Abstract
Polychlorinated biphenyls (PCBs) are persistent toxic pollutants occurring as complex mixtures in the environment. Humans are known genetically to have > 60-fold differences in hepatic cytochrome P450 1A2 (CYP1A2) levels and > 12-fold differences in aryl hydrocarbon receptor (AHR) affinity, both of which could affect PCB pharmacokinetics. Thus, we compared Ahrb1_Cyp1a2(+/+) high-affinity AHR wild-type, Ahrd_Cyp1a2(+/+) poor affinity AHR wild-type, Ahrb1_Cyp1a2(−/−) knockout, and Ahrd_Cyp1a2(−/−) knockout mouse lines. We chose a mixture of three coplanar and five noncoplanar PCBs to reproduce that seen in human tissues, breast milk, and the food supply. The mixture was given by gavage to the mother on gestational day 10.5 (GD10.5) and postnatal day 5 (PND5); tissues were collected from pups and mothers at GD11.5, GD18.5, PND6, PND13, and PND28. Ahrb1_Cyp1a2(−/−) pups showed lower weight at birth and slower rate of growth postnatally. Absence of CYP1A2 resulted in significant splenic atrophy at PND13 and PND28. Presence of high-affinity AHR enhanced thymic atrophy and liver hypertrophy in the pups. Concentrations of each congener were analyzed at all time points: maximal noncoplanar congener levels in maternal tissues were observed from GD18 until PND6, whereas the highest levels in pups were found between PND6 and PND28. Coplanar PCB concentrations were generally higher in Ahrd-containing pup tissues; these findings are consistent with earlier studies demonstrating the crucial importance of AHR-mediated inducible CYP1 in the gastrointestinal tract as a means of detoxication of oral planar polycyclic aromatic hydrocarbons.
Keywords: PCB congeners, PCB mixture, in utero exposure, immunosuppression, gas chromatography/electron capture detection, cytochrome P450 1A2, aryl hydrocarbon receptor, developmental toxicity
Polychlorinated biphenyl (PCB) production was banned worldwide between 1977 and 1984; yet, environmental contamination remains a serious problem. Human populations show striking accumulations of PCBs, with reported levels of > 1700 ng/g lipid in human breast milk samples (WHO, 1996) and 3105 ng/g of lipid in serum from residents residing near highly contaminated sites (Petrik et al., 2006). Major sources of human exposure include placental transfer and ingestion of contaminated food—especially breast milk and fatty fish (ATSDR, 2001).
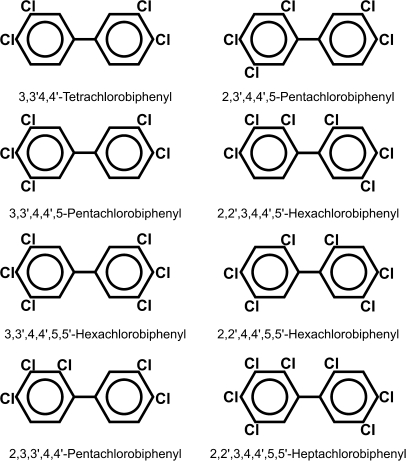
Out of 209 possible PCB congeners, our present study included a mixture of eight (Fig. 1). Congeners having chlorine atoms only in ortho positions are noncoplanar because of steric hindrance, whereas those with chlorines only in meta and/or para positions are coplanar. Coplanar PCBs are potent ligands for aryl hydrocarbon receptor (AHR) (Poland and Glover, 1977).
FIG. 1.
Chemical structures of the eight PCBs used in this study. Meta- and para-substituted congeners (first three in left row) are coplanar molecules and are able to bind AHR. The remaining five are ortho-substituted congeners and are noncoplanar.
Acute PCB exposure can cause chloracne, darkened skin and nail pigmentation, hearing loss, eye disorders, jaundice (Guo et al., 1999), and various neurological symptoms (Masuda, 2001). Chronic low-level PCB exposure—because of industrial pollution or consumption of contaminated food (Schantz et al., 1996)—can increase risk of immunosuppression (Gustavsson and Hogstedt, 1997), cardiovascular disease (Gustavsson and Hogstedt, 1997), and cancer (Negri et al., 2003). Chronic PCB exposure also can disrupt glucose metabolism (Imbeault et al., 2002), thyroid hormone signaling (Tan and Zoeller, 2007), and estrogen and testosterone signaling (Battershill, 1994). Most of these effects appear to be mediated via AHR (Simon et al., 2007).
AHR, when activated by ligand, can up- and downregulate hundreds of genes (Sartor et al., 2009), including increased expression of the three mouse Cyp1 genes. The prototypical AHR agonist is 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD; “dioxin”). Other exogenous planar AHR ligands include benzo[a]pyrene (BaP), other polycyclic hydrocarbons (PAHs), and coplanar polyhalogenated biphenyls (Nebert, 1989). Toxic equivalency factors (TEFs) are used to estimate a chemical's toxicity based on several factors including its AHR-binding affinity; the TEF for the most potent ligand, TCDD, is set at 1.0 (Bhavsar et al., 2007; Van den Berg et al., 2006).
AHR allelic differences have been reported. In mice, a single amino acid change accounts for much of the 15- to 20-fold differences in affinity (Poland et al., 1994); the C57BL/6J (B6) Ahrb1 allele encodes the high-affinity AHR and the DBA/2J (D2) Ahrd allele the poor affinity AHR (Nebert et al., 2000). In humans, there is a > 12-fold difference in TCDD binding to AHR, although DNA sequences responsible have not yet been identified (Nebert et al., 2004).
In utero exposure of laboratory animals to dioxin-like compounds, including coplanar PCBs, can result in birth defects—such as cleft palate and hydronephrosis—and immunosuppression (Selgrade, 2007). In a comparison of B6 mice and the congenic B6.D2-Ahrd line treated in utero with polybrominated biphenyls, B6 mice are at ∼20-fold greater risk than B6.D2-Ahrd mice for neonatal lethality (Curran et al., 2006).
Cytochrome P450 1A2 (CYP1A2) is one of three members of the mammalian CYP1 family (Nelson et al., 2004). This enzyme metabolizes estrogen, uroporphyrinogen, and melatonin; CYP1A2 also metabolizes environmental arylamines, as well as about two dozen drugs including caffeine, theophylline, and acetaminophen (Nebert et al., 2004).
In human populations, there is > 60-fold variation in hepatic CYP1A2 basal and inducible levels (Nebert et al., 2004); yet, no DNA sequence within or near the CYP1A2 gene unequivocally responsible for this trait has been found (Jiang et al., 2006). Intriguingly, the CYP1A2 protein is also able to sequester planar AHR ligands (Hakk et al., 2009). The protective role of maternal mouse hepatic CYP1A2 was directly demonstrated—by comparing Cyp1a2(+/+) wild-type with Cyp1a2(−/−) knockout pregnant mice treated in utero with TCDD; replacement of the mouse Cyp1a2 gene with the human CYP1A2 gene evoked the same protective effect (Dragin et al., 2006). When the CYP1A2 enzyme is absent, offspring are approximately sixfold more sensitive to dioxin-induced cleft palate, hydronephrosis, and embryolethality compared with Cyp1a2(+/+) mice; greater amounts of TCDD were shown to reach Cyp1a2(−/−) fetuses, thereby making them more susceptible to teratogenesis (Dragin et al., 2006).
In our present study, we chose to compare high-affinity Ahrb1 with poor affinity Ahrd mice combined with presence or absence of the Cyp1a2 gene; this would simulate the extremes of AHR and CYP1A2 gene expression in human populations. Rather than a single congener, we administered an environmentally relevant PCB mixture—one that mirrors levels of various congeners found in human tissues, breast milk, and the food supply. Lastly, we wished to give PCBs at appropriate developmental ages and at sufficiently low concentrations so as not to cause substantial mortality or overt birth defects, yet high enough to ensure that differences in behavioral phenotype might be seen when the offspring reach adulthood. This report describes an analysis of the concentrations of various PCB congeners in several tissues in utero, postnatally and in the mother, as well as toxic effects observed. A subsequent report (Curran, Genter, Patel, Vorhees, Williams, and Nebert, in preparation) will describe the behavioral studies.
MATERIALS AND METHODS
Chemicals.
All PCB congeners (Table 1, Fig. 1) were purchased from ULTRA Scientific (North Kingstown, RI). The PCBs were dissolved in acetone (5 mg/ml), and the resulting solutions were dissolved in corn oil; the acetone was removed under a gentle stream of argon during magnetic stirring. All other chemicals and reagents were bought from either Fisher Chemical (Fairlawn, NJ) or Sigma Chemical Company (St Louis, MO) as the highest available grades.
TABLE 1.
List of PCB Congeners in the Mixture Used in This Study
| PCB congener | Planarity | IUPAC # | Dose/kg | TEFa |
| 3,3′4,4′-Tetrachlorobiphenyl | Coplanar | 77 | 5 mg | 0.0005 |
| 3,3′,4,4′,5-Pentachlorobiphenyl | Coplanar | 126 | 25 μg | 0.1 |
| 3,3′,4,4′,5,5′-Hexachlorobiphenyl | Coplanar | 169 | 250 μg | 0.03 |
| 2,3,3′,4,4′-Pentachlorobiphenyl | Noncoplanar | 105 | 10 mg | 0.00003 |
| 2,3′,4,4′,5-Pentachlorobiphenyl | Noncoplanar | 118 | 10 mg | 0.00003 |
| 2,2′,3,4,4′,5′-Hexachlorobiphenyl | Noncoplanar | 138 | 10 mg | 0.0005 |
| 2,2′,4,4′,5,5′-Hexachlorobiphenyl | Noncoplanar | 153 | 10 mg | 0.0005 |
| 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl | Noncoplanar | 180 | 10 mg | 0.00001 |
Note. IUPAC, International Union of Pure and Applied Chemistry.
Listed in Van den Berg et al. (2006).
Animals.
We used four groups of mice—representing the extremes for AHR affinity and CYP1A2 expression in human populations (Table 2). C57BL/6J (B6) and B6.D2-Ahrd (congenic line having DBA/2J poor affinity Ahrd allele on B6 background) mice were purchased from The Jackson Laboratory (Bar Harbor, ME); both are Cyp1a2(+/+) wild type. The Ahrb1_Cyp1a2(−/−) knockout mouse line was created by the Nebert laboratory (Liang et al., 1996). A novel Ahrd_Cyp1a2(−/−) line was developed by mating Cyp1a2(−/−) mice with the B6.D2-Ahrd line. By means of at least eight backcrosses into B6 mice, all four genotypes are expressed in a > 99.8% B6 genetic background, which should reduce experimental noise. Animals were housed in the University of Cincinnati's Laboratory Animal Medicine Facilities, having a 12:12-h light-dark cycle. Standard care included rodent chow (Lab Diet 5001; Purina Mills, Richmond, IN) and water ad libitum with pregnant mothers receiving breeder chow (Lab Diet 5015; Purina Mills) from gestational day 0.5 (GD0.5) through weaning. All mouse experiments were conducted in accordance with the National Institutes of Health standards for the care and use of experimental animals and the University of Cincinnati's Medical Center Institutional Animal Care and Use Committee.
TABLE 2.
Genotypes of Mice Chosen for These Studies
| AHR allele | AHR ligand affinity | CYP1A2 | Genotype | Common name | Predicted phenotypea |
| Ahrb1 | High affinity | Absent | Ahrb1Cyp1a2(−/−) | Cyp1a2(−/−) knockout | Most vulnerable to PCB mixture |
| Ahrd | Poor affinity | Absent | AhrdCyp1a2(−/−) | Almost as vulnerable | |
| Ahrd | Poor affinity | Present | AhrdCyp1a2(+/+) | B6.D2-Ahrd | Almost as resistant |
| Ahrb1 | High affinity | Present | Ahrb1Cyp1a2(+/+) | C57BL/6J (B6) | Most resistant |
We predict that the most important factor—leading to PCB-induced poor growth rate—would be absence of maternal liver CYP1A2. Of less importance, but still to be reckoned with, is the high-affinity AHR maternal-fetal unit in which CYP1 enzymes are highly induced by the coplanar PCB ligands (and therefore PCBs are cleared from the tissues); alternatively, and competing with detoxication, more reactive PCB intermediates might be formed.
Breeding protocol.
Nulliparous females 3–5 months of age (20–25 g) were used for all matings. The morning when a vaginal plug was found was considered GD0.5; plug-positive females were removed from the breeding cage. Pregnant females were housed individually with pups until weaning on postnatal day 28 (PND28).
Dosing of animals.
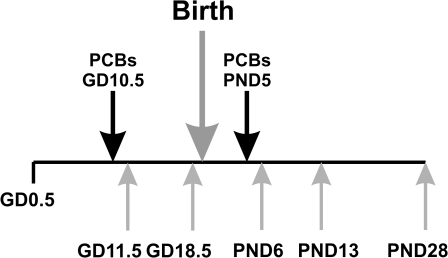
Pregnant females were given PCBs by gavage on GD10.5 and a second time on PND5 (Fig. 2); this time point was chosen to insure continual AHR activation throughout lactation and was based on our own preliminary data. Control animals received an equivalent volume of corn oil vehicle alone (15 ml/kg). Dosing was delayed until GD10.5 to avoid interfering with embryo implantation and to minimize neonatal lethality (Curran et al., 2006). Between GD10.5 and PND20 (gestation plus lactation) comprises the period of rodent brain development that most closely matches brain development in the second to early third trimesters of human development (Clancy et al., 2007). Another manuscript will describe neurobehavioral studies in these mice, tested between days PND60 and PND100 (Curran, Genter, Patel, Vorhees, Williams, and Nebert, in preparation).
