Abstract
Low temperature is one of the abiotic factors limiting plant growth and productivity. Yet, knowledge about sex-related responses to low temperature is very limited. In our study, the effects of low, non-freezing temperature on morphological, physiological, and ultrastructural traits of leaves in Populus cathayana Rehd. males and females were investigated. The results showed that 4 °C temperature caused a chilling stress, and females suffered from greater negative effects than did males. At the early growth stage of development, chilling (4 °C) significantly inhibited plant growth, decreased net photosynthesis rate (Pn), stomatal conductance (gs), transpiration (E), and chlorophyll pigments (Chl), and increased intercellular CO2 concentration (Ci), chlorophyll a/b (Chl a/b), proline, soluble sugar and H2O2 contents, and ascorbate peroxidase (APX) activity in both sexes, whereas peroxidase (POD) and glutathione reductase (GR) activities decreased and thiobarbituric acid reactive substance (TBARS) content increased only in females. Chilling stress also caused chloroplast changes and an accumulation of numerous plastoglobules and small vesicles in both sexes. However, disintegrated chloroplasts and numerous tilted grana stacks were only found in chilling-stressed females. Under chilling stress, males showed higher Chl and soluble sugar contents, and higher superoxide dismutase (SOD), POD, and GR activities than did females. In addition, males exhibited a better chloroplast structure and more intact plasma membranes than did females under chilling stress. These results suggest that sexually different responses to chilling are significant and males possess a better self-protection mechanism than do females in P. cathayana.
Keywords: Antioxidant enzymes, chlorophyll, low temperature, photosynthesis, sexual difference, ultrastructure
Introduction
Low temperature is one of the abiotic factors that affect plant survival and growth. When exposed to low temperature, many plant species express physiological or cellular perturbations, generally called low-temperature injury (Hausman et al., 2000). As a consequence, developmental, morphological, physiological, and biochemical processes are altered, affecting productivity, quality, and survival in many plants (Maestrini et al., 2009). Chilling means the presence of low, non-freezing temperature, which is below the optimum temperature for the considered species (generally ranging from +15 °C to 0 °C). During the chilling process, although being accompanied by the accumulation of protective components (e.g. carbohydrates, free proline, and carotenoids), a series of negative effects would happen (Renaut et al., 2005). Usually the most vulnerable targets are photosynthetic components, responses visible as changes in pigment complexes, reduced photosynthetic rates, destroyed chloroplast structures, or restricted electron transport and enzyme activities (Kratsch and Wise, 2000; Marian et al., 2004; Renaut et al., 2005). The generation of reactive oxygen species (ROS) is the most important event when plants suffer from chilling stress. ROS act as signal molecules in regulating many physiological processes (Pei et al., 2000). Excess ROS will create cytotoxic conditions, including oxidative damage to lipids, proteins, and nucleic acids (Mittler et al., 2002), and they will affect photosynthetic pigments, plasma membranes, and cell ultrastructure (Foyer et al., 1994; Xu et al., 2008a). Correspondingly, plants have evolved several antioxidant enzymes, such as superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), peroxidase (POD, EC 1.11.1.7), and glutathione reductase (GR, EC 1.6.4.2), to prevent and alleviate the negative effects of ROS.
Dioecious plants are an important component of terrestrial ecosystems. Many morphological, physiological and ecological differences have been observed between males and females in a number of species in responses to environmental stresses (Espirito-Santo et al., 2003; Li et al., 2007; Letts et al., 2008; Rozas et al., 2009). Because of the different resource costs of reproduction, females are more common on high-resource microsites, while males may be more tolerant to environmental stresses (Jones et al., 1999; Stehlik et al., 2008). Populus cathayana Rehd. is a dioecious, fast-growing tree species, widely distributed in the northern, central, and south-western regions of China. Our earlier studies have shown that males and females of P. cathayana demonstrate different adaptability to a series of biotic and abiotic stresses (Xu et al., 2008b, c; Zhao et al., 2009; Chen et al., 2010a; Zhang et al., 2010a, b). However, little is known about the sex-specific responses to chilling.
Recently, several proteomic and metabolic studies on poplar under chilling stress have been reported (Hausman et al., 2000; Jouve et al., 2000; Renaut et al., 2004, 2005; Pagter et al., 2008), and several low temperature-induced genes have been isolated and expressed in poplar (Maestrini et al., 2009). However, these studies have mainly focused on a single sex. In trees, chilling is a frequently occurring event during the growth season and it may have a huge impact on plant growth and/or productivity (Hausman et al., 2000). Thus, whether different sexes of poplar show different responses to chilling is still unknown.
In this study, P. cathayana was used as research material to investigate sex-specific responses to chilling at an early growth stage. Based on previous studies on low temperature stress (Li et al., 2002, 2004, 2005; Puhakainen et al., 2004), it is hypothesized that there is a set of parallel responses to chilling in the two sexes of P. cathayana, and these responses may be different between males and females. Therefore, the aim of the present work is to compare a series of changes in morphological, physiological, and ultrastructural traits between males and females of P. cathayana in response to a short period of low, non-freezing temperature during their active growth stage of development and to assess which sex suffers less negative effects to chilling.
Materials and methods
Plant materials and experimental design
The experimental plants involved 60 male and 60 female cuttings, which originated from 20 F1 individuals derived from a controlled intraspecific cross between two P. cathayana genotypes with divergent phenotypes, sampled from the Qinghai Province, China (LeDu, 36°31′ N, 102°28′ E). The mean altitude, annual rainfall, and annual temperature in the area are 3160 m, 335 mm, and 6.9 °C, respectively. The experiment was carried out at the Chengdu Institute of Biology, the Chinese Academy of Sciences in June, 2009. Cuttings were planted in 10 l plastic pots filled with 8 kg homogenized soil and 8 g slow release fertilizer (13% N, 10% P, 14% K), and they were kept in a greenhouse. After the plantlets were about 50 cm high, they were moved to a phytotron. The experimental layout was completely randomized with two main factors including sex and temperature regime. Three temperature regimes were applied (25, 15, and 4 °C) and 25 °C was the control condition. Five replicates were used in each treatment, each replicate including four cuttings of each sex. The light intensity was about 120 μmol m−2 s−1 (12/12 h light/dark), the relative air humidity was 80%, and CO2 concentration was 400±10 μmol mol−1. The treatment lasted for 14 d. At the end of the experiment, when there were significant sexual differences in the morphological traits of leaves, as shown in Fig. 1, the 4th and 5th fully expanded leaves were collected for further measurements as described below.
