Abstract
Mode of action (MOA) analysis provides a systematic description of key events leading to adverse health effects in animal bioassays for the purpose of informing human health risk assessment. Uncertainties and data gaps identified in the MOA analysis may also be used to guide future research to improve understanding of the MOAs underlying a specific toxic response and foster development of toxicokinetic and toxicodynamic models. An MOA analysis, consistent with approaches outlined in the MOA Framework as described in the Guidelines for Carcinogen Risk Assessment, was conducted to evaluate small intestinal tumors observed in mice chronically exposed to relatively high concentrations of hexavalent chromium (Cr(VI)) in drinking water. Based on review of the literature, key events in the MOA are hypothesized to include saturation of the reductive capacity of the upper gastrointestinal tract, absorption of Cr(VI) into the intestinal epithelium, oxidative stress and inflammation, cell proliferation, direct and/or indirect DNA modification, and mutagenesis. Although available data generally support the plausibility of these key events, several unresolved questions and data gaps were identified, highlighting the need for obtaining critical toxicokinetic and toxicodynamic data in the target tissue and in the low-dose range. Experimental assays that can address these data gaps are discussed along with strategies for comparisons between responsive and nonresponsive tissues and species. This analysis provides a practical application of MOA Framework guidance and is instructive for the design of studies to improve upon the information available for quantitative risk assessment.
Keywords: risk assessment, carcinogenesis, hexavalent chromium, Cr(VI), mode of action
Mode of action (MOA) analysis is a systematic description of likely key events that lead to adverse health effects following exposure to environmental toxicants (Dellarco and Wiltse, 1998). The U.S. Environmental Protection Agency (EPA) has formally described MOA analysis in the context of cancer risk assessment in an MOA Framework described in the “Guidelines for Carcinogen Risk Assessment” (U.S. EPA, 2005), and others have expanded this concept to the assessment of noncancer endpoints (Bogdanffy et al., 2001; Julien et al., 2009; Meek et al., 2003; Seed et al., 2005; U.S. EPA, 2005). MOA analysis has historically been performed to judge the likelihood that adverse outcomes observed in animals will occur in humans, as well as to support the choice of low-dose extrapolation approaches. MOA analysis may also identify data gaps that can be used to design studies aimed at improving or further substantiating the hypothesized key events in an MOA.
Hexavalent chromium (Cr(VI)) provides a useful case study for applying MOA analysis for purposes of identifying data gaps to guide future research. Our goal was to critically examine the peer-reviewed literature to identify articles pertinent to the oral carcinogenicity of Cr(VI) for purposes of proposing a plausible MOA hypothesis underlying the carcinogenic response observed in mice exposed to Cr(VI) in drinking water for 2 years (NTP, 2008b; Stout et al., 2009a). This MOA analysis was conducted in accordance with the U.S. EPA MOA Framework (U.S. EPA, 2005) and identified key data gaps that could be used to guide future research on Cr(VI). This effort, along with a review of key literature related to key events in the hypothesized MOA, is described in detail in this paper.
BACKGROUND
Chromium is a naturally occurring element that primarily exists in two oxidized states: Cr(VI) and trivalent chromium (Cr(III)). The latter form is considered a micronutrient with a putative biological role in insulin sensitivity (Anderson, 2000) and is reported to exhibit limited acute and chronic toxicity and has been shown to be noncarcinogenic (IARC, 1990; NTP, 2008a; Stout et al., 2009b). In contrast, Cr(VI) is a strong oxidizing agent and is acutely toxic under certain exposure scenarios. Based on evidence of lung cancer among workers in certain industries with occupational exposure to Cr(VI), this compound has been classified as a known human carcinogen by inhalation routes of exposure (IARC, 1990). Because of the differences in toxicity by species, health risk assessment and heath-based environmental standards for chromium are typically valence-specific. Further, valence state and environmental chemistry—specifically, oxidation-reduction (redox) chemistry, inform the mobility, bioavailability, bioaccessibility, and toxicity of chromium in the environment (James et al., 1997). Much of the Cr(VI) in the environment is attributable to anthropogenic sources—due primarily to applications in wood preservatives, pigments, anticorrosive primers, metal plating and releases as a result of some ferrometal and stainless steel operations, and the combustion of fossil fuel (IARC, 1990). Cr(VI) also occurs naturally and is prevalent in groundwater in certain areas (Oze et al., 2007). As a result, human exposures are likely to be widespread. For example, Cr(VI) exists at low concentrations in approximately one-third of the California drinking water supply (CDHS, 2009).
Until recently, Cr(VI) was not considered to pose a cancer risk by the oral route of exposure. In fact, the EPA Integrated Risk Information System file for Cr(VI), prepared in 1998, states that “[n]o data were located in the available literature that suggested that Cr(VI) is carcinogenic by the oral route of exposure” (U.S. EPA, 1998). In promulgating the federal drinking water standard (maximum contaminant level) for total chromium, the U.S. EPA concluded that “the body's normal physiology provides detoxification for CrVI which provides protection from the oral toxicity” (U.S. EPA, 1991). Reduction of Cr(VI) to Cr(III) prior to absorption is thought to offer protection against Cr(VI) carcinogenicity from oral intake at environmentally relevant exposure levels (De Flora, 2000; Proctor et al., 2002a). However, absorption from the gastrointestinal (GI) tract and reduction in the GI lumen are recognized to be competing kinetic processes (O'Flaherty et al., 2001). Thus, several authors have argued that Cr(VI) could pose a cancer risk from ingestion at sufficient doses (Costa and Klein, 2006; McCarroll et al., 2010; Sedman et al., 2006).
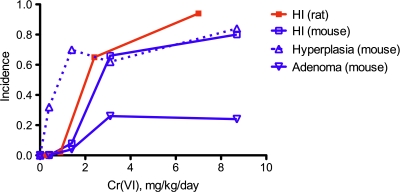
In 2008, the National Toxicology Program (NTP) completed a 2-year cancer bioassay for Cr(VI) in drinking water. In this study, investigators found that Cr(VI) caused tumors in the small intestines of mice and the oral mucosa of rats at exposures of 20–180 mg/l Cr(VI) in the form of sodium dichromate dihydrate (SDD) (NTP, 2008b; Stout et al., 2009a). Statistically significant increases relative to concurrent controls were observed at ≥ 60 mg/l Cr(VI). Interestingly, the tumors occurred along the alimentary canal (portal of entry) and were characterized as relatively rare in both species and were generally only found near the termination of the study (NTP, 2008b; Stout et al., 2009a). Because Cr(VI) was administered at concentrations that greatly exceed expected human exposures (Supplementary fig. 1), there are many questions regarding dose-response and risk to humans at environmentally relevant exposures. The small intestine tumors were observed only in mice and were dose dependent, and the tumor type and incidence followed a logical pattern from benign adenoma to malignant carcinoma. The tumors were preceded in dose and time by diffuse hyperplasia, whereas comparable responses were not observed in the rat small intestine (Table 1). The oral cavity tumors in rats were not preceded by any obvious adverse pathology. Considering that many mutagens induce tumors at multiple sites and with relatively short time to tumor development (McCarroll et al., 2010; U.S. EPA, 2007), the hyperplasia associated with the small intestinal tumors are suggestive of a nonmutagenic MOA, where mutation is unlikely to be the initiating key event in the intestinal tumorigenesis. However, the intracellular reduction of Cr(VI) can form chemical species that can potentially react directly with DNA (O'Brien et al., 2003; Salnikow and Zhitkovich, 2008; Zhitkovich, 2005). Hence, further studies are necessary to elucidate the MOA for these tumors.
TABLE 1.
Summary of Select Neoplastic and Nonneoplastic Lesions in the NTP 2-Year Bioassay. Incidence of Nonneoplastic and Neoplastic Lesions in the Intestine and Oral Cavity
| Cr(VI) drinking water concentration, mg/l |
|||||
| 0 | 5 | 20 | 60 | 180 | |
| Findings in the small intestines of female mice (and rats)a | |||||
| Duodenum | |||||
| Histiocytic infiltration | 0/50 (0/46)b | 0/50 (0/49) | 4/50 (1/48) | 33/50** (30/46)** | 40/50** (47/50)** |
| Focal hyperplasia | 0/50 | 0/50 | 1/50 | 2/50 | 0/50 |
| Diffuse hyperplasia | 0/50 | 16/50** | 35/50** | 31/50** | 42/50** |
| Adenoma | 0/50 | 0/50 | 2/50# | 13/50*** | 12/50*** |
| Carcinoma | 0/50 | 0/50 | 0/50 | 1/50# | 6/50* |
| Jejunumc | |||||
| Histiocytic infiltration | 0/50 | 0/50 | 0/50 | 2/50 | 8/50** |
| Diffuse hyperplasia | 0/50 | 2/50 | 1/50 | 0/50 | 8/50** |
| Adenoma | 0/50 | 1/50 | 0/50 | 2/50# | 5/50* |
| Carcinoma | 1/50 | 0/50 | 2/50# | 2/50# | 1/50 |
| Combined tumors in small intestined | 1/50 | 1/50 | 4/50# | 17/50*** | 22/50*** |
| Findings in rat oral mucosa or tonguee | |||||
| Female, combined papilloma and carcinoma | 1/50 | 1/50 | 0/50 | 2/50# | 11/50** |
| Male, combined papilloma and carcinoma | 0/50 | 1/50 | 0/49 | 0/50 | 7/49** |
Note. Detailed results and statistical analyses can be found in NTP (2008b) and Stout et al. (2009a).
For brevity, the intestinal results for males are not shown. Similar positive and negative findings were reported for male mice and rats, respectively.
Histiocytic infiltration was the only histopathological response reported in the male and female rat duodenum.
No lesions were reported for the jejunum in male and female rats.
No intestinal tumors were observed in male or female rats.
No tumors were observed in the mouse oral mucosa or tongue.
*p ≤ 0.05, **p ≤ 0.01 by poly-3 test, ***p ≤ 0.001 by poly-3 test; #exceeded historical control range.
APPLICATION OF MOA AND HUMAN RELEVANCE FRAMEWORKS
U.S. EPA (2005) defines MOA as “a sequence of key events and processes, starting with interaction of an agent with a cell, proceeding through operational and anatomical changes, and resulting in cancer formation.” Although there is some ambiguity concerning whether the above definition excludes pharmacokinetics from the MOA, we concur with others who clearly include toxicokinetics as key events in the MOA (Julien et al., 2009). Because of the known dependence of biochemical and toxicological properties on chromium speciation, it is impossible to place tissue responses from different chromium compounds into their proper context without consideration of toxicokinetics to arrive at comparative internal doses. EPA defines a key event as “an empirically observable and quantifiable precursor step that is itself a necessary element of the MOA or is a biologically based marker for such an element.” Importantly, the MOA Framework stresses that the MOA for each tumor site should be evaluated and that:
An agent may work by more than one mode of action, both at different sites and at the same tumor site. Thus the mode of action and human relevance cannot necessarily be generalized to other toxic endpoints or tissues or cell types without additional analyses.
Thus, it may be inappropriate to extrapolate findings from various systems, tissues, and cell types (e.g., lung carcinoma) to a previously unrecognized tumor such as intestinal carcinoma; but rather data should be evaluated (and obtained if necessary) for tumors of interest.
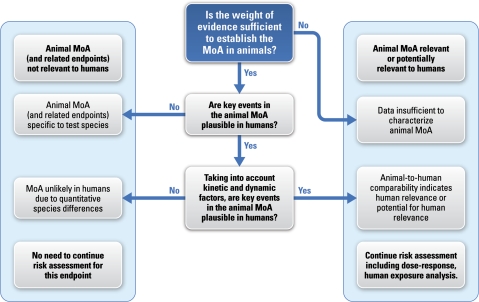
In addition to MOA analysis, it is instructive to consider the human relevance framework that has been developed and described by several investigators (Meek et al., 2003; Seed et al., 2005). As shown in Figure 1, the first step in the human relevance framework is to consider whether the MOA in animals is well established. Considering that the MOA underlying the development of intestinal tumors in mice is not known, the highlighted blue box represents the current status for understanding human relevance of these tumors. Nevertheless, there are human data available to begin considering the plausibility and relevance of our hypothesized MOA for humans.
FIG. 1.
General schematic of the human relevance framework as developed by Meek et al. (2003) and revised by Seed et al. (2005). The box highlighted in blue represents the current status of the MOA for intestinal tumors in animals. Adapted from Seed et al. (2005).