FIG. 2.
Experimental time line. The time line shows when mothers were treated and when tissues were collected. Note that the mother was gavaged a second time on PND5, so that the only source of PCBs to the pups would be via lactation.
Plasma total thyroxine assay.
T4 levels were measured using a standard enzyme immunoassay kit (ALPCO Diagnostics, Salem, NH) following the manufacturer's protocol. Blood was collected in heparinized tubes following decapitation and centrifuged at 2500 × g for 5 min at 4°C, and the plasma fraction was stored at −80°C until analysis. Samples were collected from GD18.5 fetuses (pooled from 2 to 3 fetuses per sample) and pups at PND6, PND13, and PND28 (1 pup per sample). All samples were run in duplicate. T4 levels were calculated by comparison with a standard curve.
Gas chromatography with electron capture detection.
Gas chromatography with electron capture detection (GC-ECD) was used to analyze the concentration of individual PCB congeners. Figure 2 shows a time line of treatment and tissue collection. Using a modification of previously developed methods (Imsilp et al., 2005), it was possible to detect all eight congeners included in the dosing mixture. Samples from control tissues were analyzed, and we found no interfering peaks or quantifiable PCB levels.
Tissues were collected following carbon dioxide asphyxiation, rinsed in ice-cold 1× PBS, blotted, snap frozen on dry ice, and stored at −80°C until processing. Tissues were collected at GD11.5, GD18.5, PND6, PND13, and PND28 time points. Tissues included the following: maternal brain, liver, mammary gland, and adipose tissue from the inguinal fat pad; pup brain, liver, subcutaneous fat, stomach contents, adipose tissue from the inguinal fat pad; and plasma. At GD11.5, two whole embryos were pooled for analysis. At GD18.5, brain (pooled from two fetuses) and liver (from a single fetus) were analyzed. Adipose samples from PND6 litters, as well as PND13 litters, were pooled, using both subcutaneous fat and inguinal fat pad. At PND28, there was sufficient adipose tissue available to analyze subcutaneous fat and the inguinal fat pad separately.
Tissue samples were weighed and homogenized in 2.5 ml of a 1:1 (vol/vol) solution of analytical grade hexane and acetone. PCB 65 (2,3,5,6-tetrachlorobiphenyl) was used as an internal standard with 25 μl of a 2 μg/ml solution (50 ng total) added to each sample prior to homogenization. Homogenates were centrifuged for 5 min at 2500 × g and the supernatant fractions collected; the extraction procedure was repeated three times. Tissue (∼150 mg) or plasma (∼100 μl) was used for each sample. Supernatant fractions were pooled and filtered using a solvent-resistant 0.45-μm polyvinylidene fluoride filter (Millipore; Burlington, MA). The filter was rinsed once with 1 ml hexane. Water was removed from the pooled supernatants using anhydrous sodium sulfate. Samples were then dried to dampness under a gentle stream of argon in a 40°C water bath. All sample preparation was done using glass tubes, pipettes, and syringes.
Samples were reconstituted in 1 ml analytical grade hexane, and 1 μl was injected into an Agilent 6890N gas chromatography system using a 60-m × 0.25-mm × 0.25-μm DB-5 capillary column and a microelectron capture detector. The carrier gas was helium, and the makeup gas was argon-methane (95:5%). Injector and detector temperatures were 250°C and 325°C, respectively. The column oven was programmed to increase from 145°C to 275°C at 1.5°C per min and then increased to 300°C at 8°C per min, holding at 300°C for 5 min. The injector was operated in the split/splitless mode.
Data were collected and analyzed using ChemStation software (Hewlett Packard/Agilent Technologies, Wilmington, DE). Calibration standards of 5–200 ng/ml, spiked with 50 ng of internal standard, were used to construct calibration curves. All standard curves had correlations with R2 ≥ 0.998. Limits of quantification were 0.5 ng/100 mg and 0.5 ng/100 μl of tissue and plasma, respectively. The interassay coefficient of variance was less than 4%, and check standards were run daily.
Total RNA preparation and reverse transcription.
Mice were euthanized using carbon dioxide asphyxiation followed by decapitation for tissue collection at two prenatal and three postnatal time points (Fig. 2). Tissues were rinsed in ice-cold 1× PBS, blotted, snap frozen on dry ice, and stored at −80°C until processing. Total RNA was isolated from liver, brain, and whole embryos (GD11.5)—homogenized in 1 ml/100 mg TriReagent (Molecular Research Corporation, Inc., Cincinnati, OH)—and RNA was extracted using the manufacturer's protocol. The cDNA was synthesized using reverse-iT (AbGene) and the replacement Verso kits (Thermo Scientific; Waltham, MA), oligo-dT primers, and 2 μg total RNA. The manufacturer's 2-step protocol was used for all samples.
Quantitative real-time PCR.
We carried out quantitative real-time PCR (qRT-PCR) using a DNA Engine Opticon-2 Real-Time PCR Detection System (MJ Research). Samples were prepared in 20-μl volume reactions with 1 μg cDNA added to 7 μl water, 1 μl of each primer (10μM solutions), and 10 μl iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) for each sample. Table 3 shows the primer sets used.
TABLE 3.
Primers Used in These Studies
| Gene | Forward primer | Reverse primer |
| Cyp1a1 | 5′-CAGACCTCAGCTGCCCTATC-3′ | 5′-CTTGCCCAAACCAAAGAGAG-3′ |
| Cyp1a2 | 5′-ACAACGAGGGACACCTCAC-3′ | 5′-GGGATCTCCCCAATGCAC-3′ |
| Actb | 5′-CATCCGTAAAGACCTCTATGCC-3′ | 5′-ACGCAGCTCAGTAACAGTCC-3′ |
Note. The probes for the mouse Cyp1a1 and Cyp1a2 genes detect CYP1A1 and CYP1A2 mRNA, respectively. The probe for the mouse β-actin (Actb) gene detects ACTB mRNA.
All primers were designed to cross at least two exons to avoid confounding by any contaminating genomic DNA. The “housekeeping genes” glyceraldehyde-3-phosphate dehydrogenase and β-actin (ACTB) messenger RNA (mRNAs) were both scrutinized to be sure that they were not affected by PCB treatment; subsequently, ACTB was chosen as the preferred internal standard. Results were normalized to ACTB expression for both liver and brain. Values were expressed as the CYP1A1 or CYP1A2 mRNA/ACTB mRNA ratios over corn oil–treated controls using control animals of the same age. N = 3–5 per group. Quantification of fold changes was done using the delta-delta Ct method (Karlen et al., 2007). The primer sets for target genes and the control gene were tested and, in our hands, found to have similar PCR efficiency (± 3%)—both of which were close to 100% efficiency, as determined originally by curves of standards. Hence, we were able to use the delta-delta Ct method without needing to generate standard curves each time. PCR products run on a 2% agarose gel confirmed a single band of the appropriate size.
Histology.
Animals were perfused transcardially with 4% paraformaldehyde and small sections of liver and brain removed and soaked overnight in the same preservative. Slices (4 μm) of paraffin-embedded tissue were rehydrated, stained with hematoxylin and eosin, and visualized by light microscopy.
Chemical hazard precaution.
PCBs are not only very toxic but also some are likely human carcinogens. All personnel were instructed in safe handling procedures. Laboratory coats, gloves, and masks were worn at all times, and contaminated materials were collected separately for disposal by the Hazardous Waste Unit or by independent contractors. PCB-fed mice were housed separately, and their carcasses treated as contaminated biological materials.
Statistical analysis.
SigmaPlot 9.0 and SigmaStat 3.1 (Systat Software Inc.; San Jose, CA) were used for statistical analysis of litter size and pup weights, using two-way ANOVAs to consider the factors of genotype and treatment and their interaction. Many comparisons included all treated groups versus control Ahrb1_Cyp1a2(+/+) mice, which were one-way ANOVAs, followed by Holm-Sidak multiple pairwise comparisons. Data are presented as means ± SEM. p Values of < 0.05 were regarded as statistically significant for all comparisons.
RESULTS
Selection of PCB Congeners for This Study
Rather than a single congener, we wished to study an environmentally relevant mixture of PCBs—one that most closely mimicked human exposures. The congeners selected represent those having been identified as among the most toxic and most prevalent by the World Health Organization (Van den Berg et al., 2006) and those that are commonly found in the human food supply (Costabeber et al., 2006; Dewailly et al., 1999) and in human tissues collected during cohort studies (Soechitram et al., 2004). We also included the noncoplanar congener PCBs 105 and 118 because they can be metabolized to the neurotoxic metabolite 4-hydroxy-PCB 107 (Meerts et al., 2004).
The chemical structures and dosages of all eight congeners, along with their established TEFs, are listed in Table 1 and illustrated in Figure 1. Preliminary studies were conducted using the individual congeners in order to determine a dosing regimen that (1) resulted in AHR-mediated CYP1A1 upregulation through late gestation until weaning in high-affinity AHR (Ahrb1) mice and (2) did not cause excessive neonatal lethality (defined as not more than 25% greater lethality than control litters) or teratogenesis. From these preliminary results, the final composition for the mixture of coplanar plus noncoplanar PCBs was ultimately established.
Selection of Genotypes Used in This Study
We wished to have the full range of AHR affinity and levels of CYP1A2 expression in the maternal-fetal unit. Thus, we chose combinations of two lines having the high-affinity AHR and two having the poor affinity AHR plus two lines having the Cyp1a2(+/+) wild-type gene and two having the Cyp1a2(−/−) genotype (Table 3). Based on previously published data discussed above, we postulated that the Cyp1a2(−/−)-containing maternal-fetal units would be most vulnerable to toxic effects of coplanar PCBs seen in the pups and the Cyp1a2(+/+)-containing units most resistant. However, addition of five noncoplanar congeners put a wild card into our ultimately decided upon mixture, and we therefore were not certain what to expect.
Litter Statistics and Neonatal Lethality
There were no significant differences in distribution of gender or litter size across genotype or treatment groups—with means of 7–8 pups per litter. Neonatal lethality rates were higher in PCB-treated litters compared with control litters; the highest rate (20%) was seen in PCB-treated Ahrd_Cyp1a2(−/−) mice. Neonatal lethality in PCB-treated Cyp1a2(−/−) litters was within an acceptable range: ≥ 75% of Cyp1a2(−/−) newborns survived birth and ∼67% of Cyp1a2(−/−) pups survived until weaning compared with 70–77% of Cyp1a2(+/+) pups. No cases of hydronephrosis or cleft palate were seen in any of the PCB-treated pups. No further attempt was made to determine the cause of neonatal deaths.
Birth weights were significantly lower for pups born to PCB-treated Cyp1a2(−/−) mothers regardless of Ahr genotype (Table 4). Treatment and genotype provided the two main effects; there was a treatment × genotype interaction. Although statistically significant (p < 0.001), it should be noted that the actual difference in mean birth weights across genotypes and treatment groups was < 70 mg.
TABLE 4.
Assessment of Birth Weights and Neonatal Lethality
| Genotype | Treatment | Birth weight (g) | Dystocia cases (% pregnancies) | Abnormal gestation (% pregnancies)a | Litter size | Neonatal lethality (%) |
| Ahrb1_Cyp1a2(+/+) | Corn oil | 1.33 ± 0.01 | 0 | 5.9 | 7.8 ± 0.3 | 6.8 |
| Ahrb1_Cyp1a2(+/+) | PCB | 1.32 ± 0.01 | 18 | 5.3 | 8.3 ± 0.5 | 15 |
| Ahrd_Cyp1a2(+/+) | Corn oil | 1.32 ± 0.01 | 6.7 | 0 | 8.1 ± 0.3 | 3.5 |
| Ahrd_Cyp1a2(+/+) | PCB | 1.33 ± 0.01 | 16 | 0 | 7.7 ± 0.4 | 13 |
| Ahrb1_Cyp1a2(−/−) | Corn oil | 1.30 ± 0.01 | 0 | 0 | 7.9 ± 0.4 | 8.7 |
| Ahrb1_Cyp1a2(−/−) | PCB | 1.27 ± 0.01* | 21 | 22 | 7.0 ± 0.4 | 14 |
| Ahrd_Cyp1a2(−/−) | Corn oil | 1.32 ± 0.02 | 0 | 0 | 7.6 ± 0.4 | 12 |
| Ahrd_Cyp1a2(−/−) | PCB | 1.28 ± 0.01* | 26 | 21 | 8.0 ± 0.4 | 21 |
Note. All values are expressed as means ± SEM.
Abnormal gestation refers to pregnancies that ended prematurely or were prolonged beyond the normal 19.5-day gestational length.
Significantly different from all other groups. p < 0.001; N was ≥ 10 litters per group.
Overall, PCB-treated mothers experienced higher rates of dystocia and length of gestation abnormalities compared with corn oil–treated mothers regardless of genotype (Table 4). All PCB-treated groups experienced significantly greater incidences of dystocia (∼16 to 26% of all pregnancies) than controls; only Cyp1a2(−/−) mothers experienced more abnormalities in the length of gestation (∼21 to 22% of pregnancies) regardless of their Ahr genotype.
