heerden et al., 2003a; Feng and Cao, 2005). Chilling-induced inhibition of photosynthesis observed here in P. dilatatum leaves under adaxial illumination after one night of chilling is thus in agreement with previous observations. However, in photosynthesis recovered following a second chilling treatment (Fig. 1A), reflecting the modest chilling sensitivity of this species. The recovery of photosynthesis after the second night of stress, demonstrated by the values for Vcmax and Jmax (Table 4), is not observed in many chilling-sensitive C3 species (van Heerden et al., 2003a; Feng and Cao, 2005). Moreover, photosynthesis was only decreased after two nights of chilling under abaxial illumination (Fig. 1A). The results presented in this study (Fig. 1A, B; Tables 4, 5) together with the data that we have obtained previously in plants grown with CO2 enrichment (Soares et al., 2008) demonstrate that these stresses initially exert their greatest effects on photosynthesis under adaxial illumination but the resultant inhibition is followed by a rapid recovery upon a second stress exposure. In contrast, under abaxial illumination the P. dilatatum leaf is initially more resistant to dark chilling. This evidence demonstrates that each side of the leaf has a unique sensitivity to dark chilling.
The absence of effects on stomatal conductance after one night of chilling under adaxial illumination (Fig. 1B) suggests that the decrease in the photosynthetic rate is associated with non-stomatal limitations to photosynthesis, as also indicated by the results in Table 4. Moreover, after one night of chilling, the dorsoventral differences in the Ci values on each side of the leaf disappeared. Under optimal conditions of irradiance and temperature, the mean Ci values were 157±15.2 when light was oriented to the adaxial surface, and 251±22.3 when light was oriented to the abaxial surface. After one night of chilling the mean Ci values were similar on both sides of the leaf, being 230±20.1 and 226±19.8, respectively. However, after the second night of chilling the dorsoventral asymmetry in mean Ci values was partially restored; the mean Ci values were then 209±19.8 when light was oriented to the adaxial surface, and 259±23.5 when light was oriented to the abaxial surface.
Dark chilling is known to decrease the activity of several photosynthetic enzymes in C3 and C4 plants (e.g. Jones et al., 1998; Pittermann and Sage, 2001; van Heerden et al., 2003a) and also cause decreased electron transport capacity in C3 plants (e.g. Bertamini et al., 2005; Strauss et al., 2007). The higher decrease in Vpmax observed in P. dilatatum leaves after one night of chilling compared with Vcmax, Jmax, and Amax, together with the failure of this parameter to recover after two nights of chilling stress under adaxial illumination (Tables 4, 5), suggests that the maximal activity of the first carboxylation step in C4 plants has a higher sensitivity to dark chilling. Moreover, the decrease in Vcmax and Jmax values under abaxial illumination together with the absence of an effect on Vpmax after the second night of chilling (Table 4) suggest that the maximal activity of the first carboxylation enzyme of C4 plants on the abaxial surface is less sensitive to dark chilling. It is important to note that while photosynthesis on both sides of the leaf is determined under adaxial illumination, this is not the case under abaxial illumination where photosynthesis on the abaxial side of the leaf alone can be detected. These results strongly suggest that PEPC activity on each side of the P. dilatatum leaf has a different sensitivity to dark chilling.
(ii) Dark chilling effects on photosynthesis are leaf-side specific, the abaxial side being more sensitive to inhibition:
Dark chilling had a greater impact on photosynthesis on the abaxial sides of the leaves under adaxial illumination. In marked contrast, under abaxial illumination the dark chilling effect on the abaxial side of the leaf was observed only on the second consecutive night of chilling (Fig. 2C). These results demonstrate that the acclimation of photosynthesis to dark chilling is most rapid on the surface that is directly exposed to light. Although the effects of dark chilling on metabolism, physiology, and membrane fluidity are expected to be similar on each side of the leaf irrespective of the light orientation, the higher light intensity arriving on the abaxial surface under abaxial illumination may increase the activities of photosynthetic enzymes in such a way as to overcome any metabolic limitations incurred during the chilling treatment. The activation of photosynthetic activity may be insufficient to overcome metabolic constraints resulting from the second chilling treatment. Taken together, these results suggest that the orientation of light is an important determinant of the ability of each side of the P. dilatatum leaf to respond to the stress imposed by dark chilling.