Summary of the Key Events in the Hypothesized Cr(VI) MOA for Mouse Intestinal Tumors
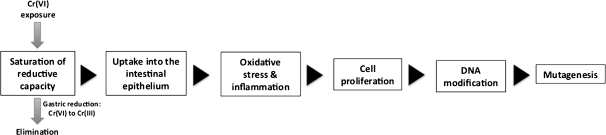
We postulate that the MOA for mouse intestinal tumors is a combination of proliferative pressure and direct and/or indirect (e.g., epigenetic) DNA modification. The MOA is hypothesized to be a direct consequence of exceeding the extracellular reductive capacity of the lumen of the upper GI tract (i.e., mouth and stomach). Sustained saturation of the reductive capacity of the upper GI tract results in continuous delivery of Cr(VI) to the intestinal lumen at doses sufficient to initiate subsequent key events. The second proposed key event is absorption of Cr(VI) from the intestinal lumen into the epithelial tissues of the small intestine. Both the first and the second key events are empirically measurable pharmacokinetic parameters that are critical to understanding target tissue dose and construction of pharmacokinetic and pharmacodynamic models. It is important to consider that NTP also recently conducted a 2-year bioassay for Cr(III), administered as chromium picolinate, and neither neoplastic nor nonneoplastic effects were observed despite very high doses (NTP, 2008b; Stout et al., 2009b). Hence, if Cr(VI) is reduced to Cr(III) before cellular absorption, toxicity is unlikely to occur. Following absorption into epithelial tissues, the third proposed key event is oxidative stress, which in turn is expected to lead to tissue damage and inflammation. The fourth hypothesized key event is cell proliferation, consistent with the diffuse hyperplasia in the NTP studies (NTP, 2007, 2008b). The fifth hypothesized key event involves DNA modification that may occur as a result of intracellular reduction of Cr(VI) to potentially DNA reactive and oxidative species, which in turn can lead to genetic or epigenetic changes to DNA. DNA modification might also occur as a result of prolonged proliferative pressure. Finally, the sixth hypothesized key event involves mutagenesis. The sequence of key events in our hypothesized MOA is outlined in Figure 2. Support for each proposed key event is provided below. It should be noted that although these key events are presented in a linear fashion, it is recognized that some events may occur concomitantly. Finally, it should be noted that this MOA differs from that recently proposed by McCarroll et al., (2010) which suggests that DNA damage and mutagenesis occur prior to cell proliferation.
FIG. 2.
Hypothesized MOA for Cr(VI) carcinogenesis in the GI tract.
Description of the Key Events in the Proposed MOA for Small Intestine Tumors
Key Event 1: Saturation of Cr(VI) Reductive Capacity in the Upper GI Tract
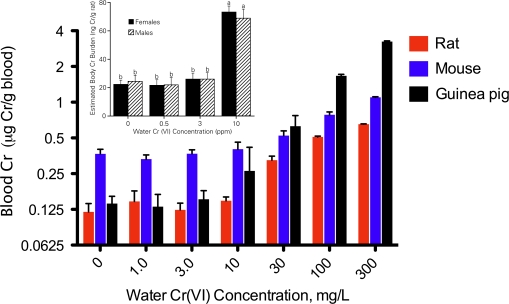
Cr(VI) is much more readily absorbed into cells than Cr(III), and thus, extracellular reduction of Cr(VI) to Cr(III) in the gut lumen before cellular absorption is a critical kinetic process limiting toxicity (De Flora, 2000; De Flora et al., 1997; Donaldson and Barreras, 1966; Febel et al., 2001; Kerger et al., 1996; U.S. EPA, 1998). At high drinking water concentrations, it is anticipated that exceedence of reduction capacity can lead to increased tissue uptake. Several lines of evidence suggest that all the drinking water concentrations in the NTP (2008b) study may have exceeded the ability of the rodent stomach to reduce Cr(VI). For instance, toxicokinetic data from the NTP (2007) study as well as Sutherland et al. (2000) suggest that dose-dependent transitions in chromium disposition occur in rodents somewhere between 3 and 10 mg/l Cr(VI) in drinking water (Fig. 3). Recently, several authors (Collins et al. 2010; Stern 2010; Stout et al., 2009a) have concluded that toxicokinetic data collected in NTP (2008b) indicate that reductive capacity was not exceeded because “tissue concentration data were consistent with a linear or supralinear dose-response” (Stout et al., 2009a). These authors anticipate a positive increase in the slope of chromium tissue concentration that would be indicative of saturation of reductive capacity and increased accumulation of chromium. Stern (2010) plotted the mouse data in Figure 3 as a line graph and concluded that the data were linear or supralinear and that a threshold for Cr(VI) reduction had not been achieved (Supplementary fig. 2A). However, Figure 3 and Supplementary figure 2B suggest that the change in slope anticipated by these authors at or near carcinogenic drinking water concentrations may have begun at the lowest concentration, i.e., 5 mg/l Cr(VI), employed in the 2-year bioassay (NTP, 2008b), indicating saturation of reductive capacity at all doses.
FIG. 3.
Total chromium (Cr) concentration in the blood of rats, mice, and guinea pigs following 21 days of exposure to the indicated drinking water concentrations, milligrams per liter Cr(VI) as SDD (data are taken from NTP, 2007). For each species, these data suggest a dose-dependent transition in the disposition of chromium somewhere between 3 and 10 mg/l Cr(VI) in drinking water. The inset shows a similar dose-dependent transition in total Cr body burden between 3 and 10 mg/l Cr(VI) in drinking water administered as potassium dichromate for 44 weeks, which was reproduced with kind permission from Springer Science & Business Media: Biological Trace Element Research, Rats Retain Chromium in Tissues Following Chronic Ingestion of Drinking Water Containing Hexavalent Chromium, 74, 2000, 41–53, Sutherland, Zhitkovich, Kluz, and Costa, Figure 5, Copyright 2000 by Humana Press Inc.
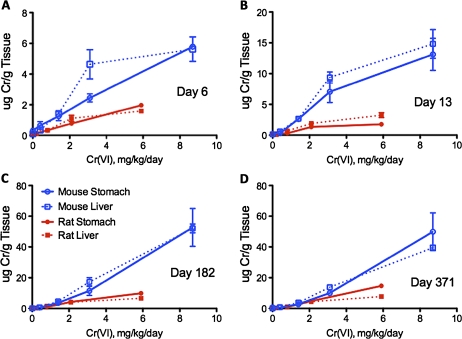
Tissue data from both NTP studies (NTP, 2007, 2008b) indicate that mice had higher concentrations of chromium in the stomach, liver, and blood than rats (Figs. 3 and 4) and suggest that mice may have lower capacity to reduce Cr(VI) to Cr(III) in the stomach lumen than rats or an enhanced ability to absorb chromium in the intestines. Differences in tissue chromium concentration may explain why hyperplasia was observed in the mouse duodenum at all doses in the 2-year NTP (2008b) study but not in the rat duodenum. Such differences in tissue concentrations may reflect differences in extracellular reductive capacities in the GI tract in the two species and/or differences in surface area or membrane transport. GI reductive capacity is determined, in part, by gastric fluid composition, pH, and gastric acid production rate (De Flora et al., 1997; Donaldson and Barreras, 1966). Measures of gastric fluid reduction rates and capacities between rodent species are currently not available but would be informative for understanding the pharmacokinetics and human relevance given the different tissue responses in the small intestines of mice and rats. Interestingly, Stern (2010) performed trend tests on the data in Figure 4 and found that a linear relationship was observed with the entire dose range as well as with just the two lowest concentrations—and again concluded that these data suggest that saturation of reductive capacity was not achieved in NTP (2008b). However, he did not report such a test for the data in Figure 3, which contains lower concentrations (see Supplementary fig. 2A).
FIG. 4.
Total chromium in the glandular stomach and liver in male rats and female mice after 6 (A), 13 (B), 182 (C), and 371 (D) days of exposure. Tissue concentration data are mean micrograms of Cr per gram tissue ± SE reported in tabular form in NTP (2008b). The milligrams per kilogram per day dose is the average daily ingested dose of Cr(VI) over the study duration as reported in Stout et al. (2009a).
Notably, the lowest drinking water concentration tested in the NTP (2008b) study exceeds the 95th percentile for Cr(VI) concentrations in U.S. drinking water by approximately 300-fold (Supplementary fig. 1); thus, whether Cr(VI) is reduced to Cr(III) in the stomach at concentrations expected in the environment is critical for assessing human relevance for risk assessment. Thus, quantification of key event 1 is important for understanding the differential responses observed in rats and mice as well as to evaluate the potential human relevance of the intestinal tumors observed in mice.
Although data exist for the reductive capacity of human gastric fluid, these data are limited and no comparable data exist for rodents. Proctor et al. (2002b) found that in simulated human gastric fluid, the rate of Cr(VI) reduction was similar across variables of pH, concentration of Cr(VI), fasted or fed conditions, and in real human gastric fluid as compared with simulated fluid. However, at lower pH (1.5 as compared with 4.5 or 7), the reductive capacity (total mass of Cr(VI) reduced per volume of fluid) of simulated gastric fluid was significantly increased. The reductive capacity of real human stomach fluid was 10-fold higher than that of simulated fluid at the same pH (approximately 1.5 consistent with fasting conditions) (Proctor et al. 2002b). Collins et al. (2010), Stern (2010), and Stout et al. (2009a) suggest that the reductive capacity of the rodent stomach can be extrapolated from data on the reductive capacity of the human gastric fluid using bodyweight scaling because factors affecting reduction are under metabolic control (i.e., dependent on gastric acid secretion) as compared with pH, which is known to vary by species (Supplementary table 2). However, differences in the anatomy and physiology of the rodent stomach, as compared with that of humans, are significant (Supplementary table 2), and reduction rate and capacity may not be readily extrapolated on the basis of bodyweight scaling. Thus, development of physiologically based pharmacokinetic (PBPK) models, based on measured species-specific reduction rates and capacities for gastric fluid, as well as measures of target tissue dose, are needed to more reliably quantify interspecies extrapolations.
Key Event 2: Uptake into the Small Intestine Epithelial Mucosa
Cr(VI) that escapes reduction in the stomach is likely to be absorbed into the epithelial cells of the intestinal mucosa, due in part to the high surface area of the tissue. Cr(VI) primarily exists as chromate at neutral pH and as hydrochromate below a pH of 6 (Zhitkovich, 2005); the former has isostructural similarity with physiological sulfate and phosphate ions and thus readily enters cells through the anion transport channels (De Flora, 2000; Markovich, 2001; Salnikow and Zhitkovich, 2008; Zhitkovich, 2005). It is generally believed that Cr(VI) uptake is mediated by the solute carrier (SLC) 4A anion transporter family and SLC4A1 in particular. This has been surmised based on findings that erythrocytes express high levels of the SLC4A1 anion transporter and readily take up Cr(VI) (Cohen et al., 1993; Markovich, 2001). The SLC4A1 protein product, anion exchanger 1 (AE1, also known as band 3), is inhibited by stilbene disulfonates (Cohen et al., 1993; Little et al., 1996; Markovich, 2001). These inhibitors have also been shown to reduce Cr(VI) uptake into cells in vitro; however, the inhibitors are not specific to AE1 (Kudrycki et al., 1990). Studies in rats indicate that SLC4A1 is abundantly expressed in erythrocytes and kidney but comparatively limited elsewhere. Nevertheless, within the rat GI tract, SLC4A1 expression is highest in the duodenum (Kudrycki et al., 1990). The SLC4A2 family member is more abundantly expressed than SLC4A1 in the rat small intestines (Kudrycki et al., 1990; Rossmann et al., 2000). Unlike SLC4A1, SLC4A2 function is regulated by both intracellular and extracellular pH and has several N-terminal variants (Alper, 2009; Alper et al., 1999). Thus, differences in Cr(VI) uptake across species might be because of differences in the expression and regulation (e.g., by intracellular pH) of SLC4A genes in the GI tract. Therefore, research studies designed to examine SLC gene expression and function (e.g., ability to transport sulfate and/or Cr(VI)) may be important to characterize interspecies difference in Cr(VI) uptake into the small intestine epithelial mucosa and to determine target tissue dose for pharmacokinetic and pharmacodynamic models.
In contrast to Cr(VI), Cr(III) only enters the cell passively or perhaps by endocytosis. As will be discussed for the third hypothesized key event, the toxicity of Cr(VI) is generally believed to be the result of intracellular reduction of Cr(VI) to Cr(III). Thus, intracellular absorption as Cr(VI) is a necessary key event. This is supported by the results of the recently completed NTP 2-year bioassay where rats and mice were exposed to high concentrations of Cr(III) in drinking water but no biologically significant neoplastic or nonneoplastic lesions were found despite the accumulation of chromium in tissues (NTP, 2008a; Stout et al., 2009b). Hence, there are distinctly different effects depending on the chromium species ingested.
Key Event 3: Oxidative Stress Leading to Tissue Damage and Inflammation
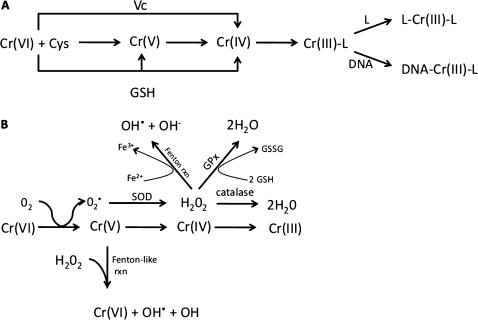
Within the cell, enzymes may play a role in reducing Cr(VI) to Cr(III). However, much of the intracellular reduction involves binding of Cr(VI) to low molecular weight thiols such as glutathione (GSH) and cysteine, as well as antioxidants like ascorbate (Fig. 5A) (De Flora et al., 1985; O'Brien et al., 2003; Zhitkovich, 2005). The cellular abundance of these reactants can differ across species, and reaction rates are estimated to differ greatly for ascorbate, GSH, and cysteine (O'Brien et al., 2003). Reduction of Cr(VI) can also lead to the formation of less stable intermediates such as Cr(V) and Cr(IV) as well as thiol radicals (O'Brien et al., 2003; Yao et al., 2008). Another potential pathway involves reduction of Cr(VI) to Cr(V) by molecular oxygen, which results in the generation of reactive oxygen species (ROS) (Liu and Shi, 2001). This in turn leads to the formation of hydrogen peroxide that can be removed by catalase, conjugation with GSH (perhaps competing with GSH-mediated Cr(VI) reduction), as well as by Fenton reactions with iron. Peroxide can also undergo Fenton reactions with Cr(V), thereby reforming Cr(VI) and hydroxide radicals (Liu and Shi, 2001). Thus, sustained exposure to Cr(VI) might lead to oxidative stress (Fig. 5B).