Growth Rates
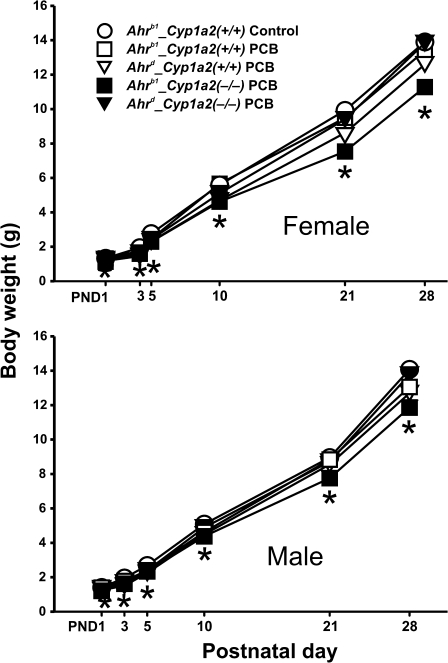
In utero exposure to the PCB mixture caused a statistically significant decrease in growth rate for Ahrb1_Cyp1a2(−/−) pups regardless of sex (Fig. 3). p Values increased from < 0.05 at PND3 to < 0.01 at PND5 and < 0.001 from PND10 through PND28.
FIG. 3.
Body weights from PND1 through PND28. (A) Females. (B) Males. PCB treatment always appeared to decrease growth rates slightly throughout the lactational period. Data are presented as mean values ± SEM. N ≥ 10 per time point. *p Value < 0.001.
Plasma T4 Levels
One of the best characterized effects of in utero PCB exposure in human populations is a reduction in circulating thyroid hormones (Takser et al., 2005; Wang et al., 2005). In rodent studies, this effect is seen—regardless of whether coplanar or noncoplanar congeners are administered (Van Birgelen et al., 1995).
There were no significant differences in total plasma T4 at GD18 when levels are normally at their lowest. By PND6, we found that PCB-treated Ahrb1_Cyp1a2(−/−) pup's T4 levels were decreased to 80% of that in untreated Ahrb1_Cyp1a2(+/+) pups (p < 0.05). At PND13 and PND28, there was a trend of PCB-treated Ahrb1_Cyp1a2(−/−) pups having lower T4 levels than all other genotypes treated or untreated; however, the comparisons were not statistically significant at the p < 0.05 level (data not shown).
Histology
In PND28 animals of all treated versus nontreated genotypes, we looked for PCB-induced changes in liver and in overall brain structure and development. Using coded samples so that the observer was unaware of the genotype or treatment, histological examination showed no overt changes detectable in either liver or several areas of brain (data not shown).
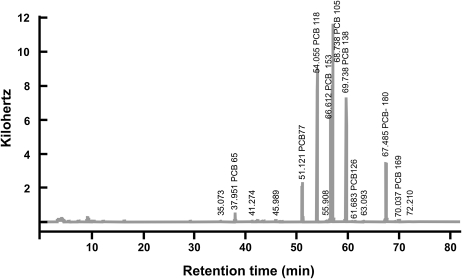
Detection of PCB Congeners in Tissues of Mother and Pup
Figure 4 depicts a typical GC-ECD chromatogram—enhanced to highlight individual retention times for each PCB congener. Analysis of PCB congeners was carried out on GD11.5, GD18.5, PND6, PND13, and PND28 (Tables 5–14). Several of the coplanar PCBs were below the level of detection in many tissues, whereas all the noncoplanar PCBs were always detected—until PND28. In general, all the coplanar congeners were highest in maternal inguinal fat pad, whereas all the noncoplanar congeners were highest in both maternal inguinal fat pad and mammary tissue. In the postnatal pup, all eight PCBs were highest in adipose tissue.
FIG. 4.
GC-ECD sample chromatogram. GC-ECD was used to quantify PCB congeners. Depicted is a sample chromatogram showing the results from adipose tissue taken from an Ahrb_Cyp1a2(−/−) mother at GD11.5; a software program was used to identify and quantify each of the eight congeners.
TABLE 5.
Coplanar PCB Congener Analysis on GD11.5
| Genotype | Tissue | PCB 77 | PCB 126 | PCB 169 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 12.8 ± 2.0 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 2.7 ± 0.4 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 17.0 ± 1.8 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 28.3 ± 2.7 | 3.6 ± 1.6 | 25.2 ± 6.5 |
| AhrdCyp1a2(+/+) | 93.5 ± 12 | 1.8 ± 0.4 | 9.9 ± 1.6 | |
| Ahrb1Cyp1a2(−/−) | 15.2 ± 2.0 | 1.8 ± 0.4 | 10.3 ± 1.2 | |
| AhrdCyp1a2(−/−) | 109 ± 8.5 | 2.3 ± 0.7 | 13.7 ± 1.7 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 96.7 ± 10 | < 0.5 | 7.0 ± 1.1 |
| AhrdCyp1a2(+/+) | 262 ± 18 | 1.8 ± 0.4 | 11.8 ± 1.2 | |
| Ahrb1Cyp1a2(−/−) | 156 ± 9.2 | 2.2 ± 0.7 | 13.2 ± 2.7 | |
| AhrdCyp1a2(−/−) | 284 ± 4.1 | 1.7 ± 0.9 | 13.7 ± 2.1 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 96.4 ± 9.7 | < 0.5 | 8.8 ± 1.2 |
| AhrdCyp1a2(+/+) | 297 ± 43 | 2.6 ± 0.4 | 15.2 ± 3.1 | |
| Ahrb1Cyp1a2(−/−) | 127 ± 4.6 | 2.0 ± 0.6 | 11.5 ± 2.1 | |
| AhrdCyp1a2(−/−) | 289 ± 7.7 | 2.4 ± 0.5 | 16.2 ± 0.9 | |
| Ahrb1Cyp1a2(+/+) | Placenta | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 5.3 ± 2.8 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 5.3 ± 1.8 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 15.3 ± 1.2 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Whole embryoa | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 6.3 ± 1.1 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 4.4 ± 3.2 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 7.7 ± 0.5 | < 0.5 | < 0.5 |
Note. For all 10 tables (Tables 5–14), congeners are ranked according to their planarity and chlorination patterns, with PCB 77 being a coplanar tetrachlorobiphenyl and PCB 180 a noncoplanar heptachlorobiphenyl. All values denote the means of three to five per group ± SEM and represent nanograms per 100 mg tissue wet weight. Limits of quantification were 0.5 ng/100 mg or 0.5 ng/100 μl of tissue or plasma, respectively. For all intergenotype, intercongener, and group comparisons, p values are available upon request. For GD11.5 collection, these tissues were collected 24 h after the initial PCB dose.
Whole embryo represents N = 2–3, and two to three placentas were pooled from each mother.
On GD11.5, 24 h after the first gavage (Tables 5 and 6), all three coplanar congeners were lowest in Ahrb1_Cyp1a2(+/+) maternal inguinal fat pad compared with the other three genotypes. On the other hand, all three coplanar congeners were highest in Ahrd_Cyp1a2(−/−) maternal mammary tissue compared with the other three genotypes. In placenta and whole embryo, all three coplanar congeners were lowest in Ahrb1_Cyp1a2(+/+). The coplanar PCB 126 and PCB 169 were below the limits of detection in maternal brain, the placenta, and the whole embryo—for all four genotypes. All five noncoplanar congeners were lowest in Ahrb1_Cyp1a2(+/+) maternal brain, the placenta, and the whole embryo.
TABLE 6.
Noncoplanar PCB Congener Analysis on GD11.5
| Genotype | Tissue | PCB 105 | PCB 118 | PCB 138 | PCB 153 | PCB 180 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | 14.0 ± 2.5 | 16.2 ± 2.6 | 23.2 ± 3.7 | 16.1 ± 2.1 | 30.4 ± 2.7 |
| AhrdCyp1a2(+/+) | 27.8 ± 3.5 | 27.6 ± 3.5 | 32.3 ± 3.9 | 30.6 ± 3.1 | 44.6 ± 4.7 | |
| Ahrb1Cyp1a2(−/−) | 25.4 ± 0.8 | 25.5 ± 0.4 | 30.6 ± 0.8 | 26.0 ± 1.9 | 41.0 ± 2.0 | |
| AhrdCyp1a2(−/−) | 26.7 ± 3.5 | 28.0 ± 3.1 | 33.4 ± 4.4 | 28.3 ± 2.7 | 46.1 ± 6.7 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 150 ± 34 | 135 ± 17 | 159 ± 18 | 125 ± 13 | 222 ± 34 |
| AhrdCyp1a2(+/+) | 183 ± 28 | 157 ± 16 | 184 ± 17 | 149 ± 16 | 244 ± 21 | |
| Ahrb1Cyp1a2(−/−) | 150 ± 21 | 123 ± 9.4 | 145 ± 11 | 110 ± 5.9 | 184 ± 14 | |
| AhrdCyp1a2(−/−) | 177 ± 18 | 154 ± 9.5 | 176 ± 12 | 141 ± 11 | 237 ± 14 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 293 ± 41 | 258 ± 60 | 214 ± 28 | 173 ± 21 | 154 ± 19 |
| AhrdCyp1a2(+/+) | 395 ± 23 | 358 ± 51 | 287 ± 18 | 235 ± 18 | 206 ± 13 | |
| Ahrb1Cyp1a2(−/−) | 381 ± 47 | 323 ± 66 | 267 ± 36 | 207 ± 25 | 189 ± 32 | |
| AhrdCyp1a2(−/−) | 376 ± 3.0 | 326 ± 5.3 | 263 ± 4.1 | 220 ± 12 | 194 ± 11 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 339 ± 43 | 302 ± 38 | 254 ± 34 | 203 ± 27 | 184 ± 27 |
| AhrdCyp1a2(+/+) | 441 ± 60 | 393 ± 54 | 332 ± 47 | 267 ± 38 | 243 ± 35 | |
| Ahrb1Cyp1a2(−/−) | 323 ± 38 | 278 ± 33 | 230 ± 30 | 180 ± 21 | 163 ± 25 | |
| AhrdCyp1a2(−/−) | 400 ± 6.9 | 365 ± 6.6 | 301 ± 7.3 | 244 ± 6.3 | 219 ± 11 | |
| Ahrb1Cyp1a2(+/+) | Placenta | 7.62 ± 2.0 | 8.37 ± 2.3 | 9.8 ± 3.1 | 9.06 ± 2.7 | 15.2 ± 5.4 |
| AhrdCyp1a2(+/+) | 16.7 ± 2.4 | 18.2 ± 2.4 | 19.9 ± 3.0 | 19.2 ± 2.7 | 29.2 ± 4.6 | |
| Ahrb1Cyp1a2(−/−) | 33.1 ± 8.4 | 32.8 ± 7.8 | 33.3 ± 8.1 | 29.3 ± 7.3 | 43.2 ± 13 | |
| AhrdCyp1a2(−/−) | 23.7 ± 1.3 | 23.7 ± 2.0 | 26.3 ± 1.7 | 24.7 ± 0.2 | 41.4 ± 1.7 | |
| Ahrb1Cyp1a2(+/+) | Whole embryoa | 6.2 ± 0.4 | 7.3 ± 0.3 | 8.0 ± 0.8 | 6.7 ± 0.6 | 8.5 ± 0.5 |
| AhrdCyp1a2(+/+) | 11.5 ± 2.2 | 11.5 ± 1.9 | 11.6 ± 2.0 | 10.6 ± 1.6 | 16.2 ± 2.4 | |
| Ahrb1Cyp1a2(−/−) | 17.2 ± 6.2 | 16.1 ± 5.4 | 15.1 ± 3.8 | 12.7 ± 3.4 | 17.2 ± 2.3 | |
| AhrdCyp1a2(−/−) | 10.8 ± 0.8 | 11.2 ± 0.8 | 10.7 ± 0.7 | 9.36 ± 0.6 | 14.4 ± 0.4 |
Note. For GD11.5 collection, these tissues were collected 24 h after the initial PCB dose.
Whole embryo represents N = 2–3, and two to three placentas were pooled from each mother.
On GD18.5, 8 days after the first gavage (Tables 7 and 8), the coplanar PCB 77 was highest in Ahrd_Cyp1a2(−/−) maternal brain, liver, inguinal fat pad and mammary tissue, and fetal brain and liver compared with that in the other three genotypes. Except for PCB 169 levels being considerably higher than PCB 126 levels (because of 10-fold greater PCB 169 concentration in the dosing solution), these two other coplanar congeners were not different among the tissues or genotypes. PCB 126 and PCB 169 were below the limits of detection in maternal and fetal brain and fetal liver—for all four genotypes; these data confirm that the dose given on GD10.5 was beginning to disappear. All five noncoplanar congeners were unremarkable, except for being highest in Ahrb1_Cyp1a2(+/+) fetal liver.
TABLE 7.