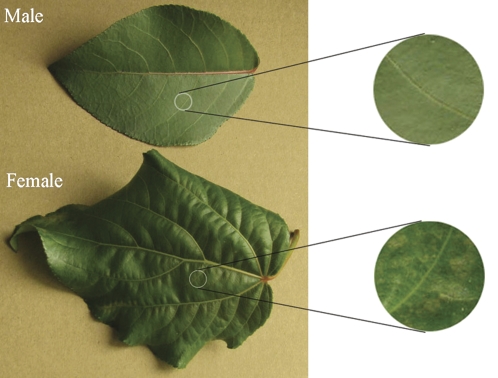
Fig. 1.
The morphological traits of P. cathayana leaves after 14 d of chilling stress.
Gas exchange and chlorophyll pigments
Net photosynthesis rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration (E) were measured using a portable photosynthesis measuring system, Li-Cor 6400 (Li-Cor Inc. Lincoln, Nebraska, USA). The environmental parameters were as follows: leaf temperature 25, 15, or 4 °C, leaf–air vapour pressure deficit 1.5±0.5 kPa, relative air humidity 80%, photosynthetic photon flux density (PPFD) 1200 μmol m−2 s−1, and ambient CO2 concentration 400±5 μmol mol−1.
Samples (1 g leaves) were homogenized in chilled 80% (v/v) acetone in darkness overnight, and the homogenates were centrifuged at 10 000 g for 10 min at 4 °C. The absorbance of the acetone extracts was measured at 663, 645, and 470 nm. The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids (Caro), and total chlorophyll (Chl) were calculated as described by Lichtenthaler (1987).
Relative electrolyte leakage
Fifteen freshly cut leaf discs (0.5 cm in diameter) were incubated in tubes with 10 ml of deionized water at 25 °C for 6 h. The electrical conductivity of the bathing solution (C1) was determined using a conductivity instrument (LC116, Mettler-Toledo Instruments Co., Ltd, Shanghai, China). Then the tubes were incubated in a boiling water bath (100 °C) for 25 min and cooled to room temperature, and the total electrical conductivity (C2) was measured. Ion leakage was calculated using the following equation: relative electrolyte leakage (REL)=(C1/C2)×100.
Lipid peroxidation and protein content
Lipid peroxidation was estimated by measuring the content of thiobarbituric acid reactive substances (TBARS) in leaf homogenates according to Zhang et al. (2010b). Leaves (0.2 g) were homogenized in 5 ml of 4:1 (v/v) of ethanol:water and centrifuged at 12 000 g for 10 min. A 2 ml sample of supernatant was added to a test tube with 2 ml of reaction solution comprised of 20% (w/v) TCA, 0.01% butylated hydroxytoluene, and 0.6% thiobarbituric acid. The mixture was heated in boiling water for 15 min and then quickly cooled in an ice bath. After centrifugation at 12 000 g for 10 min, the absorbance of the supernatant was determined at 450, 532, and 600 nm using a spectrometer (Unicam UV-330, Unicam, Cambridge, UK). The TBARS content was calculated using the following formula: C=6.45(OD532–OD600)–0.56OD450. The protein concentration was determined according to the Bradford method (Bradford, 1976).
Proline and soluble sugars
Proline was quantified by means of a colorimetric reaction with ninhydrin according to the method of Singh et al. (1973). Proline was extracted from 0.1 g of fresh leaves with 3 ml of methanol:chloroform:water (12:5:1, by vol.). After centrifugation at 10 000 g for 5 min, the supernatant was used for proline estimation. A volume of 1 ml of supernatant was transferred to a test tube and heated in a water bath until methanol was evaporated, after which 0.33 ml ninhydrin solution (0.01 g of ninhydrin, 0.166 ml sulphuric acid, and 0.25 ml glacial acetic acid), 0.33 ml of glacial acetic acid, and 0.33 ml of water were added to the sample. The tubes were then cooled to room temperature and 2 ml of toluene was added. After 30 s of shaking, two phases were separated, and the absorbance of the upper phase was read at 520 nm using a spectrometer (Unicam UV-330).
Soluble sugars were estimated by the anthrone method. Leaves (about 0.2 g) were homogenized in a mortar, added to 50 ml distilled water and extracted in boiling water for 20 min. Thereafter, the extract was cooled to room temperature and centrifuged. For the reaction, 1 ml of the supernatant was added to 5 ml anthrone reagent, mixed and heated in boiling water for 10 min, and cooled using ice water. The absorbance was determined using glucose as the standard at 620 nm.
Superoxide radicals and hydrogen peroxide
Measurement of followed the method of Zhang et al. (2010b). First, samples reacted with 1 ml hydroxylamine hydrochloride for 1 h. Then, 1 ml p-aminobenzene sulphonic acid and 1 ml α-naphthylamine were added. The solution was kept at 25 °C for 20 min. The mixture was measured under 530 nm using NaNO2 as the standard curve. Controls or blanks in the presence of SOD and MnCl2 (scavengers of ) were simultaneously examined by adding 100 U of SOD and 10 mM of MnCl2 into the sample buffer. The H2O2 content was determined as a H2O2–titanium complex resulting from the reaction of tissue-H2O2 with titanium tetrachloride. H2O2 measurements for controls or blanks were performed in the presence of 10 mM ascorbic acid (AsA) in the sample buffer.
Antioxidant enzyme activities
Samples (0.5 g leaves) were ground in liquid nitrogen and extracted with 50 mM potassium phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 1% (w/v) PVP, 0.1 mM PMSF, and 0.2% (v/v) Triton X100 for the measurements of POD, SOD, and GR. For APX, 20 mM ascorbate was included in the extraction buffer. The extracts were centrifuged at 12 000 g at 4 °C for 15 min. The supernatants were used for the enzyme activity assays. All operations were performed at 0–4 °C.
SOD activity was determined by measuring its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) as described by Beauchamp and Fridovich (1971). The reaction mixture with a total volume of 3 ml contained 0.3 ml each of 20 μM riboflavin, 150 mM L-methionine, 600 μM NBT, and enzyme extract containing 100 μg protein. The reaction was started with the addition of riboflavin and carried out for 20 min under irradiance of 170 μmol photons m−2 s−1 provided by a white fluorescent lamp. A system devoid of enzymes served as a negative control. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT when monitored at 560 nm.
APX activity assay was conducted as described by Nakano and Asada (1981). The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 1 mM sodium ascorbate, and enzyme extract containing 100 μg proteins in a final volume of 1 ml. The reaction was started by the addition of 0.5 mM H2O2. The decrease in the concentration of ascorbate was recorded at 290 nm. The enzyme activity was calculated from the initial rate of the reaction using the extinction coefficient of ascorbate (2.8 mM−1cm−1 at 290 nm).