FIG. 5.
Simplified Cr(VI) reduction schemes. (A) Reduction of Cr(VI) by low molecular weight ligands (L). Adapted from Zhitkovich (2005). (B) Reduction of Cr(VI) by molecular oxygen (adapted from Liu and Shi 2001). Note that reactions are simplified and do not show mass balance. SOD, superoxide dismutase; Cys, cysteine; GSSG, oxidized GSH; GPx, glutathione peroxidase; Vc, ascorbate.
Understanding chromium disposition is complicated by several factors. For instance, Cr(V) and Cr(IV) are typically short-lived species. Unlike Cr(VI), Cr(V) and Cr(III) are paramagnetic and can be traced by magnetic resonance imaging albeit not easily distinguished (Liu and Shi, 2001). As recently noted by Nickens et al. (2010), there are several limitations inherent to in vitro studies on chromium. For example, many cultured cells have abnormally low antioxidant levels, which, coupled with dose rate issues when applying Cr(VI) and other constituents, obfuscate conclusions regarding Cr(VI) and oxidative stress. Moreover, the redox status and compliment of anion transporters likely differ across cell type as well as condition of confluence and terminal differentiation (Markovich, 2001). Thus, in vivo data from target tissues of interest are necessary to better understand the role of oxidative stress in the MOA underlying intestinal tumors in mice.
Direct evidence for oxidative stress in intestinal tissue (from any species) is limited. Acute exposure of rats to Cr(VI) by oral gavage led to decreased activities of intestinal mucosal enzymes, decreased levels of sulfhydryls, increased lipid peroxidation, and mixed alterations of antioxidant enzymes (e.g., GSH reductase and glutathione-S-transferase); however, these bolus doses (100 mg/kg potassium chromate) were greater than the highest doses in the NTP study (∼17 to 25 mg/kg SDD) (Arivarasu et al., 2008). Mice exposed to 5 or 20 mg/l Cr(VI) as SDD in drinking water for 9 months exhibited no evidence of oxidative DNA damage in the intestine (De Flora et al., 2008), suggesting that if oxidative stress occurred in the small intestines it was not sufficient to induce oxidative DNA damage. It is also conceivable that prolonged exposure to Cr(VI) can lead to adaptive changes that ameliorate oxidative damage. For example, differences in oxidative markers were observed in the rat intestinal mucosa following acute (3 days) and subchronic (30 days) exposure to potassium dichromate (Sengupta et al., 1990). Generally, acute exposure led to significant decreases in GSH and activity of antioxidant enzymes, whereas prolonged exposure led to a more mixed response with the activity of some enzymes being unaltered while others were increased or decreased.
Oxidative stress is well recognized to induce inflammation. Broadly, oxidative stress leads to activation of nuclear factor-κB (NF-κB) and subsequent downstream pathways resulting in the release of cytokines. This broad mechanism is implicated in airway inflammation, intestinal inflammation, and certain cancers (Kruidenier and Verspaget, 2002; Rahman and MacNee, 2000; Roberts et al., 2009). Cr(VI)-induced inflammation may play a critical role in lung cancer. A single intranasal exposure to particulate Cr(VI) (∼1 mg/kg) was shown to induce lung inflammation that required nearly 21 days to fully resolve (Beaver et al., 2009). Repeated (about once every 2 weeks) intranasal administration of particulate Cr(VI) resulted in an inflammatory reaction following each exposure, as well as signs of chronic inflammation in the bronchiolar and alveolar regions of the lung (Beaver et al., 2009). In contrast, nasal instillation of soluble Cr(VI) at 0.25 or 0.75 mg/kg for 3 consecutive days resulted in no overt signs of inflammation at 1 and 21 days postexposure (O'Hara et al., 2006). O'Hara et al. (2006) also showed that these concentrations significantly downregulated the antioxidant gene heme oxygenase-1 in the lung, suggesting that Cr(VI) may induce only mild inflammatory responses at these exposures (consistent with pathology data), but concomitantly alter the regulation of antioxidant enzymes. It should be noted that similar exposures have been shown to induce DNA damage and apoptosis in rodent lungs; some of these effects were partially mitigated by oral administration of N-acetylcysteine (D'Agostini et al., 2002; Izzotti et al., 1998, 2002). Taken together, these data suggest that exposure to Cr(VI) is likely to induce oxidative stress in tissues that come in direct contact with the compound, including the lung via inhalation and the highly absorbent intestinal mucosa following oral ingestion of high concentrations of Cr(VI) in drinking water.
Clear evidence for chronic inflammation in the duodenum was not observed in the mouse intestines at the doses tested in the 2-year NTP study. However, histiocytic infiltration was observed in the duodenum of both rats and mice in the 90-day and 2-year NTP studies (Tables 1 and 2). This infiltration was characterized as small clusters of macrophages as opposed to the more normal randomly distributed pattern. The NTP study authors stated that the biological significance of histiocytic infiltration is not known. Considering that histiocytic infiltration was observed as early as 90 days, the prolonged presence of leukocytes in tissues might be a sign of chronic mild inflammation. This might be important, as cytokines released from macrophages have been implicated in carcinogenesis within the large intestines (He et al., 2006; Li et al., 2009).
TABLE 2.
Spatiotemporal Depiction of Diffuse Hyperplasia and Histiocytic Infiltration
| Duration | 13 Weeks |
104 Weeks |
|||||||
| Mg/l Cr(VI) | ≥ 22 | ≥ 44 | ≤ 350 | ≥ 5 | ≥ 60 | ≥ 60 | ≥ 180 | ≥ 180 | ≥ 180 |
| Location | Duodenum |
Duodenum |
Jejunum |
||||||
| Pathology | DH | HI | Neo | DH | HI | Neo | DH | HI | Neo |
| Species | |||||||||
| Rat | — | √ | — | — | √ | — | — | — | — |
| Mouse | √ | √ | — | √ | √ | √ | √ | √ | √ |
DH, diffuse hyperplasia; HI, histiocytic infiltration; Neo, neoplasm.
Both redox status and inflammation can activate similar pathways—particularly via NF-κB and activation protein-1 (Rahman and MacNee, 2000; Roberts et al., 2009). The latter protein is a heterodimer of c-fos and jun protooncogenes with important roles in cell proliferation and differentiation. These and other transcription factors are regulated, in part, by cellular oxidative and nitrosative status (Marshall et al., 2000). In recent reviews on the carcinogenicity of chromium, it is noted that dysregulation of genes involved in cell death, DNA repair, and cell cycle can induce an epigenetic state that ultimately favors DNA damage (Holmes et al., 2008; Nickens et al., 2010). Nickens et al. (2010) posit that cell death pathways, inherently protective from cancer, may become disrupted by inflammatory responses to Cr(VI). Thus, redox status and inflammation can promote cell proliferation and inhibit programed cell death, thereby increasing the chance of genetic damage.
Key Event 4: Cell Proliferation
Data from the NTP Cr(VI) studies indicate that diffuse intestinal hyperplasia occurred in the duodenum of mice at all doses examined and as early as 90 days of exposure. Although tumor incidence did not increase in the 2-year study except in the highest doses (Table 1), it is conceivable that proliferation (1) preceded tumorigenesis, (2) was incidental to tumorigenesis, and/or (3) was elevated because of tumorigenesis. However, the NTP (2008b) study authors stated that the diffuse hyperplasia observed in the mouse small intestines was “consistent with regenerative hyperplasia secondary to previous epithelial cell injury.” Similar language was used to describe the observed intestinal hyperplasia in the 90-day study. Focal hyperplasia, a potentially preneoplastic lesion (Stout et al., 2009a), was not statistically significantly elevated at any dose but was present in some animals (Table 1). Figure 6 highlights the relationship between intestinal neoplasms, intestinal hyperplasia, and histiocytic infiltration. Although histiocytic infiltration was noted in both rodent species in the 90-day and 2-year NTP bioassays, intestinal hyperplasia was not observed in rats in either study at any doses. In contrast, diffuse hyperplasia occurred at similar incidence in the three highest doses; yet adenoma incidence was only significantly elevated in the two highest doses relative to controls. The presence of hyperplasia with and without tumor formation suggests that Cr(VI) induced cell proliferation independent of mutagenesis. These data suggest that the proliferation observed in mice is a necessary precursor for the development of intestinal neoplasms and plays a role in initiation and/or promotion but is not necessarily indicative of tumorigenesis.
FIG. 6.
A comparison of histiocytic infiltration (HI), diffuse hyperplasia, and adenomas in the duodenum of female mice and rats. The milligrams per kilogram per day dose is the average daily ingested dose of Cr(VI) over the study duration as reported in Stout et al. (2009a). The incidence data are as reported in Table 1 herein. Note that hyperplasia and tumors were not observed in rats.
Key Event 5: DNA Modification
The fifth key event in the hypothesized MOA for the mouse intestinal tumors involves DNA modification. An important part of MOA analysis is an evaluation of the mutagenicity and genotoxicity of the compound of interest. Carcinogens can be broadly classified as either DNA-reactive carcinogens or epigenetic carcinogens (Baccarelli and Bollati, 2009; Boobis et al., 2009; Williams, 2008). The ability of chromium to directly induce DNA damage has been studied extensively and is summarized below. However, evidence of direct interaction with DNA in and of itself does not demonstrate that a specific tumor arises as a result of a DNA-reactive MOA. In fact, there is growing evidence that chromium may act via epigenetic mechanisms.
Direct DNA modification.
The reactivity of chromium with DNA has been the subject of numerous studies and several recent reviews (O'Brien et al., 2003; Salnikow and Zhitkovich, 2008; Zhitkovich, 2005). Known and/or expected forms of DNA damage associated with Cr(VI) exposure include DNA adducts, single- and double-strand DNA breaks, inter- and intrastrand cross-links, oxidative DNA damage, and replication blockage. It is important to note that Cr(VI) itself is unreactive toward DNA, but following uptake and intracellular reduction, reduced chromium valence states, particularly Cr(V) and Cr(III), can interact with DNA (Nickens et al., 2010; O'Brien et al., 2003; Zhitkovich, 2005). As was shown in Figure 5A, binary Cr(III)-ligand complexes occur intracellularly, and these can subsequently form ternary complexes with themselves (e.g., GSH-Cr(III)-GSH) or with DNA (e.g., GSH-Cr(III)-DNA). The ternary DNA complexes may comprise the majority of Cr-DNA adducts (Arakawa et al., 2000; O'Brien et al., 2003; Zhitkovich, 2005). Shuttle vector assays indicate that GSH and amino acid chromium adducts predominantly lead to base pair substitutions, whereas ascorbate-Cr adducts lead to equal amounts of mutations and large genetic changes (Zhitkovich, 2005). These complexes can also lead to DNA-protein cross-links (DPX). DNA interstrand cross-links are thought to comprise much of the remaining direct Cr-DNA lesions (O'Brien et al., 2003; Zhitkovich, 2005). Several lines of evidence suggest that most Cr-DNA interaction occurs on the phosphate backbone (Arakawa et al., 2000; O'Brien et al., 2003). Repair of Cr-DNA lesions has recently been shown to increase genotoxicity, as cells deficient in either base or nucleotide excision repair were more resistant to Cr(VI) mutagenesis in vitro (Brooks et al., 2008). ROS may also contribute to chromium-mediated genotoxicity. However, its overall importance remains uncertain (Nickens et al., 2010; O'Brien et al., 2003; Zhitkovich, 2005).
An important consideration for DNA reactivity is mutagenic efficiency, which refers to the probability of a DNA adduct to induce a heritable mutation in a normal cell (Jarabek et al., 2009). In this regard, DNA damage in differentiated cells is less likely to lead to heritable mutations (Jarabek et al., 2009). An important consideration is whether Cr(VI) reaches target cells in the intestine that possess proliferative potential. Physically, unreduced Cr(VI) might come into direct contact with proliferative crypt cells, but it is not known whether these cells express the necessary transporters to readily absorb Cr(VI). Conceivably, immature crypt cells may be unable to transport Cr(VI), whereas villous cells may readily absorb Cr(VI) resulting in oxidative stress, cytotoxicity, and regenerative hyperplasia. Indeed, there is some evidence that certain transporters might be more abundantly expressed in differentiated cells (Markovich, 2001; Silberg et al., 1995). As noted by Jarabek et al., (2009), some forms of DNA damage are more prone to induce cytotoxicity than mutagenesis. DNA damage caused by accumulation of Cr(VI) in mature differentiated cells along the villous could lead to cytotoxicity as opposed to mutagenesis. Thus, it is conceivable that Cr(VI) has relatively low mutagenic efficiency.
Even among DNA-reactive compounds, there is evidence for nonlinearities and thresholds that limit carcinogenicity (Hoel and Portier, 1994; Williams, 2008). Table 3 lists several factors that limit the carcinogenicity of DNA-reactive compounds and summarizes what is currently known or hypothesized about their potential role in the MOA for the intestinal tumors observed in mice. Several of these factor relate to pharmacokinetics including delivery to target tissues and target cells, uptake into target cells, and intracellular (de)toxification. Additionally, many inherent aspects about DNA structure (e.g., so-called junk DNA), DNA repair, and transformation also limit the possibility of carcinogenicity of DNA-reactive compounds—particularly in cells with a short life span. However, as discussed in the next section, Cr(VI) might induce epigenetic effects that in turn impact the inherent barriers to carcinogenicity.
TABLE 3.