Coplanar PCB Congener Analysis on GD18.5
| Genotype | Tissue | PCB 77 | PCB 126 | PCB 169 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 4.6 ± 0.9 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 26.4 ± 1.8 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 4.3 ± 1.3 | 18.4 ± 1.5 | 33.9 ± 2.9 |
| AhrdCyp1a2(+/+) | 18.2 ± 5.4 | 2.9 ± 0.8 | 5.4 ± 1.1 | |
| Ahrb1Cyp1a2(−/−) | 1.5 ± 0.9 | < 0.5 | 5.1 ± 0.8 | |
| AhrdCyp1a2(−/−) | 69.5 ± 7.9 | < 0.5 | 3.6 ± 0.7 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 34.4 ± 12 | 2.8 ± 0.7 | 52.9 ± 7.2 |
| AhrdCyp1a2(+/+) | 149 ± 29 | 5.4 ± 1.3 | 63.1 ± 10.5 | |
| Ahrb1Cyp1a2(−/−) | 44.3 ± 6.8 | 8.9 ± 1.0 | 62.9 ± 8.9 | |
| AhrdCyp1a2(−/−) | 434 ± 19 | 8.0 ± 0.6 | 47.9 ± 5.8 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 15.2 ± 6.9 | 1.9 ± 0.2 | 44.1 ± 2.7 |
| AhrdCyp1a2(+/+) | 95.7 ± 23 | 3.1 ± 1.1 | 50.4 ± 9.9 | |
| Ahrb1Cyp1a2(−/−) | 17.0 ± 5.2 | 5.9 ± 0.7 | 44.3 ± 8.1 | |
| AhrdCyp1a2(−/−) | 371 ± 18 | 6.2 ± 0.5 | 38.3 ± 4.1 | |
| Ahrb1Cyp1a2(+/+) | Fetal brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 2.7 ± 1.0 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 18.8 ± 3.1 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Fetal liver | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 3.59 ± 1.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 21.3 ± 2.3 | < 0.5 | < 0.5 |
Note. These tissues were collected 8 days after the first gavage on GD10.5.
TABLE 8.
Noncoplanar PCB Congener Analysis on GD18.5
| Genotype | Tissue | PCB 105 | PCB 118 | PCB 138 | PCB 153 | PCB 180 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | 29.4 ± 3.2 | 30.8 ± 2.5 | 31.1 ± 2.5 | 23.1 ± 2.3 | 32.4 ± 2.4 |
| AhrdCyp1a2(+/+) | 33.1 ± 2.6 | 33.2 ± 4.8 | 30.6 ± 4.8 | 23.5 ± 3.4 | 34.4 ± 6.0 | |
| Ahrb1Cyp1a2(−/−) | 33.4 ± 2.4 | 32.4 ± 3.3 | 29.2 ± 2.8 | 22.5 ± 2.7 | 32.1 ± 3.6 | |
| AhrdCyp1a2(−/−) | 34.4 ± 2.2 | 32.6 ± 1.5 | 30.4 ± 1.6 | 23.3 ± 1.1 | 33.0 ± 2.2 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 164 ± 4.5 | 129 ± 5.0 | 148 ± 4.0 | 76.1 ± 21 | 136 ± 5.5 |
| AhrdCyp1a2(+/+) | 127 ± 16.7 | 89.7 ± 13 | 98.9 ± 16 | 64.4 ± 10 | 94.0 ± 16 | |
| Ahrb1Cyp1a2(−/−) | 145 ± 16 | 97.5 ± 12 | 132 ± 14 | 69.1 ± 10 | 121 ± 14 | |
| AhrdCyp1a2(−/−) | 126 ± 13 | 87.9 ± 9.3 | 109 ± 11 | 65.4 ± 7.0 | 107 ± 13 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 639 ± 38 | 580 ± 38 | 570 ± 37 | 448 ± 36 | 482 ± 34 |
| AhrdCyp1a2(+/+) | 691 ± 56 | 614 ± 49 | 586 ± 51 | 475 ± 40 | 515 ± 49 | |
| Ahrb1Cyp1a2(−/−) | 740 ± 91 | 657 ± 84 | 626 ± 79 | 502 ± 70 | 541 ± 67 | |
| AhrdCyp1a2(−/−) | 678 ± 43 | 599 ± 34 | 559 ± 39 | 445 ± 29 | 478 ± 43 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 514 ± 31 | 477 ± 31 | 485 ± 34 | 386 ± 32 | 432 ± 38 |
| AhrdCyp1a2(+/+) | 575 ± 49 | 516 ± 38 | 507 ± 44 | 411 ± 35 | 486 ± 47 | |
| Ahrb1Cyp1a2(−/−) | 495 ± 56 | 449 ± 53 | 436 ± 53 | 353 ± 47 | 412 ± 56 | |
| AhrdCyp1a2(−/−) | 566 ± 42 | 508 ± 33 | 496 ± 41 | 401 ± 31 | 465 ± 53 | |
| Ahrb1Cyp1a2(+/+) | Fetal brain | 20.8 ± 1.5 | 21.5 ± 1.0 | 21.3 ± 1.1 | 16.3 ± 1.0 | 19.8 ± 1.0 |
| AhrdCyp1a2(+/+) | 18.1 ± 3.3 | 16.7 ± 3.1 | 15.3 ± 3.2 | 12.3 ± 2.6 | 14.8 ± 3.3 | |
| Ahrb1Cyp1a2(−/−) | 20.4 ± 2.5 | 19.3 ± 2.5 | 17.9 ± 2.7 | 14.2 ± 2.4 | 17.9 ± 2.8 | |
| AhrdCyp1a2(−/−) | 25.4 ± 5.2 | 24.3 ± 4.6 | 22.1 ± 4.4 | 17.3 ± 3.5 | 20.5 ± 3.8 | |
| Ahrb1Cyp1a2(+/+) | Fetal liver | 47.2 ± 5.5 | 34.9 ± 5.0 | 41.9 ± 5.3 | 26.3 ± 4.0 | 34.1 ± 4.5 |
| AhrdCyp1a2(+/+) | 37.4 ± 7.7 | 26.6 ± 6.1 | 29.0 ± 6.8 | 19.4 ± 4.9 | 26.6 ± 7.2 | |
| Ahrb1Cyp1a2(−/−) | 36.9 ± 5.3 | 23.7 ± 3.9 | 28.3 ± 4.8 | 16.7 ± 3.3 | 24.2 ± 4.8 | |
| AhrdCyp1a2(−/−) | 37.6 ± 3.3 | 25.3 ± 2.1 | 30.0 ± 1.9 | 17.9 ± 1.4 | 25.3 ± 2.4 |
Note. These tissues were collected 8 days after the first gavage on GD10.5.
On PND6, 24 h after the second gavage (Tables 9 and 10), the coplanar PCB 77 was highest in Ahrd-containing mouse lines for all tissues examined: maternal brain, liver, inguinal fat pad, and mammary tissue and pup brain, liver, adipose tissue, and stomach. The other two coplanar congeners, and PCB 126 and PCB 169, were highest in Ahrb1_Cyp1a2(+/+) maternal liver and inguinal fat pad compared with that in the other three genotypes. The coplanar PCB 126 and PCB 169 were below the limits of detection in maternal and pup brain—for all four genotypes. All five noncoplanar congeners were remarkably similar when comparing the four genotypes in any of the eight tissues examined.
TABLE 9.
Coplanar PCB Congener Analysis on PND6
| Genotype | Tissue | PCB 77 | PCB 126 | PCB 169 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | 0.7 ± 0.7 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 9.8 ± 2.0 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 3.2 ± 0.8 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 10.0 ± 1.1 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 11.5 ± 1.8 | 3.1 ± 2.0 | 18.1 ± 1.9 |
| AhrdCyp1a2(+/+) | 43.2 ± 8.8 | 0.7 ± 0.7 | 2.3 ± 0.6 | |
| Ahrb1Cyp1a2(−/−) | 10.0 ± 4.3 | < 0.5 | 2.7 ± 1.6 | |
| AhrdCyp1a2(−/−) | 36.6 ± 6.0 | < 0.5 | 1.3 ± 0.7 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 122 ± 19 | 8.1 ± 0.2 | 43.6 ± 1.4 |
| AhrdCyp1a2(+/+) | 271 ± 27 | 5.4 ± 1.8 | 34.5 ± 7.9 | |
| Ahrb1Cyp1a2(−/−) | 200 ± 7.9 | 1.7 ± 0.2 | 39.7 ± 5.4 | |
| AhrdCyp1a2(−/−) | 304 ± 11 | 4.4 ± 2.0 | 22.2 ± 1.7 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 26.4 ± 14 | 0.8 ± 0.2 | 14.3 ± 4.1 |
| AhrdCyp1a2(+/+) | 172 ± 36 | 4.1 ± 3.1 | 14.9 ± 5.5 | |
| Ahrb1Cyp1a2(−/−) | 64.8 ± 22 | < 0.5 | 22.4 ± 6.8 | |
| AhrdCyp1a2(−/−) | 164 ± 36 | 1.2 ± 0.3 | 12.0 ± 0.7 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | 1.2 ± 0.7 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 11.6 ± 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 2.2 ± 0.1 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 10.8 ± 1.0 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | 30.5 ± 3.8 | 2.9 ± 2.0 | 24.7 ± 6.3 |
| AhrdCyp1a2(+/+) | 59.5 ± 8.2 | 0.6 ± 0.3 | 4.2 ± 1.6 | |
| Ahrb1Cyp1a2(−/−) | 21.5 ± 1.6 | 0.8 ± 0.2 | 11.4 ± 4.5 | |
| AhrdCyp1a2(−/−) | 121 ± 28 | 0.7 ± 0.4 | 31.5 ± 7.9 | |
| Ahrb1Cyp1a2(+/+) | Pup total adipose tissue | 52.5 ± 10 | 1.4 ± 0.7 | 26.0 ± 3.3 |
| AhrdCyp1a2(+/+) | 182 ± 30 | 2.8 ± 0.4 | 22.8 ± 9.1 | |
| Ahrb1Cyp1a2(−/−) | 109 ± 9.7 | 1.0 ± 0.3 | 18.8 ± 10 | |
| AhrdCyp1a2(−/−) | 339 ± 59 | 2.8 ± 1.4 | 20.5 ± 11 | |
| Ahrb1Cyp1a2(+/+) | Pup stomach | 48.4 ± 20 | 1.6 ± 0.9 | 15.6 ± 2.9 |
| AhrdCyp1a2(+/+) | 169 ± 29 | 2.7 ± 1.3 | 10.5 ± 4.9 | |
| Ahrb1Cyp1a2(−/−) | 101 ± 13 | 0.7 ± 0.3 | 18.9 ± 2.7 | |
| AhrdCyp1a2(−/−) | 225 ± 26 | 1.8 ± 0.5 | 21.7 ± 4.3 |
Note. These tissues were collected 24 h after the second gavage on PND5.
TABLE 10.
Noncoplanar PCB Congener Analysis on PND6
| Genotype | Tissue | PCB 105 | PCB 118 | PCB 138 | PCB 153 | PCB 180 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | 17.7 ± 1.4 | 17.2 ± 1.6 | 18.6 ± 1.4 | 16.9 ± 1.3 | 32.1 ± 1.6 |
| AhrdCyp1a2(+/+) | 26.2 ± 2.9 | 24.5 ± 3.2 | 25.6 ± 3.6 | 21.9 ± 3.6 | 40.0 ± 4.2 | |
| Ahrb1Cyp1a2(−/−) | 24.2 ± 2.2 | 22.7 ± 2.0 | 22.5 ± 2.9 | 20.4 ± 3.2 | 36.9 ± 3.2 | |
| AhrdCyp1a2(−/−) | 17.2 ± 2.2 | 15.6 ± 2.2 | 17.8 ± 3.3 | 17.2 ± 2.9 | 30.5 ± 5.2 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 79.2 ± 8.8 | 60.8 ± 7.4 | 70.6 ± 5.4 | 41.9 ± 4.4 | 81.8 ± 4.0 |
| AhrdCyp1a2(+/+) | 95.6 ± 14 | 70.6 ± 13 | 76.6 ± 13 | 51.0 ± 10 | 86.6 ± 15 | |
| Ahrb1Cyp1a2(−/−) | 82.7 ± 14 | 59.5 ± 13 | 72.2 ± 14 | 45.4 ± 14 | 85.3 ± 20 | |
| AhrdCyp1a2(−/−) | 71.6 ± 13 | 50.3 ± 10 | 62.1 ± 10 | 39.0 ± 8.3 | 76.5 ± 15 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 538 ± 29 | 507 ± 18 | 536 ± 16 | 450 ± 13 | 524 ± 22 |
| AhrdCyp1a2(+/+) | 586 ± 54 | 545 ± 59 | 488 ± 41 | 414 ± 44 | 445 ± 33 | |
| Ahrb1Cyp1a2(−/−) | 642 ± 16 | 585 ± 13 | 569 ± 16 | 460 ± 11 | 521 ± 25 | |
| AhrdCyp1a2(−/−) | 464 ± 52 | 463 ± 12 | 441 ± 42 | 376 ± 29 | 428 ± 29 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 267 ± 66 | 246 ± 61 | 240 ± 52 | 189 ± 41 | 268 ± 33 |
| AhrdCyp1a2(+/+) | 377 ± 48 | 346 ± 44 | 338 ± 42 | 249 ± 34 | 320 ± 28 | |
| Ahrb1Cyp1a2(−/−) | 285 ± 49 | 259 ± 46 | 249 ± 45 | 188 ± 36 | 252 ± 28 | |
| AhrdCyp1a2(−/−) | 256 ± 27 | 215 ± 16 | 223 ± 15 | 182 ± 28 | 250 ± 31 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | 29.6 ± 4.5 | 27.6 ± 3.4 | 27.1 ± 4.8 | 22.6 ± 3.9 | 31.6 ± 7.4 |
| AhrdCyp1a2(+/+) | 32.4 ± 4.8 | 32.2 ± 5.2 | 28.9 ± 5.2 | 24.0 ± 4.4 | 30.0 ± 4.8 | |
| Ahrb1Cyp1a2(−/−) | 25.9 ± 3.5 | 24.4 ± 3.1 | 19.1 ± 2.7 | 17.5 ± 2.1 | 22.0 ± 3.5 | |
| AhrdCyp1a2(−/−) | 19.5 ± 2.1 | 18.0 ± 0.8 | 17.3 ± 1.6 | 14.9 ± 1.3 | 21.1 ± 3.6 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | 235 ± 52 | 209 ± 47 | 210 ± 42 | 164 ± 34 | 254.8 ± 45 |
| AhrdCyp1a2(+/+) | 156 ± 28 | 123 ± 26 | 117 ± 21 | 101 ± 23 | 145 ± 26 | |
| Ahrb1Cyp1a2(−/−) | 271 ± 25 | 235 ± 23 | 220 ± 19 | 178 ± 16 | 246.0 ± 18 | |
| AhrdCyp1a2(−/−) | 269 ± 40 | 233 ± 37 | 243 ± 38 | 198 ± 32 | 297 ± 40 | |
| Ahrb1Cyp1a2(+/+) | Pup total adipose tissue | 508 ± 25 | 454 ± 23 | 417 ± 19 | 317 ± 11 | 343 ± 12 |
| AhrdCyp1a2(+/+) | 434 ± 81 | 378 ± 74 | 351 ± 73 | 269 ± 52 | 279 ± 67 | |
| Ahrb1Cyp1a2(−/−) | 626 ± 42 | 547 ± 39 | 487 ± 38 | 371 ± 30 | 384 ± 38 | |
| AhrdCyp1a2(−/−) | 543 ± 43 | 475 ± 40 | 406 ± 35 | 326 ± 29 | 318 ± 36 | |
| Ahrb1Cyp1a2(+/+) | Pup stomach | 329 ± 11 | 291 ± 2.0 | 272 ± 8.0 | 206 ± 10 | 253 ± 15 |
| AhrdCyp1a2(+/+) | 353 ± 51 | 307 ± 49 | 270 ± 46 | 205 ± 38 | 242 ± 36 | |
| Ahrb1Cyp1a2(−/−) | 348 ± 23 | 305 ± 22 | 271 ± 20 | 211 ± 15 | 253 ± 24 | |
| AhrdCyp1a2(−/−) | 347 ± 47 | 300 ± 41 | 287 ± 41 | 224 ± 34 | 273 ± 42 |
Note. These tissues were collected 24 h after the second gavage on PND5.