POD activity was assayed in 2 ml of 100 mM potassium phosphate buffer (pH 6.5) containing 40 mM guaiacol, 10 mM H2O2, and enzyme extract including 100 μg proteins at 25 °C in a spectrophotometer at 436 nm. Activity was based on the rate of tetraguaiacol production using an extinction coefficient of 25.5 mM−1 cm−1.
GR activity was determined according to Jablonski and Anderson (1978). The reaction mixture consisted of 10 mM GSSG, 1 mM EDTA, and 200 mM phosphate buffer. The supernatant was preincubated at 25 °C for 5 min. The reaction was initiated by an addition of 1 mM NADPH, and the rate of oxidation of NADPH was monitored at 340 nm. The enzyme activity is expressed as μmol NADPH min−1 mg−1 protein.
Transmission electron microscopy
Small leaf sections (2 mm in length), avoiding the midrib, were selected for the transmission electron microscope analysis. The sections were fixed in 2.5% (v/v) glutaral pentanedial in 0.2 M of PBS (sodium phosphate buffer, pH 7.0) for 3 h at 22 °C and post-fixed in 2% osmium tetraoxide (OsO4) for 2 h. The tissues were then sequentially dehydrated in 30%, 50%, 70%, and 90% acetone, and embedded in Epon 812 for 2 h. Ultra-thin sections (80 nm) were sliced, stained with uranyl acetate and lead citrate, and mounted on copper grids for viewing in the H-600IV TEM (Hitachi, Tokyo, Japan) at an accelerating voltage of 60 kV.
Statistical analysis
For gas exchange measurements, ten leaves were used. For physiological and biochemical measurements, three biological replicates were used, and each replicate was assayed in triplicate. To assess differences in total growth and the physiological and biochemical properties between the sexes, multivariate analysis of variance (MANOVA) was used. In the MANOVA, the overall mean of the groups (partitioned to a series of sum of squares) was compared by Test Statistics (Pillai's Trace, Wilks’ Lambda, Lawley-Hotelling and Roy's Largest Root), and between-group variance was expressed as F-statistics. Once the MANOVA tests were established, post hoc comparisons were tested using the Tukey test for means of individual parameters at a significance level of P ≤0.05. All statistical analyses were performed using SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL, USA).
Results
Generally, the results of MANOVA showed that the effects of sex, low temperature, and their interaction were highly significant (P ≤0.001; Table 1). The F values, as the ratio of the between-groups variance to within-group variance, were relatively high (2682) for the effect of sex, indicating that there were significant differences between the sexes. Because the MANOVA technique just gives an overall test of the equality of mean vectors of several groups and in order to provide information on which variables are responsible for the differences in the mean, Tukey tests were performed at a significance level of P ≤0.05 in the following analyses.
Table 1.
Results of multivariate analysis of variance (MANOVA) on all growth, physiological and biochemical data in P. cathayana males and females
| Effect | Test statistic | Value | F | Hypothesis df | Error df | Significance |
| Sex | Pillai's Trace | 1.000 | 2.682E3a | 11.000 | 2.000 | 0.000 |
| Wilks’ Lambda | 0.000 | 2.682E3a | 11.000 | 2.000 | 0.000 | |
| Hotelling's Trace | 1.475E4 | 2.682E3a | 11.000 | 2.000 | 0.000 | |
| Roy's Largest Root | 1.475E4 | 2.682E3a | 11.000 | 2.000 | 0.000 | |
| Temperature | Pillai's Trace | 1.996 | 132.460 | 22.000 | 6.000 | 0.000 |
| Wilks’ Lambda | 0.000 | 1.647E3a | 22.000 | 4.000 | 0.000 | |
| Hotelling's Trace | 3.372E5 | 1.533E4 | 22.000 | 2.000 | 0.000 | |
| Roy's Largest Root | 3.369E5 | 9.189E4b | 11.000 | 3.000 | 0.000 | |
| Sex×Temperature | Pillai's Trace | 1.974 | 20.527 | 22.000 | 6.000 | 0.001 |
| Wilks’ Lambda | 0.000 | 1.480E2a | 22.000 | 4.000 | 0.000 | |
| Hotelling's Trace | 1.743E4 | 792.203 | 22.000 | 2.000 | 0.001 | |
| Roy's Largest Root | 1.739E4 | 4.743E3b | 11.000 | 3.000 | 0.000 |
Exact statistic.
The statistic is an upper bound of F that yields a lower bound for the significance level.
Sexual differences in morphological traits
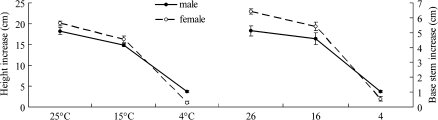
Female leaves began to curl from the leaf margin after 6 d at 4 °C and showed clear damage after 10 d, forming spots along the leaf vein, as shown in Fig. 1. However, male leaves at 4 °C and leaves of both sexes at 15 °C did not show any visible changes even at the end of the 14-d experiment. In addition, chilling significantly inhibited plant growth (height and base stem increase), but sexual differences were not significant (Fig. 2).
Fig. 2.
The height and base stem increases in males and females of P. cathayana at 25, 15, and 4 °C during 14 d of treatment. (This figure is available in colour at JXB online.)
Sexual differences in gas exchange and chlorophyll pigments
Chilling significantly decreased Pn, gs, E, Chl b, and Chl, and increased Chl a/b and Ci in both sexes. Females showed a significant decrease in Chl a, while Caro significantly increased in males at 4 °C. Males possessed higher gs, E, Chl a, and Chl, and lower Chl a/b than did females at 4 °C, while all these parameters had no significant sexual differences at 15 °C. In addition, all parameters showed a significant temperature effect, but Pn, Ci, and Caro had no significant interaction effect of temperature and sex (Table 2).
Table 2.
