Abstract
Stem cells remain in specialized niches over the lifespan of the organism in many organs to ensure tissue homeostasis and enable regeneration. How the niche is maintained is not understood, but is likely as important as intrinsic stem cell self-renewal capacity for tissue integrity. We here demonstrate a high degree of phenotypic plasticity of the two main niche cell types, ependymal cells and astrocytes, in the neurogenic lateral ventricle walls in the adult mouse brain. In response to a lesion, astrocytes give rise to ependymal cells and ependymal cells give rise to niche astrocytes. We identify EphB2 forward signaling as a key pathway regulating niche cell plasticity. EphB2 acts downstream of Notch and is required for the maintenance of ependymal cell characteristics, thereby inhibiting the transition from ependymal cell to astrocyte. Our results show that niche cell identity is actively maintained and that niche cells retain a high level of plasticity.
Stem cell maintenance depends on cell intrinsic self-renewal capacity as well as the specific molecular environment created by neighboring cells forming the stem cell niche. Stem cell populations are protected by, for example, a certain degree of lineage plasticity, with the possibility of dedifferentiation of progenitor cells to replace lost stem cells (Simon and Frisén, 2007). How niche cells are maintained is largely unknown, although this is likely to be as important as intrinsic stem cell self-renewal capacity for tissue homeostasis and regeneration.
Several studies have demonstrated altered morphology of the neural stem cell niche in the lateral ventricle wall in adult rodents after experimental manipulations (Barnabé-Heider et al., 2008; Carlén et al., 2009; Conover et al., 2000; Kuo et al., 2006; Luo et al., 2008), providing a model system for the study of niche remodeling. The lateral ventricle is lined by a single layer of multiciliated ependymal cells. In the subventricular zone, located subjacent to the ependymal layer, there are both differentiated niche cells and cells of different maturational stages from a subpopulation of ventricle-contacting self-renewing astrocytes with characteristics of neural stem cells to neuroblasts which migrate to the olfactory bulb (Zhao et al., 2008).
The main niche cells in the lateral ventricle wall are ependymal cells and astrocytes, which are tightly interconnected through adherence and gap junctions (Doetsch et al., 1997; Mirzadeh et al., 2008). Subventricular zone astrocytes can be divided into proliferating stem cells and non-proliferating niche cells, which are interconnected with each other as well as with ependymal cells to form a unique architectonic structure (Mirzadeh et al., 2008). Several studies have illustrated the importance of the niche and how damage or loss of ependymal cells impacts the maintenance and proliferation of stem/progenitor cells and the migration of neuroblasts (Barnabé-Heider et al., 2008; Lim et al., 2000; Sawamoto et al., 2006).
The ependymal layer was thought to be incapable of regeneration and that loss of this niche cell type would be permanent. This appears to be the case after loss of larger areas of ependymal cells (Carlén et al., 2009; Kuo et al., 2006). However, two studies indicated that ependymal cells are replaced after minor lesions or during aging (Luo et al., 2006; Luo et al., 2008). These studies suggested that astrocytes give rise to new ependymal cells and also that some astrocytes relocate to the ependymal layer after a lesion or during aging (Luo et al., 2006; Luo et al., 2008). However, the origin of new ependymal cells or astrocytes in the ependymal layer has not been directly demonstrated and the molecular mechanisms of these restructuring processes have remained uncharacterized.
Blocking of the ephrin-B/EphB interaction by infusion of soluble ectodomains of ligands or receptors into the lateral ventricles resuls in similar remodeling of the niche as induced by a lesion or during aging (Conover et al., 2000), providing a first indication that this class of molecules may regulate niche cell plasticity. Eph tyrosine kinase receptors and their ephrin ligands control cell-cell interactions in many developing and adult tissues (Pasquale, 2008), and have been identified as important regulators of both proliferation, differentiation, survival and migration of stem/progenitor cells (Chumley et al., 2007; Depaepe et al., 2005; Genander and Frisén, 2010; Genander et al., 2009; Genander et al., 2010; Hara et al., 2010; Holmberg et al., 2005; Holmberg et al., 2006; Jiao et al., 2008; Qiu et al., 2008; Ricard et al., 2006).
Here we report that EphB signaling regulates niche cell plasticity. By combining genetic fate mapping with lesioning of the lateral ventricle wall, we found that ependymal cells and niche astrocytes are mutually convertible during niche remodeling. Downregulation of EphB2 resulted in this cellular conversion, and blockade of EphB/ephrin-B signaling resulted in a cell fate shift between ependymal cells and niche astrocytes. Furthermore, we show that EphB activity acts downstream of Notch signaling and is sufficient to rescue ependymal cell loss induced by suppressed Notch signaling. These data demonstrate an unanticipated plasticity of niche cells and a novel role for Eph receptor signaling in regulating cell lineage conversion.
Results
Astrocytes replace ependymal cells
In order to study the reactions to an injury to the adult mouse lateral ventricle wall neural stem cell niche we made intraventricular injections of neuraminidase, which cleaves sialic acid of sialoglycoproteins on ependymal cells (Del Carmen Gomez-Roldan et al., 2008; Grondona et al., 1996; Luo et al., 2008). To induce a limited injury and circumvent excessive scar formation we injected 100 ng neuraminidase, which results in severe damage in the ependymal layer in the injected side of the lateral ventricle (Luo et al., 2008), but mild ependymal denudation in the contralateral ventricle. We always analyzed the contralateral side to the injection to exclude secondary effects by tissue penetration of the injection needle.
A previous study suggested that lost ependymal cells are replaced by subventricular zone astrocytes after this type of lesion, although this was inferred indirectly and no lineage analysis was performed (Luo et al., 2008). We performed genetic fate mapping to investigate if there is any fate plasticity of niche cells and whether astrocytes can give rise to ependymal cells. We used Cx30-CreER mice, which express tamoxifen-dependent Cre recombinase (CreERT2) under the control of the astrocyte specific Connexin-30 promoter (Slezak et al., 2007); our unpublished data). The Cx30-CreER line was crossed with the R26R-YFP reporter line, allowing for YFP expression in CreER expressing astrocytes after tamoxifen administration (Figures 1A and 1B). Only astrocytes, identified by GFAP and glutamine synthetase expression, showed reporter expression short time after tamoxifen administration (Figures 1C and 1D). Some recombined cells expressed the proliferation marker Ki67 (4.5 ± 1.0%, 154 recombined cells were examined, mean ± S.D., Figure 1E). No recombined doublecortin-positive neuroblasts were seen early after the termination of tamoxifen administration, but starting a few days later an increasing proportion of neuroblasts showed recombination (our unpublished data). Ependymal cells were never recombined (360 recombined cells were examined, Figure 1F).
Figure 1. Subventricular zone astrocytes give rise to ependymal cells after a lesion.