On PND13, 8 days after the second gavage (Tables 11 and 12), the coplanar PCB 77 was highest in Cyp1a2(−/−)-containing mouse lines for maternal inguinal fat pad and mammary tissues; PCB 77 was highest in Ahrd_Cyp1a2(−/−) maternal brain and liver, as well as pup brain, liver, fat, and plasma compared with that of the other three genotypes. The coplanar PCB 126 and PCB 169 were below the limits of detection in maternal and pup brain and plasma—for all four genotypes. Again, all five noncoplanar congeners were remarkably similar when comparing the four genotypes in any of the eight tissues examined, except that Ahrb1Cyp1a2(−/−) pups showed higher levels in all tissues at this time point.
TABLE 11.
Coplanar PCB Congener Analysis on PND13*
| Genotype | Tissue | PCB 77 | PCB 126 | PCB 169 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 0.5 ± 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 1.7 ± 0.6 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | < 0.5 | 4.5 ± 1.4 | 2.4 ± 0.7 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | 0.9 ± 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 1.5 ± 0.1 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 3.3 ± 2.1 | 1.0 ± 0.6 | 35.6 ± 11 |
| AhrdCyp1a2(+/+) | 10.4 ± 0.8 | 1.2 ± 0.8 | 34.9 ± 1.8 | |
| Ahrb1Cyp1a2(−/−) | 33.9 ± 6.1 | 5.3 ± 2.9 | 69.4 ± 11 | |
| AhrdCyp1a2(−/−) | 35.9 ± 12 | 0.9 ± 0.6 | 15.0 ± 8.8 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | < 0.5 | < 0.5 | 1.8 ± 1.1 |
| AhrdCyp1a2(+/+) | 1.2 ± 0.2 | < 0.5 | 1.9 ± 0.3 | |
| Ahrb1Cyp1a2(−/−) | 3.1 ± 0.6 | < 0.5 | 10.8 ± 2.4 | |
| AhrdCyp1a2(−/−) | 3.2 ± 0.6 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 3.1 ± 1.0 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 13.6 ± 6.9 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | 1.0 ± 0.7 | 22.6 ± 4.1 | 31.9 ± 3.1 |
| AhrdCyp1a2(+/+) | 11.6 ± 2.7 | 1.2 ± 0.6 | 2.3 ± 0.3 | |
| Ahrb1Cyp1a2(−/−) | 0.9 ± 0.5 | 1.2 ± 0.4 | 11.6 ± 1.7 | |
| AhrdCyp1a2(−/−) | 46.5 ± 16 | < 0.5 | 1.4 ± 1.2 | |
| Ahrb1Cyp1a2(+/+) | Pup total adipose tissue | 2.4 ± 0.7 | 2.1 ± 0.5 | 50.0 ± 4.8 |
| AhrdCyp1a2(+/+) | 106 ± 16 | 6.4 ± 0.5 | 40.7 ± 4.1 | |
| Ahrb1Cyp1a2(−/−) | 19.1 ± 1.0 | 14.4 ± 1.0 | 96.0 ± 6.3 | |
| AhrdCyp1a2(−/−) | 408 ± 32 | 3.6 ± 1.5 | 24.7 ± 12 | |
| Ahrb1Cyp1a2(+/+) | Pup plasma | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | 2.0 ± 0.2 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 8.2 ± 0.8 | < 0.5 | < 0.5 |
Note. These tissues were collected 8 days after the second gavage to the mother on PND5.
TABLE 12.
Noncoplanar PCB Congener Analysis on PND13
| Genotype | Tissue | PCB 105 | PCB 118 | PCB 138 | PCB 153 | PCB 180 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | 8.96 ± 4.2 | 8.07 ± 3.9 | 10.0 ± 4.1 | 7.69 ± 2.9 | 11.6 ± 3.4 |
| AhrdCyp1a2(+/+) | 5.22 ± 0.70 | 4.36 ± 0.93 | 5.00 ± 1.2 | 4.31 ± 1.1 | 6.55 ± 1.9 | |
| Ahrb1Cyp1a2(−/−) | 9.45 ± 3.9 | 8.48 ± 3.9 | 10.1 ± 4.3 | 8.33 ± 3.2 | 13.9 ± 4.5 | |
| AhrdCyp1a2(−/−) | 3.65 ± 1.3 | 3.26 ± 1.4 | 3.97 ± 1.3 | 3.63 ± 1.1 | 6.59 ± 2.2 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 16.0 ± 4.8 | 10.8 ± 3.4 | 37.6 ± 7.9 | 13.3 ± 3.8 | 52.0 ± 7.8 |
| AhrdCyp1a2(+/+) | 17.8 ± 3.6 | 11.4 ± 3.0 | 36.4 ± 7.5 | 13.4 ± 3.6 | 47.0 ± 10 | |
| Ahrb1Cyp1a2(−/−) | 41.3 ± 19 | 25.9 ± 12 | 58.3 ± 12 | 24.3 ± 8.6 | 70.7 ± 2.5 | |
| AhrdCyp1a2(−/−) | 8.65 ± 0.75 | 4.50 ± 0.54 | 26.4 ± 1.4 | 6.31 ± 0.69 | 39.1 ± 4.4 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 188 ± 79 | 213 ± 79 | 333 ± 96 | 303 ± 82 | 448 ± 88 |
| AhrdCyp1a2(+/+) | 249 ± 38 | 270 ± 46 | 400 ± 66 | 356 ± 58 | 469 ± 21 | |
| Ahrb1Cyp1a2(−/−) | 393 ± 170 | 353 ± 120 | 507 ± 95 | 440 ± 49 | 625 ± 26 | |
| AhrdCyp1a2(−/−) | 235 ± 54 | 278 ± 53 | 424 ± 59 | 382 ± 45 | 525 ± 47 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 21.1 ± 9.3 | 24.3 ± 10 | 38.7 ± 15 | 32.0 ± 12 | 66.7 ± 24 |
| AhrdCyp1a2(+/+) | 27.1 ± 3.6 | 30.5 ± 5.0 | 44.1 ± 7.5 | 36.1 ± 5.9 | 74.7 ± 8.2 | |
| Ahrb1Cyp1a2(−/−) | 113 ± 36 | 113 ± 33 | 155 ± 36 | 132 ± 24 | 246 ± 27 | |
| AhrdCyp1a2(−/−) | 11.3 ± 2.3 | 14.2 ± 2.9 | 24.8 ± 4.6 | 21.3 ± 3.8 | 53.6 ± 11.1 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | 23.2 ± 3.8 | 21.1 ± 2.9 | 23.5 ± 4.0 | 18.2 ± 3.3 | 23.9 ± 4.3 |
| AhrdCyp1a2(+/+) | 27.8 ± 6.1 | 23.9 ± 5.8 | 21.0 ± 5.3 | 16.3 ± 3.9 | 19.0 ± 4.8 | |
| Ahrb1Cyp1a2(−/−) | 47.9 ± 4.5 | 41.4 ± 5.6 | 37.8 ± 3.6 | 29.0 ± 2.9 | 37.0 ± 3.7 | |
| AhrdCyp1a2(−/−) | 23.7 ± 8.5 | 20.9 ± 6.9 | 18.1 ± 6.2 | 14.6 ± 5.0 | 16.6 ± 5.3 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | 98.6 ± 8.6 | 79.6 ± 6.3 | 77.6 ± 6.6 | 48.2 ± 4.8 | 68.2 ± 7.1 |
| AhrdCyp1a2(+/+) | 64.7 ± 1.3 | 51.3 ± 7.4 | 52.8 ± 11 | 36.9 ± 11 | 49.1 ± 13 | |
| Ahrb1Cyp1a2(−/−) | 231 ± 33 | 181 ± 28 | 175 ± 24 | 123 ± 22 | 161 ± 21 | |
| AhrdCyp1a2(−/−) | 77.8 ± 25 | 58.1 ± 19 | 58.1 ± 19 | 39.0 ± 14 | 50.7 ± 18 | |
| Ahrb1Cyp1a2(+/+) | Pup total adipose tissue | 624 ± 70 | 558 ± 57 | 562 ± 56 | 442 ± 42 | 482 ± 42 |
| AhrdCyp1a2(+/+) | 594 ± 120 | 518 ± 88 | 501 ± 72 | 412 ± 37 | 429 ± 48 | |
| Ahrb1Cyp1a2(−/−) | 740 ± 26 | 691 ± 38 | 651 ± 26 | 497 ± 12 | 551 ± 12 | |
| AhrdCyp1a2(−/−) | 636 ± 34 | 566 ± 27 | 535 ± 26 | 428 ± 25 | 459 ± 30 | |
| Ahrb1Cyp1a2(+/+) | Pup plasma | 17.9 ± 3.8 | 16.7 ± 3.3 | 17.8 ± 3.3 | 15.3 ± 3.4 | 16.9 ± 2.9 |
| AhrdCyp1a2(+/+) | 15.6 ± 1.0 | 13.7 ± 0.84 | 11.5 ± 0.8 | 9.7 ± 0.7 | 9.6 ± 1.0 | |
| Ahrb1Cyp1a2(−/−) | 35.7 ± 2.1 | 34.1 ± 1.3 | 28.8 ± 1.2 | 24.3 ± 0.8 | 28.2 ± 0.4 | |
| AhrdCyp1a2(−/−) | 11.8 ± 0.9 | 11.2 ± 0.9 | 9.6 ± 0.8 | 8.8 ± 0.4 | 7.9 ± 0.4 |
Note. These tissues were collected 8 days after the second gavage to the mother on PND5.
On PND28, 23 days after the second gavage (Table 13), all three coplanar congeners were below the limits of detection in maternal brain, liver, mammary tissue (with exception of Ahrd_Cyp1a2(−/−)), and in pup brain—for all four genotypes. Again, this finding indicates that the second dose on PND5 was being largely cleared from the tissues. Individual coplanar congeners were undetectable in various tissues of all four genotypes: PCB 126 in maternal inguinal fat pad and in pup liver and adipose tissue and PCB 77 in pup brain. Again, the coplanar PCB 77 was highest in Ahrd_Cyp1a2(−/−) maternal inguinal fat pad and mammary tissue, as well as in pup liver, adipose tissue, and subcutaneous fat compared with that in the other three genotypes.
TABLE 13.
Coplanar PCB Congener Analysis on PND28
| Genotype | Tissue | PCB 77 | PCB 126 | PCB 169 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | 8.5 ± 1.3 | |
| AhrdCyp1a2(−/−) | 6.5 ± 4.0 | < 0.5 | 8.4 ± 4.4 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 5.2 ± 3.2 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | < 0.5 | < 0.5 | 3.6 ± 1.9 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | |
| AhrdCyp1a2(−/−) | 20.0 ± 5.3 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Pup subcutaneous adipose tissue | < 0.5 | < 0.5 | 11.5 ± 1.7 |
| AhrdCyp1a2(+/+) | < 0.5 | < 0.5 | 18.5 ± 2.9 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | 18.2 ± 0.2 | |
| AhrdCyp1a2(−/−) | 182 ± 60 | < 0.5 | 19.2 ± 1.9 | |
| Ahrb1Cyp1a2(+/+) | Pup inguinal fat pad | < 0.5 | < 0.5 | 12.7 ± 0.4 |
| AhrdCyp1a2(+/+) | < 0.5 | 2.2 ± 1.3 | 24.4 ± 6.2 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | 4.2 ± 0.8 | 41.2 ± 13 | |
| AhrdCyp1a2(−/−) | 243 ± 37 | < 0.5 | 22.0 ± 2.3 |
Note. These tissues were collected 23 days after the second gavage of the mother on PND5.