Growth with CO2 enrichment decreased the photosynthetic rates only on the adaxial sides of the P. dilatatum leaves under adaxial illumination (Soares et al., 2008). A similar response was observed in maize plants grown with CO2 enrichment (Driscoll et al., 2006). In both studies, photosynthesis fell below the levels of detection on the adaxial sides of the leaves under abaxial illumination, while the abaxial sides of the leaves have rates similar to controls. These results presented on this study and those presented by Driscoll et al. (2006) and Soares et al. (2008) suggest that the effects of environmental stress are different on each leaf surface, revealing the importance of these studies in our current understanding of the dorsoventral regulation of photosynthesis in monocotyledonous C4 species.
(iii) Differential sensitivity of stomatal conductance to dark chilling suggests leaf-side-specific stress signalling:
The data presented here demonstrate that the two sides of P. dilatatum leaves are not functionally symmetrical, as the C4 leaf structure might suggest (Cavaco, 2000). Moreover, we show here that each side of the leaf can respond differently to environmental stress. In agreement with this view, stomatal conductance is less sensitive to the effects of dark chilling on the adaxial side of the leaf under adaxial illumination (Fig. 2B). This observation is similar to that obtained in plants subjected to water deficit, a stress that, like chilling requires cellular osmotic adjustment (Turner and Singh, 1984; Lu, 1988; Wang et al., 1998). The lower sensitivity of the adaxial stomata in leaves exposed to water deficit has been explained by a lower sensitivity of the guard cells on this leaf surface to abscisic acid and to calcium ions (De Silva et al., 1986; Wang et al., 1998). While we have no direct evidence for a specific effect of dark chilling on the stomata per se, the different responses of stomata on the adaxial and abaxial surfaces are intriguing, particularly as it may be a common feature of the response of C4 cereal leaves to stress situations.
The significance of independent, dorsoventral systemic stress signalling pathways
We have previously described the dorsoventral regulation of photosynthesis with respect to light orientation under optimal growth conditions in two monocotyledonous species that belong to the NADP-ME subtype, P. dilatatum and maize, and we have discussed the practical relevance of the findings at a whole-plant level within the context of the natural diurnal light environment that C4 plants face every day (Driscoll et al., 2006; Soares et al., 2008; Soares-Cordeiro et al., 2009). The data presented here show that each side of the leaf responds differently in terms of inhibition by dark chilling and the ability to recover from the stress. We consider that these differential responses are possible because of the structure of the P. dilatatum leaves, which restricts gas flow. The data presented here confirm the two-compartment model for monocotyledonous C4 leaves developed in order to explain the dorso-ventral differnces in the regulation of photosynthesis (Long et al., 1989). The finding that dark chilling, like growth with CO2 enrichment (Soares et al., 2008), modifies not only the dorsoventral regulation of photosynthesis with respect to light orientation but also the acclimation ability of each side of the leaf to the stress, is intriguing. While more studies are required to unravel the physiological relevance of these findings at the whole plant level, it is apparent that the two-compartment, two-airspace system of C4 cereal leaves would enable leaf-side-specific CO2-signalling and defence-signalling pathways. This type of compartmentation would also facilitate the independent regulation of stomatal movements on each leaf surface, in a manner that may not primarily be related to photosynthesis (von Caemmerer et al., 2004; Baroli et al., 2008). Moreover, differential dorsoventral stress signalling pathways would allow independent acclimation processes to occur on each side of the leaf. While the significance of leaf-side-specific stress responses remains to be elucidated, the leaf-side-specific regulation of photosynthesis may be controlled by the developmental genetic programme that governs the adaxial–abaxial patterning of leaves (Chitwood et al., 2009).
Acknowledgments
Ana Sofia Soares-Cordeiro was supported by the Fundação para a Ciência e a Tecnologia (Ph.D grant no. SFRH/13728/2003), the Fundação Calouste Gulbenkian, and the Society for Experimental Biology. The authors thank Dr GL Lockett, Margot Forage Germplasm Centre, New Zealand for providing the P. dilatatum Poiret cv. Raki seeds. We thank Professor Dr Dinis Pestana and Master Sandra Aleixo, Sciences Faculty, University of Lisbon for help with the application of the C4 photosynthesis model and assistance with Mathematica software.