Factors that Limit the Carcinogenicity of DNA-Reactive Carcinogens and Potential Relevance for the MOA of Mouse Small Intestine Tumors Induced by Cr(VI)
| Factors that limit carcinogenicity (Williams, 2008) | Limits Cr(VI) carcinogenicity? | Basis |
| Incomplete absorption/rapid excretion | Yes | Cr(VI) is reduced to Cr(III) in GI tract; Cr(III) is poorly absorbed and excreted in the feces |
| Binding to extracellular molecules | Yes | Cr(VI) reduction to Cr(III) is mediated by binding to organic molecules |
| Dilution upon systemic absorption | Not applicable | Point of contact effect, systemic absorption is not applicable |
| Low probability of reaching target stem cells | Yes | Cr(VI) may be partially or entirely reduced to Cr(III) before reaching the small intestine |
| Unknown | Cr(VI) might not directly contact or be absorbed by proliferative crypt cells of the small intestine | |
| Limited cellular uptake/efficient elimination at target site | Yes | Extracellular reduction of Cr(VI) to Cr(III) limits cellular uptake |
| Unknown | Crypt cells might not express necessary transporters for absorption | |
| Limited bioactivation or/efficient detoxification in target cells | Unknown | Intracellular reduction of Cr(VI) to Cr(III) might generate free radicals as well as Cr(III)-L species capable of interacting with DNA |
| Reaction with non-DNA nucleophiles | Unknown | (See previous) |
| Reaction with nonutilized regions of DNA | Yes | General phenomenon |
| Efficient DNA repair | Unknown | Cr(VI) might induce epigenetic changes that inhibit DNA repair |
| Low probability of producing transforming mutations in multiple critical genes | Unknown | Potential epigenetic factors |
| Infrequency of neoplastic development from preneoplastic lesion | Unknown | Persistent diffuse hyperplasia might increase the chance of neoplastic development |
Epigenetic DNA modifications.
Three recent reviews on the genotoxicity and carcinogenicity of Cr(VI) have suggested that chromium induces epigenetic changes (Holmes et al., 2008; Nickens et al., 2010; Salnikow and Zhitkovich, 2008). Most of the evidence for this form of DNA modification derives from studies that indicate that chromium increases genomic instability, which is characterized by chromosomal instability and/or microsatellite instability (MSI). The latter can be observed in tumor cells where there is either shortening or lengthening of repetitive DNA sequences that are prone to replication error (Geigl et al., 2008; Grady and Carethers, 2008). MSI is often a result of the loss or hindrance of DNA mismatch repair (MMR) genes such as MutL homolog 1 (MLH1). Indeed, some tumors exhibit hypermethylation of MLH1, and thus, epigenetic silencing of MLH1 can result in an MSI phenotype without mutation in MMR genes (Geigl et al., 2008; Grady and Carethers, 2008).
Lung biopsies taken from workers occupationally exposed to chromate exhibit fewer p53 point mutations than normally expected in lung tumors (20 vs. ∼50%). However 79% (30/38) of these tumors had signs of MSI as compared with 15% (4/26) in tumors from unexposed individuals (Hirose et al., 2002; Kondo et al., 1997). Relative to lung tumors from nonexposed individuals, tumors from chromate workers exhibit reduced expression of MLH1 as well as signs of MLH1 hypermethylation (Takahashi et al., 2005). In vitro exposure of cultured cells to Cr(VI) was shown to increase cellular methylation of histone H3 lysine 9 (H3K9), which was inhibited by pretreatment of cells with the antioxidant ascorbate (Sun et al., 2009). Immunoprecipitation of methylated H3K9 following Cr(VI) exposure was shown to coprecipitate the MLH1 promoter; moreover, the treatment of cells with Cr(VI) produced time- and dose-dependent decreases in messenger RNA (mRNA) levels of MLH1 (Sun et al., 2009). It has also been shown that chromium can cross-link histone remodeling enzymes, thereby influencing gene expression (Schnekenburger et al., 2007).
Salnikow and Zhitkovich (2008) have also posited that Cr(VI)-induced carcinogenesis might involve genomic instability arising through effects on MMR genes (Salnikow and Zhitkovich, 2008). These authors reported that several cell lines deficient in MMR genes were resistant to Cr(VI)-induced toxicity (Peterson-Roth et al., 2005). Zhitkovich and colleagues argue that exposure to Cr(VI) might selectively inhibit the growth or promote the death of normal cells but that cells deficient in MMR “due to spontaneous mutagenesis and epigenetic changes” would continue to proliferate and acquire more mutations because of their insensitivity to Cr(VI) (Peterson-Roth et al., 2005). This “selective outgrowth” would result in an MSI phenotype as observed in the tumors from chromate workers (Hirose et al., 2002; Kondo et al., 1997; Takahashi et al., 2005). However, one recent study reported that human bronchial epithelial cells immortalized by repeated passage in low concentrations of Cr(VI) did not show involvement of MLH1 or MSI (Rodrigues et al., 2009).
In vivo evidence of DNA modification.
Although the evidence for chromium-induced DNA damage in vitro is convincing, the evidence for Cr(VI)-induced genotoxicity in vivo is comparatively weak, particularly when administered via drinking water (Nickens et al., 2010). Recent reviews on the carcinogenicity of Cr(VI) cite several subchronic studies as providing evidence for in vivo genotoxicity (McCarroll et al., 2010; Sedman et al., 2006; Supplementary table 1). As noted in Supplementary table 1, many of these subchronic studies cited in these two reviews employed relatively high doses of Cr(VI)—some higher than those used in the 2-year NTP cancer bioassay (NTP, 2008b). Of the studies noted by McCarroll et al. (2010) and Sedman et al. (2006), five involved oral exposure of rats to Cr(VI) and reported positive responses for genotoxicity in tissues such as bone marrow, liver, brain, or blood cells (Bagchi et al., 1995a,b, 1997; Bigaliev et al., 1977; Coogan et al., 1991); yet, no tumors occurred in any of these tissues in rats in the 2-year NTP study. Three of these studies attributed the genotoxicity to oxidative mechanisms, not direct DNA reactivity (Bagchi et al., 1995a,b, 1997).
Four studies in mice (excluding micronucleus studies) were previously cited as demonstrating genotoxicity (McCarroll et al., 2010; Sedman et al., 2006). Two of the four studies reported gene mutation following ip administration (Itoh and Shimada, 1996; Knudsen, 1980), a route of exposure that has very little relevance when evaluating effects of Cr(VI) following oral exposure, as ip administration bypasses the protective reductive mechanisms described earlier for Key Event 1. A third study, Bagchi et al. (2002), reported DNA damage in liver and brain tissue within 24–96 h after oral gavage of relatively high doses of ≥ 6 mg/kg Cr(VI), which the authors attributed to oxidative mechanisms. The fourth study, Dana Devi et al. (2001), reported increased DNA damage in leukocytes via the comet assay within 24 h of exposure. In the NTP study, chronic exposure to doses that are comparable to those Dana Devi et al. (2001) reported to induce DNA damage in leukocytes did not result in tumors among mice in any tissue other than the small intestines; moreover, in the NTP study, there was no apparent toxicity to leukocytes (NTP, 2008b). Further, the gavage doses used by Dana Devi et al. (2001) (∼0.18 to 24 mg/kg Cr(VI)), with the possible exception of the lowest dose, were likely sufficient to exceed the reductive capacity of the GI tract and thus may have resulted in increased absorption and oxidative stress as reported by Bagchi et al. (1995a,b, 1997, 2002). Importantly, the comet assay measures DNA damage (not mutation), and oxidative DNA damage has been shown increase tail lengths in leukocytes (Collins et al., 2008); thus, the findings by Dana Devi et al. (2001) may relate to oxidative stress as opposed to direct Cr(VI) DNA damage. In this regard, in vitro exposures of human lymphocytes to Cr(III) and Cr(VI) suggest that comet tails induced by the latter are likely the result of oxidative mechanisms as evidenced by the shortening of tail moments by incubation with catalase and their lengthening by posttreatment with endonuclease III, which nicks DNA at oxidized bases (Blasiak and Kowalik, 2000).
Several studies have examined micronucleus formation in bone marrow (Supplementary table 1). All studies involving ip administration were positive and employed doses of ≥ 10 mg/kg potassium chromate (∼3 mg/kg of Cr(VI)). As noted above, this route of exposure has very limited application for understanding the MOA from drinking water exposure to Cr(VI). Among several oral exposure studies, only one has reported positive findings of chromosomal aberrations in bone marrow of Swiss mice (Sarkar et al., 1993). It is noteworthy that many of the studies reporting positive findings for genetic damage from ip exposure to Cr(VI) have employed doses (on a milligrams per kilogram basis) greater than the lowest dose in the NTP study and in some cases doses that exceed the highest doses tested by NTP (Supplementary table 1).
Several studies that have directly compared the micronucleus formation of Cr(VI) by administration in drinking water (or oral gavage) and ip injection suggest that only ip administration results in genotoxicity. Shindo et al. (1989) exposed two strains of mice (CD-1 and MS/Ae) to several concentrations of Cr(VI) via oral gavage and by ip injection. In both strains of mice, there was a dose-dependent decrease in polychromatic erythrocytes (PCEs) and an increase in micronucleated PCEs following ip administration but not following gavage (Shindo et al., 1989). Similarly, De Flora et al. (2006) showed that ip administration of 17.7 mg/kg Cr(VI) to 8-month-old male BDF1 mice resulted in a significant increase in micronucleated PCEs, whereas gavage of the same dose did not. De Flora et al. (2006) showed that 8-month-old male mice exposed to 10 or 20 mg Cr(VI)/l in drinking water for 20 days showed no increase in micronucleated normochromatic erythrocytes (NCEs) at 5, 12, and 20 days of exposure or micronucleated PCEs at day 20. They also exposed 2-month-old mice to 5, 50, and 500 mg Cr(VI)/l in drinking water for up to 210 days. No changes in micronucleated NCEs were observed after 14, 28, 56, and 146 days of exposure. A slight but statistically insignificant increase was observed in micronucleated PCEs in the highest dose group relative to controls (i.e., 2.38 vs. 1.83% PCE). De Flora et al. (2006) also showed that pregnant Swiss albino mice exposed to 5 and 10 mg of Cr(VI)/l in drinking water throughout pregnancy showed no significant increase in micronucleated PCEs in the bone marrow. Similarly, no increases in micronucleated PCEs were found in the livers or peripheral blood of the fetuses. In contrast, rats administered an ip dose of 50 mg/kg on day 17 of pregnancy demonstrated significant increases in bone marrow micronucleated PCEs and in the liver and peripheral blood of the fetuses (De Flora et al., 2006). These studies, employing direct comparisons of oral and ip exposure routes, strongly suggest that gastric reduction of Cr(VI) can protect against genotoxicity in blood cells and other tissues.
Mirsalis et al. (1996) reported that 1–20 mg Cr(VI)/l did not induce micronucleus formation in mice exposed by drinking water (ad libitum 48 h) or gavage (two treatments for 2 consecutive days). Similar findings were observed in the NTP 90-day drinking water study (NTP, 2007). Male and female B6C3F1 mice exposed to ∼20 to 350 mg/l Cr(VI) showed no significant increase in micronucleated NCE. NTP (2007) also carried out a comparative study where three strains of male mice were exposed for 90 days to roughly 20, 45, and 90 mg/l Cr(VI). In this study, unlike in the 2-year study, B6C3F1 mice showed an increase in micronuclei formation, but it was judged to be equivocal because no dose group showed a significant increase over the control. In BALB/c mice, no increase was observed, whereas in a transgenic C57BL/6 mouse strain, there was a significant increase of micronucleated NCEs at the highest dose group (90 mg/l).
To date, the only study that has addressed Cr(VI)-induced genotoxicity in the target tissue of interest (i.e., small intestine) measured DPX and oxidative DNA damage (De Flora et al., 2008). In this study, De Flora et al. (2008) exposed female SKH-1 hairless mice to 5 and 20 mg Cr(VI)/l in drinking water for 9 months and examined genotoxic damage in blood cells and the GI tract. They found no significant changes in either DPX or 8-hydroxy-2′-deoxyguanosine (8-OH-dG) formation in the forestomach, glandular stomach, or duodenum after 9 months of exposure. The investigators also treated mucosal scrapings from the forestomach, glandular stomach, and duodenum of untreated animals with Cr(VI) in vitro and found significant increases in DPX and 8-OH-dG formation. Together, these findings underscore that Cr(VI) can be genotoxic in vitro without inducing genotoxicity in vivo when ingested orally in drinking water because Cr(VI), below certain concentrations, can be reduced to Cr(III) before entering cells. It should be noted that the lack of oxidative DNA damage at ≤ 20 mg/l Cr(VI) does not preclude changes in redox status in the tissue, as changes in redox can occur without inducing DNA damage.
Two studies have reported that coexposure to intense artificial ultraviolet radiation and Cr(VI) (as potassium chromate)in drinking water at 0.5, 2.5, and 5 mg/l resulted in an increased occurrence of skin tumors in hairless mice (Davidson et al., 2004; Uddin et al., 2007). At 5 mg/l, an increase in the chromium content of the skin was also reported. Although some have posited that this indicates that Cr(VI) was systemically absorbed and distributed to the skin, others have speculated that dermal exposure to Cr(VI) during the experiments might have occurred (Salnikow and Zhitkovich, 2008). Regardless, these findings are of questionable relevance to humans because Cr(VI)-exposed workers have not been shown to have an increase in skin cancer despite substantial systemic and dermal exposures (ATSDR, 2008; IARC, 1990; U.S. EPA, 1998).