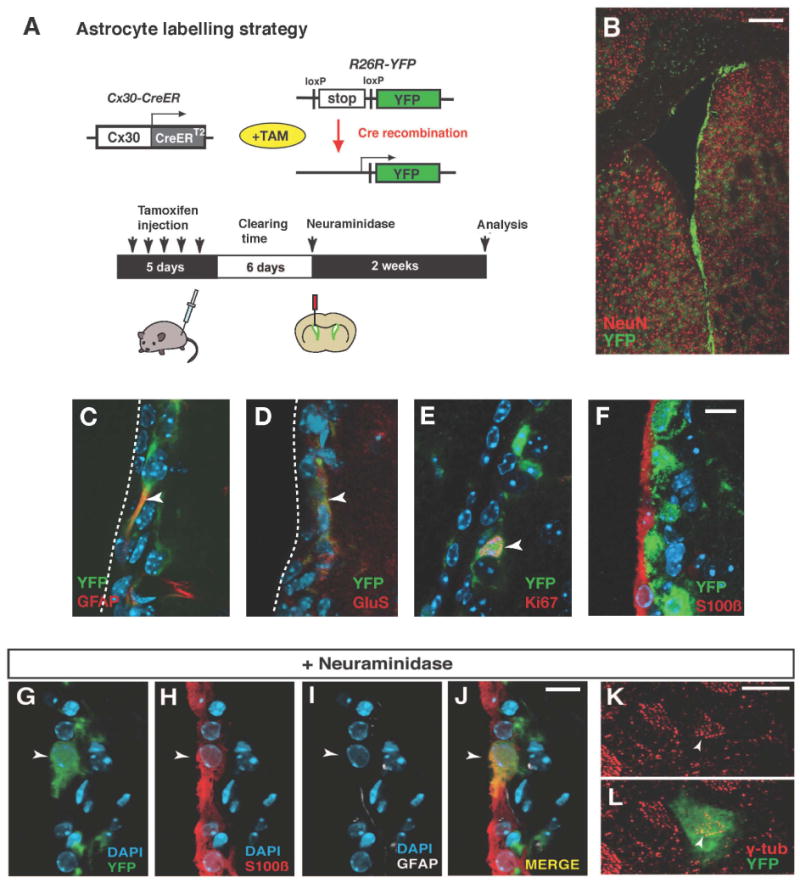
(A) Experimental strategy for lineage tracing of astrocytes. Transgenic mice expressing tamoxifen inducible Cre recombinase under the control of the connexin30 promoter (Cx30-CreER) were crossed with R26R-YFP reporter mice. (B) Distribution of recombined cells in the subventricular zone and brain parenchyma after 5 days injection of tamoxifen. (C-F) Recombined cells are positive for the astrocytic markers GFAP (C) and glutamine synthetase (GluS, D) and some for the proliferation marker Ki67 (E), but none the ependymal cell marker S100ß (F). (G-L) Astrocytes give rise to ependymal cells after a neuraminidase lesion. A recombined cell expressing S100ß but not GFAP is integrated in the ependymal layer (arrowheads in G-J). (K and L) Whole mount analysis reveals the localization of gamma-tubulin on the apical surface of a recombined astrocyte-derived cell (arrowhead), as in neighboring non-recombined ependymal cells. Scale bars indicate 200 μm in (B), 10 μm in (F), (J) and (K).
To directly assess whether astrocytes can give rise to ependymal cells in response to an injury to the niche, we induced recombination in Cx30-CreER × R26R-YFP mice, and after 6 days of tamoxifen clearing, we injected neuraminidase into the lateral ventricle (Figure 1A). Waiting 6 days between the last tamoxifen injection and the injury ensures that all recombination occurs prior to the insult and that even if other cells than astrocytes would start to express the Cx30-CreER transgene in response to the injury it would not result in recombination. Tamoxifen and its active metabolite 4-hydroxytamoxifen have a half-life of 6-12 hours in the mouse (Robinson et al., 1991), and CreER protein is no longer detectable in the nucleus of cells after six days without tamoxifen (Meletis et al., 2008).
Two weeks after lesioning we detected recombined astrocyte-derived cells that were interposed in the ependymal layer and that were positive for the ependymal cell marker S100ß (1.1 ± 0.2% of recombined cells in the lateral ventricular wall were S100ß positive, total 1577 recombined cells were examined, 0% in unlesioned animals, mean ± S.D., n=3 mice, Figures 1G-1J. Of these, 75% had lost GFAP and were positive for the cilia associated marker CD133/prominin (Figure S1A). Whole mount preparations of the lateral ventricle wall from animals receiving neuraminidase showed intercalation of YFP-positive recombined cells in the ependymal layer (Figures 1K and 1L). We detected basal body patches on the apical surface of interposed recombined cells, which is a characteristic of ependymal cells, establishing that astrocytes give rise to ependymal cells after this type of injury (Figures 1K and 1L). Adminstration of BrdU in the drinking water during the full two weeks after the lesion did not result in labeling of any astrocyte-derived ependymal cells (data not shown). This indicates that non-proliferating niche astrocytes give rise to ependymal cells without going through a cell division.
Ependymal cells acquire astrocyte characteristics after an injury to the niche
Next, we asked whether ependymal cells also respond to a lesion to the niche. Adenovirus injected into the lateral ventricle exclusively infect ependymal cells (Akli et al., 1993; Bajocchi et al., 1993; Davidson and Bohn, 1997; Doetsch et al., 1999a; Johansson et al., 1999), and this property can be used for genetic labeling and fate mapping of ependymal cells (Carlén et al., 2009). Ependymal cells were genetically labeled by injection of a Cre recombinase expressing adenovirus into the lateral ventricle of R26R-YFP reporter mice, allowing for specific and inheritable labeling of ependymal cells (Figure 2A, Carlén et al., 2009). YFP-expressing recombined ependymal cells lined the lateral ventricle wall (Figure 2B) and were positive for the ependymal marker S100ß and CD133 (Figures 2C and 2D). The recombination was specific to ependymal cells and no recombined cell expressed GFAP, Glutamine synthetase, DCX or the proliferation marker Ki67 (Figures 2E-2H; Carlén et al., 2009). A similar labeling specificity was obtained after injection of adenovirus expressing GFP (data not shown). To further ensure specificity of the ependymal cell labeling, we used a second strategy in parallel: we electroporated a Cre-expressing vector under the control of the ependymal cell-specific Foxj1 promoter into the lateral ventricle of adult R26R-YFP mice, which gave similar results (Figure S2; Carlen et al., 2009).
Figure 2. Ependymal cells acquire an astrocytic phenotype after a lesion.
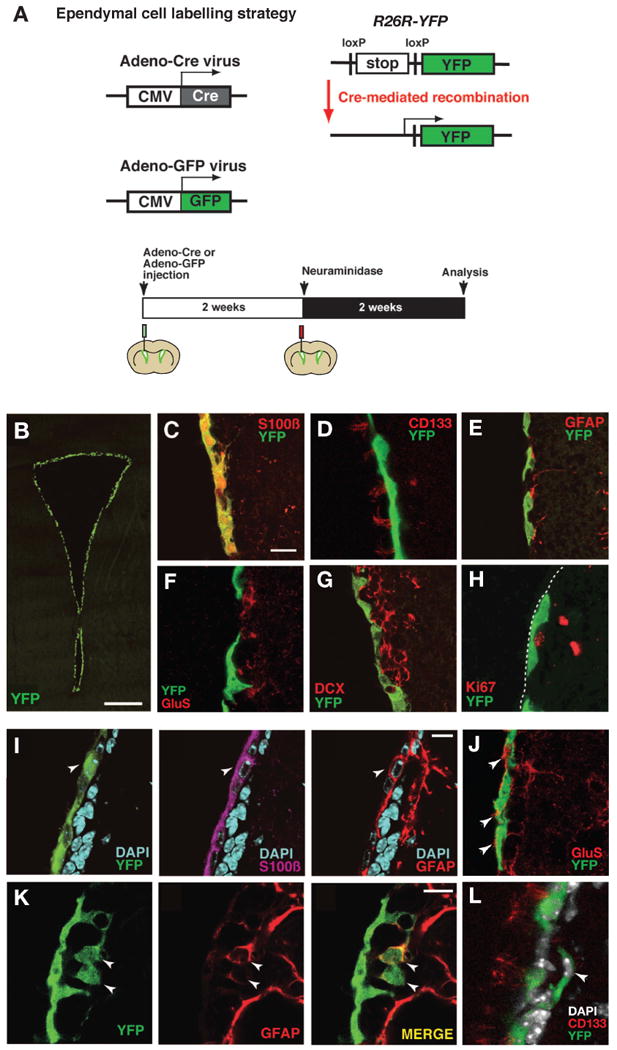
(A) Illustration of the strategy for genetic fate mapping of ependymal cells. (B-H) Recombined cells are distributed along the surface of the lateral wall (B), and labeled with S100ß (C) and CD133 (D), but not with GFAP (E), glutamine synthetase (GluS, F), DCX (G) or Ki67 (H). (I and J) Expression of the astrocyte markers GFAP (I) and GluS (J) in ependymal cells after neuraminidase treatment. Arrowheads in (I) indicate a recombined cell expressing both S100ß and GFAP and arrowheads in (J) indicate GluS expressing ependymal cell-derived astrocytes. (K and L) Delamination of ependymal cell-derived cells from the ependymal layer after a neuraminidase induced lesion. Arrowheads point to GFAP-positive recombined cells in the subventricular zone (K) and a CD133-negative cell (L). The luminal side of the ependymal layer is indicated by a broken line in (H). Scale bars represent 200 μm in B, 10 μm in (C), (I) and (K).