The gas exchange parameters and chlorophyll pigment concentrations in male and female leaves of P. cathayana as affected by temperature (25, 15, and 4 °C)
| Parameters | 25 °C males | 25 °C females | 15 °C males | 15 °C females | 4 °C males | 4 °C females | Fs | Ft | Fs×t |
| Pn (μmol m−1 s−1) | 16.19±0.75 bc | 16.79±0.70 c | 15.72±0.64 bc | 13.90±0.50 b | 2.99±0.13 a | 1.10±0.19 a | 0.027* | 0.000** | 0.059 |
| gs (mol m−1 s−1) | 0.45±0.01 d | 0.51±0.05 d | 0.44±0.02 cd | 0.34±0.02 c | 0.17±0.01 b | 0.04±0.00 a | 0.035* | 0.000** | 0.017* |
| Ci (μmol mol−1) | 400.29±4.97 a | 406.67±7.60 ab | 416.97±7.41 ab | 419.44±3.90 ab | 433.13±6.79 bc | 445.33±6.22 c | 0.304 | 0.001** | 0.836 |
| E (mmol m−1 s−1) | 5.98±0.10 d | 6.44±0.24 d | 3.19±0.27 c | 2.96±0.24 c | 1.72±0.11 b | 0.66±0.07 a | 0.188 | 0.000** | 0.021* |
| Chl a (mg g−1 Fw) | 25.60±1.18 bc | 25.44±1.01 bc | 25.99±0.92 c | 25.89± 1.17 c | 24.11±1.32 b | 20.98±0.79 a | 0.002** | 0.000** | 0.001** |
| Chl b (mg g−1 Fw) | 20.08±1.16 c | 15.39±0.71 b | 14.25±0.72 b | 15.62±0.77 b | 8.78±0.15 a | 5.98±0.39 a | 0.014* | 0.000** | 0.001** |
| Caro (mg g−1 Fw) | 4.04±0.11 a | 4.30±0.25 ab | 4.57±0.35 ab | 3.66±0.14 a | 5.79±0.19 b | 5.59±0.33 b | 0.174 | 0.000** | 0.125 |
| Chl (mg g−1 Fw) | 45.70±1.08 d | 40.82±0.70 c | 40.26±1.14 c | 41.42±0.94 cd | 32.86±0.31 b | 26.97±1.18 a | 0.001** | 0.000** | 0.001** |
| Chl a/b | 1.28±0.83 a | 1.66±0.08 ab | 1.85±0.15 b | 1.65±0.04 ab | 2.75±0.07 c | 3.52±0.10 d | 0.002** | 0.000** | 0.000** |
Each value is the mean ±SE. Fs, sex effect; Ft, temperature effect; Fs×t, sex and temperature interaction effect; Fw, fresh weight; *, 0.01<P ≤0.05; **, P ≤0.01.
Sexual differences in relative electrolyte leakage and biochemical traits
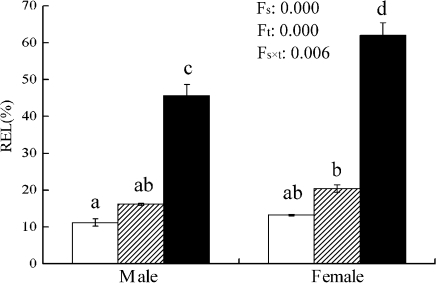
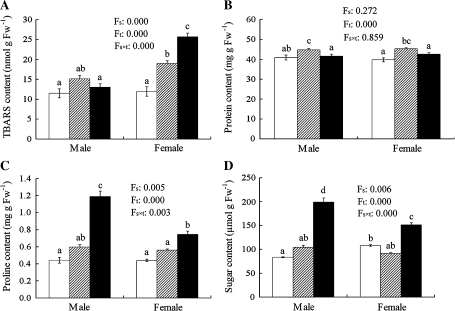
As shown in Fig. 3, the relative electrolyte leakage of cellular membrane was lower in both male and female poplar leaves at 25 °C. However, it sharply increased at 4 °C, and the sexual difference was significant, chilling-stressed females showing higher REL than chilling-stressed males. Significant sexual differences were also recorded in TBARS, proline and soluble sugar contents under chilling. At 4 °C, proline and soluble sugar contents significantly increased in both sexes, while TBARS increased only in females. Males showed higher proline and soluble sugar contents, and a lower TBARS content at 4 °C. However, there was no significant sexual difference in the total protein content (Fig. 4).
Fig. 3.
Relative electrolyte leakage (REL) of cellular membrane in male and female leaves of P. cathayana as affected by temperature (open squares, 25 °C; hatched squares, 15 °C; closed squares, 4 °C). Fs, sex effect; Ft, temperature effect; Fs×t, sex and temperature interaction effect.
Fig. 4.
The contents of TBARS (A), proteins (B), proline (C), and soluble sugars (D) in male and female leaves of P. cathayana as affected by temperature (open squares, 25 °C; hatched squares, 15 °C; closed squares, 4 °C. Fs, sex effect; Ft, temperature effect; Fs×t, sex and temperature interaction effect; Fw, fresh weight. The bars with different letters are significantly different from each other (P ≤0.05). Each value is the mean ±SE (n=3).
Sexual differences in reactive oxygen species and antioxidant enzymes
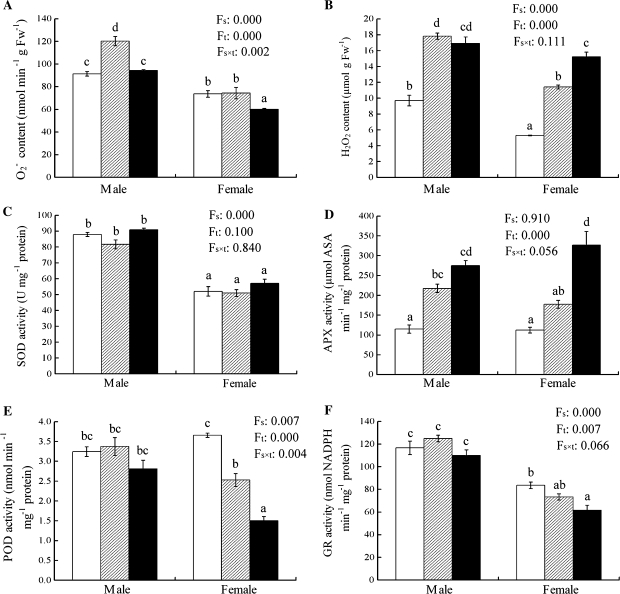
Chilling significantly increased the H2O2 content and APX activity in both sexes, while the production rate and SOD activity showed only small changes, except for the increase in males at 15 °C. The activities of POD and GR significantly decreased along with a decreasing temperature in females but not in males. Overall, males showed a higher production rate, and higher SOD, POD, and GR activities than did females under chilling stress. However, there were smaller sexual differences in the H2O2 content and APX activity at 4 °C (Fig. 5).
Fig. 5.
The production rate of (A), H2O2 content (B), and the activities of SOD, APX, POD, and GR in male and female leaves of P. cathayana as affected by temperature (open squares, 25 °C; hatched squares, 15 °C; closed squares, 4 °C). Fs, sex effect; Ft, temperature effect; Fs×t, sex and temperature interaction effect; Fw, fresh weight. The bars with different letters are significantly different from each other (P ≤0.05). Each value is the mean ±SE (n=3).