Glossary
Abbreviations
- ϕ
apparent quantum yield
- θ
curvature degree
- A
CO2 assimilation rate
- Amax
maximal rate of photosynthesis
- CBF
CRT/CRE BINDING FACTOR
- chla
chlorophyll a
- chlb
chlorophyll b
- Ci
intercellular CO2 concentration
- COR
COLD-REGULATED
- I
Irradiance
- Jmax
maximal electron transport rate
- Kc
Michaelis–Menten constant of Rubisco for CO2
- Ko
Michaelis–Menten constant of Rubisco for O2
- Kp
Michaelis–Menten constant of PEPC for CO2
- NADP-ME
NADP-malic enzyme
- PEPC
phosphoenolpyruvate carboxylase
- Rd
mitochondrial respiration
- Rm
mesophyll mitochondrial respiration
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase
- Vcmax
maximal ribulose-1,5-bisphosphate carboxylation rate
- Vpmax
maximal phosphoenolpyruvate carboxylase carboxylation rate
References
- Badger MR, Collatz GJ. Studies on the kinetic mechanism of ribulose-1,5-bisphosphste carboxylase and oxygenase reactions, with particular reference to the effect of temperature on kinetic parameters. Carnegie Institution of Washington Year Book. 1977;76:355–361. [Google Scholar]
- Baroli I, Price GD, Badger MR, von Caemmerer S. The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiology. 2008;146:737–747. doi: 10.1104/pp.107.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Wierer R, Hatheway WH, Larcher W. Photosynthesis of Coffea arabica after chilling. Physiologia Plantarum. 1985;64:449–454. [Google Scholar]
- Berry JA, Farquhar GD. The CO2-concentrating function of C4 photosynthesis. A biochemical model. In: Hall DO, Coombs J, Goodwin TW, editors. Proceedings of the Fourth International Congress on Photosynthesis. London, UK: Biochemical Society; 1978. pp. 119–131. [Google Scholar]
- Bertamini M, Muthuchelian K, Rubinigg M, Zorer R, Nedunchezhian N. Low-night temperature (LNT) induced changes of photosynthesis in grapevine (Vitis vinifera L.) plants. Plant Physiology and Biochemistry. 2005;43:693–699. doi: 10.1016/j.plaphy.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Bertamini M, Muthuchelian K, Rubinigg M, Zorer R, Velasco R, Nedunchezhian N. Low-night temperature increased the photoinhibition of photosynthesis in grapevine (Vitis vinifera L. cv. Riesling) leaves. Environmental and Experimental Botany. 2006;57:25–31. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cavaco AMV. Faculty of Sciences. Portugal: University of Lisbon; 2000. Cold-acclimation of the C4 gramineae Paspalum dilatatum cv. Raki: a physiological and biochemical approach. PhD thesis. [Google Scholar]
- Cavaco AM, Bernardes da Silva A, Arrabaça MC. Effects of long-term chilling on growth and photosynthesis of the C4 gramineae Paspalum dilatatum. Physiologia Plantarum. 2003;119:87–96. [Google Scholar]
- Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, Timmermans MC. Pattern formation via small RNA mobility. Genes and Development. 2009;23:549–554. doi: 10.1101/gad.1770009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DLR, Cox RC, Hetherington AM, Mansfield TA. The role of abscisic acid and calcium in determining the behaviour of adaxial and abaxial stomata. New Phytologist. 1986;104:41–51. doi: 10.1111/j.1469-8137.1986.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Driscoll SP, Prins A, Olmos E, Kunert KJ, Foyer CH. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. Journal of Experimental Botany. 2006;57:381–390. doi: 10.1093/jxb/erj030. [DOI] [PubMed] [Google Scholar]
- Evans JR. Carbon fixation profiles do reflect light absorption profiles in leaves. Australian Journal of Plant Physiology. 1995;22:865–873. [Google Scholar]
- Evans JR, Vogelmann TC. Profiles of 14C fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant, Cell and Environment. 2003;26:547–560. [Google Scholar]
- Feng Y-L, Cao KF. Photosynthesis and photoinhibition after night chilling in seedlings of two tropical tree species grown under three irradiances. Photosynthetica. 2005;43:567–574. [Google Scholar]
- Flexas J, Badger M, Chow WS, Medrano H, Osmond CB. Analysis of the relative increase in photosynthetic O2 uptake when photosynthesis in grapevine leaves is inhibited following low night temperatures and/or water stress. Plant Physiology. 1999;121:675–684. doi: 10.1104/pp.121.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BJ, Whitehead HCM, Rowley JA. Effect of light intensity and temperature on photosynthesis rate, leaf starch content and ultrastructure of. Paspalum dilatatum. Australian Journal of Plant Physiology. 1975;2:185–195. [Google Scholar]
- Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiology. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C-H, Oku T, Shimazaki K-I. Properties of proton pumping in response to blue light and fusicoccin in guard cell protoplasts isolated from adaxial epidermis of Vicia leaves. Plant Physiology. 1995;109:187–194. doi: 10.1104/pp.109.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann P. Effect of changes in growth temperature on photosynthesis and carotenoid composition in Zea mays leaves. Physiologia Plantarum. 1996;97:554–562. [Google Scholar]
- Haldimann P, Fracheboud Y, Stamp P. Carotenoid composition in Zea mays developed at sub-optimal temperature and different light intensities. Physiologia Plantarum. 1995;95:409–414. [Google Scholar]
- Ivory D, Whiteman P. Effect of temperature on growth of five subtropical grasses. II. Effect of low night temperature. Australian Journal of Plant Physiology. 1978;5:149–157. [Google Scholar]
- Jones TL, Tucker DE, Ort DR. Chilling delays circadian pattern of sucrose phosphate synthase and nitrate reductase activity in tomato. Plant Physiology. 1998;118:149–158. doi: 10.1104/pp.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S-S, Kim JM, Lee CB. A comparative study on the effect of chilling treatment in the light and in the dark on subsequent photosynthesis in cucumber. Australian Journal of Plant Physiology. 2001;28:489–496. [Google Scholar]
- Lambers H, Stuart Chapin S III, Pons TL. Plant physiological ecology. New York, USA: Springer; 1998. pp. 26–29. [Google Scholar]
- Lichtenhaler HK, Wellburn AR. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemistry Society Transactions. 1983;11:591–592. [Google Scholar]
- Long SP, Farage PK, Bolhár-Nordenkampf HR, Rohrhofer U. Separating the contribution of the upper and lower mesophyll to photosynthesis in Zea mays L. leaves. Planta. 1989;177:207–216. doi: 10.1007/BF00392809. [DOI] [PubMed] [Google Scholar]
- Lu Z. The sensitivity of adaxial and abaxial stomatal resistance in wheat leaf to soil water stress. Acta Phytophysiologica Sinica. 1988;14:223–227. [Google Scholar]
- Ludlow MM, Wilson GL. Photosynthesis of tropical pasture plants. II. Temperature and illuminance history. Australian Journal of Biological Sciences. 1971;24:1065–1075. [Google Scholar]
- Lyons MJ. Chilling injury in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1973;24:445–466. [Google Scholar]
- Martin B, Ort DR, Boyer JS. Impairment of photosynthesis by chilling-temperatures in tomato. Plant Physiology. 1981;68:329–334. doi: 10.1104/pp.68.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozaki K, Yamaguchi-Shinozaki K. Identification of cold-inducible downstreamgenes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant Journal. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Massad R-S, Tuzet A, Bethenod O. The effect of temperature on C4-type leaf photosynthesis parameters. Plant, Cell and Environment. 2007;30:1191–1204. doi: 10.1111/j.1365-3040.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC. The role of the mesophyll in stomatal responses to light and CO2. Plant, Cell and Environment. 2008;31:1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- Naidu SL, Moose SP, AL-Shoaibi AK, Raines CA, Long SP. Cold tolerance of C4 photosynthesis in Miscanthus× gigantis: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiology. 2003;132:1688–1697. doi: 10.1104/pp.103.021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Robertson EJ, Fryer MJ, Leech RM, Baker NR. Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant, Cell and Environment. 1995;18:1–12. [Google Scholar]
- Nishio JN, Sun J, Vogelmann TC. Carbon fixation gradients across spinach leaves do not follow internal light gradients. Plant Cell. 1993;5:953–961. doi: 10.1105/tpc.5.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitskaya L, Trevanion S, Driscoll SD, Foyer CH, Noctor G. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant, Cell and Environment. 2002;25:821–836. [Google Scholar]
- Pasternak D, Wilson GL. After-effects of night temperatures on stomatal behaviour and photosynthesis of sorghum. New Phytologist. 1972;71:683–689. [Google Scholar]
- Pearson M, Davies WJ, Mansfield TA. Asymmetric responses of adaxial and abaxial stomata to elevated CO2: impacts on the control of gas exchange by leaves. Plant, Cell and Environment. 1995;18:837–843. [Google Scholar]
- Peisker M. Conditions for low, and oxygen-independent, CO2 compensation concentrations in C4 plants as derived from a simple model. Photosynthetica. 1979;13:198–207. [Google Scholar]
- Pemadasa MA. Movements of abaxial and adaxial stomata. New Phytologist. 1979;82:69–80. [Google Scholar]