Neuraminidase was injected in the same ventricle as the adenovirus (Figure 2A). To exclude the possibility of additional viral-mediated recombination after lesioning, the neuraminidase injection was made 2 weeks after the adenovirus injection, a time when all adenovirus has been cleared from the ventricle (Carlen et al., 2009). All analyses were performed on the contralateral side to the injection to avoid any tissue disturbance or non-specific labeling by the injection. Similar to the fate mapping of subventricular zone astrocytes, all animals were analyzed 2 weeks after neuraminidase injection. In control animals receiving vehicle, all YFP-positive recombined cells were localized within the ependymal layer and expressed S100ß, while none of them expressed GFAP (data not shown; Carlén et al., 2009). In contrast, there were numerous GFAP-positive recombined cells in the neuraminidase treated animals (4.7 ± 0.9% of recombined cells were GFAP-positive, mean ± S.D., total 1156 recombined cells were examined, n=3 mice; Figure 2I). The ependymal-to-astrocyte transition after lesion was further indicated by the up-regulation of glutamine synthetase, a functional astrocyte marker, in recombined cells (Figure 2J). The majority of GFAP-immunoreactive recombined cells remained within the ependymal layer and co-expressed S100ß (79% of GFAP-positive recombined cells were S100ß-positive; Figure 2I), but 21% were dislocated to the subventricular zone, did not express S100ß or CD133 (Figures 2K and 2L), extended long GFAP-positive processes and were phenotypically indistinguishable from astrocytes normally residing in this area (Figure 2K and 2L). Similar results were obtained in neuraminidase-treated mice in which ependymal cells were labeled with adeno-GFP virus or by pFoxj1-Cre electroporation (Figure S2). We could not detect Ki67-positive proliferating ependymal or ependymal-derived cells nor the generation of DCX-positive neuroblasts from recombined cells (data not shown), indicating that ependymal cells did not obtain neurogenic potential after this type of lesion.
Thus, we find that ependymal cells can convert into niche astrocytes and that astrocytes can replace ependymal cells after a lesion to the niche. The proportion of ependymal cells converting to astrocytes was more than four fold higher than the number of astrocytes converting into ependymal cells (4.7% comapared to 1.1%). There are slightly more astrocytes than ependymal cells in the lateral ventricle wall, but even taking this into account, there were approximately three-fold more ependymal cell-derived astrocytes (3.0 ± 0.7 cells per section) than there were astrocyte-derived ependymal cells (1.3 ± 0.3 cells per section) after a lesion when comparing the absolute numbers of these events.
Disruption of the EphB/ephrin-B interaction induces niche cell lineage conversion
How is the phenotype of niche cells regulated at the molecular level? Several Eph tyrosine kinase receptors and their ephrin ligands are expressed in the adult subventricular zone and are involved in regulating the migration of neuroblasts as well as the proliferation of neural stem/progenitor cells (Chumley et al., 2007; Conover et al., 2000; Holmberg et al., 2005; Ricard et al., 2006). Conover et al. (2000) demonstrated changes in the structure of the niche after inhibiting EphB signaling, reminiscent of the effect of a lesion to the niche (Luo et al. 2008; see above), suggesting that EphB/ephrin-B signaling may be involved in regulating niche cell plasticity.
We first examined the expression pattern of EphB receptors and B class ephrins in the adult subventricular zone. By using EphB2-Fc and ephrin-B2-Fc fusion proteins, which bind to all B-class ephrins and Eph receptors, respectively, we confirmed that both ephrin-B ligands and EphB receptors were present in the adult ventricular wall (Figures S3A-S3C). In situ hybridization revealed that EphB1 and EphB2 mRNA and ephrin-B1 and ephrin-B2 mRNA are expressed in the adult lateral ventricular wall (Figures S3D-S3G; data not shown), in line with previous reports (Conover et al., 2000; Ricard et al., 2006). Immunohistochemical analysis and/or analysis of reporter mice revealed that EphB1 is expressed in ependymal cells and subventricular zone astrocytes and EphB2 is expressed in ependymal cells and ßIII-tubulin-positive neuroblasts (Figures S3H to S3K). We detected ephrin-B1 expression in ependymal cells and neuroblasts and ephrin-B2 in ependymal cells and subventricular zone astrocytes (Figures S3L to S3O).
Conover et al. (2000) demonstrated a reduction in the number of ependymal cells and an increase in the number of astrocytes after disruption of EphB/ephrin-B signaling by injection of soluble ligand or receptor ectodomains fused to Fc. Moreover, a striking finding in this study was an almost ten-fold increase in the number of ventricle-contacting astrocytes. The origin of the new ventricle-contacting astrocytes was not assessed, but it was suggested that the disruption of the EphB/ephrin-B interaction promoted astrocytes located in the subventricular zone to enter the ependymal layer (Conover et al., 2000). Our finding that ependymal cells have a considerable degree of fate plasticity and can give rise to astrocytes prompted us to assess the origin of astrocytes within the ependymal layer after disruption of EphB/ephrin-B interaction.
We genetically labeled subventricular zone astrocytes or ependymal cells, as described above, and infused soluble non-clustered EphB2-Fc protein into the lateral ventricle, which blocks endogenous EphB/ephrin-B interactions and signaling (Conover et al., 2000; Holmberg et al., 2005). EphB2-Fc was infused for 3 days or 1 week into the lateral ventricle using an osmotic pump (Figures 3A and 3F). While Fc-fragment infusion did not alter the position or marker expression of labeled astrocytes or ependymal cells (Figures 3B, 3C, 3G and 3H), we observed lineage conversion of niche cells after EphB2-Fc infusion (Figures 3D, 3E, 3I and 3J). In Cx30-CreER;R26R-YFP mice treated with EphB2-Fc, 1.8 ± 0.4% (mean ± S.D., total 1335 cells were examined, n=3 mice) of the YFP-positive astrocyte-derived cells in the lateral ventricle wall expressed S100ß and resided within the ependymal layer (Figure 3D). Of these cells, 60% had lost GFAP expression, were CD133/prominin-immunoreactive and had multiple cilia (Figure 3E; Figure S1B). Whole mount preparations showed the intercalation of astrocyte-derived recombined cells in the ependymal layer and the formation of ependymal cell-characteristic basal bodies (Figures 3C and 3E), similar to what was observed after neuraminidase treatment (Figure 1). Administration of BrdU in the drinking water during the entire period of EphB2-Fc delivery failed to label any astrocyte-derived ependymal cells, indicating that they derive from non-dividing niche astrocytes.
Figure 3. Blocking of the EphB/ephrin-B interaction induces phenotypic switches of niche cells.