On PND28, all five noncoplanar congeners (Table 14) were remarkably similar when comparing the four genotypes in any of the eight tissues examined, with a few modest exceptions. Four of the five noncoplanar congeners were below the limits of detection in maternal brain; Ahrd_Cyp1a2(+/+) was the exception, with four of the five noncoplanar PCBs detectable. PCB 118 is known to be readily metabolized, and it was below the level of detection in maternal liver for all genotypes except Ahrb1_Cyp1a2(−/−). For pup inguinal fat pad, all five noncoplanar congeners were highest in Ahrb1_Cyp1a2(−/−), and in pup subcutaneous fat, two of the five noncoplanars, PCB 105 and PCB 118, were highest in Ahrd_Cyp1a2(−/−).
TABLE 14.
Noncoplanar PCB Congener Analysis on PND28
| Genotype | Tissue | PCB 105 | PCB 118 | PCB 138 | PCB 153 | PCB 180 |
| Ahrb1Cyp1a2(+/+) | Maternal brain | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| AhrdCyp1a2(+/+) | < 0.5 | 3.5 ± 2.3 | 7.6 ± 5.0 | 6.7 ± 4.7 | 18.4 ± 13 | |
| Ahrb1Cyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | < 0.5 | 2.9 ± 0.8 | |
| AhrdCyp1a2(−/−) | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | |
| Ahrb1Cyp1a2(+/+) | Maternal liver | 0.6 ± 0.4 | < 0.5 | 5.8 ± 3.2 | 1.8 ± 1.1 | 17.9 ± 4.2 |
| AhrdCyp1a2(+/+) | 6.3 ± 3.2 | < 0.5 | 18.0 ± 7.7 | 9.8 ± 5.7 | 32.7 ± 9.7 | |
| Ahrb1Cyp1a2(−/−) | 4.7 ± 0.8 | 2.5 ± 1.2 | 14.8 ± 3.3 | 5.2 ± 1.5 | 27.4 ± 2.7 | |
| AhrdCyp1a2(−/−) | 4.0 ± 2.3 | < 0.5 | 13.5 ± 4.9 | 5.1 ± 2.6 | 23.0 ± 5.4 | |
| Ahrb1Cyp1a2(+/+) | Maternal inguinal fat pad | 3.9 ± 2.0 | 7.2 ± 3.0 | 27.8 ± 9.1 | 36.5 ± 10 | 119 ± 17 |
| AhrdCyp1a2(+/+) | 44.2 ± 31 | 57.2 ± 39 | 126 ± 73 | 134 ± 64 | 272 ± 81 | |
| Ahrb1Cyp1a2(−/−) | 31.7 ± 11 | 41.9 ± 16 | 95.7 ± 26 | 112 ± 23 | 233 ± 15 | |
| AhrdCyp1a2(−/−) | 34.7 ± 22 | 48.1 ± 29 | 112 ± 56 | 118 ± 49 | 230 ± 59 | |
| Ahrb1Cyp1a2(+/+) | Maternal mammary tissue | 14.1 ± 9.7 | 19.2 ± 13 | 43.5 ± 26 | 48.2 ± 29 | 104 ± 60 |
| AhrdCyp1a2(+/+) | 10.0 ± 0.5 | 12.7 ± 0.7 | 22.7 ± 3.2 | 25.6 ± 2.9 | 59.3 ± 3.3 | |
| Ahrb1Cyp1a2(−/−) | 19.0 ± 7.8 | 24.3 ± 11 | 47.4 ± 21 | 51.3 ± 22 | 103 ± 34 | |
| AhrdCyp1a2(−/−) | 18.4 ± 11 | 22.7 ± 14 | 46.3 ± 27 | 44.0 ± 24 | 75.5 ± 36 | |
| Ahrb1Cyp1a2(+/+) | Pup brain | 5.7 ± 1.4 | 5.9 ± 1.3 | 8.0 ± 2.1 | 6.0 ± 2.4 | 6.9 ± 2.0 |
| AhrdCyp1a2(+/+) | 12.1 ± 3.7 | 12.1 ± 4.1 | 15.1 ± 7.5 | 10.9 ± 5.4 | 13.6 ± 7.6 | |
| Ahrb1Cyp1a2(−/−) | 15.2 ± 1.4 | 13.4 ± 1.3 | 13.0 ± 1.2 | 8.8 ± 0.6 | 12.5 ± 2.2 | |
| AhrdCyp1a2(−/−) | 14.9 ± 2.9 | 12.5 ± 2.8 | 10.9 ± 2.5 | 10.1 ± 2.0 | 9.4 ± 2.7 | |
| Ahrb1Cyp1a2(+/+) | Pup liver | 24.3 ± 1.3 | 18.2 ± 1.0 | 29.9 ± 1.9 | 14.5 ± 0.9 | 23.0 ± 1.7 |
| AhrdCyp1a2(+/+) | 41.2 ± 3.8 | 29.5 ± 5.6 | 38.0 ± 8.6 | 21.2 ± 5.7 | 27.9 ± 7.6 | |
| Ahrb1Cyp1a2(−/−) | 60.8 ± 5.9 | 39.4 ± 3.1 | 43.1 ± 5.6 | 26.4 ± 2.4 | 35.7 ± 2.8 | |
| AhrdCyp1a2(−/−) | 40.2 ± 7.4 | 23.3 ± 5.1 | 28.8 ± 4.3 | 15.8 ± 2.3 | 20.3 ± 2.8 | |
| Ahrb1Cyp1a2(+/+) | Pup subcutaneous adipose tissue | 198 ± 10 | 192 ± 6.9 | 236 ± 14 | 180 ± 6.1 | 183 ± 13 |
| AhrdCyp1a2(+/+) | 342 ± 44 | 311 ± 49 | 336 ± 48 | 259 ± 37 | 291 ± 60 | |
| Ahrb1Cyp1a2(−/−) | 369 ± 23 | 312 ± 21 | 293 ± 15 | 233 ± 13 | 218 ± 3.6 | |
| AhrdCyp1a2(−/−) | 407 ± 26 | 343 ± 22 | 321 ± 17 | 259 ± 17 | 236 ± 17 | |
| Ahrb1Cyp1a2(+/+) | Pup inguinal fat pad | 200 ± 16 | 199 ± 11 | 247 ± 10 | 200 ± 8.9 | 224 ± 8.2 |
| AhrdCyp1a2(+/+) | 386 ± 71 | 341 ± 62 | 355 ± 63 | 289 ± 50 | 302 ± 55 | |
| Ahrb1Cyp1a2(−/−) | 584 ± 94 | 502 ± 74 | 487 ± 75 | 408 ± 58 | 428 ± 100 | |
| AhrdCyp1a2(−/−) | 430 ± 59 | 376 ± 49 | 370 ± 51 | 306 ± 31 | 298 ± 32 |
Note. These tissues were collected 23 days after the second gavage of the mother on PND5.
The distribution of noncoplanar congeners from mother to pup closely matches their chlorination patterns: the more readily metabolized pentachlorobiphenyls were found at lower concentrations in the mothers and higher concentrations in their pups. In contrast, PCB 180, which is known to have an exceptionally long half-life (Oberg et al., 2002), was still found at relatively high concentrations in maternal tissues. It is well known that if adjacent carbon atoms have no chlorine atoms, then that PCB congener is more efficiently metabolized than congeners having no adjacent carbon atoms occupied with chlorine atoms. The pentachlorobiphenyls PCB 105 and 118 reached the highest levels in pups with lower concentrations of PCBs 138, 153, and 180; it is not clear why pentachlorinated congeners would persist in a tissue longer than hexa- and hepta-chlorinated congeners. Ahrb1_Cyp1a2(+/+) dams were more effective at clearing both planar and noncoplanar congeners, continuing a trend first noted at GD11.5 for Ahrb1-containing mice.
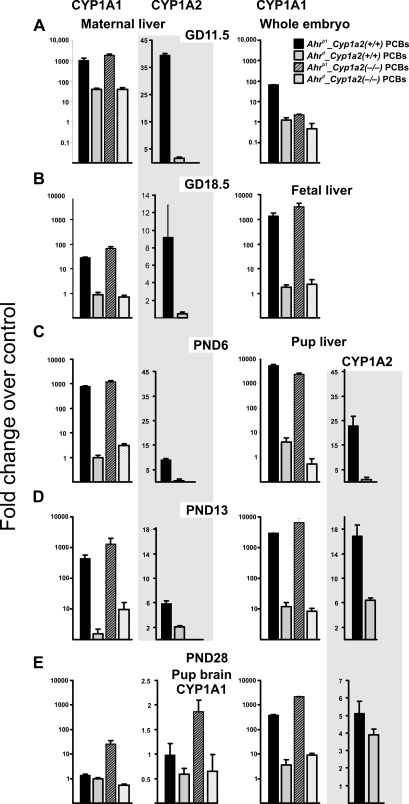
CYP1A1 and CYP1A2 mRNA Levels
Figure 5 depicts CYP1A1 and CYP1A2 mRNA levels—at the same five collection time points as carried out for congener analysis in Tables 5–14 as quantified by qRT-PCR. CYP1A1 mRNA was highly induced in Ahrb1_Cyp1a2(+/+) maternal liver on GD11.5, GD18.5, PND6, and PND13 but not PND28. CYP1A1 mRNA was highly induced in Ahrb1_Cyp1a2(−/−) maternal liver at all five time points, including PND28. CYP1A1 mRNA was not significantly induced in Ahrd_Cyp1a2(+/+) or Ahrd_Cyp1a2(−/−) maternal liver at any of the five time points.
FIG. 5.
CYP1A1 and CYP1A2 mRNA levels, quantified by qRT-PCR. (Top row) GD11.5. CYP1A1 induction was significantly higher in Ahrb1-containing maternal liver compared with Ahrd-containing mothers. CYP1A2 mRNA levels were 40-fold higher in Ahrb1_Cyp1a2(+/+) maternal liver compared with Ahrd_Cyp1a2(+/+) mothers. In this and in subsequent panels, all values represent mean values of fold induction over corn oil–treated B6 controls ± SEM. N = 3–5 per group. Interindividual and group statistics are available upon request. (Second row) GD18.5. CYP1A1 induction was significantly higher in Ahrb1-containing maternal and pup liver compared with Ahrd-containing mothers and pups. CYP1A2 mRNA levels were ∼10-fold higher in Ahrb1_Cyp1a2(+/+) maternal liver compared with Ahrd_Cyp1a2(+/+) mothers and ∼100-fold higher in Ahrb1_Cyp1a2(+/+) pup liver compared with Ahrd_Cyp1a2(+/+) pups. (Third row) PND6. CYP1A1 induction was ∼1000-fold higher in Ahrb1-containing maternal and pup liver compared with Ahrd-containing mothers and pups. CYP1A2 mRNA levels were approximately eightfold higher in Ahrb1_Cyp1a2(+/+) maternal liver compared with Ahrd_Cyp1a2(+/+) mothers and > 10-fold higher in Ahrb1_Cyp1a2(+/+) pup liver compared with Ahrd_Cyp1a2(+/+) pups. (Fourth row) PND13. CYP1A1 induction was significantly higher in Ahrb1-containing maternal and pup liver compared with Ahrd-containing mothers and pups. CYP1A2 mRNA levels were approximately sixfold higher in Ahrb1_Cyp1a2(+/+) maternal liver compared with Ahrd_Cyp1a2(+/+) mothers and > 15-fold higher in Ahrb1_Cyp1a2(+/+) pup liver compared with Ahrd_Cyp1a2(+/+) pups. (Bottom row) PND28. CYP1A1 induction was significantly higher in Ahrb1_Cyp1a2(−/−) maternal liver compared with mothers from the other three genotypes. CYP1A1 induction was highest in Ahrb1-containing pup liver compared with Ahrb1-containing maternal liver and Ahrd-containing pup liver. In pup brain, CYP1A1 was significantly induced in Ahrb1_Cyp1a2(−/−) pups compared with pups from the other three genotypes. CYP1A2 mRNA was significantly higher in both Ahrb1_Cyp1a2(+/+) and Ahrd_Cyp1a2(+/+) pup liver. In this figure and the next, “significantly” denotes p values anywhere between < 0.05 and < 0.0001.
CYP1A1 mRNA showed an increased trend, but not statistically significant (p > 0.05), in Ahrb1_Cyp1a2(+/+) whole embryo on GD11.5 (Fig. 5A). CYP1A1 mRNA was highly induced in Ahrb1_Cyp1a2(+/+) and Ahrb1_Cyp1a2(−/−) fetal liver on GD18.5 (Figs. 5A and 5B), as well as in pup liver on PND6, PND13, and PND28 (Figs. 5C, 5D, and 5E). CYP1A1 mRNA showed an increased trend, but was not statistically significant (p > 0.05), in Ahrb1_Cyp1a2(+/+) pup brain at PND28, whereas CYP1A1 mRNA was statistically significantly induced in Ahrb1_Cyp1a2(−/−) pup brain at PND28 (Fig. 5E).