Key Event 6: Mutagenesis
Although the specific genetic alterations that resulted in tumor formation in the small intestine of mice in the NTP study are not known, several lines of evidence suggest that the genetic damage might be the result of epigenetic changes that lead to genomic instability. Experimentally, exposure of transgenic gpt+ Chinese hamster V79 fibroblasts to soluble potassium chromate has been shown to induce mutant colonies with apparent transgene (gpt) deletions, many of which turned out to be silenced through hypermethylation in the promoter region; moreover, many mutants could be reverted by inhibition of DNA methylation (Klein et al., 2002). In contrast, transgene deletions induced by exposure to insoluble chromate were not hypermethylated (Klein et al., 2002). These data suggest that Cr(VI) can exert epigenetic gene silencing by aberrant DNA methylation. MSI is a common trait in 15–20% of human colorectal cancers (Geigl et al., 2008; Grady and Carethers, 2008). MSI is often a result of the loss or hindrance of DNA MMR, and nearly a third of individuals with colorectal cancers characterized by MSI have hereditary mutations in genes involved with MMR such as MLH1; moreover, 15–20% of the sporadic cases with MSI exhibit hypermethylation of MLH1 (Geigl et al., 2008; Grady and Carethers, 2008). MSI, including MLH1 hypermethylation, has also been found in a number of human tumors of the small intestine (Ruemmele et al., 2009). As previously described, lung biopsies taken from workers occupationally exposed to chromate exhibit an MSI phenotype and signs of MLH1 hypermethylation (Hirose et al., 2002; Kondo et al., 1997; Takahashi et al., 2005).
An important uncertainty in Cr(VI)-induced intestinal carcinogenesis is whether DNA modification occurs early or late in the process. According to Grady and Carethers (2008), genomic instability occurs early in colorectal tumorigenesis. However, in the context of environmental exposure to Cr(VI), this should not be confused with occurring early in the MOA. In the MOA proposed herein, pharmacokinetic and pharmacodynamic processes precede DNA modification and tumorigenesis. In the context of risk assessment, mutagenesis has been argued to be an early key event “that initiates a cascade of other key events such as cytotoxicity or cell proliferation” for chemicals that have a “mutagenic MOA” (U.S. EPA, 2007). Because studies on Cr(III) administered as chromium picolinate show increased cellular chromium levels but no carcinogenic effects (NTP, 2008a), intracellular chromium cannot unequivocally be considered to be a mutagen that acts through a “linear” MOA. Considering further that intestinal tumors induced by Cr(VI) in mice appear to associate with prolonged diffuse hyperplasia, it seems more likely that prolonged proliferative pressure may increase the chance of a spontaneous mutation and/or otherwise promote clonal expansion of initiated cells (including those with genomic instability). It is worth noting that analysis of DNA from the tumors observed in the NTP 2-year bioassay (NTP, 2008b) has the potential to provide important information regarding the MOA. As Kondo et al. (1997) remarked regarding lung tumors in chromate workers, “it is necessary to test samples from chromate lung cancer patients for mutations in other cancer-associated genes, gene instability, loss of heterozygosity, and chromosomal aberrations in an effort to elucidate the mechanisms involved in carcinogenesis in chromate workers.” However, to the best of our knowledge, such an analysis has not been undertaken for tumors observed in the NTP rodent study.
Plausibility
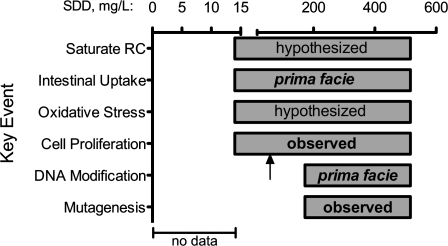
The MOA Framework (U.S. EPA, 2005) includes evaluation of the proposed MOA using causality criteria originally proposed by Sir Bradford Hill (Hill, 1965). Plausibility of the proposed MOA, consideration of alternative MOAs, and comparison with MOAs for chemicals that cause similar tumors are important factors that touch upon the Hill criteria. In regard to plausibility, the MOA presented herein is consistent with general principles of carcinogenesis and the findings of the 2-year NTP study, which suggest that (1) there is no evidence of cancer from Cr(VI) exposure in drinking water outside the alimentary canal, (2) neoplasms in the small intestine occurred at doses that likely greatly exceeded the reductive capacity of the proximal portions of alimentary canal, and (3) proliferation occurred relatively early after exposure and at lower doses than tumorigenesis. Considering that no neoplasms were found in tissues other than those lining the alimentary canal, systemic absorption of Cr(VI) seems unlikely to pose a carcinogenic risk. Similarly, there is little evidence of cancers outside the respiratory tract following inhalation exposures in animals or humans (De Flora, 2000), and occupational exposures to Cr(VI) have not been shown to cause GI tract cancers despite evidence of substantial incidental ingestion (Gatto et al., 2010). Figure 7 outlines the causal and temporal relationships as well as data gaps for proposed key events in the MOA.
FIG. 7.
Causal and temporal associations supporting Key Events in the hypothesized MOA for intestinal tumors. Each bar represents where Key Events have been observed, inferred, or hypothesized. It is noted that DNA modification and mutagenesis could occur below the doses where tumors were observed. The arrow indicates concentration (62.5 mg/l SDD) where cell proliferation was observed in the 90-day NTP (2007) study.
Figure 6 depicts the dose-response concordance of the key events in the hypothesized MOA. On a milligrams per kilogram basis, mice and rats ingested roughly equivalent doses of Cr(VI) in the 2-year NTP bioassays. Whereas there is a dose-dependent increase in histiocytic infiltration in both species, dose-dependent increases in hyperplasia and adenomas in the small intestine were only observed in mice. The figure indicates that diffuse hyperplasia occurs at similar incidence levels in tumorigenic and some nontumorigenic doses, indicating that the observed proliferation may be antecedent to carcinogenesis. The findings also suggest that proliferation itself may not be sufficient to cause carcinogenesis but that coupled with additional and prolonged duodenal exposure to unreduced Cr(VI) increases the risk of carcinogenesis. From a temporal standpoint, the early onset of hyperplasia (by 90 days) and long time to tumor is more consistent with Cr(VI) carcinogenesis arising secondary to the onset of tissue damage and cell proliferation than direct Cr(VI)-induced mutagenesis.
Alternative MOA Constructs for Framing Available Data
Alternative MOAs include mutation as an early key event as described by McCarroll et al. (2010). Based on currently available data, these investigators concluded that the weight of evidence supports a mutagenic MOA based on the genetic activity profile for Cr(VI) and evidence that DNA damage was observed in circulating lymphocytes within 24 h following oral gavage of Cr(VI) at doses similar to that administered in the NTP study (Dana Devi et al., 2001). Although there are some similarities in the MOA proposed by McCarroll et al. (2010) and that proposed herein, differences include the sequence of key events (e.g., occurrence of mutagenesis as an initiating or later key event), the inclusion of critical pharmacokinetic steps (saturation of GI reductive capacity and absorption into intestinal epithelium), and consideration of epigenetic changes consistent with intestinal cancers (e.g., MMR genes).
Similarities with Other Duodenal Carcinogens in 2-Year Bioassays
In their summary of the findings of the 2-year bioassay of Cr(VI) in drinking water, Stout et al. (2009a) noted that captan was the only other compound examined by NTP that resulted in both benign and malignant intestinal neoplasms of epithelial origin attributed to chemical exposure (NCI, 1977). As such, the MOA for captan (and the structurally similar folpet) may provide important insights for understanding the intestinal tumors observed in mice exposed to Cr(VI) in drinking water. Like Cr(VI), captan and folpet are clearly mutagenic in vitro, but evidence for in vivo mutagenicity is equivocal or negative (Arce et al., 2010; Bernard and Gordon, 2000). Captan and folpet react readily with thiols (e.g., GSH, cysteine, and proteins) and induce blunted villi and villous cytotoxicity, regenerative crypt cell proliferation, and neoplasms (adenomas and carcinomas) in the mouse duodenum and, to a lesser extent, jejunum (Cohen et al., 2010). In 2004, the U.S. EPA changed their cancer classification of captan from “a probable human carcinogen” (Category B) to “not likely” after an independent peer review concluded that captan acted through a nonmutagen threshold MOA that “required prolonged irritation of the duodenal villi as the initial key event” (Gordon, 2007; U.S. EPA, 2004). The similarities between captan, folpet, and Cr(VI) make it plausible that they share a common MOA, i.e., cytotoxicity in villous cells, thereby placing sustained proliferative pressure on crypt cells that increase the risk of carcinogenesis. Notably, an explanation for the absence of intestinal tumors in rats following exposure to captan and folpet is not known; yet, Cohen et al. (2010) concluded that the MOA in mice might be relevant to humans under chronic high-dose exposures.
Relevance to Humans
The data set available to assess relevance to humans is relatively robust for Cr(VI)-induced carcinogenicity with the most reliable findings derived from occupational epidemiology studies of Cr(VI)-exposed workers. Although occupational exposure occurs primarily by inhalation, historical worker exposures in some industries were extremely high and caused GI disorders including ulcers, diarrhea, and abdominal pain (NIOSH 1975; PHS 1956). It is probable that at the extreme exposures experienced by workers in some historical industries, oral exposures from incidental ingestion of inspired particulates and hand to mouth contact were significant as evidenced by yellow staining of teeth and tongues among workers of the historical chromate production industry (PHS, 1956).
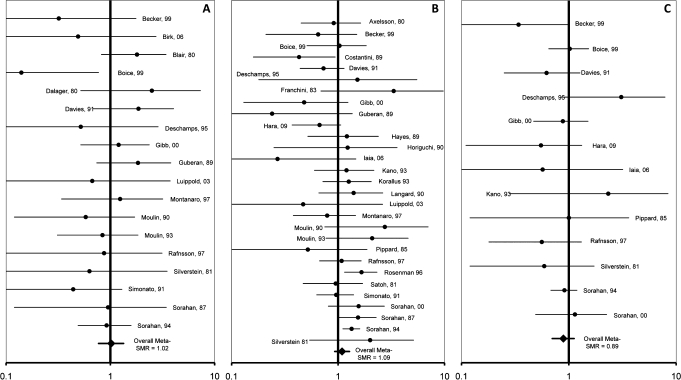
Well over 100 epidemiological studies in Cr(VI)-exposed workers have been published. Although these studies mostly focus on lung cancer, dozens have also examined associations between chromium exposure and cancers outside the respiratory system. A systematic review of literature reporting on GI tract cancers among workers with known occupational exposure to Cr(VI) and meta-analysis of 32 studies published since 1950 did not find an association (Gatto et al., 2010). Meta-standardized mortality ratios (meta-SMRs) calculated for cancers of the oral cavity, esophagus, stomach, colon, and rectum of Cr(VI)-exposed workers were generally around 1.0, and none were significantly elevated—even among more highly exposed subcohorts (Fig. 8). Gatto et al. (2010) identified only three studies reporting small intestine cancer among Cr(VI)-exposed workers, all with insufficient sample size to develop a meta-SMR. All three studies reporting on small intestine cancers were based on a very small number of cases (n < 3) with no significant increases reported (Gatto et al., 2010), an observation that is not consistent for an association between Cr(VI) exposure and small intestine tumors in humans.
FIG. 8.
Standardized mortality ratio (SMR) risk estimates from epidemiologic studies that evaluated occupational exposure to Cr(VI) and risk of oral (A), stomach (B), and colon cancers (C).
Several epidemiologic studies have investigated cancer incidence or mortality among populations with environmental exposures to chromium or Cr(VI) by drinking water or contaminated soil and found no increases in cancer risk (Armienta-Hernandez and Rodriguez-Castillo, 1995; Bednar and Kies, 1991; Fryzek et al., 2001; GGHB, 1991; GTBH, 1989). However, these studies are ecologic and limited by the quality and availability of reliable information on exposure and/or outcome. Studies of Chinese villagers possibly exposed to very high concentrations of Cr(VI) in drinking water have reported an increase in stomach cancer mortality (Beaumont et al., 2008; Zhang and Li, 1987). However, other investigations of the same population found no dose-response for Cr(VI) exposure and stomach cancer mortality using three different exposure metrics (Kerger et al., 2009). Kerger et al. (2009) concluded that the increased risk was because of differences in demographic factors for rural and urban communities. It is important to consider that the original mortality and exposure data, upon which all these epidemiologic studies are based, are of questionable usefulness because the mortality data are crude and cannot be corrected for age or gender. Furthermore, the person-years at risk can only be roughly estimated, and there are significant concerns regarding the classification of exposure status. Follow-up for cancer mortality in this study is only 4–14 years following initial exposure, and latency for stomach cancer is expected to be greater than 20 years. For example, latency for stomach cancer associated with asbestos exposure is 20–40 years (Levine, 1985), with Helicobacter pylori infection is 50 years (Correa, 2004), and with ionizing radiation is 20–30 years (Mossman, 1984). Therefore, temporal ambiguity is a very significant concern in the studies of the exposed Chinese population. Given these, and likely other serious limitations, findings from the Chinese villagers studies should not be considered reliable evidence regarding the human relevance of GI tract cancers from drinking water exposure to Cr(VI).
Although there is an abundance of quality studies investigating GI tract cancers in humans exposed to Cr(VI) in occupational settings, differences in toxicokinetics between drinking water and occupational exposure, as well as in the pattern, duration, and frequency of exposure, present uncertainty as to whether data from occupational studies are useful to assess risk from long-term drinking water exposure. Also, studies of populations exposed environmentally to Cr(VI) are generally null regarding GI tract cancers. Given the breadth of epidemiologic data, findings from these studies indicate that human relevance is not likely or only relevant at very high levels of exposure.