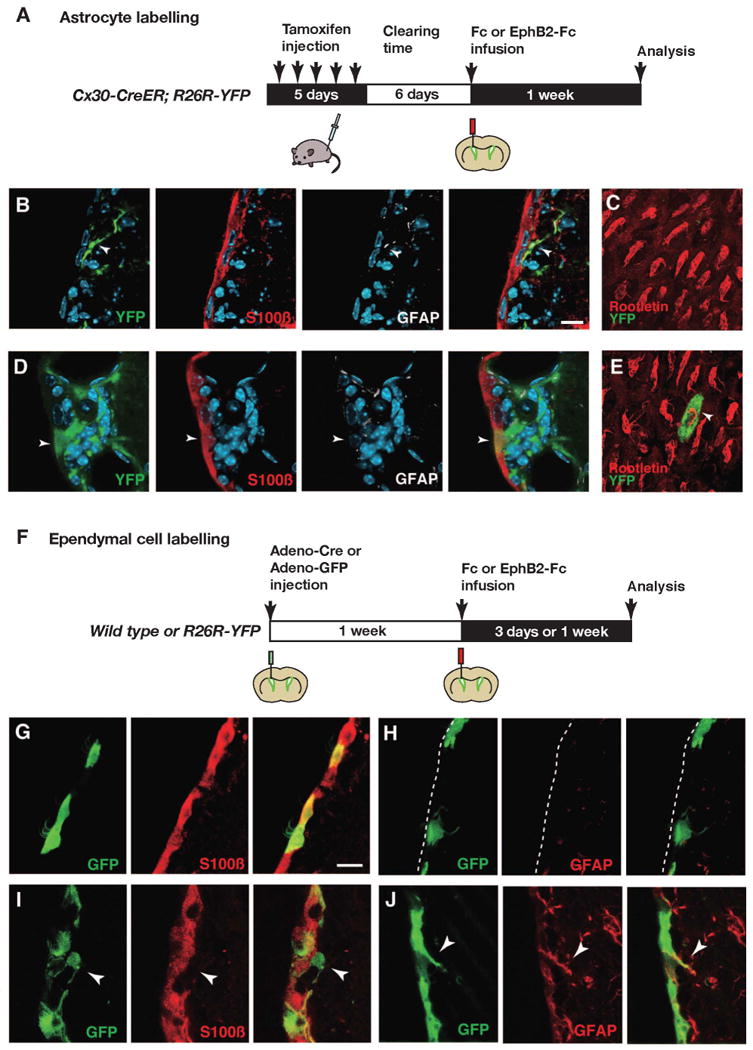
(A) Experimental outline for assessing the effect on astrocytes of blocking the EphB/ephrin-B interaction. (B-E) In Fc-infused mice, YFP-positive recombined astrocytes do not display the ependymal cell marker S100ß or rootletin (B and C), while in EphB2-infused mice recombined cells express S100ß together with rootletin (D and E). (C and E) En face views of the ependymal layer in Fc (C) and EphB2-Fc-infused mice (E). Basal bodies of cilia in ependymal cells are visualized with an anti-rootletin antibody. (F-J) Fate conversion of ependymal cells into astrocytes by blocking of EphB/ephrin-B interactions. (F) Ependymal cells were labeled with adeno-Cre or adeno-GFP virus, and Fc or EphB2-Fc soluble protein was infused into the lateral ventricle one week later. (G and H) In an animal infused with Fc protein, S100ß expression is retained in GFP-positive ependymal cells (G), and GFAP expression is not detected (H). (I and J) In an animal infused with EphB2-Fc protein, some GFP-labeled ependymal cells have lost S100ß expression and have delaminated from the ependymal layer (arrowheads in I), and express GFAP (arrowheads in J). The luminal side of the ependymal layer is indicated by a broken line in (H). The scale bars indicate 10 μm in (B) and (G).
The experiment above shows that disruption of EphB/ephrin-B signaling results in astrocyte-derived cells that contact the ventricle. However, the majority of these cells lose their astrocytic phenotype and take on ependymal cell features, suggesting that the large increase in astrocytes within the ependymal layer reported (Conover et al., 2000) could have a different cellular origin. EphB2-Fc ventricle infusion also affected adeno-GFP pre-labeled ependymal cells (Figures 3G-3J). Already after a three-day infusion of EphB2-Fc protein, 5.9% of GFP labeled cells expressed GFAP and extended long processes into the subventricular zone (total 2055 recombined cells were examined, Figure 3J). The majority of these GFAP expressing cells remained within the ependymal layer, but 1.5% of all GFP labeled cells had delaminated into the subventricular zone and had lost S100ß expression (Figure 3I). We could not detect Ki67 nor Dcx in ependyma-derived GFP labeled cells after EphB2-Fc infusion (data not shown), indicating that neither cell proliferation nor neuronal differentiation was induced. The absolute number of ependymal cell-derived astrocytes was 2.8-fold higher than the number of astrocyte-derived ependymal cells after infusion of EphB2-Fc (ependymal-derived astrocyes were 3.9 ± 0.6 cells per section; astrocyte-derived ependymal cells were 1.3 ± 0.5 cells per section). These results demonstrate that blockade of the EphB/ephrin-B interaction in the lateral ventricle wall results in similar astrocyte-to-ependymal and ependymal-to-astrocyte phenotypic switch of niche cells as seen after neuraminidase-induced lesioning.
EphB forward signaling mediates subventricular zone niche cell maintenance
EphB-ephrin-B signaling can act bidirectionally in juxtaposed cells, via receptor-mediated forward and ligand-mediated reverse signaling (Egea and Klein, 2007; Pasquale, 2008). We ectopically expressed dominant negative mutants to distinguish between the role of EphB2-mediated forward and ephrin-B2-mediated reverse signaling for the maintenance of ependymal cell phenotype. The tyrosine residues Y604 and Y610 in the autoinhibitory region of EphB2 are essential for receptor phosphorylation, and substitution of these tyrosines abolishes ligand-dependent signal transduction (Holland et al., 1997). We electroporated an expression vector with EphB2Y604F,Y610F, in which Y604 and Y610 were substituted by phenylalanine, into the lateral ventricle wall together with a ß-galactosidase (ß-gal) reporter construct (Barnabé-Heider et al., 2008). Ependymal cells were labeled by an adeno-GFP virus injection one week prior to the electroporation (Figures 4A-4C). We could not detect GFAP in labeled ependymal cells in control animals electroporated with only the ß-gal reporter construct (n=3 animals; Figures 4D-4G). However, co-electroporation of ß-gal and EphB2Y604F,Y610F expression vectors induced GFAP expression in GFP and ß-gal co-labeled ependymal cells (49.5 ± 13.3% of GFP and ß-gal-labeled ependymal cells were GFAP-positive, mean ± S.D., total 51 cells were examined, n=3 animals), and some of these cells had translocated into the subventricular zone (Figures 4H-4K; data not shown). We next asked whether increased EphB2 signaling altered the ependymal cell phenotype. We did not detect any alteration in morphology or marker expression after ectopic expression of EphB2 by electroporation or in adult EphB2F620D/F620D mice (data not shown), which carry mutant constitutively active ligand-independent EphB2 receptors (Holmberg et al., 2006).
Figure 4. EphB2 forward signaling maintains the ependymal cell phenotype.