CYP1A2 mRNA was significantly induced in maternal liver of Ahrb1_Cyp1a2(+/+) at GD11.5, GD18.5, PND6, and PND13 (Figs. 5A, 5B, 5C, and 5D). CYP1A2 mRNA was not detectable in whole embryo at GD11.5 but was significantly increased in Ahrb1_Cyp1a2(+/+) fetal liver at GD18.5 (Fig. 5B). CYP1A2 mRNA was also significantly induced in Ahrb1_Cyp1a2(+/+) pup liver at PND6, PND13, and PND28. CYP1A2 mRNA was not detectable in Ahrd_Cyp1a2(+/+) embryonic or fetal liver (GD11.5 and GD18.5) or neonatal liver on PND6, but there was a trend of increase that was not significant (p > 0.05) at PND13, and the induction was statistically significant (p < 0.05) at PND28.
Evidence of Immunosuppression and AHR Activation
Oral BaP is well known to cause immunosuppression, even at quite low daily doses (Uno et al., 2004). We therefore wondered if the PCB mixture, perhaps especially the coplanar PCBs operating via AHR-mediated downstream effects, might cause toxic effects of the immune system in the neonate and weanling—following the two gavage treatments of the PCB mixture at GD10.5 and PND5.
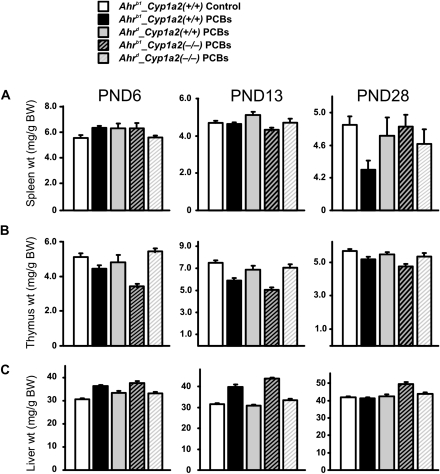
Whereas no significant splenic atrophy was seen at PND6 (Fig. 6A), a statistically significant decrease was observed on PND13 in Ahrb1_Cyp1a2(−/−) pups. Statistically significant splenic atrophy was found at PND28 in Ahrb1_Cyp1a2(+/+) pups, whereas a trend of decreased spleen weight that was not statistically significant (p > 0.05) was noted in all the three other genotypes (Fig. 6A). Immunosuppression in the offspring thus appears to be associated with the Ahrb1 phenotype combined with the presence of maternal hepatic CYP1A2.
FIG. 6.
Spleen, thymus, and liver weights depicted as milligrams per gram body weight (BW). Wet weights (N ≥ 10 per group) were recorded at three postnatal time points. In this and subsequent panels, weights from PCB-treated animals of all four genotypes were compared with weights from corn oil–treated B6 controls at the same ages. Interindividual and group statistics are available upon request. (Top row) There were no significant differences in spleen weights at PND6. At PND13, spleen weights were significantly decreased in Ahrb1_Cyp1a2(−/−) pups. At PND28, spleen weights were significantly decreased in Ahrb1_Cyp1a2(+/+) pups. (Middle row) At PND6, there were significant decreases in thymus weights in Ahrb1-containing mice. At PND13, thymus wet weights were significantly decreased in all genotypes, but the greatest decrease was seen in Ahrb1-containing pups. At PND28, thymus wet weights were significantly decreased only in Ahrb1_Cyp1a2(+/+) pups. (Bottom row) At PND6, there were significant increases in liver weight in all PCB-treated groups compared with controls. At PND13, liver weights were only increased in Ahrb1-containing pups. At PND28, liver weights were increased significantly only in Ahrb1_Cyp1a2(−/−) pups. Liver histology showed no differences in any of the genotypes throughout this time period (data not shown).
Thymic atrophy was statistically significant (p < 0.001) in both Ahrb1-containing mouse lines at PND6, PND13, and PND28 (Fig. 6B). Statistically significant (p < 0.05) decreases in thymus weights were also seen in both Ahrd-containing mouse lines at PND13. The fact that thymic atrophy was not so striking at PND28 suggests that the concentrations of coplanar PCBs might have been diminished by this time—which is consistent with the data in Tables 13 and 14. Thymic atrophy can be caused by chronic AHR activation, which would occur by repeated administration of the PCB mixture. This would explain why the Ahrb1 phenotype is more important than the presence or absence of maternal hepatic CYP1A2.
The liver weight to total body weight ratio was dramatically increased in all four PCB-treated genotypes at PND6 (p < 0.001) compared with that in controls receiving no PCBs (Fig. 6C); this ratio is also caused by chronic AHR activation. The liver weight to total body weight ratio was significantly (p < 0.001) increased in the two Ahrb1-containing mouse lines at PND13 (p < 0.001) but only in Ahrb1_Cyp1a2(−/−) weanlings at PND28 (p < 0.001).
Mothers at PND28 were sacrificed and organ weights obtained. Spleen weights were trending downward in Cyp1a2(−/−) mothers, but the data were not significantly different (p > 0.05) from that in Cyp1a2(+/+) mothers. Thymus weights were trending lower, and liver weight to total body weight ratios were trending upward in Ahrb1-containing mothers, but again these results were not significantly different (p > 0.05) from that in Ahrd-containing mothers (data not illustrated).
DISCUSSION
In the present study, we have shown that genetic differences in AHR affinity, as well as the presence or absence of the CYP1A2 enzyme, influence the maternal-fetal unit—with regard to PCB congener pharmacokinetics and degrees of toxic response to the developing neonate and weanling mouse. Our initial goal was to decide upon a PCB mixture that not only reflected that found in human breast milk and other tissues and a dose that would activate AHR but also a PCB dosage exposure that was neither substantially lethal nor causing overt birth defects such as cleft palate and hydronephrosis. Our long-range goal was to study behavioral phenotypes in these offspring when they reached adulthood at PND60, and this is the subject of the next paper (Curran, Genter, Patel, Vorhees, Williams, and Nebert, in preparation).
We were aware of studies using Aroclor mixtures that have repeatedly described neurological deficits across rodent species (Chishti et al., 1996; Kang et al., 2002). However, when attempts were made to identify individual congeners responsible for these effects, it became clear that single congeners did not produce the wide-ranging effects reported when studying Aroclor mixtures in laboratory animal studies, as well as effects observed in human cohorts (Ulbrich and Stahlmann, 2004); this suggests that a mixture of congeners, rather than individual congeners, might be responsible for PCB-induced developmental neurotoxicity. In fact, other researchers reported that a mixture of coplanar plus noncoplanar PCB congeners was required to elicit changes in thyroid hormone signaling in the developing brain (Gauger et al., 2004). For all these reasons, we decided to use the eight PCB complex mixture listed in Table 1, with chemical structures depicted in Figure 1.
Preliminary studies also confirmed that we needed to provide a booster dose of the PCB mixture—in order to maintain high CYP1A1 mRNA inducibility (as a measure of chronic AHR activation) and sufficient PCB congener concentrations in the various tissues of the pup. Hence, we chose the regimen of one dose of PCBs given on GD10.5 and a second dose on PND5; collection time points for analysis included GD11.5, GD18.5, PND6, PND13, and PND28. The time line for our entire experimental paradigm is summarized in Figure 2.
We then wished to test differences in response to the PCB mixture in mice having the high-affinity versus poor-affinity AHR combined with the presence versus absence of CYP1A2. Previous work from this laboratory had convincingly shown that presence of maternal hepatic CYP1A2 was able to sequester TCDD such that the fetus was protected approximately sixfold more than fetuses from mothers lacking hepatic CYP1A2; this led, in Cyp1a2(−/−) mothers, to an approximately six times larger dose of TCDD required to cause cleft palate and hydronephrosis (Dragin et al., 2006). It is also well known that Ahrd-containing mice require a 15- to 20-fold higher dose of TCDD in order to achieve the same level of CYP1 inducibility as that seen in Ahrb1-containing mice (Poland et al., 1974). Combining these facts, we hypothesized that the Ahrb1_Cyp1a2(−/−) mother-fetal unit would be most vulnerable, the Ahrd_Cyp1a2(−/−) unit would be almost as vulnerable, the Ahrb1_Cyp1a2(+/+) unit would be most resistant, and the Ahrd_Cyp1a2(+/+) would be almost as resistant—with regard to toxicity induced by the PCB mixture (Table 3).
True to our predictions, small but statistically significant PCB-induced decreases in newborn birth weights were found in both Cyp1a2(−/−)-containing lines (Table 4) and a small but statistically significant PCB-induced decreased growth rate was seen in Ahrb1_Cyp1a2(−/−) pups between PND1 and PND28 (Fig. 3). In general, noncoplanar congener concentrations in mother and offspring tissues between GD11.5 and PND28 were not associated with the Ahr or Cyp1a2 genotype (Tables 5–14)—except for Ahrb1_Cyp1a2(−/−) PND13 pups exhibiting higher levels in all tissues (Table 12), all five noncoplanar congeners highest in Ahrb1_Cyp1a2(−/−) PND28 pup inguinal fat pad (Table 14), and PCB 105 and PCB 118 highest in Ahrd_Cyp1a2(−/−) PND28 pup subcutaneous fat (Table 14).
However, the coplanar PCB congeners did show an intriguing pattern: at various collection time points, the Ahrd_Cyp1a2(−/−) maternal brain, liver, adipose, and mammary tissues (as well as placenta, fetal, and neonatal tissues) retained the most coplanar PCB 77—until PND28 when most coplanar congeners had been metabolically cleared from the tissues (Table 13). Thus, protection of the fetus by maternal hepatic CYP1A2 in Ahrb1_Cyp1a2(+/+) mice, after the PND5 dose given to the mother, continues through lactation and is associated with the high-affinity AHR. Our data are consistent with previous findings that the greatest exposure to pups is during lactation—not during gestation (Seegal et al., 1997).
Figure 5 depicts CYP1A1 and CYP1A2 mRNA levels—at the same five collection time points as carried out for congener analysis in Tables 5–14—as quantified by qRT-PCR. CYP1A1 mRNA was highly induced in Ahrb1_Cyp1a2(+/+) maternal liver on GD11.5, GD18.5, PND6, and PND13 but not PND28; disappearance on PND28 is consistent with the coplanar PCBs being mostly sequestered by maternal liver CYP1A2. In support of this hypothesis, CYP1A1 mRNA was highly induced in Ahrb1_Cyp1a2(−/−) maternal liver at all five time points, including PND28, because of no maternal liver CYP1A2 to tie up the coplanar PCB inducers. CYP1A1 mRNA was not significantly induced in Ahrd_Cyp1a2(+/+) or Ahrd_Cyp1a2(+/+) maternal liver at any of the five time points; these observations are consistent with what is generally seen in Ahrd-containing mouse lines (Nebert et al., 1972).
Western blots of CYP1A1 and CYP1A2 protein could have been carried out in all the tissues in which qRT-PCR measurements of CYP1A1 and CYP1A2 mRNA levels were obtained because it is always possible that protein levels might not reflect mRNA levels. However, in several dozen studies from this laboratory (Uno et al., 2008 and references therein), there has never been an instance in which CYP1 protein levels did not accurately reflect CYP1 mRNA levels.
An important next step in future studies would be to determine the concentration, identity, and transfer of PCB metabolites produced during gestation and lactation. Does the reduction in lower molecular weight congeners represent true clearance or simply the biotransformation into potentially toxic metabolites (Kimura-Kuroda et al., 2005)? This is an important question because hydroxylated and methylsulfonated metabolites can cross the placenta (Park et al., 2009; Soechitram et al., 2004), and some have reported half-lives nearly as long as the parent congeners (Hovander et al., 2006; Linderholm et al., 2010). The small amounts of tissue available in a rodent study precluded the analysis at this time, but the use of radiolabeled congeners offers an option for tracking such metabolites more effectively.
CYP1A1 mRNA showed an increased trend that was not statistically significant (p > 0.05), in Ahrb1_Cyp1a2(+/+) pup brain at PND28, whereas CYP1A1 mRNA was still statistically significantly induced in Ahrb1_Cyp1a2(−/−) pup brain at PND28 (Fig. 5E). This observation is consistent with hepatic CYP1A2 acting as a “sink” (Dragin et al., 2006)—when maternal CYP1A2 is absent, more PCBs reach the offspring.
The Figure 5 data confirm that the effect of CYP1A induction by coplanar PCBs can be seen not only in maternal liver but also in embryonic, fetal, and neonatal liver, as well as in PND28 brain. In other words, we achieved our goal of the PCB mixture regimen having a significant effect on the maternal-fetal unit—including AHR activation in postnatal brain. These data give us encouragement to proceed with the behavioral studies (to be described elsewhere).
Interestingly, decreases in PND13 and PND28 spleen weight (Fig. 6A)—as well as PND6, PND13, and PND28 thymus weight (Fig. 6B)—are more closely related to the Ahr genotype than the presence or absence of CYP1A2. This finding is consistent with recent studies (Shi et al., 2010) showing that intestinal inducible CYP1A1 is by far the most critical in detoxifying oral BaP—thereby preventing this PAH from distributing itself to distal tissues. Distal tissues, in this case, would include the intrauterine contents as well as pups receiving PCB-laced milk via lactation when the mother receives oral PAHs; distal tissues thus would also include pup spleen. Our present study indicates that this phenomenon extends from BaP to PCBs that are able to be metabolized by CYP1 enzymes; this finding further indicates that intestinal AHR affinity (and therefore inducible gut CYP1A1) is more crucial than the effect of maternal liver CYP1A2 acting as a sink to sequester AHR ligands such as coplanar PCBs. Unlike BaP, however, some more highly chlorinated PCBs (e.g., PCB 126) are not good CYP1 substrates, although they are excellent inducers of the CYP1 enzymes. Thus, an alternative explanation includes the possibility that persistent induction of CYP1 enzymes could lead to oxidative stress, thereby imposing more toxicity to the mice.