The key events in the proposed MOA for intestinal tumors in mice are thought to be potentially relevant to humans, meaning that from prolonged and relatively high Cr(VI) exposures the proposed key events are qualitatively relevant. However, quantitative relevance is questionable because, like rodents, systemic bioavailability of Cr(VI) is limited by reduction of Cr(VI) to Cr(III) in the human stomach (De Flora, 2000; Febel et al., 2001; Finley et al., 1997). Finley et al. (1997) reported no increase in urinary or blood chromium of humans consuming Cr(VI) in water at the current federal drinking water standard of 0.1 mg/l Cr(VI), suggesting that drinking water exposure at this level is entirely reduced to Cr(III) before systemic absorption. At higher concentrations (approximately ≥ 5 mg/l Cr(VI)), Finley et al. (1997) reported that chromium concentrations in blood and urine were increased. These data suggest that in humans, similar to rodents, there is a saturation level for stomach reductive capacity. Another explanation is that at the higher concentrations, greater amounts of Cr(III) were absorbed (Finley et al., 1997). A better understanding of Cr(VI) disposition in rodents through the development of pharmacokinetic models could inform the disposition of Cr(VI) in humans as well assess whether the MOA for mouse tumors is relevant to humans.
It is noteworthy, though perhaps coincidental, that the hypothesized MOA for Cr(VI)-induced intestinal tumors in mice shares certain aspects with human intestinal carcinogenesis. Inflammation and irritation are thought to play key roles in human diseases of the intestines such as Crohn's disease (Coussens and Werb, 2002; Ryan, 1996; Thun et al., 2004). Thus, if Cr(VI) concentrations were sufficiently high to exceed reductive capacity of the upper alimentary canal for an extended time, possible key events in the mouse MOA (irritation, inflammation, hyperplasia, and genetic damage) might be qualitatively relevant to humans. Similarly, MSI and MMR pathways are implicated in human cancers of the large and small intestines, and chromium may very well affect these genes and/or induce genomic instability (Geigl et al., 2008; Grady and Carethers, 2008; Heyer et al., 1999; Holmes et al., 2008; Nickens et al., 2010; Sun et al., 2009). These and other heritable genetic variations are associated with intestinal cancer and may be predictive of cancer risk (Geigl et al., 2008; Tokuoka et al., 2009; Walther et al., 2009). The influence of chromium on these genes within the intestines is not known.
DATA GAPS IN THE MOA
This critical review of the scientific literature for purposes of developing a plausible MOA underlying intestinal tumors observed in mice exposed to Cr(VI) in drinking water resulted in the identification of several data gaps and uncertainties in the MOA that could be addressed through targeted research (Table 4). The differences in the intestinal response to Cr(VI) in rats and mice suggest differences in pharmacokinetics—specifically reduction of Cr(VI) to Cr(III) in the lumen of the upper GI tract and the intestinal uptake of Cr(VI) through anion transporters. Target tissue data for chromium are needed to better understand if the differential responses are pharmacokinetic or pharmacodynamic in nature, or both. Gastric fluids from humans, mice, and rats are needed to measure reduction rate and capacity—which can be important for parameterizing species-specific PBPK models. Such models capable of reasonably estimating dosimetry in rodents and predicting dosimetry in humans could be very informative in the context of understanding potential human health risks. From an experimental perspective, one could use such models to predict the species-specific administered dose of Cr(VI) needed to induce key events in the MOA and then test those predictions experimentally.
TABLE 4.
Data Gaps in the MOA for Cr(VI)-induced Intestinal Cancers and Studies Needed to Address These Gaps
| Key event | Data gap | Studies needed |
| (i) Sustained saturation of the reductive capacity of the upper alimentary | Are there species differences in the reductive capacity of the upper alimentary canal? Are there dose-dependent transitions in the kinetics of Cr(VI)? Is there a dose at which Cr(VI) will be completely reduced in the stomach? | Pharmacokinetic data on the disposition of chromium in rats and mice. Measures of the reduction rate and capacity of gastric fluid. Development of a PBPK model capable of predicting chromium disposition in multiple species. |
| (ii) Uptake of Cr(VI) from the intestinal lumen | Did unreduced Cr(VI) get passed into the lumen of the duodenum at all doses? Did unreduced Cr(VI) reach the jejunum? Are there species differences in anion transporters that affect Cr(VI) uptake? Is Cr(VI) taken up by proliferating crypt cells? | Pharmacokinetic data including measures of chromium in the glandular stomach, duodenum, and jejunum, as well as the liver, kidney, femur, blood (plasma and erythrocytes), urine, and feces. Markers of Cr(VI) absorption in crypt cells as compared with villi. |
| (iii) Oxidative stress, tissue damage, and inflammation | Does unreduced Cr(VI) reaching the intestines get taken into the epithelium and reduced intracellularly? Does this lead to oxidative stress and/or inflammation in the intestines? | Measures of oxidative stress, ratio of oxidized to reduced GSH, inflammatory cytokines, and changes in related genes in target tissues. |
| (iv) Cell proliferation | Do lower doses than those used in the NTP study induce intestinal diffuse hyperplasia? What is the source of proliferation (cytotoxicity, mitogenesis, etc.)? | Examination of histopathological and gene expression changes indicative of cell proliferation at lower drinking water concentrations. |
| (v) DNA modification | Can chromium or oxidative DNA damage be detected in DNA samples from target tissues and cells? Are the tumors in the NTP study the result of chromium-mediated DNA damage, oxidative DNA damage, proliferative pressure, or some combination? Does chromium induce epigenetic changes? | Measures of chromium in genomic DNA samples from target tissues. Measure 8-OH-dG damage in target tissues. Assessment of transcriptional changes related to DNA repair genes in target tissues. Analysis of histone and DNA methylation status in target tissues. Expression level of MMR genes implicated in chromium toxicity. |
| (vi) Mutagenesis | Does Cr(VI) exposure induce measurable increases in DNA mutations? Are there hot spots for Cr(VI)-induced mutation? | In vivo mutation analysis of select codons (e.g., ras codons 12, 13, and 61) or exons (e.g., p53 exons 5–8) in frequently mutated targets. |
There are currently no data to determine whether the intestinal tissue in mice experienced oxidative stress. However, it is assumed to occur because of the intracellular reduction of Cr(VI) (as explained in Key Event 3: Oxidative Stress Leading to Tissue Damage and Inflammation section). Direct measures of oxidative stress, such as the ratio of reduced to oxidized GSH and transcript changes related to oxidative stress pathways, could provide important information to better understand the potential role of oxidative stress. The role of inflammatory responses in the MOA could be assessed through measures of cytokines in target tissues. Because histopathology was performed by the NTP following exposures at 90 days and 2 years, the data available for cell proliferation as a key event are more complete. However, a no effect level for hyperplasia in the mouse duodenum was not identified; thus, one important data gap is the identification of a dose below which cell proliferation does not occur in mice. Additionally, the species differences in intestinal proliferation might involve unknown toxicodynamic differences that could be informed by transcript changes related to cell cycle.
Although De Flora et al. (2008) found no genotoxicity in target tissues of the small intestine at drinking water exposures of 5 and 20 mg/l Cr(VI), several important data gaps remain for DNA modification as a key event. Specifically, it is not known whether direct chromium-mediated genotoxicity occurred in the NTP study and whether genotoxicity is an early initiating event or occurs much later in the sequence of events. Examining genomic DNA samples from target tissue for the presence of chromium adducts following administration of Cr(VI) would be informative regarding the type of DNA modification or damage that occurred. Given the potential involvement of oxidative stress, measures of oxidative DNA damage at doses that caused tumors in mice would also be informative. Again, changes in gene transcripts might inform the types of DNA modification and repair pathways induced by Cr(VI) exposure.
Recent studies implicating epigenetic changes in DNA following chromium exposure highlight the need to look for such changes in the intestinal tissue—especially because intestinal cancer is associated with some of the same changes (e.g., MMR genes). If a similar evaluation of the mouse intestinal tumors from the NTP (2008b) study was performed as in the lung tumors from chromate workers (Kondo et al., 1997), the role of epigenetic changes might be better informed. Short of repeating a 2-year bioassay, changes in mRNA transcripts related to MMR pathways, as well as changes in DNA and histone methylation, might be observable in a subchronic study and provide information regarding the mechanisms of mutagenicity.
An important unresolved question is whether mutagenesis is an early key event in the MOA or whether it occurs after saturation of Cr(VI) reduction and the induction of cell proliferation. Thus, it might be worthwhile to measure in vivo mutation in various hot spots such as p53 or the ras codons implicated in colon cancer or genes related to genomic instability. Such an approach was recently performed in rats following subchronic exposure to formaldehyde (Meng et al., 2010).
Studies designed to address the above data gaps would not only refine the hypothesized MOA but could also lead to new insights or even support other proposed MOAs. To further elucidate the MOA underlying the intestinal tumors in mice (Fig. 2), pharmacokinetic and pharmacodynamic data can be compared between the responsive mouse intestine and the unresponsive rat intestine. Similarly, pharmacokinetic and pharmacodynamic data from the mouse duodenum and jejunum could be compared with and correlated with the diffuse hyperplasia and tumor formation that were elevated further down the small intestine as Cr(VI) drinking water concentration increased. Potentially, the pattern of key events that occur in the jejunum (e.g., uptake, redox, transcript changes, and proliferation) at higher concentrations would parallel the pattern of events that occur in the duodenum at lower concentrations and further substantiate the MOA.
DISCUSSION
The U.S. EPA MOA Framework has been used to conduct an analysis of the likely key events leading to the development of intestinal tumors in mice chronically exposed to Cr(VI) in drinking water. The available data suggest that the hypothesized MOA in mice (Fig. 2) is plausible; however, additional data are needed to support (or refute) this MOA and to help explain the interspecies differences in response, as well as better understand human relevance (Fig. 1). Studies aimed at filling the data gaps in the MOA in rodents (Table 4) could provide important information concerning human relevance and low-dose extrapolation methods appropriate for risk assessment. Studies designed to address the data gaps described above are currently underway and should be completed by the end of 2010.
With regard to low-dose extrapolation, it is well recognized that mechanisms of toxicity and carcinogenicity of compounds can change as a function of dose (Slikker et al., 2004). Considering that the lowest tumorigenic Cr(VI) drinking water concentration in the NTP (2008b) study (20 mg/l) is 1000 times greater than the 95th percentile of U.S. Cr(VI) drinking water concentrations (Supplementary fig. 1), it is critical to understand the MOA at the tumorigenic doses in order to understand the risk at environmental exposure levels. Even if direct DNA reactivity is a component in the intestinal tumor formation in mice, the absence of such tumors in rats suggests that there are one or more barriers to the induction of intestinal tumor formation in some species and that it is worthwhile to identify those barriers.
U.S. EPA (2005) indicates that even chemicals with direct DNA reactivity might act through a nonlinear MOA and thus support nonlinear low-dose extrapolation if such can be reasonably demonstrated and/or expected:
A nonlinear approach should be selected when there are sufficient data to ascertain the mode of action and conclude that it is not linear at low doses and the agent does not demonstrate mutagenic or other activity consistent with linearity at low doses. Special attention is important when the data support a nonlinear mode of action but there is also a suggestion of mutagenicity. Depending on the strength of the suggestion of mutagenicity, the assessment may justify a conclusion that mutagenicity is not operative at low doses and focus on a nonlinear approach, or alternatively, the assessment may use both linear and nonlinear approaches. (emphasis added)
Notably, a review of the dose-response shapes of 315 carcinogens previously studied by NTP and the National Cancer Institute suggested that the dose-response for these carcinogens is often nonlinear—even for carcinogens that test positive in bacterial mutation assays (Hoel and Portier, 1994). These authors concluded that the dose-response for other endpoints (e.g., toxicokinetics, cell proliferation, and gene expression) should be evaluated for informing the low-dose risk estimation. Indeed, some of the proposed research described above could inform the most appropriate low-dose extrapolation approach for the oral carcinogenicity of Cr(VI).
To our knowledge, this is the first practical example of using MOA analysis to formally guide research for the purpose of risk assessment. As demonstrated here using Cr(VI) as a case study, MOA analysis has been shown to be a powerful tool to guide future research by focusing on those studies that will be most informative concerning potential human health risks. It is not surprising that the data gaps identified in the hypothesized MOA underlying the oral carcinogenicity of Cr(VI) would require computational toxicology methods consistent with those outlined in the National Academy of Sciences report titled Toxicity Testing in the 21st Century: A Vision and a Strategy (NAS, 2007). Such studies combine traditional techniques, such as histopathology and biochemical measures, with high throughput and high content approaches like toxicogenomics. We believe that gathering such data is essential to extrapolating the findings observed in the NTP cancer bioassay to humans and will provide for a more scientifically defensible assessment of human health risks.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Cr(VI) Panel of the American Chemistry Counsel.
Supplementary Material
Acknowledgments
We appreciate the thoughtful insights of Dr Ted Simon in the development of the MOA, the recommendations of the many scientists participating in the MOA Research Study to date, and contributions from the Toxicological Sciences peer reviewers. We are very grateful for the considerable contributions provided by the Toxicology Excellence for Risk Assessment Expert Panel overseeing the Cr(VI) MOA Research Program. The panel report is available at http://www.tera.org/Peer/Chromium/Chromium.htm.