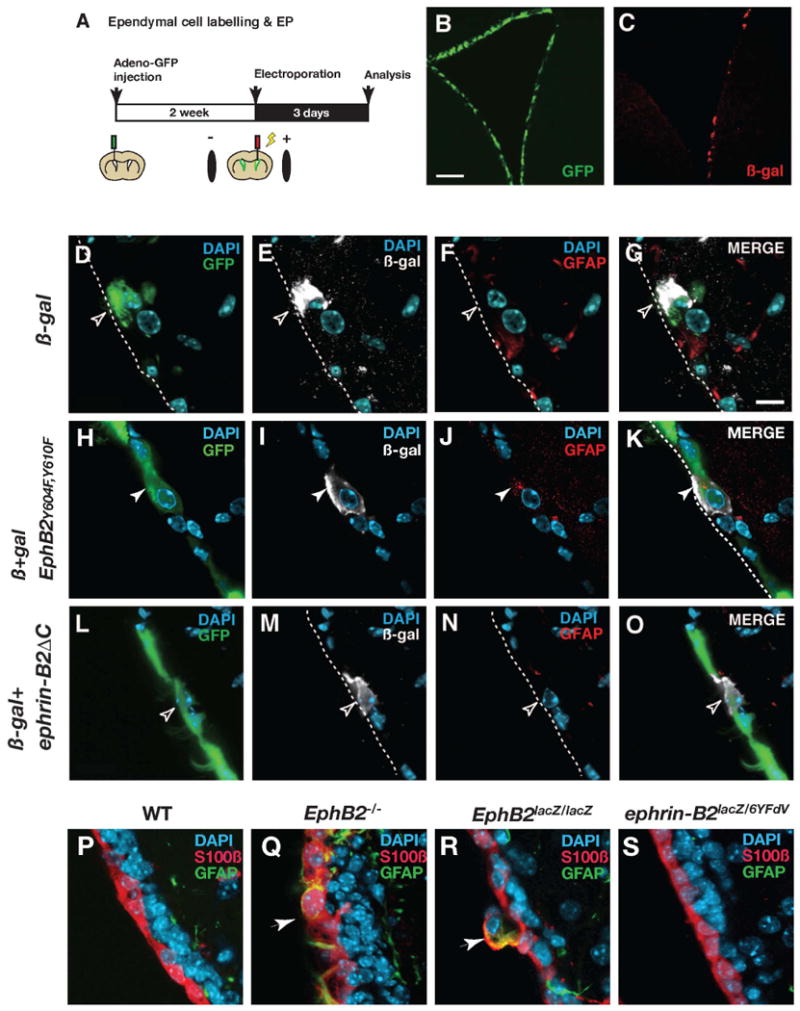
(A) Illustration of the experimental procedure. After labeling ependymal cells with adeno-GFP virus, plasmid vectors were electroporated into the lateral wall. (B and C) GFP expression by adenovirus transduction (B) and ß-galactosidase (ß-gal) expression by plasmid electroporation (C). (D-G) In a control animal electroporated with ß-gal expression vector, a cell which is double-positive for GFP and ß-gal does not express GFAP (open arrowheads). (H-K) In an animal electroporated with a vector expressing EphB2Y604F,Y610F together with the ß-gal expression vector, a cell which is double-positive for GFP and ß-gal expresses GFAP in the ependymal layer (white arrowheads). (L-O) In an animal electroporated with a vector expressing ephrin-B2ΔC together with the ß-gal expression vector, a cell which is double-positive for GFP and ß-gal does not express GFAP (open arrowheads). (P-S) Analysis of the ependymal layer in EphB2 or ephrin-B2-deficient mice. There are no GFAP and S100ß co-expressing cells in the ependymal layer of wild type mice (WT, P), but they are present (indicated by arrows) in both EphB2-/- (Q) and EphB2lacZ/lacZ mice (R). The ventricle wall appears indistinguishable from the WT in ephrin-B2lacZ/6YFdV mice (S). The luminal side of the ependymal layer is indicated by broken lines. Scale bars indicate 200 μm in (B) and 10 μm in (D).
To address whether ephrin-B2-mediated reverse signaling also contributes to astrocytic conversion of ependymal cells, we designed an expression vector with a cytoplasmic deletion mutant of ephrin-B2 (ephrin-B2ΔC), which is incapable of reverse signaling (Figures S4). However, ephrin-B2ΔC overexpression did not induce GFAP up-regulation nor ependymal cell translocation to the subventricular zone (n=3 animals; Figures 4L-4O). These results indicate that maintenance of ependymal cell phenotype depends on EphB2-mediated forward signaling.
We next analyzed mice carrying mutations in EphB2 or ephrin-B2. There was a statistically significant increase in the number of GFAP expressing cells within the ependymal layer in both EphB2-/- mice (2.26 ± 1.07% of S100ß+ ependymal cells co-expressed GFAP, compared to 0% in WT, p<0.001, Figures 4P and 4Q) and EphB2lacZ/lacZ mice (3.96 ± 2.72% of S100ß+ ependymal cells co-expressed GFAP, compared to 0% in WT, p<0.01, Figures 4P and 4R), the latter in which the EphB2 intracellular domain is replaced by ß-galactosidase resulting in a receptor capable of activating reverse signaling but incapable of forward signaling (Henkemeyer et al., 1996). We did not find any apparent phenotype in the lateral ventricle wall of ephrin-B2lacZ/6YFdV mice (Figure 4S), in which ephrin-B2 reverse signaling is impaired (Dravis et al., 2004; Chenaux and Henkemeyer. unpublished).
Reduced ependymal EphB2 expression after a lesion contributes to the generation of astrocytes
We next asked whether EphB/ephrin-B expression is altered by injury. Quantitative analysis revealed significantly reduced EphB2 protein levels in ependymal cells in the lateral ventricle wall after neuraminidase lesioning, compared to PBS-injected controls (Figures 5A-5G). The similar cellular restructuring of the lateral ventricle wall niche after a lesion or blocking of the EphB/ephrin-B interaction, together with the reduction of EphB2 levels after a lesion, suggested the possibility that EphB/ephrin-B signaling regulates niche cell phenotype.
Figure 5. Reduced ependymal EphB2 expression after a lesion contributes to the generation of astrocytes.
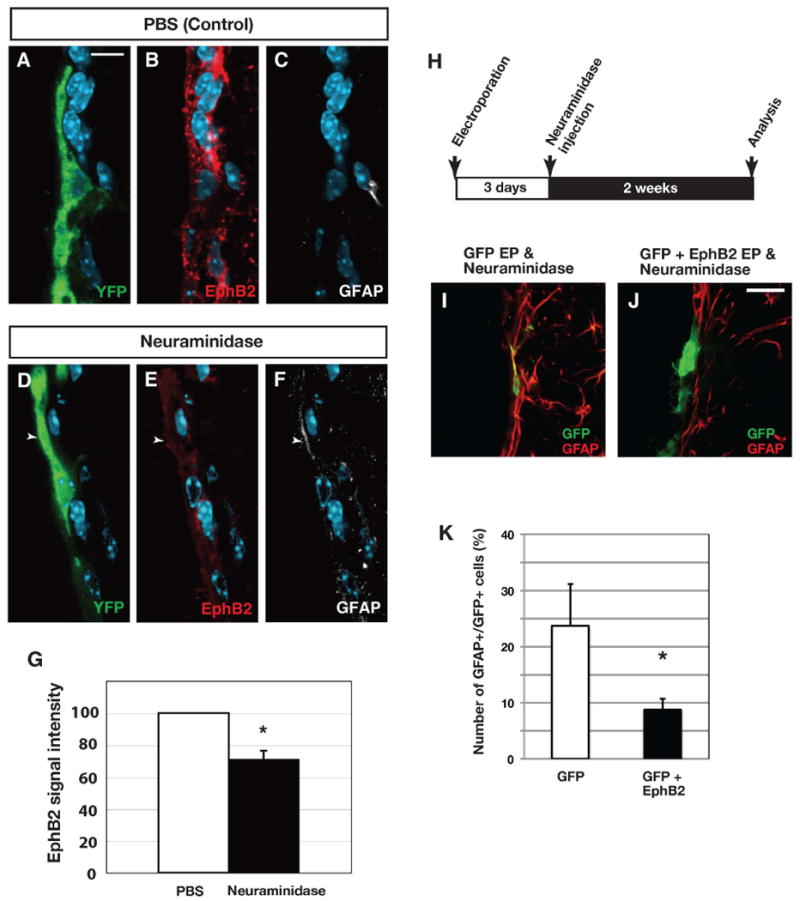
(A-G) Immunoreactivity of EphB2 in ependymal cells after neuraminidase treatment in R26R-YFP mice. Ependymal cells were labeled by injection of an adeno-Cre virus into the lateral ventricle. Compared to control (A-C), EphB2 expression is reduced in recombined ependymal cells 2 weeks after neuraminidase treatment (D-F). Note that GFAP expression is increased in the recombined cells (arrowhead). (G) Quantification of EphB2-immunoreactivity reveals a decrease after a neuraminidase lesion. (H-K) Overexpression of EphB2 inhibits astrocytic conversion of ependymal cells. (H) Experimental procedures of EphB2 overexpression combined with neuraminidase treatment. Electroporation of EphB2-expression vector partly blocks the transition of ependymal cells to astrocytes (I-K). Bars show mean + SD, each for n=3 mice. *=p<0.05, Student's t-test. Scale bar represents 10 μm in (A) and 20 μm in (J),
To directly test this possibility we ectopically expressed EphB2 in ependymal cells at the time of the lesion by electroporation of an EphB2 expression plasmid prior to the neuraminidase injection (Figure 5H). This resulted in significant reduction of the number of electroporated cells that displayed the astrocyte marker GFAP after the lesion (Figures 5I-5K). We conclude that EphB2 expression is reduced after a neuraminidase lesion and the inhibition of astrocyte generation by ependymal cells by ectopic EphB2 expression indicates that the reduced levels of EphB2 contribute to the ependymal to astrocyte conversion.