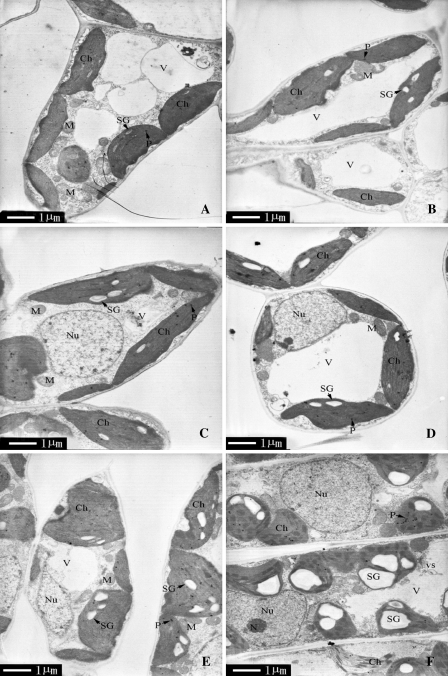
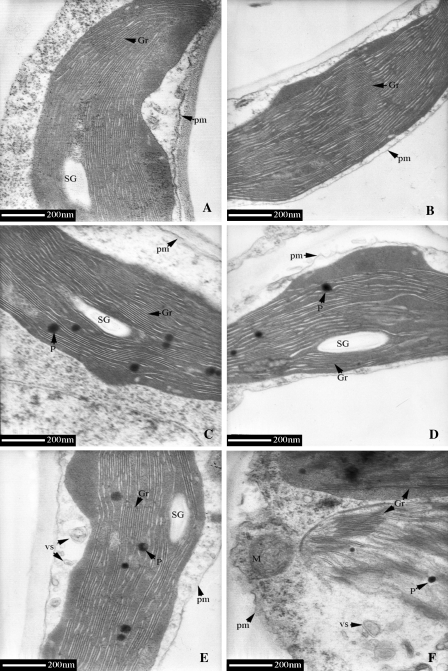
Sexual differences in ultrastructural morphology
As shown in Fig. 6, the leaves of both sexes contained large central vacuoles, oblong chloroplasts with well-arranged thylakoid membranes in distinct grana regions (8–16 thylakoids per granum) at 15 °C and 25 °C. However, the chilling injury of chloroplasts was noticeable: chloroplasts changed into a subcircular shape, and the thylakoids became considerably swollen and distorted at 4 °C. Meanwhile, more plastoglobules and many vesicles of different sizes appeared at 4 °C. There was a greater accumulation of starch grains in chloroplasts and more invaginations of plasma membrane in females than in males (Fig. 7). In addition, disintegrated chloroplasts and numerous tilted granal stacks were found only in females at 4 °C (Figs 6F, 7F). However, the changes in mitochondria and nuclei were relatively small in both sexes under chilling stress.
Fig. 6.
Transmission electron micrographs of mesophyll cells of P. cathayana males and females under different temperatures. (A) 25 °C, males; (B) 25 °C, females; (C) 15 °C, males; (D) 15 °C, females; (E) 4 °C, males; (F) 4 °C, females. Ch, chloroplast; Gr, granum; M, mitochondrion; N, nucleolus; Nu, nucleus; P, plastoglobule; SG, starch grain; V, vacuole; vs, small vesicle.
Fig. 7.
Ultrastructure of chloroplast in P. cathayana males and females under different temperatures. (A) 25 °C, males; (B) 25 °C, females; (C) 15 °C, males; (D) 15 °C, females; (E) 4 °C, males; (F) 4 °C, females. Gr, granum; M, mitochondrion; P, plastoglobule; SG, starch grain; V, vacuole; vs, small vesicle; pm, plasmalemma.
Discussion
Our results showed that there are significant differences in morphological, physiological, and biochemical responses to chilling between P. cathayana males and females. An early morphological response is the avoidance mechanism by adjusting growth rate, visible as a reduction in shoot height and base stem. Our results on the set of plant responses to chilling are consistent with many previous studies (Hendrichson et al., 2004; Renaut et al., 2005; Cocozza et al., 2009). However, the responses differed between males and females. For example, clear chilling-caused spots were found in females but not in males during the 14-d treatment.
Chilling response is based on complex metabolic and structural changes in plant cells involving regulation at various levels. REL reflects the extent of cellular membrane damage and TBARS is an important product of membrane lipid peroxidation. In this study, chilling significantly increased REL and TBARS in both sexes and induced females to exhibit a higher REL and TBARS content than did males, suggesting that 14-d chilling caused great cellular membrane damage and females suffered from more serious peroxidation than did males. This result is consistent with our earlier study on sexual differences of poplars in responses to drought, elevated temperatures, photoperiod transition, rust disease, and salinity (Xu et al., 2008b; Zhao et al., 2009; Chen et al., 2010b; Zhang et al., 2010b). In addition, the greater increases in proline and soluble sugars in chilling-stressed males than in chilling-stressed females suggest that males possess a better osmoregulation mechanism than do females, because soluble sugars and proline are major osmoregulation substances in the expanded leaves of many plants (Morgan, 1984). Sugars also play an important role in energy acquisition, and proline is vital in preventing protein denaturation, being a source for carbon and nitrogen, and acting as a hydroxyl radical scavenger (Larher et al., 2003; Xiao et al., 2009). At certain extent, the greater increases of soluble sugars and proline detected in males are a sign of better protection against environmental stress in male cells compared with female cells.
Changes in photosynthesis have been reported in many previous studies when plants were exposed to chilling stress (Johnsen et al., 2007; DeRidder and Crafts-Brandner, 2008; Suzuki et al., 2008). Usually, decreases of gs and E in chilling-stressed poplars are due to stomatal closure. However, an increase of Ci in both sexes during chilling was observed, indicating that the decrease in Pn is not due to a decrease in available CO2 caused by a decrease in stomatal conductance, but possibly due to non-stomatal factors, such as a decline in the chlorophyll content, inhibition of Rubisco activity, and damage to the photosynthetic system (Peeler et al., 1988; Haldimann, 1998; Zhou et al., 2007). Actually, the total chlorophyll content significantly decreased in both sexes, and chilling-stressed males exhibited higher Chl and lower Chl a/b than did chilling-stressed females. The decrease of chlorophyll pigments during chilling is considered to be an adaptation to avoid the possible photoinhibition/photodynamic damage during winter (Kudoh and Sonoike, 2002). Higher Chl a/b could be due to increased rates of conversion of Chl b to Chl a through Chl b reductase (Folly and Engel, 1999, Sheumann et al., 1999). In addition, the increase in Caro content was significant only in chilling-stressed males. Caro is thought to be correlated with an increase in chilling tolerance and it could act as a photoprotectant and/or enhancer of cold acclimation in juvenile tissues (Renaut et al., 2005). Thus, the decline of Chl can be responsible for the decline of Pn, while the accumulation of Caro plays an important role in protecting plant cells from chilling stress.