References
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am. J. Physiol. 1999;277:G321–G332. doi: 10.1152/ajpgi.1999.277.2.G321. [DOI] [PubMed] [Google Scholar]
- Anderson RA. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000;26:22–27. [PubMed] [Google Scholar]
- Arakawa H, Ahmad R, Naoui M, Tajmir-Riahi HA. A comparative study of calf thymus DNA binding to Cr(III) and Cr(VI) ions. Evidence for the guanine N-7-chromium-phosphate chelate formation. J. Biol. Chem. 2000;275:10150–10153. doi: 10.1074/jbc.275.14.10150. [DOI] [PubMed] [Google Scholar]
- Arce GT, Gordon EB, Cohen SM, Singh P. Genetic toxicology of folpet and captan. Crit. Rev. Toxicol. 2010;40:546–574. doi: 10.3109/10408444.2010.481663. [DOI] [PubMed] [Google Scholar]
- Arivarasu NA, Fatima S, Mahmood R. Oral administration of potassium dichromate inhibits brush border membrane enzymes and alters anti-oxidant status of rat intestine. Arch. Toxicol. 2008;82:951–958. doi: 10.1007/s00204-008-0311-0. [DOI] [PubMed] [Google Scholar]
- Armienta-Hernandez MA, Rodriguez-Castillo R. Environmental exposure to chromium compounds in the valley of Leon, Mexico. Environ. Health Perspect. 1995;103(Suppl. 1):47–51. doi: 10.1289/ehp.103-1519325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Draft: Toxicological Profile for Chromium. 2008. Agency for Toxic Substances and Disease Registry. Available at: http://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=62&tid=17. Accessed November 13, 2010. [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Balmoori J, Bagchi M, Ye X, Williams CB, Stohs SJ. Comparative effects of TCDD, endrin, naphthalene and chromium (VI) on oxidative stress and tissue damage in the liver and brain tissues of mice. Toxicology. 2002;175:73–82. doi: 10.1016/s0300-483x(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Hassoun EA, Bagchi M, Muldoon DF, Stohs SJ. Oxidative stress induced by chronic administration of sodium dichromate [Cr(VI)] to rats. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995a;110:281–287. doi: 10.1016/0742-8413(94)00103-h. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Hassoun EA, Bagchi M, Stohs SJ. Chromium-induced excretion of urinary lipid metabolites, DNA damage, nitric oxide production, and generation of reactive oxygen species in Sprague-Dawley rats. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995b;110:177–187. doi: 10.1016/0742-8413(94)00093-p. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ. Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic. Biol. Med. 1997;22:471–478. doi: 10.1016/s0891-5849(96)00352-8. [DOI] [PubMed] [Google Scholar]
- Beaumont JJ, Sedman RM, Reynolds SD, Sherman CD, Li LH, Howd RA, Sandy MS, Zeise L, Alexeeff GV. Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology. 2008;19:12–23. doi: 10.1097/EDE.0b013e31815cea4c. [DOI] [PubMed] [Google Scholar]
- Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, Patierno SR. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ. Health Perspect. 2009;117:1896–1902. doi: 10.1289/ehp.0900715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar CM, Kies C. Inorganic contaminants in drinking water correlated with disease occurrence in Nebraska. Water Resour. Bull. Am. Water Resour. Assoc. 1991;27:631–635. [Google Scholar]
- Bernard B, Gordon E. An evaluation of the common mechanism approach to the food quality protection act: captan and four related fungicides, a practical example. Int. J. Toxicol. 2000;19:43–61. [Google Scholar]
- Bigaliev A, Turebaev M, Elemesova M. Cytogenetic study of the in vivo mutagenic properties of chromium compounds. In: Dubinin NP, editor. Genet Posledstviya Zagryanenia Okruzhayushehei Sredy (Proceedings of the Symposium) Moscow, Russia: Nauka; 1977. pp. 173–176. [Google Scholar]
- Blasiak J, Kowalik J. A comparison of the in vitro genotoxicity of tri- and hexavalent chromium. Mutat. Res. 2000;469:135–145. doi: 10.1016/s1383-5718(00)00065-6. [DOI] [PubMed] [Google Scholar]
- Bogdanffy MS, Daston G, Faustman EM, Kimmel CA, Kimmel GL, Seed J, Vu V. Harmonization of cancer and noncancer risk assessment: proceedings of a consensus-building workshop. Toxicol. Sci. 2001;61:18–31. doi: 10.1093/toxsci/61.1.18. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Daston GP, Preston RJ, Olin SS. Application of key events analysis to chemical carcinogens and noncarcinogens. Crit. Rev. Food Sci. Nutr. 2009;49:690–707. doi: 10.1080/10408390903098673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, O'Brien TJ, Ceryak S, Wise JP, Sr, Wise SS, Wise JP, Jr., Defabo E, Patierno SR. Excision repair is required for genotoxin-induced mutagenesis in mammalian cells. Carcinogenesis. 2008;29:1064–1069. doi: 10.1093/carcin/bgn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDHS. Chromium-6 in Drinking Water: An Overview of Sampling Results. 2009. Available at: http://www.cdph.ca.gov/certlic/drinkingwater/pages/chromium6sampling.aspx. Accessed November 13, 2010. [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Gordon EB, Singh P, Arce GT, Nyska A. Carcinogenic mode of action of folpet in mice and evaluation of its relevance to humans. Crit. Rev. Toxicol. 2010;40:531–545. doi: 10.3109/10408441003742903. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Stout MD, Levine KE, Kissling GE, Melnick RL, Fennell TR, Walden R, Abdo K, Pritchard JB, Fernando RA, Burka LT, Hooth MJ. Exposure to hexavalent chromium resulted in significantly higher tissue chromium burden compared to trivalent chromium following similar oral doses to male F344/n rats and female B6C3F1 mice. Toxicol. Sci. 2010;118:368–379. doi: 10.1093/toxsci/kfq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- Coogan TP, Motz J, Snyder CA, Squibb KS, Costa M. Differential DNA-protein crosslinking in lymphocytes and liver following chronic drinking water exposure of rats to potassium chromate. Toxicol. Appl. Pharmacol. 1991;109:60–72. doi: 10.1016/0041-008x(91)90191-g. [DOI] [PubMed] [Google Scholar]
- Correa P. Is gastric cancer preventable? Gut. 2004;53:1217–1219. doi: 10.1136/gut.2004.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Klein CB. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006;36:155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostini F, Izzotti A, Bennicelli C, Camoirano A, Tampa E, De Flora S. Induction of apoptosis in the lung but not in the liver of rats receiving intra-tracheal instillations of chromium(VI) Carcinogenesis. 2002;23:587–593. doi: 10.1093/carcin/23.4.587. [DOI] [PubMed] [Google Scholar]
- Dana Devi K, Rozati R, Saleha Banu B, Jamil K, Grover P. In vivo genotoxic effect of potassium dichromate in mice leukocytes using comet assay. Food Chem. Toxicol. 2001;39:859–865. doi: 10.1016/s0278-6915(01)00019-9. [DOI] [PubMed] [Google Scholar]
- Davidson T, Kluz T, Burns F, Rossman T, Zhang Q, Uddin A, Nadas A, Costa M. Exposure to chromium (VI) in the drinking water increases susceptibility to UV-induced skin tumors in hairless mice. Toxicol. Appl. Pharmacol. 2004;196:431–437. doi: 10.1016/j.taap.2004.01.006. [DOI] [PubMed] [Google Scholar]
- De Flora S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- De Flora S, Camoirano A, Bagnasco M, Bennicelli C, Corbett GE, Kerger BD. Estimates of the chromium(VI) reducing capacity in human body compartments as a mechanism for attenuating its potential toxicity and carcinogenicity. Carcinogenesis. 1997;18:531–537. doi: 10.1093/carcin/18.3.531. [DOI] [PubMed] [Google Scholar]
- De Flora S, D'Agostini F, Balansky R, Micale R, Baluce B, Izzotti A. Lack of genotoxic effects in hematopoietic and gastrointestinal cells of mice receiving chromium(VI) with the drinking water. Mutat. Res. 2008;659:60–67. doi: 10.1016/j.mrrev.2007.11.005. [DOI] [PubMed] [Google Scholar]
- De Flora S, Iltcheva M, Balansky RM. Oral chromium(VI) does not affect the frequency of micronuclei in hematopoietic cells of adult mice and of transplacentally exposed fetuses. Mutat. Res. 2006;610:38–47. doi: 10.1016/j.mrgentox.2006.06.011. [DOI] [PubMed] [Google Scholar]
- De Flora S, Morelli A, Basso C, Romano M, Serra D, De Flora A. Prominent role of DT-diaphorase as a cellular mechanism reducing chromium(VI) and reverting its mutagenicity. Cancer Res. 1985;45:3188–3196. [PubMed] [Google Scholar]
- Dellarco VL, Wiltse JA. US Environmental Protection Agency's revised guidelines for carcinogen risk assessment: incorporating mode of action data. Mutat. Res. 1998;405:273–277. doi: 10.1016/s0027-5107(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Donaldson RM, Jr., Barreras RF. Intestinal absorption of trace quantities of chromium. J. Lab. Clin. Med. 1966;68:484–493. [PubMed] [Google Scholar]
- Febel H, Szegedi B, Huszar S. Absorption of inorganic, trivalent and hexavalent chromium following oral and intrajejunal doses in rats. Acta Vet. Hung. 2001;49:203–209. doi: 10.1556/004.49.2001.2.10. [DOI] [PubMed] [Google Scholar]
- Finley BL, Kerger BD, Katona MW, Gargas ML, Corbett GC, Paustenbach DJ. Human ingestion of chromium (VI) in drinking water: pharmacokinetics following repeated exposure. Toxicol. Appl. Pharmacol. 1997;142:151–159. doi: 10.1006/taap.1996.7993. [DOI] [PubMed] [Google Scholar]
- Fryzek JP, Mumma MT, McLaughlin JK, Henderson BE, Blot WJ. Cancer mortality in relation to environmental chromium exposure. J. Occup. Environ. Med. 2001;43:635–640. doi: 10.1097/00043764-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Kelsh MA, Mai DH, Suh M, Proctor DM. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer Epidemiol. 2010;34:388–399. doi: 10.1016/j.canep.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Geigl JB, Obenauf AC, Schwarzbraun T, Speicher MR. Defining 'chromosomal instability'. Trends Genet. 2008;24:64–69. doi: 10.1016/j.tig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Greater Glasgow Health Board (GGHB) Assessment of the Risk to Human Health from Land Contaminated by Chromium Waste. Department of Public Health, Greater Glasgow Health Board; 1991. [Google Scholar]
- Gordon E. Captan: transition from 'B2' to 'not likely'. How pesticide registrants affected the EPA Cancer Classification Update. J. Appl. Toxicol. 2007;27:519–526. doi: 10.1002/jat.1265. [DOI] [PubMed] [Google Scholar]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greater Tokyo Bureau of Hygiene (GTBH) Report Concerning the Effect of Chromium in a Health Survey. Greater Tokyo Bureau of Hygiene; 1989. [Google Scholar]
- He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797–802. doi: 10.1136/gut.2005.078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer J, Yang K, Lipkin M, Edelmann W, Kucherlapati R. Mouse models for colorectal cancer. Oncogene. 1999;18:5325–5333. doi: 10.1038/sj.onc.1203036. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Kondo K, Takahashi Y, Ishikura H, Fujino H, Tsuyuguchi M, Hashimoto M, Yokose T, Mukai K, Kodama T, et al. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol. Carcinog. 2002;33:172–180. doi: 10.1002/mc.10035. [DOI] [PubMed] [Google Scholar]
- Hoel DG, Portier CJ. Nonlinearity of dose-response functions for carcinogenicity. Environ. Health Perspect. 1994;102(Suppl. 1):109–113. doi: 10.1289/ehp.94102s1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Wise JP., Sr Carcinogenicity of hexavalent chromium. Indian J. Med. Res. 2008;128:353–372. [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Chromium, nickel and welding. IARC Monogr. Eval. Carcinog. Risks Hum. 1990;49:1–648. [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Shimada H. Micronucleus induction by chromium and selenium, and suppression by metallothionein inducer. Mutat. Res. 1996;367:233–236. doi: 10.1016/s0165-1218(96)90082-8. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Bagnasco M, Camoirano A, Orlando M, De Flora S. DNA fragmentation, DNA-protein crosslinks, postlabeled nucleotidic modifications, and 8-hydroxy-2'-deoxyguanosine in the lung but not in the liver of rats receiving intratracheal instillations of chromium(VI). Chemoprevention by oral N-acetylcysteine. Mutat. Res. 1998;400:233–244. doi: 10.1016/s0027-5107(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Cartiglia C, Balansky R, D'Agostini F, Longobardi M, De Flora S. Selective induction of gene expression in rat lung by hexavalent chromium. Mol. Carcinog. 2002;35:75–84. doi: 10.1002/mc.10077. [DOI] [PubMed] [Google Scholar]
- James BR, Petura JC, Vitale RJ, Mussoline GR. Oxidation-reduction chemistry of chromium: relevance to the regulation and remediation of chromate-contaminated soils. J. Soil Contam. 1997;6:569–580. [Google Scholar]
- Jarabek AM, Pottenger LH, Andrews LS, Casciano D, Embry MR, Kim JH, Preston RJ, Reddy MV, Schoeny R, Shuker D, et al. Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit. Rev. Toxicol. 2009;39:659–678. doi: 10.1080/10408440903164155. [DOI] [PubMed] [Google Scholar]
- Julien E, Boobis AR, Olin SS. The key events dose-response framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit. Rev. Food Sci. Nutr. 2009;49:682–689. doi: 10.1080/10408390903110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerger BD, Butler WJ, Paustenbach DJ, Zhang J, Li S. Cancer mortality in Chinese populations surrounding an alloy plant with chromium smelting operations. J. Toxicol. Environ. Health A. 2009;72:329–344. doi: 10.1080/15287390802529898. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Paustenbach DJ, Corbett GE, Finley BL. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicol. Appl. Pharmacol. 1996;141:145–158. doi: 10.1006/taap.1996.0271. [DOI] [PubMed] [Google Scholar]