There is a substantial increase in the number of astrocytes in the subventricular zone after both a neuraminidase lesion and after EphB2-Fc infusion. Ependymal cells are not the only source of such cells, but many of these astrocytes arise from duplication of subventricular zone astrocytes (Figure S5)(Conover et al., 2000).
EphB2 mediates Notch-dependent maintenance of ependymal cell identity
Constitutive Notch signaling is required for ependymal cell maintenance in the adult forebrain (Carlén et al., 2009), raising the question whether EphB-mediated signaling could be a downstream target of Notch signaling in ependymal cells. To address this issue, we first examined EphB2 expression in ependymal cells after genetic deletion of Rbpj (also known as CSL, CBF1, Suppressor of Hairless and Lag-1), a key mediator of canonical signaling by all Notch receptors (Louvi and Artavanis-Tsakonas, 2006). Intraventricular injection of an adenovirus expressing Cre recombinase into RbpjloxP/loxP mice (Tanigaki et al., 2002) results in the selective ablation of Rbpj and canonical Notch signaling in ependymal cells (Carlén et al., 2009). One week after virus injection we observed a significant decrease in EphB2-immunoreactivity in Cre-expressing ependymal cells, while EphB2 expression was not abrogated in RbpjloxP/+ mice (Figures 6A-6E). Next, we tested whether enhanced Notch activity increases EphB2 expression by ectopically expressing the intracellular domain of the Notch-1 receptor (NICD), which acts as a constitutively active ligand-independent receptor. Three days after electroporation of a NICD expression vector together with a GFP expression vector, NICD over expressing ependymal cells showed significantly higher levels of EphB2-immunoreactivity, while GFP reporter plasmid alone did not alter the level of EphB2 (Figures 6F-6J). These gain and loss of function experiments indicate that the expression of EphB2 in ependymal cells is regulated by Rbpj-dependent canonical Notch signaling. We did not find evidence for Notch signaling being regulated by a neuraminidase lesion (Figures S6A-S6C). We did not find evidence for altered levels of ephrin-B1 or ephrin-B2 in ependymal cells in response to modulation of Notch signaling (Figures S6D-S6I).
Figure 6. EphB2 expression is regulated by Notch signaling and maintains ependymal cell phenotype.
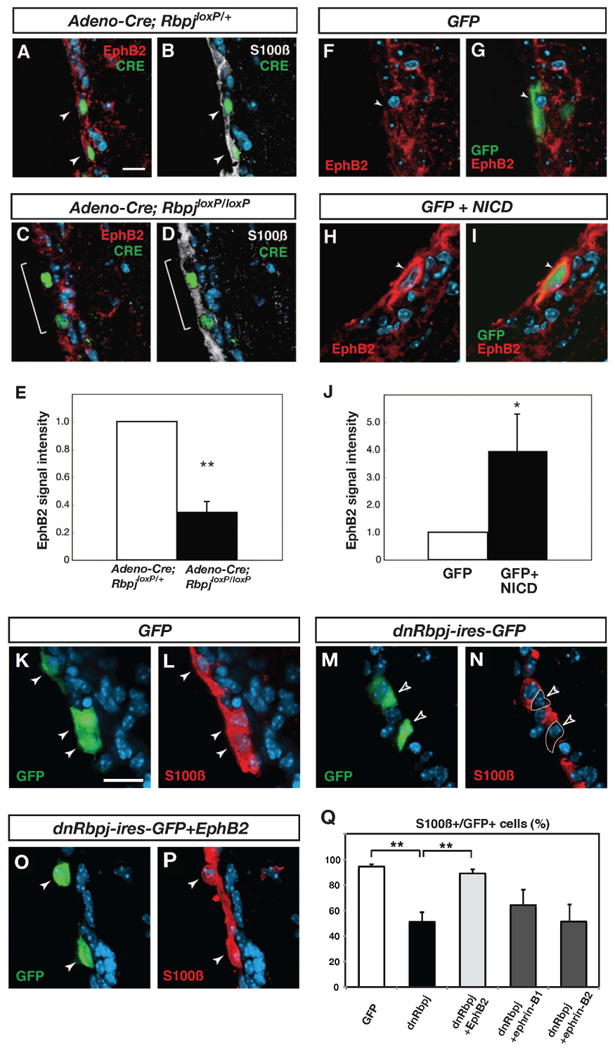
(A and B) In a mouse heterozygous for a conditional allele of Rbpj (RbpjloxP/+), EphB2 expression is maintained in ependymal cells after an adeno-Cre virus injection (indicated by white arrowheads). (C and D) In a mouse homozygous for the conditional allele of Rbpj (RbpjloxP/loxP), a decrease in EphB2 expression is evident 1 week after injection of an adeno-Cre virus (indicated by a white bracket). (E) Quantification of EphB2-immunoreactivity in ependymal cells. (F and G) Expression of EphB2 in a control animal electroporated with a GFP expression vector. (H and I) High expression of EphB2 was detected in electroporated cells 3 days after electroporation of NICD. (J) Quantification of EphB2-immunoreactivity in ependymal cells.
(K, L and Q) In mice electroporated with a GFP expression vector, the majority of GFP-expressing cells are S100ß-immunoreactive ependymal cells (white arrowheads). (M, N and Q) In mice electroporated with a dominant negative Rbpj expression vector (dnRbpj-ires-GFP), the number of S100ß-positive GFP-labeled cells is decreased to 55.9%. Open arrowheads indicate GFP-positive, S100ß-negative cells. (O, P and Q) In mice electroporated with dnRbpj-ires-GFP and EphB2 expression vectors, the number of S100ß-positive GFP-labeled cells is restored to 89.3%. White arrowheads indicate GFP and S100ß double-positive cells. The bars in (E, J, and Q) show mean + SD. *=p<0.05, **=p<0.01, Student's t-test. Scale bars represent 10 μm in (A) and 20 μm in (K).
Deletion of Rbpj in ependymal cells results in cell fate change and ependymal cell loss (Carlén et al., 2009). If EphB2 is a an important functional target of Notch signaling in ependymal cells, overexpression of EphB2 could prevent the fate change of ependymal cells caused by Rbpj depletion. To test this we electroporated an expression vector for a dominant-negative form of Rbpj (dnRbpj) alone or together with an EphB2 expression vector into the lateral ventricle wall of adult mice. The dnRbpj vector also expressed GFP bi-cistronically, allowing us to identify transfected cells. First, we electroporated a construct containing only the GFP reporter. After 3 days of electroporation, 94.6 ± 1.7% of GFP-expressing cells were double-labeled with S100ß (mean ± S.D., total 191 cells were examined, n=3 animals; Figures 6K, 6L and 6Q), validating that electroporation targeted predominantly ependymal cells (Barnabé-Heider et al., 2008). However, the number of GFP-expressing cells that were positive for the ependymal cell marker S100ß were decreased to 55.9 ± 2.5% by dnRbpj electroporation (total 91 cells were examined, n=3, Figures 6M, 6N and6Q), confirming that blocking Rbpj activity resulted in the loss of ependymal cell identity, as we previously reported (Carlén et al., 2009). In contrast, co-electroporation of an EphB2 expression vector together with dnRbpj largely prevented the ependymal cell fate change (89.3 ± 3.1% of GFP-expressing cells were S100ß-positive; total 143 cells were examined, n=3, Figures 6O-6Q). Neither ectopically expressed ephrin-B1 nor ephrin-B2 influenced the effect of blocking canonical Notch signaling (Figure 6Q). Thus, EphB2 over expression is sufficient to rescue the effect of loss of canonical Notch signaling on ependymal phenotype.