The accumulation of ROS in plant cells is a common phenomenon under chilling stress (Keshavkant and Naithani, 2001; Lukatkin, 2002). ROS can act as signalling molecules for stress responses. Yet an excess accumulation of ROS would cause a threat to plant cells. Interestingly, it was found that did not exhibit accumulation while H2O2 significantly increased under chilling stress, which indicates that the oxidative stress for chilling poplar cells is mainly caused by an excess accumulation of H2O2 but not . Meanwhile, chilling stress also significantly increased the APX activity in poplar leaves, although it did not show a significant sexual difference. APX is widely distributed in plant cells, being present in chloroplasts, mitochondria, peroxisomes, and the cytosol (Jiménez et al., 1997; Diaz-Vivancos et al., 2006). It plays an important role in eliminating H2O2 when plants are under oxidative stress. Li et al. (2009) reported that an over-expressed poplar peroxisomal APX gene in tobacco could enhance plants’ stress tolerance. Therefore, the increase of APX activity observed in our study may be an essential response to chilling and can be due to the induction of excess H2O2 accumulation. However, the activities of POD and GR gradually declined with decreasing temperature in females but not in males. Under chilling stress, increases of POD and GR activities have been reported in many plant species (Hodges et al., 1997; Lee and Lee, 2000; Payton et al., 2001; Xu et al., 2008a), while decreases in their activities have also been reported (Kang and Saltveit, 2001; Keshavkant and Naithani, 2001; Rivero et al., 2002; Huang and Guo, 2005). Such inconsistent responses of antioxidant enzymes may be attributed to different treatment duration and different species. In this study, it was found that the changes can be attributed to different genders as well. Generally, most antioxidant enzyme activities increase or change little in chilling-tolerant cultivars, while some decreases occur in chilling-sensitive cultivars (Huang and Guo, 2005; Guo et al., 2006, 2007). Thus, the decline of POD and GR activities under a chilling condition also indicates that P. cathayana females are more chilling-sensitive than males. In addition, males always exhibited a higher production rate and SOD activity than did females, suggesting that males are able to achieve a better control of toxicity than females. Overall, higher antioxidant enzyme activities accompanied with a lower REL and TBARS content in chilling-stressed males than in chilling-stressed females suggest that males have a better protection mechanism operating in detoxifying ROS than do females.
The ultrastructural morphological injuries were apparent in poplar leaves under chilling stress. Because the chloroplast is the focal site of photosynthesis, it is the first and most severely impacted organelle under chilling injury (Kimball and Salisbury, 1973). It is typical that the shape and size of the chloroplasts change, thylakoids become swollen and distorted, numerous plastoglobules accumulate, and plasma membranes invaginate under chilling stress (Kratsch and Wise, 2000; Stefanowska et al., 2002; Xu et al., 2008a). This study was consistent with these results, and it was also found that males suffered less serious injuries in chloroplasts than did females, as chilling-stressed males showed better-developed granal stacks and less invagination of plasma membrane than did chilling-stressed females; for instance, chloroplast disintegration was observed in chilling-stressed females but not in chilling-stressed males. There have been many reports showing that starch grains would reduce or disappear under chilling stress (Murphy and Wilson, 1981; Musser et al., 1984; Saropulos and Drennan, 2007), and there have also been reports indicating that starch grains increase in chilling-stressed plants (Karpilova et al., 1980; Verheul et al., 1996; Mamun et al., 2006). Such different responses to chilling in the size and number of starch grains could depend on the plant species and development stage (Kratsch and Wise, 2000). In this study, it was found that there was more starch grain accumulation in the chloroplasts of chilling-stressed females than in chilling-stressed males at the same development stage, which again proved the presence of sex-dependent responses to chilling.
In conclusion, our results showed that the sexually different responses of P. cathayana to chilling in morphological, physiological, and ultrastructural traits are significant and females suffer more negative effects to chilling than do males. At 4 °C, females suffered more growth cessation and greater chilling injuries in leaf morphology, cellular membranes, and chloroplast ultrastructure than did males. Chilling-stressed males showed a better osmotic adjustment ability, higher antioxidant enzyme activities, and chlorophyll pigment contents than did chilling-stressed females. Therefore, when P. cathayana is planted in cold regions, more effective protective measurements should be provided for females (e.g. low temperature acclimation) to improve their chance for survival.
Acknowledgments
The research was supported by the Key Program of the National Natural Science Foundation of China (No. 30930075), and the Program of ‘Knowledge Innovation Engineering’ of the Chinese Academy of Sciences (No. KSCX2-YW-Z-1019) and West Light Foundation of the Chinese Academy of Sciences.
Glossary
Abbreviations
- APX
ascorbate peroxidase
- Caro
carotenoids
- Chl
chlorophyll
- Ci
intercellular CO2 concentration
- E
transpiration
- GR
glutathione reductase
- gs
stomatal conductance
- NBT
nitroblue tetrazolium
- Pn
net photosynthesis rate
- POD
peroxidase
- REL
relative electrolyte leakage
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
References
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen FG, Chen LH, Zhao HX, Korpelainen H, Li CY. Sex-specific responses and tolerances of Populus cathayana to salinity. Physiologia Plantarum. 2010a;140:163–173. doi: 10.1111/j.1399-3054.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- Chen LH, Zhang S, Zhao HX, Korpelainen H, Li CY. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant, Cell and Environment. 2010b;33:1767–1778. doi: 10.1111/j.1365-3040.2010.02182.x. [DOI] [PubMed] [Google Scholar]
- Cocozza C, Lasserre B, Giovannelli A, Castro G, Fragnelli G, Tognetti R. Low temperature induces different cold sensitivity in two poplar clones (Populus× canadensis Monch ‘I-214’ and P. deltoides Marsh. ‘Dvina’) Journal of Experimental Botany. 2009;60:3655–3664. doi: 10.1093/jxb/erp212. [DOI] [PubMed] [Google Scholar]