- Pittermann J, Sage RF. The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A.S. Hischc. to long- and short-term chilling. Journal of Experimental Botany. 2001;52:829–838. doi: 10.1093/jexbot/52.357.829. [DOI] [PubMed] [Google Scholar]
- Shen J-R, Terashima I, Katoh S. Cause of dark, chilling-induced inactivation of photosynthetic oxygen-evolving system in cucumber leaves. Plant Physiology. 1990;93:1354–1357. doi: 10.1104/pp.93.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Roughan PG, Basset HCM. Selective inhibition of mesophyll chloroplast development in some C4-pathway species by low night temperatures. Planta. 1974;118:57–73. doi: 10.1007/BF00390503. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Bowles DJ. Plants in a cold climate. Philosophical Transactions of the Royal Society of London B. 2002;357:831–847. doi: 10.1098/rstb.2002.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AS, Driscoll SP, Olmos E, Harbinson J, Arrabaça MC, Foyer CH. Adaxial/abaxial specification in the regulation of photosynthesis with respect to light orientation and growth with CO2 enrichment in the C4 species Paspalum dilatatum. New Pytologist. 2008;177:186–198. doi: 10.1111/j.1469-8137.2007.02218.x. [DOI] [PubMed] [Google Scholar]
- Soares-Cordeiro AS, Driscoll SP, Pellny TK, Olmos E, Arrabaça MC, Foyer CH. Variations in the dorso-ventral organization of leaf structure and Kranz anatomy coordinate the control of photosynthesis and associated signalling at the whole leaf level in monocotyledonous species. Plant, Cell and Environment. 2009;32:1833–1844. doi: 10.1111/j.1365-3040.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- Strauss AJ, Krüger GHJ, Strasser RJ, van Heerden PDR. The role of low soil temperature in the inhibition of growth and PSII function during dark-chilling in soybean genotypes of contrasting tolerance. Physiologia Plantarum. 2007;131:89–105. doi: 10.1111/j.1399-3054.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Nishio JN. Why abaxial illumination limits photosynthetic carbon fixation in spinach leaves? Plant and Cell Physiology. 2001;42:1–8. doi: 10.1093/pcp/pce001. [DOI] [PubMed] [Google Scholar]
- Sun J, Nishio JN, Vogelmann TC. Green light drives CO2 fixation deep within leaves. Plant and Cell Physiology. 1998;39:1020–1026. [Google Scholar]
- Sung DY, Kaplan F, Lee KJ, Guy CL. Acquired tolerance to temperature extremes. Trends in Plant Science. 2003;8:179–187. doi: 10.1016/S1360-1385(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Terashima I, Inoue Y. 1985a. Vertical gradient in photosynthetic properties of spinach chloroplasts dependent on intra-leaf light environment. Plant and Cell Physiology. 24:781–785. [Google Scholar]
- Terashima I, Inoue Y. 1985b. Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach: biochemical and ultrastructural differences. Plant and Cell Physiology. 26:63–75. [Google Scholar]
- Travis AJ, Mansfield TA. Light saturation of stomatal opening on the adaxial and abaxial epidermis of Commelina communis. Journal of Experimental Botany. 1981;32:1169–1179. [Google Scholar]
- Turner NC. Response of adaxial and abaxial stomata to light. New Phytologist. 1970;69:647–653. [Google Scholar]
- Turner NC, Singh DP. Responses of adaxial and abaxial stomata to light and water deficits in sunflower and sorghum. New Phytologist. 1984;96:187–195. [Google Scholar]
- van Heerden PDR, Krüger GHJ, Loveland JE, Parry MAJ, Foyer CH. 2003a. Dark-chilling imposes metabolic restrictions on photosynthesis in soybean. Plant, Cell and Environment. 26:323–337. [Google Scholar]
- van Heerden PDR, Tsimilli-Michael M, Krüger GHJ, Strasses RJ. 2003b. Dark-chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiologia Plantarum. 117:476–491. doi: 10.1034/j.1399-3054.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. Techniques in plant sciences. Canberra: CSIRO; 2000. Biochemical models of leaf photosynthesis. In: pp. 91–122. [Google Scholar]
- von Caemmerer S, Furbank RT. Modelling C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego, USA: Academic Press; 1999. pp. 173–211. [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. Journal of Experimental Botany. 2004;55:1157–1166. doi: 10.1093/jxb/erh128. [DOI] [PubMed] [Google Scholar]
- Wang X-Q, Wu W-H, Assmann SM. Differential responses of adaxial and abaxial guard cell of broad bean to abscisic acid and calcium. Plant Physiology. 1998;118:1421–1429. doi: 10.1104/pp.118.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Huang LF, Du YS, Yu JQ. Greenhouse and field cucumber genotypes use different mechanisms to protect against dark-chilling. Functional Plant Biology. 2004;31:1215–1223. doi: 10.1071/FP04045. [DOI] [PubMed] [Google Scholar]