Ablation of canonical Notch signaling induces ependymal cell generation by astrocytes
Intrigued by the reciprocal fate change, with ependymal cells generating astrocytes and vice versa, after both a neuraminidase lesion and inhibition of EphB signaling we asked whether also blocking Notch signaling in astrocytes would result in their generation of ependymal cells. Notch signaling maintains neural stem cell features of subventricular zone astrocytes and blocking Notch signaling results in the loss of these cells and precocious neurogenesis (Imayoshi et al., 2010). However, whether Notch signaling in some astrocytes may influence their transition to the ependymal phenotype had not been assessed.
We crossed Cx30-CreER mice with mice carrying loxP flanked Rbpj and induced recombination-mediated labeling and Rbpj depletion in SVZ astrocytes in the adult mice by tamoxifen. The lateral ventricle wall appeared largely normal (Figures 7A-7F) and we found only very few recombined ependymal cells in RbpjloxP/+ mice (0.23% compared to 0% in wild type mice). In contrast, 5.25% of recombined cells in RbpjloxP/loxP mice 2-3 months after induction of recombination were located in the ependymal layer and expressed ependymal markers such as S100ß and CD133 (Figures 7G-7L). Thus, blocking both the EphB and Notch signaling pathways results in reciprocal fate conversion of ependymal cells and astrocytes.
Figure 7. Astrocytes give rise to ependymal cells after blocking canonical Notch signaling.
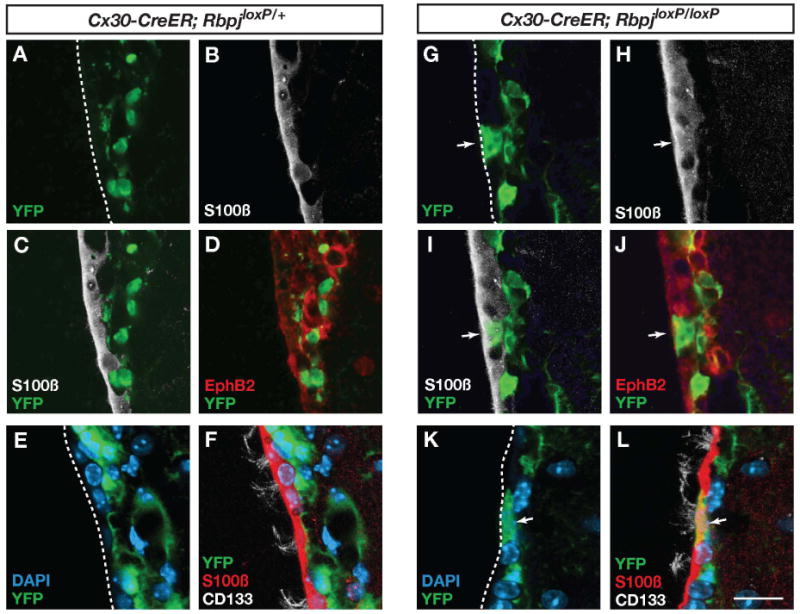
(A-F) The ventricle wall appears largely intact after recombination in Cx30-CreER;RbpjloxP/+ mice. (G-L) Recombination in Cx30-CreER;RbpjloxP/loxP mice results in the apperance of astrocyte derived ependymal cells (arrows) expressing S100ß, EphB2 and CD133. The images in (A-D, G-J) are from two months and the images in (E, F, K and L) are from 3 months after induction of recombination by injection of tamoxifen. The number of recombined ependymal cells per section was 0.12 ± 0.17 in Cx30-Cre;Rbpjloxp/+ mice (total 572 recombined cells analyzed), 4.3 ± 2.5 in Cx30-Cre;Rbpjloxp/loxP mice (total 750 recombined cells analyzed). Scale bar represents 20 μm in (L).
Discussion
Much interest has focused on how stem cells are maintained by intrinsic self-renewal capacity and environmental signals in specialized niches. However, less is known regarding how niches are maintained. We show here that ependymal cells and astrocytes have considerable phenotypic plasticity and can replace each other. The finding that the same signaling pathways, EphB and Notch, reciprocally regulate ependymal and astrocytic phenotypes is intriguing. It may provide an ingenious way to ensure the maintenance of the stem cell niche, as the modulation of these signaling pathways in the situation of a lesion provides a safeguard for controlling the cell composition of the niche. Extensive remodeling occurs in the ventricle wall stem cell niche in the adult brain in response to injury (Barnabé-Heider et al., 2008; Carlén et al., 2009; Conover et al., 2000; Kuo et al., 2006; Luo et al., 2008). The neuronal production is rapidly regained after insults, demonstrating the capacity of the stem cell lineage and niche to regenerate (Doetsch et al., 1999b). It was suggested, based on the lack of proliferation of forebrain ependymal cells, that the increase in the number of astrocytes in the ependymal layer after a lesion was the result of subventricular zone astrocytes migrating towards the ventricle lumen (Luo et al., 2008). However, ependymal cells are, in spite of their quiescence, rather plastic and respond to stroke by giving rise to astrocytes and neuroblasts (Carlén et al., 2009). Ependymal cells in the adult spinal cord are even more plastic and display neural stem cell properties (Barnabé-Heider et al., 2010; Meletis et al., 2008). We foundnd that most astrocytes intercalated within the ependymal layer derive from ependymal cells. However, astrocytes do also enter the ependymal layer, but the majority of these cells loses astrocytic features and differentiates to ependymal cells with expression of typical molecular markers, a cuboidal shape and multiple cilia. Both the conversion of astrocyte to ependymal cell and vice versa occurs without cell division. Thus, the main subventricular zone niche cells show a high degree of plasticity and are phenotypically mutually interconvertible.
Ependymal cells and subventricular zone astrocytes both originate from radial glial cells (Spassky et al., 2005), which act as neural stem cells in the developing central nervous system. Many genes associated with neural stem/progenitor cells, including nestin, Sox2, Prominin-1/CD133 and Notch-1, are commonly expressed in ependymal cells and subventricular zone astrocytes (Doetsch et al., 1997; Ellis et al., 2004; Ferri et al., 2004; Johansson et al., 1999; Sakakibara et al., 2002; Weigmann et al., 1997). The maintenance of genetic programs similar to that of a common precursor may contribute to the fate plasticity of ependymal cells and astrocytes.
The ependymal layer was thought to be incapable of regeneration and that loss of this niche cell type would be permanent. This is indeed the case after loss of larger patches of ependymal cells (Carlén et al., 2009; Kuo et al., 2006). A previous report suggested that subventricular zone astrocytes could replace lost ependymal cells after minor lesions (Luo et al., 2008), which we here confirmed by genetic fate mapping. We never saw groups of astrocyte-derived ependymal cells, but they were always present as individual cells within the ependymal layer. It is unclear why ependymal cells can be replaced after small but not large lesions, but may suggest that neighboring ependymal cells are required for successful generation of new ependymal cells. It is noteworthy in this context that the ephrin/Eph contact is restricted to direct cell contacts, since both the receptor and ligand are membrane bound, and that ependymal cells express both ephrin-B ligands and EphB receptors (Figure S3). Our finding that EphB2 signaling is required for maintaining the ependymal cell phenotype suggests that contact between ependymal cells may promote EphB2 signaling and acquiring the full ependymal phenotype. Moreover, ependymal cells express both Notch1 and its cognate ligands Delta-like 1 and Jagged-1 and constitutive Notch signaling is also required for ependymal cell maintenance (Carlén et al., 2009). Thus, contact with neighboring ependymal cells may be required for attaining and/or maintaining the ependymal cell phenotype, which could explain why small, but not large, lesions are repaired.