- DeRidder BP, Crafts-Brandner SJ. Chilling stress response of post-emergent cotton seedlings. Physiologia Plantarum. 2008;134:430–439. doi: 10.1111/j.1399-3054.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P, Rubio M, Mesonero V, Periago PM, Barceló AR, Martínez-Gómez P, Hernández JA. The apoplastic antioxidant system in Prunus: response to long-term plum pox virus infection. Journal of Experimental Botany. 2006;57:3813–3824. doi: 10.1093/jxb/erl138. [DOI] [PubMed] [Google Scholar]
- Espirito-Santo MM, Madeira BG, Neves FS, Faria ML, Fagundes M, Fernandes GW. Sexual differences in reproductive phenology and their consequences for the demography of Baccharis dracunculifolia (Asteraceae), a dioecious tropical shrub. Annals of Botany. 2003;91:13–19. doi: 10.1093/aob/mcg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folly P, Engel N. Chlorophyll b to chlorophyll a conversion precedes chlorophyll degradation in Hordeum vulgare L. Journal of Biological Chemistry. 1999;274:21811–21816. doi: 10.1074/jbc.274.31.21811. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiologia Plantarum. 1994;92:696–717. [Google Scholar]
- Guo Z, Ou W, Lu S, Zhong Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiology and Biochemistry. 2006;44:828–836. doi: 10.1016/j.plaphy.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Guo ZF, Huang MY, Lu SY, Yaqing Z, Zhong Q. Differential response to paraquat induced oxidative stress in two rice cultivars on antioxidants and chlorophyll a fluorescence. Acta Physiologiae Plantarum. 2007;29:39–46. [Google Scholar]
- Haldimann P. Low growth temperature-induced changes to pigment composition and photosynthesis in Zea mays genotypes differing in chilling sensitivity. Plant, Cell and Environment. 1998;21:200–208. [Google Scholar]
- Hausman JF, Evers D, Thiellement H, Jouve L. Compared responses of poplar cuttings and in vitro raised shoots to short-term chilling treatments. Plant Cell Reports. 2000;19:954–960. doi: 10.1007/s002990000229. [DOI] [PubMed] [Google Scholar]
- Hendrichson L, Ball MC, Wood JT, Chow WS, Furbank RT. Low temperature effects on photosynthesis and growth of grapevine. Plant, Cell and Environment. 2004;27:795–809. [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. Journal of Experimental Botany. 1997;48:1105–1113. [Google Scholar]
- Huang M, Guo Z. Responses of antioxidative system to chilling stress in two rice cultivars differing in sensitivity. Biologia Plantarum. 2005;49:81–84. [Google Scholar]
- Jablonski PP, Anderson JW. Light-dependent reduction of oxidised glutathione by ruptured chloroplasts. Plant Physiology. 1978;61:221–225. doi: 10.1104/pp.61.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, delRio LA, Sevilla F. Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiology. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen K, Maier C, Sanchez F, Anderson P, Butnor J, Waring R, Linder S. Physiological girdling of pine trees via phloem chilling: proof of concept. Plant, Cell and Environment. 2007;30:128–134. doi: 10.1111/j.1365-3040.2006.01610.x. [DOI] [PubMed] [Google Scholar]
- Jones MH, Macdonald SE, Henry GHR. Sex- and habitat-specific responses of a high arctic willow, Salix arctica, to experimental climate change. Oikos. 1999;87:129–138. [Google Scholar]
- Jouve L, Franck T, Gaspar T, Cattivelli L, Hausman JF. Poplar acclimation to cold during in vitro conservation at low non-freezing temperature: metabolic and proteic changes. Journal of Plant Physiology. 2000;157:117–123. [Google Scholar]
- Kang HM, Saltveit ME. Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiologia Plantarum. 2001;113:548–556. [Google Scholar]
- Karpilova I, Chugunova N, Bil’ K, Chermnykh L. Ontogenetic changes of chloroplast ultrastructure, photosynthates and photosynthate outflow from the leaves in cucumber plants under conditions of reduced night temperature. Soviet Plant Physiology. 1980;29:113–120. [Google Scholar]
- Keshavkant S, Naithani SC. Chilling-induced oxidative stress in young sal (Shorea robusta) seedlings. Acta Physiologiae Plantarum. 2001;23:457–466. [Google Scholar]
- Kimball SL, Salisbury FB. Ultrastructural changes of plants exposed to low temperatures. American Journal of Botany. 1973;60:1028–1033. [Google Scholar]
- Kratsch HA, Wise RR. The ultrastructure of chilling stress. Plant, Cell and Environment. 2000;23:337–350. [Google Scholar]
- Kudoh H, Sonoike K. Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta. 2002;215:541–548. doi: 10.1007/s00425-002-0790-9. [DOI] [PubMed] [Google Scholar]
- Larher FR, Aziz A, Gibon Y, Trotel-Aziz P, Sulpice R, Bouchereau A. An assessment of the physiological properties of the so-called compatible solutes using in vitro experiments with leaf discs. Plant Physiology and Biochemistry. 2003;41:657–666. [Google Scholar]
- Lee DH, Lee CB. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Science. 2000;159:75–85. doi: 10.1016/s0168-9452(00)00326-5. [DOI] [PubMed] [Google Scholar]
- Letts MG, Phelan CA, Johnson DRE, Rood SB. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiology. 2008;28:1037–1048. doi: 10.1093/treephys/28.7.1037. [DOI] [PubMed] [Google Scholar]
- Li CY, Junttila O, Palva ET. Environmental regulation and physiological basis of freezing tolerance in woody plants. Acta Physiologiae Plantarum. 2004;26:213–222. [Google Scholar]
- Li CY, Puhakainen T, Welling A, Viherä-Aarnio A, Ernstsen A, Junttila O, Heino P, Palva ET. Cold acclimation in silver birch (Betula pendula). Development of freezing tolerance in different tissues and climatic ecotypes. Physiologia Plantarum. 2002;116:478–488. [Google Scholar]
- Li CY, Xu G, Zang RG, Korpelainen H, Berninger F. Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitudinal gradient. Tree Physiology. 2007;27:399–406. doi: 10.1093/treephys/27.3.399. [DOI] [PubMed] [Google Scholar]
- Li CY, Yang YQ, Junttila O, Palva ET. Sexual differences in cold acclimation and freezing tolerance development in sea buckthorn (Hippophae rhamnoides L.) ecotypes. Plant Science. 2005;168:1365–1370. [Google Scholar]
- Li Y, Hai R, Du X, Jiang X, Lu H. Over-expression of a Populus peroxisomal ascorbate peroxidase (PpAPX) gene in tobacco plants enhances stress tolerance. Plant Breeding. 2009;128:404–410. [Google Scholar]
- Lichtenthaler HK. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Lukatkin AS. Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants. 1. Reactive oxygen species formation during plant chilling. Russian Journal of Plant Physiology. 2002;49:622–627. [Google Scholar]