That pup splenic atrophy is more closely related to the Ahr genotype—than the presence or absence of CYP1A2—would further suggest that both Cyp1a2(+/+) and Cyp1a2(−/−) mothers received sufficient amounts of coplanar PCBs to elicit immunosuppression and activate the AHR receptor. Presumably, consistent with the (Dragin et al., 2006) study, at some lower levels of coplanar PCBs being administered, we would be able to see an effect in pups of Cyp1a2(−/−) mothers but no effect in pups of Cyp1a2(+/+) mothers. But, perhaps not; a dose-response study would clarify this question.
The liver weight to total body weight ratio was dramatically increased—a sign of chronic AHR activation—in all four PCB-treated genotypes at PND6 compared with controls receiving no PCBs (Fig. 6C). The liver weight to total body weight ratio was significantly increased in the two Ahrb1-containing mouse lines at PND13 but only in Ahrb1_Cyp1a2(−/−) weanlings at PND28. Intriguingly, this is the mouse line that we had predicted would be most vulnerable to the PCBs' regimen (Table 2), and this is the same mouse line that had the highest amounts of coplanar PCB 126 and PCB 169 in PND28 pup inguinal fat (Tables 13 and 14).
Finally, can we extrapolate our mouse data to human populations? One might query whether the combination—of amount of exposure to coplanar PCBs, > 60-fold variation in basal and inducible hepatic CYP1A2 levels, and > 12-fold differences in AHR affinity—might be relevant to human risk assessment of PCB-induced birth defects. The answer to this question is not yet proven, but the present study, especially combined with the previous study (Dragin et al., 2006), provides the basis for speculation about an “at-risk” subset in human populations. For example, the genotype of the affected fetus need not necessarily carry a teratogenic risk; rather, instead, a susceptible maternal genotype might be more crucial to risk of birth defects.
Hence, the highest risk for PCB-induced birth defects is likely to be in one whose maternal liver has genetically very low CYP1A2 activity, combined with a fetus who expresses the highest AHR inducibility. This notion follows from the observation that, although fetal CYP1A2 does not contribute to PCB-induced teratogenesis because it is not expressed in the embryo or fetus (Nebert, 1989), fetal high-affinity AHR is probably essential for coplanar PCB-induced toxicity and teratogenesis, just as it is for TCDD-mediated teratogenesis (Peters et al., 1999; Thomae et al., 2004).
It should be noted that, for human cohort studies, determination of the precise level of environmental PCB exposure would be expected to be a confounding factor in such genotype-phenotype association studies. In addition, to date, no DNA variant sites in or near either the human CYP1A2 or the AHR gene have been shown unequivocally to reflect variations in the CYP1A2 or AHR phenotype (Jiang et al., 2006; Nebert et al., 2004).
FUNDING
National Institutes of Health Grants (R21 ES015335 to D.W.N., C.V.V., M.T.W.; T32 ES007051 to C.P.C.; P30 ES06096 to D.W.N.).
Acknowledgments
We thank our colleagues for valuable discussions and help during the course of this work and careful readings of the manuscript—especially Ying Chen (now at University of Colorado, Denver), Larry G. Hansen (University of Illinois, Professor Emeritus), and many colleagues including John Lipscomb at the U.S. Environmental Protection Agency and Stephen Winslow at Shaw Environmental (who allowed C.P.C. to use their equipment). Portions of this work were presented at the Society of Toxicology 46th Annual Meeting in March 2007, Charlotte, NC, and the Teratology Society 49th Annual Meeting in June 2009, Puerto Rico.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) ToxFaq: Polychlorinated Biphenyls. 2001. U.S. Department of Health and Human Services, Public Health Service. Atlanta, GA. [Pamphlet] [Google Scholar]
- Battershill JM. Review of the safety assessment of polychlorinated biphenyls (PCBs) with particular reference to reproductive toxicity. Hum. Exp. Toxicol. 1994;13:581–597. doi: 10.1177/096032719401300901. [DOI] [PubMed] [Google Scholar]
- Bhavsar SP, Hayton A, Reiner EJ, Jackson DA. Estimating dioxin-like polychlorinated biphenyl toxic equivalents from total polychlorinated biphenyl measurements in fish. Environ. Toxicol. Chem. 2007;26:1622–1628. doi: 10.1897/06-621r.1. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–660. [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Costabeber I, Dos Santos JS, Xavier AA, Weber J, Leaes FL, Junior SB, Emanuelli T. Levels of polychlorinated biphenyls (PCBs) in meat and meat products from the state of Rio Grande do Sul, Brazil. Food Chem. Toxicol. 2006;44:1–7. doi: 10.1016/j.fct.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Curran CP, Miller KA, Dalton TP, Vorhees CV, Miller ML, Shertzer HG, Nebert DW. Genetic differences in lethality of newborn mice treated in utero with coplanar versus non-coplanar hexabromobiphenyl. Toxicol. Sci. 2006;89:454–464. doi: 10.1093/toxsci/kfj048. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ. Health Perspect. 1999;107:823–828. doi: 10.1289/ehp.99107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Dalton TP, Miller ML, Shertzer HG, Nebert DW. For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. J. Biol. Chem. 2006;281:18591–18600. doi: 10.1074/jbc.M601159200. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Perspect. 2004;112:516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL, Yu ML, Hsu CC, Rogan WJ. Chloracne, goiter, arthritis, and anemia after polychlorinated biphenyl poisoning: 14-year follow-up of the Taiwan Yucheng cohort. Environ. Health Perspect. 1999;107:715–719. doi: 10.1289/ehp.99107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am. J. Ind. Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hakk H, Diliberto JJ, Birnbaum LS. The effect of dose on 2,3,7,8-TCDD tissue distribution, metabolism and elimination in Cyp1a2(-/-) knockout and C57BL/6N parental strains of mice. Toxicol. Appl. Pharmacol. 2009;241:119–126. doi: 10.1016/j.taap.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fangstrom B, Kocan A, Petrik J, Trnovec T, Bergman A. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environ. Sci. Technol. 2006;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Imbeault P, Chevrier J, Dewailly E, Ayotte P, Despres JP, Mauriege P, Tremblay A. Increase in plasma pollutant levels in response to weight loss is associated with the reduction of fasting insulin levels in men but not in women. Metabolism. 2002;51:482–486. doi: 10.1053/meta.2002.31338. [DOI] [PubMed] [Google Scholar]
- Imsilp K, Wiedenmann L, Bordson GO, Morrow CK, Cope R, Hansen LG. Time- and tissue-dependent polychlorinated biphenyl residues in hairless mice after exposure to polychlorinated biphenyl-contaminated soil. Arch. Environ. Contam. Toxicol. 2005;49:105–118. doi: 10.1007/s00244-004-0116-y. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Dragin N, Jorge-Nebert LF, Martin MV, Guengerich FP, Aklillu E, Ingelman-Sundberg M, Hammons GJ, Lyn-Cook BD, Kadlubar FF, et al. Search for an association between the human CYP1A2 genotype and CYP1A2 metabolic phenotype. Pharmacogenet. Genomics. 2006;16:359–367. doi: 10.1097/01.fpc.0000204994.99429.46. [DOI] [PubMed] [Google Scholar]
- Kang JH, Jeong W, Park Y, Lee SY, Chung MW, Lim HK, Park IS, Choi KH, Chung SY, Kim DS, et al. Aroclor 1254-induced cytotoxicity in catecholaminergic ‘CATH.a' cells related to the inhibition of NO production. Toxicology. 2002;177:157–166. doi: 10.1016/s0300-483x(02)00142-7. [DOI] [PubMed] [Google Scholar]
- Karlen Y, McNair A, Perseguers S, Mazza C, Mermod N. Statistical significance of quantitative PCR. BMC Bioinformatics. 2007;8:131. doi: 10.1186/1471-2105-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, Kuroda Y. Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Brain Res. Dev. Brain Res. 2005;154:259–263. doi: 10.1016/j.devbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, Nebert DW. Cyp1a2(-/-) null mutant mice develop normally but show deficient drug metabolism. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm L, Biague A, Mansson F, Norrgren H, Bergman A, Jakobsson K. Human exposure to persistent organic pollutants in West Africa—a temporal trend study from Guinea-Bissau. Environ. Int. 2010;36:675–682. doi: 10.1016/j.envint.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Masuda Y. Fate of PCDF/PCB congeners and change of clinical symptoms in patients with Yusho PCB poisoning for 30 years. Chemosphere. 2001;43:925–930. doi: 10.1016/s0045-6535(00)00452-5. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Lilienthal H, Hoving S, van den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Developmental exposure to 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl (4-OH-CB107): long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol. Sci. 2004;82:207–218. doi: 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- Nebert DW. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit. Rev. Toxicol. 1989;20:153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Goujon FM, Gielen JE. Aryl hydrocarbon hydroxylase induction by polycyclic hydrocarbons: autosomal dominant trait in the mouse. Nat. New Biol. 1972;236:107–110. doi: 10.1038/newbio236107a0. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Negri E, Bosetti C, Fattore E, La VC. Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: a systematic review of the epidemiological evidence. Eur. J. Cancer Prev. 2003;12:509–516. doi: 10.1097/00008469-200312000-00010. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Oberg M, Sjodin A, Casabona H, Nordgren I, Klasson-Wehler E, Hakansson H. Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl in the rat. Toxicol. Sci. 2002;70:171–182. doi: 10.1093/toxsci/70.2.171. [DOI] [PubMed] [Google Scholar]
- Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ. Health Perspect. 2009;117:1600–1606. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott BD. Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor Ahr(-/-) null mice. Toxicol. Sci. 1999;47:86–92. doi: 10.1093/toxsci/47.1.86. [DOI] [PubMed] [Google Scholar]
- Petrik J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J. Serum PCBs and organochlorine pesticides in Slovakia: age, gender, and residence as determinants of organochlorine concentrations. Chemosphere. 2006;65:410–418. doi: 10.1016/j.chemosphere.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E. Chlorinated biphenyl induction of aryl hydrocarbon hydroxylase activity: a study of the structure-activity relationship. Mol. Pharmacol. 1977;13:924–938. [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the mouse aryl hydrocarbon receptor. Mol. Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- Poland AP, Glover E, Robinson JR, Nebert DW. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically “nonresponsive” to other aromatic hydrocarbons. J. Biol. Chem. 1974;249:5599–5606. [PubMed] [Google Scholar]
- Sartor MA, Schnekenburger M, Marlowe JL, Reichard JF, Wang Y, Fan Y, Ma C, Karyala S, Halbleib D, Liu X, et al. Genomewide analysis of aryl hydrocarbon receptor binding targets reveals an extensive array of gene clusters that control morphogenetic and developmental programs. Environ. Health Perspect. 2009;117:1139–1146. doi: 10.1289/ehp.0800485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Sweeney AM, Gardiner JC, Humphrey HE, McCaffrey RJ, Gasior DM, Srikanth KR, Budd ML. Neuropsychological assessment of an aging population of Great Lakes fisheaters. Toxicol. Ind. Health. 1996;12:403–417. doi: 10.1177/074823379601200312. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol. Appl. Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Selgrade MK. Immunotoxicity: the risk is real. Toxicol. Sci. 2007;100:328–332. doi: 10.1093/toxsci/kfm244. [DOI] [PubMed] [Google Scholar]
- Shi Z, Dragin N, Galvez-Peralta M, Jorge-Nebert LF, Miller ML, Wang B, Nebert DW. Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Mol. Pharmacol. 2010;78:46–57. doi: 10.1124/mol.110.063438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Britt JK, James RC. Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul. Toxicol. Pharmacol. 2007;48:148–170. doi: 10.1016/j.yrtph.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Soechitram SD, Athanasiadou M, Hovander L, Bergman A, Sauer PJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ. Health Perspect. 2004;112:1208–1212. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Mergler D, Baldwin M, de GS, Smargiassi A, Lafond J. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ. Health Perspect. 2005;113:1039–1045. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SW, Zoeller RT. Integrating basic research on thyroid hormone action into screening and testing programs for thyroid disruptors. Crit. Rev. Toxicol. 2007;37:5–10. doi: 10.1080/10408440601123396. [DOI] [PubMed] [Google Scholar]
- Thomae TL, Glover E, Bradfield CA. A maternal Ahr(-/-) null genotype sensitizes embryos to chemical teratogenesis. J. Biol. Chem. 2004;279:30189–30194. doi: 10.1074/jbc.M403690200. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch. Toxicol. 2004;78:252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, Nebert DW. Basal and inducible CYP1 mRNA quantitation and protein localization throughout mouse gastrointestinal tract. Free Radic. Biol. Med. 2008;44:570–583. doi: 10.1016/j.freeradbiomed.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Birgelen AP, Smit EA, Kampen IM, Groeneveld CN, Fase KM, Van der KJ, Poiger H, Van den BM, Koeman JH, Brouwer A. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur. J. Pharmacol. 1995;293:77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De VM, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Su PH, Jong SB, Guo YL, Chou WL, Papke O. In utero exposure to dioxins and polychlorinated biphenyls and its relationship to thyroid function and growth hormone in newborns. Environ. Health Perspect. 2005;113:1645–1650. doi: 10.1289/ehp.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Levels of PCBs, PCDDs and PCDFs in human milk. Second round of WHO-coordinated exposure study. 6-1-1996. 1996. World Health Organization, European Centre for Environment and Health, Copenhagen, Denmark. [Pamphlet] [Google Scholar]