We find that Notch signaling positively regulates EphB2 expression in ependymal cells. Regulating the expression of EphB2 is an important part of the effect of Notch signaling, as ectopic expression of EphB2 can rescue the loss of ependymal phenotype seen after ablation of Notch signaling. However, it is clear that EphB2 is not the sole effecter of Notch signaling in this context, as ablation of Rbpj results in ependymal cell progeny entering the cell cycle and subsequently differentiating to neurons (Carlén et al., 2009), neither of which is seen after inhibition of EphB2 signaling. Notch signaling is a well-documented determinant of neuron-glial fate choice. After stroke, Notch signaling is reduced in many ependymal cells, and ependymal cells give rise to both astrocytes and neuroblasts. When Notch signaling is completely blocked by deletion of Rbpj in ependymal cells, they do not give rise to any astrocytes but only neurons, demonstrating the pivotal role for Notch signaling in the fate of ependymal cell derived cells (Carlén et al, 2009). We find that Notch signaling intensity is unaltered in the neuraminidase lesion model (Figures S6A-6C), providing a likely explanation for why the ependymal progeny fail to give rise to neurons in this paradigm and is restricted to the astrocytic lineage.
The role of ephrins and Eph receptors has been most extensively studied in cell migration and axon guidance (Egea and Klein, 2007; Pasquale, 2008). As a migrating cell will be exposed to new molecular environments, it is often challenging to distinguish whether an effect of modulated Eph signaling may be direct or secondary to altered cell positioning. That Eph signaling has other direct effect than regulating cell positioning is clear from studies of intestinal progenitor cells, where the signaling pathways regulating cell migration and proliferation diverge at the level of the receptor protein (Genander et al., 2009). We find in the current study that the ephrin-Eph interaction in the lateral ventricle wall regulate both cell phenotype as well as positioning with regard to the ependymal layer or subventricular zone. It is difficult to completely dissociate these effects, but the finding that ependymal cells differentiate to astrocytes within the ependymal layer appears difficult to explain as secondary to a cell migration effect of EphB signaling. Regulation of stem cell niche plasticity is a novel role for this family of tyrosine kinase receptors. Ephrins and Eph receptors are expressed in many adult stem cell systems (Holmberg et al., 2006), suggesting that they may regulate niche function and plasticity in several organs.
Experimental Procedures
Animals
Adult male C57BL6 mice (>3 months of age), Cx30-CreER (Slezak et al., 2007), R26R-LacZ (Soriano, 1999) and R26R-YFP (Srinivas et al., 2001) mice on C57BL6 background and RbpjloxP/loxP (Tanigaki et al., 2002) on CD1 background were studied. Tamoxifen (2 mg) in corn oil was injected intraperitoneally in Cx30-CreER mice once daily for 5 days. Brains from all Eph and ephrin mutant mice were coded by genotype in the M.H. laboratory and all analyses were done blind to genotype in the J.F. laboratory.
Intraventricular injection
Mice were anesthetized with avertin or isoflurane and 3 μl of Adeno-Cre or Adeno-GFP virus (2×108 PFU in saline, Vector Biolabs) was injected unilaterally into the lateral ventricle with a sterotaxic frame (0 mm anterior/posterior, 1 mm lateral to Bregma and 1.5 mm ventral to the dura). Neuraminidase (100 ng dissolved in 3 μl PBS, Roche Diagnostics) was injected intraventricularly.
Infusion of recombinant proteins
Recombinant EphB2-Fc or Fc proteins (200 μg/ml in PBS; Sigma or R&D systems) were delivered with an osmotic pump (1007D, Azlet) connected to a canula (Brain Infusion KIT3, Azlet) inserted into the lateral ventricle as described previously (Holmberg et al., 2005).
Image analysis and cell counting
Brain sections were collected from 1.1-0.1 mm anterior to Bregma, containing the anterior part of the lateral ventricle. All images and quantitative data were captured from the contralateral side to the virus injection/protein infusion. Five to eight sections were counted in each animal, and data was collected from three independent experiments. The expression of each marker was analyzed using Zeiss LSM510 confocal microscopy with 40× objective lens (optical slice was 0.9-1.0 μm thick), and image analyses were performed using ImageJ software. Signal intensities were determined by measuring mean pixel density in fluorescent images. EphB2 intensity in the ependymal cells was normalized with pixel values of neuroblasts. Three independent images were analyzed in each experiment, and compared to control samples. Statistical analysis was performed by Student's two-tailed paired t-test or Mann-Whitney's U-test.
Supplementary Material
Acknowledgments
We are grateful to Drs. U. Lendahl for NICD, dnRbpj and 12xRbpj-dsGFP expression vectors, T. Pawson for EphB2 and EphB2Y604F,Y610F vectors, M. Genander for ephrin-B1 vector, F. Farnebo for ephrin-B2 expression vector, K. Tanigaki and T. Honjo for RbpjloxP/loxPmice, F. Pfrieger for Cx30-CreER mice, T. Li for the anti-Rootletin antibody, A. Simon and members of the Frisén laboratory for valuable discussions. This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Swedish Agency for Innovation Systems, the Karolinska Institute, Tobias Stiftelsen, Knut och Alice Wallenbergs Stiftelse and NIH grant R01 MH066332. T.N. is supported by fellowships from the Uehara Memorial Foundation and Scandinavia-Japan Sasakawa Foundation and C.G. by a postdoctoral fellowship from the Wenner-Gren Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using adenoviral vectors. Nature Genetics. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in the intact and injured adult spinal cord. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2010.07.014. In press. [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F, Meletis K, Eriksson M, Bergmann O, Sabelstrom H, Harvey MA, Mikkers H, Frisén J. Genetic manipulation of adult mouse neurogenic niches by in vivo electroporation. Nat Methods. 2008;5:189–196. doi: 10.1038/nmeth.1174. [DOI] [PubMed] [Google Scholar]
- Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Bohn MC. Recombinant adenovirus: a gene transfer vector for study and treatment of CNS disease. Exp Neurol. 1997;144:125–130. doi: 10.1006/exnr.1996.6398. [DOI] [PubMed] [Google Scholar]
- Del Carmen Gomez-Roldan M, Perez-Martin M, Capilla-Gonzalez V, Cifuentes M, Perez J, Garcia-Verdugo JM, Fernandez-Llebrez P. Neuroblast proliferation on the surface of the adult rat striatal wall after focal ependymal loss by intracerebroventricular injection of neuraminidase. J Comp Neurol. 2008;507:1571–1587. doi: 10.1002/cne.21618. [DOI] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends in cell biology. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Genander M, Frisén J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.08.005. In press. [DOI] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Holmberg J, Frisén J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells. 2010;28:1196–1205. doi: 10.1002/stem.442. [DOI] [PubMed] [Google Scholar]
- Grondona JM, Perez-Martin M, Cifuentes M, Perez J, Jimenez AJ, Perez-Figares JM, Fernandez-Llebrez P. Ependymal denudation, aqueductal obliteration and hydrocephalus after a single injection of neuraminidase into the lateral ventricle of adult rats. Journal of neuropathology and experimental neurology. 1996;55:999–1008. doi: 10.1097/00005072-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Hara Y, Nomura T, Yoshizaki K, Frisen J, Osumi N. Impaired hippocampal neurogenesis and vascular formation in ephrin-A5-deficient mice. Stem Cells. 2010;28:974–983. doi: 10.1002/stem.427. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisén J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes and Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci U S A. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–3813. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Qiu R, Wang X, Davy A, Wu C, Murai K, Zhang H, Flanagan JG, Soriano P, Lu Q. Regulation of neural progenitor cell state by ephrin-B. J Cell Biol. 2008;181:973–983. doi: 10.1083/jcb.200708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug metabolism and disposition: the biological fate of chemicals. 1991;19:36–43. [PubMed] [Google Scholar]
- Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, Ueda S, Uchiyama Y, Noda T, Okano H. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Simon A, Frisén J. From stem cell to progenitor and back again. Cell. 2007;128:825–826. doi: 10.1016/j.cell.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Slezak M, Göritz C, Niemiec A, Frisén J, Chambon P, Metzger D, Pfrieger FW. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55:1565–1576. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the Rosa26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