- Maestrini P, Cavallini A, Rizzo M, Giordani T, Bernardi R, Durante M, Natali L. Isolation and expression analysis of low temperature-induced genes in white poplar (Populus alba) Journal of Plant Physiology. 2009;166:1544–1556. doi: 10.1016/j.jplph.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Mamun EA, Alfred S, Cantrill LC, Overall RL, Sutton BG. Effects of chilling on male gametophyte development in rice. Cell Biology International. 2006;30:583–591. doi: 10.1016/j.cellbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Marian CO, Krebs SL, Arora R. Dehydrin variability among rhododendron species: a 25 kDa dehydrin is conserved and associated with cold acclimation across diverse species. New Phytologist. 2004;161:773–780. doi: 10.1111/j.1469-8137.2003.01001.x. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Morgan JM. Osmoregulation and water-stress in higher-plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1984;35:299–319. [Google Scholar]
- Murphy C, Wilson JM. Ultrastructural features of chilling-injury in Episcia reptans. Plant, Cell and Environment. 1981;4:261–265. [Google Scholar]
- Musser RL, Thomas SA, Wise RR, Peeler T, Naylor AW. Chloroplast ultrastructure, chlorophyll fluorescence, and pigment composition in chilling-stressed soybeans. Plant Physiology. 1984;74:749–754. doi: 10.1104/pp.74.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Pagter M, Liu FL, Jensen CR, Petersen KK. Effects of chilling temperatures and short photoperiod on PSII function, sugar concentrations and xylem sap ABA concentrations in two Hydrangea species. Plant Science. 2008;175:547–555. [Google Scholar]
- Payton P, Webb R, Kornyeyev D, Allen R, Holaday AS. Protecting cotton photosynthesis during moderate chilling at high light intensity by increasing chloroplastic antioxidant enzyme activity. Journal of Experimental Botany. 2001;52:2345–2354. doi: 10.1093/jexbot/52.365.2345. [DOI] [PubMed] [Google Scholar]
- Peeler TC, Naylor AW. A comparison of the effects chilling on leaf gas-exchange in pea (Pisum sativum L.) and cucumber (Cucumis sativus L.) Plant Physiology. 1988;86:143–146. doi: 10.1104/pp.86.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Puhakainen T, Li CY, Boije-Malm M, Kangasjarvi J, Heino P, Palva ET. Short-day potentiation of low temperature-induced gene expression of a C-repeat-binding factor-controlled gene during cold acclimation in silver birch. Plant Physiology. 2004;136:4299–4307. doi: 10.1104/pp.104.047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaut J, Hoffmann L, Hausman JF. Biochemical and physiological mechanisms related to cold acclimation and enhanced freezing tolerance in poplar plantlets. Physiologia Plantarum. 2005;125:82–94. [Google Scholar]
- Renaut J, Lutts S, Hoffmann L, Hausman JF. Responses of poplar to chilling temperatures: proteomic and physiological aspects. Plant Biology. 2004;6:81–90. doi: 10.1055/s-2004-815733. [DOI] [PubMed] [Google Scholar]
- Rivero RM, Ruiz JM, Garcia PC, Lopez-Lefebre LR, Sanchez E, Romero L. Response of oxidative metabolism in watermelon plants subjected to cold stress. Functional Plant Biology. 2002;29:643–648. doi: 10.1071/PP01013. [DOI] [PubMed] [Google Scholar]
- Rozas V, DeSoto L, Olano JM. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytologist. 2009;182:687–697. doi: 10.1111/j.1469-8137.2009.02770.x. [DOI] [PubMed] [Google Scholar]
- Saropulos AS, Drennan DSH. Ultrastructural alterations in mesophyll and bundle sheath chloroplasts of two maize cultivars in response to chilling at high irradiance. Biologia Plantarum. 2007;51:690–698. [Google Scholar]
- Sheumann V, Schoch S, Rudiger W. Chlorophyll b reduction during senescence of barley seedlings. Planta. 1999;209:364–370. doi: 10.1007/s004250050644. [DOI] [PubMed] [Google Scholar]
- Singh TN, Paleg LG, Aspinall D. Stress metabolism. I. Nitrogen metabolism and growth in the barley plant during water stress. Australian Journal of Biological Sciences. 1973;26:45–56. [Google Scholar]
- Stefanowska M, Kuras M, Kacperska A. Low temperature-induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L. var. oleifera L.) leaves. Annals of Botany. 2002;90:637–645. doi: 10.1093/aob/mcf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik I, Friedman J, Barrett SCH. Environmental influence on primary sex ratio in a dioecious plant. Proceedings of the National Academy of Sciences, USA. 2008;105:10847–10852. doi: 10.1073/pnas.0801964105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nagasuga K, Okada M. The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant and Cell Physiology. 2008;49:433–442. doi: 10.1093/pcp/pcn020. [DOI] [PubMed] [Google Scholar]
- Verheul MJ, Picatto C, Stamp P. Growth and development of maize (Zea mays L.) seedlings under chilling conditions in the field. European Journal of Agronomy. 1996;5:31–43. [Google Scholar]
- Xiao XW, Yang F, Zhang S, Korpelainen H, Li CY. Physiological and proteomic responses of two contrasting Populus cathayana populations to drought stress. Physiologia Plantarum. 2009;136:150–168. doi: 10.1111/j.1399-3054.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- Xu PL, Guo YK, Bai JG, Shang L, Wang XJ. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiologia Plantarum. 2008a;132:467–478. doi: 10.1111/j.1399-3054.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY. Drought inhibits photosynthetic capacity more in females than in males of. Populus cathayana. Tree Physiology. 2008b;28:1751–1759. doi: 10.1093/treephys/28.11.1751. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang F, Xiao XW, Zhang S, Korpelainen H, Li CY. Sex-specific responses of Populus cathayana to drought and elevated temperatures. Plant, Cell and Environment. 2008c;31:850–860. doi: 10.1111/j.1365-3040.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen FG, Peng SM, Ma WJ, Korpelainen H, Li CY. Comparative physiological, ultrastructural and proteomic analyses reveal sexual differences in the responses of Populus cathayana under drought stress. Proteomics. 2010a;10:2661–2677. doi: 10.1002/pmic.200900650. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lu S, Xu X, Korpelainen H, Li CY. Changes in antioxidant enzyme activities and isozyme profiles in leaves of male and female Populus cathayana infected with. Melampsora larici-populina. Tree Physiology. 2010b;30:116–128. doi: 10.1093/treephys/tpp094. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Li Y, Duan BL, Korpelainen H, Li CY. Sex-related adaptive responses of Populus cathayana to photoperiod transitions. Plant, Cell and Environment. 2009;32:1401–1411. doi: 10.1111/j.1365-3040.2009.02007.x. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Huang LF, Zhang YL, Shi K, Yu JQ, Nogues S. Chill-induced decrease in capacity of RuBP carboxylation and associated H2O2 accumulation in cucumber leaves are alleviated by grafting onto figleaf gourd. Annals of Botany. 2007;100:839–848. doi: 10.1093/aob/mcm181. [DOI] [PMC free article] [PubMed] [Google Scholar]