- Klein CB, Su L, Bowser D, Leszczynska J. Chromate-induced epimutations in mammalian cells. Environ. Health Perspect. 2002;110(Suppl. 5):739–743. doi: 10.1289/ehp.02110s5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen I. The mammalian spot test and its use for the testing of potential carcinogenicity of welding fume particles and hexavalent chromium. Acta Pharmacol. Toxicol. (Copenh) 1980;47:66–70. doi: 10.1111/j.1600-0773.1980.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Kondo K, Hino N, Sasa M, Kamamura Y, Sakiyama S, Tsuyuguchi M, Hashimoto M, Uyama T, Monden Y. Mutations of the p53 gene in human lung cancer from chromate-exposed workers. Biochem. Biophys. Res. Commun. 1997;239:95–100. doi: 10.1006/bbrc.1997.7425. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease—radicals or ridiculous? Aliment. Pharmacol. Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J. Biol. Chem. 1990;265:462–471. [PubMed] [Google Scholar]
- Levine DS. Does asbestos exposure cause gastrointestinal cancer? Dig. Dis. Sci. 1985;30:1189–1198. doi: 10.1007/BF01314055. [DOI] [PubMed] [Google Scholar]
- Li GQ, Xie J, Lei XY, Zhang L. Macrophage migration inhibitory factor regulates proliferation of gastric cancer cells via the PI3K/Akt pathway. World J. Gastroenterol. 2009;15:5541–5548. doi: 10.3748/wjg.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MC, Gawkrodger DJ, MacNeil S. Chromium- and nickel-induced cytotoxicity in normal and transformed human keratinocytes: an investigation of pharmacological approaches to the prevention of Cr(VI)-induced cytotoxicity. Br. J. Dermatol. 1996;134:199–207. [PubMed] [Google Scholar]
- Liu KJ, Shi X. In vivo reduction of chromium (VI) and its related free radical generation. Mol. Cell. Biochem. 2001;222:41–47. [PubMed] [Google Scholar]
- Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 2001;81:1499–1533. doi: 10.1152/physrev.2001.81.4.1499. [DOI] [PubMed] [Google Scholar]
- Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- McCarroll N, Keshava N, Chen J, Akerman G, Kligerman A, Rinde E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: chromium (VI) Environ. Mol. Mutagen. 2010;51:89–111. doi: 10.1002/em.20525. [DOI] [PubMed] [Google Scholar]
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. A framework for human relevance analysis of information on carcinogenic modes of action. Crit. Rev. Toxicol. 2003;33:591–653. doi: 10.1080/713608373. [DOI] [PubMed] [Google Scholar]
- Meng F, Bermudez E, McKinzie PB, Andersen ME, Clewell HJ, Parsons BL. Measurement of tumor-associated mutations in the nasal mucosa of rats exposed to varying doses of formaldehyde. Regul. Toxicol. Pharmacol. 2010;57:274–283. doi: 10.1016/j.yrtph.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Mirsalis JC, Hamilton CM, O'Loughlin KG, Paustenbach DJ, Kerger BD, Patierno S. Chromium (VI) at plausible drinking water concentrations is not genotoxic in the in vivo bone marrow micronucleus or liver unscheduled DNA synthesis assays. Environ. Mol. Mutagen. 1996;28:60–63. doi: 10.1002/(SICI)1098-2280(1996)28:1<60::AID-EM9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mossman KL. Ionizing radiation and cancer. Cancer Invest. 1984;2:301–310. doi: 10.3109/07357908409018444. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (NAS) Toxicity Testing in the 21stCentury—A Vision and a Strategy. Washington, DC: National Research Council of the National Academies, National Academies Press; 2007. [Google Scholar]
- National Cancer Institute (NCI) Bioassay of captan for possible carcinogenicity, CAS No. 133-06-2. Natl. Cancer Inst. Carcinog. Tech. Rep. Ser. 1977;15:1–96. [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) Criteria for a Recommended Standard: Occupational Exposure to Chromium (VI). HEW Publication No. (NIOSH) Toxic. Rep. Ser. 1975:76–129. [Google Scholar]
- National Toxicology Program (NTP) NTP technical report on the toxicity studies of sodium dichromate dihydrate (CAS No. 7789-12-0) administered in drinking water to male and female F344/N rats and B6C3F1 mice and male BALB/c and am3-C57BL/6 mice. Toxic. Rep. Ser. 2007;72:1–74. NIH Publication No. 07-5964. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Draft NTP technical report on the toxicology and carcinogenesis studies of chromium picolinate monohydrate (CAS NO. 27882-76-4) in F344/N rats and B6C3F1 mice (feed studies) Toxic. Rep. Ser. 2008a;556:1–84. NIH Publication No. 08-5897. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) NTP technical report on the toxicology and carcinogenesis studies of sodium dichromate dihydrate (CAS No. 7789-12-0) in F344/N rats and B6C3F1 mice (drinking water studies) Toxic. Rep. Ser. 2008b;546 NIH Publication No. 08-5887. 1–192. [PubMed] [Google Scholar]
- Nickens KP, Patierno SR, Ceryak S. Chromium genotoxicity: a double-edged sword. Chem. Biol. Interact. 2010;188:276–288. doi: 10.1016/j.cbi.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- O'Flaherty EJ, Kerger BD, Hays SM, Paustenbach DJ. A physiologically based model for the ingestion of chromium(III) and chromium(VI) by humans. Toxicol. Sci. 2001;60:196–213. doi: 10.1093/toxsci/60.2.196. [DOI] [PubMed] [Google Scholar]
- O'Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium (VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J. Cell. Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oze C, Bird DK, Fendorf S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6544–6549. doi: 10.1073/pnas.0701085104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol. Cell. Biol. 2005;25:3596–3607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DM, Hays SM, Ruby MV, Liu S, Sjong A, Goodman M, Paustenbach DJ. Rate of hexavalent chromium reduction by human gastric fluid. Toxicol. Sci. 2002a;66:347. [Google Scholar]
- Proctor DM, Otani JM, Finley BL, Paustenbach DJ, Bland JA, Speizer N, Sargent EV. Is hexavalent chromium carcinogenic via ingestion? A weight-of-evidence review. J. Toxicol. Environ. Health A. 2002b;65:701–746. doi: 10.1080/00984100290071018. [DOI] [PubMed] [Google Scholar]
- Public Health Service (PHS) Health of Workers in Chromate Producing Industry, A Study. 1953 Public Health Service Publication 192. Federal Security Agency, Public Health Service, Division of Occupational Health of the Bureau of State Services. [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, Slikker W. Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CF, Urbano AM, Matoso E, Carreira I, Almeida A, Santos P, Botelho F, Carvalho L, Alves M, Monteiro C, et al. Human bronchial epithelial cells malignantly transformed by hexavalent chromium exhibit an aneuploid phenotype but no microsatellite instability. Mutat. Res. 2009;670:42–52. doi: 10.1016/j.mrfmmm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Rossmann H, Alper SL, Nader M, Wang Z, Gregor M, Seidler U. Three 5'-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann. N. Y. Acad. Sci. 2000;915:81–91. doi: 10.1111/j.1749-6632.2000.tb05226.x. [DOI] [PubMed] [Google Scholar]
- Ruemmele P, Dietmaier W, Terracciano L, Tornillo L, Bataille F, Kaiser A, Wuensch PH, Heinmoeller E, Homayounfar K, Luettges J, et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am. J. Surg. Pathol. 2009;33:691–704. doi: 10.1097/PAS.0b013e3181983ef7. [DOI] [PubMed] [Google Scholar]
- Ryan JC. Premalignant conditions of the small intestine. Semin. Gastrointest. Dis. 1996;7:88–93. [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem. Res. Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Sharma A, Talukder G. Differential protection of chlorophyllin against clastogenic effects of chromium and chlordane in mouse bone marrow in vivo. Mutat. Res. 1993;301:33–38. doi: 10.1016/0165-7992(93)90053-x. [DOI] [PubMed] [Google Scholar]
- Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol. Cell. Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman RM, Beaumont J, McDonald TA, Reynolds S, Krowech G, Howd R. Review of the evidence regarding the carcinogenicity of hexavalent chromium in drinking water. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2006;24:155–182. doi: 10.1080/10590500600614337. [DOI] [PubMed] [Google Scholar]
- Seed J, Carney EW, Corley RA, Crofton KM, DeSesso JM, Foster PM, Kavlock R, Kimmel G, Klaunig J, Meek ME, et al. Overview: using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit. Rev. Toxicol. 2005;35:664–672. doi: 10.1080/10408440591007133. [DOI] [PubMed] [Google Scholar]
- Sengupta T, Chattopadhyay D, Ghosh N, Das M, Chatterjee GC. Effect of chromium administration on glutathione cycle of rat intestinal epithelial cells. Indian J. Exp. Biol. 1990;28:1132–1135. [PubMed] [Google Scholar]
- Shindo Y, Toyoda Y, Kawamura K, Kurebe M, Shimada H, Hattori C, Satake S. Micronucleus test with potassium chromate(VI) administered intraperitoneally and orally to mice. Mutat. Res. 1989;223:403–406. doi: 10.1016/0165-1218(89)90096-7. [DOI] [PubMed] [Google Scholar]
- Silberg DG, Wang W, Moseley RH, Traber PG. The Down regulated in Adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J. Biol. Chem. 1995;270:11897–11902. doi: 10.1074/jbc.270.20.11897. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr., Andersen ME, Bogdanffy MS, Bus JS, Cohen SD, Conolly RB, David RM, Doerrer NG, Dorman DC, Gaylor DW, et al. Dose-dependent transitions in mechanisms of toxicity: case studies. Toxicol. Appl. Pharmacol. 2004;201:226–294. doi: 10.1016/j.taap.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Stern AH. A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure. Environ. Res. 2010;110:798–807. doi: 10.1016/j.envres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stout MD, Herbert RA, Kissling GE, Collins BJ, Travlos GS, Witt KL, Melnick RL, Abdo KM, Malarkey DE, Hooth MJ. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ. Health Perspect. 2009a;117:716–722. doi: 10.1289/ehp.0800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MD, Nyska A, Collins BJ, Witt KL, Kissling GE, Malarkey DE, Hooth MJ. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem. Toxicol. 2009b;47:729–733. doi: 10.1016/j.fct.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhou X, Chen H, Li Q, Costa M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009;237:258–266. doi: 10.1016/j.taap.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Zhitkovich A, Kluz T, Costa M. Rats retain chromium in tissues following chronic ingestion of drinking water containing hexavalent chromium. Biol. Trace Elem. Res. 2000;74:41–53. doi: 10.1385/BTER:74:1:41. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kondo K, Hirose T, Nakagawa H, Tsuyuguchi M, Hashimoto M, Sano T, Ochiai A, Monden Y. Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol. Carcinog. 2005;42:150–158. doi: 10.1002/mc.20073. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found. Symp. 2004;256:6–21. discussion 22–28, 49–52, 266–269. [PubMed] [Google Scholar]
- Tokuoka M, Ishii H, Mimori K, Inoue H, Doki Y, Mori M. Genetic susceptibility to gastrointestinal cancer: minireview of the genomewide studies. Ann. Surg. Oncol. 2009;16:1783–1788. doi: 10.1245/s10434-009-0425-5. [DOI] [PubMed] [Google Scholar]
- Uddin AN, Burns FJ, Rossman TG, Chen H, Kluz T, Costa M. Dietary chromium and nickel enhance UV-carcinogenesis in skin of hairless mice. Toxicol. Appl. Pharmacol. 2007;221:329–338. doi: 10.1016/j.taap.2007.03.030. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) National primary drinking water regulations—synthetic organic chemicals and inorganic chemicals; monitoring for unregulated contaminants; national primary drinking water regulations implementation; national secondary drinking water regulations. Final rule. Fed. Regist. 1991;56:3526–3597. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) Toxicological Review of Hexavalent Chromium in Support of Summary Information on the Integrated Risk Information System (IRIS) Washington, DC: U.S. Environmental Protection Agency; 1998. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) Captan; cancer reclassification; amendment of reregistration eligibility decision; notice of availability. Fed. Regist. 2004;69:68357–68360. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) Guidelines for Carcinogen Risk Assessment. Washington, DC: Risk Assessment Forum: U.S. Environmental Protection Agency; 2005. EPA/630/P-03/001F. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA) Draft Framework for Determining a Mutagenic Mode of Action for Carcinogenicity: Using EPA's 2005 Cancer Guidelines and Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Washinton, DC: U.S. Environmental Protection Agency; 2007. [Google Scholar]
- Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat. Rev. Cancer. 2009;9:489–499. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- Williams GM. Application of mode-of-action considerations in human cancer risk assessment. Toxicol. Lett. 2008;180:75–80. doi: 10.1016/j.toxlet.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Yao H, Guo L, Jiang BH, Luo J, Shi X. Oxidative stress and chromium(VI) carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2008;27:77–88. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- Zhang JD, Li XL. Chromium pollution of soil and water in Jinzhou. Zhonghua Yu Fang Yi Xue Za Zhi. 1987;21:262–264. [PubMed] [Google Scholar]
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.