Abstract
Excessive proliferation of vascular smooth muscle cells (VSMCs), which migrate from the tunica media to the subendothelial region, is one of the primary lesions involved in atherogenesis in diabetes. Here, we investigated whether high glucose potentiated the proliferation and chemotaxis of VSMCs by activating SDF-1α/CXCR4/PI-3K/Akt signalling. The expression of SDF-1α, CXCR4 and PCNA was up-regulated in tunica media of thoracic aortas by streptozotocin-induced hyperglycaemic Sprague–Dawley rats. Exposure of primary VSMCs to high glucose (25 mM) led to the up-regulated expression of SDF-1α and CXCR4, activated PI-3K/Akt signalling, and consequently promoted the proliferation and chemotaxis of VSMCs. Interestingly, the administration of SDF-1 siRNA or neutralizing antibody against SDF-1α abolished high glucose-induced up-regulation of CXCR4. Moreover, pretreatment with SDF-1α neutralizing antibody, CXCR4 specific inhibitor (AMD3100) or PI-3K inhibitor (LY294002) attenuated the high glucose-potentiated proliferation and chemotaxis in VSMCs. These results suggested that high glucose activated the SDF-1α/CXCR4/PI-3K/Akt signalling pathway in VSMCs in an autocrine manner, which enhanced the proliferation and chemotaxis of VSMCs.
Keywords: chemotaxis, CXCR4, glucose, proliferation, SDF-1α, vascular smooth muscle cell
One of the main features in diabetic patients is that many vascular complications, including atherosclerosis, worsen with the progression of the patient's condition [UK Prospective Diabetes Study (UKPDS) Group 1998], and the abnormal elevated level of blood glucose (hyperglycaemia) is considered one of the major causes of vascular complications in diabetes mellitus (The Diabetes Control and Complications Trial Research Group 1993). The formation of organized atherosclerotic plaques in patients with chronic hyperglycaemia involves a complex series of events, including abnormal proliferation and chemotaxis of vascular smooth muscle cells (VSMCs). In early atherosclerosis, smooth muscle cells migrate from the tunica media to the intima of the arterial wall.
The regulation of chemotaxis and proliferation of VSMCs, and their responses to proinflammatory signals are important factors in the pathogenesis of atherosclerosis (Kodali et al. 2006). Recent investigations have demonstrated that functional chemokine receptors, including C-C chemokine receptor 5 (CCR5) and C-X-C chemokine receptor 4 (CXCR4), are expressed on VSMCs (Hayes et al. 1998; Schecter et al. 2003). Upon activation, VSMCs switch from a predominant contractile phenotype to a synthetic secretory phenotype (Li et al. 1999); by secreting various chemokines and cytokines, the activated VSMCs can recruit macrophages and lymphocytes to the vessel wall and respond to these proteins in an autocrine manner. CXCR4 is a CXC chemokine receptor that was initially found to be essential for the entry of HIV-1 into host cells (Feng et al. 1996). After binding with its ligand, stromal cell-derived factor-1-alpha (SDF-1α), the activated SDF-1α/CXCR4 signalling pathway promotes some biological effects, such as cell proliferation, chemotaxis and migration, which have been demonstrated in numerous studies. Recently, a study by Sakihama et al. indicated that the interaction between SDF-1α and CXCR4 plays a key role in the development of transplant arteriosclerosis (Sakihama et al. 2004). Moreover, Zernecke et al. confirmed that the interaction between SDF-1α and its receptor CXCR4 is involved in neointimal hyperplasia by the recruitment of BM-derived SMC progenitor cells (Zernecke et al. 2005). Therefore, SDF-1α seems to be a promising molecular target in cardiovascular medicine (Schober et al. 2006; Gao & Li 2007). Although the earlier studies mainly focused on the role of the SDF-1α/CXCR4 axis in the recruitment of progenitor/inflammatory cells during the atherosclerosis process, the role of the SDF-1α/CXCR4 axis in the fate of VSMCs remains unclear.
Exposure of cells to high glucose activates multiple signalling pathways (Huang & Sheibani 2008). It is well known that PI-3K enzymes regulate many cellular responses, including proliferation, migration, intracellular vesicular transport, cytoskeletal rearrangements and anti-apoptosis (Vanhaesebroeck et al. 1999). The PI-3K signalling pathway involves the production of 3-phosphoinositides, which bind to the lipid-binding domains of a wide variety of proteins, including protein kinases (e.g. Akt) and regulators of small GTPases. The plectrin homology lipid-binding domain of Akt binds to the lipid products of PI-3K; Akt is then recruited to the plasma membrane and is phosphorylated at T308 and S473 to yield a fully activated kinase (Vanhaesebroeck & Alessi 2000). Recently, Zheng et al. confirmed that SDF-1α/CXCR4/eNOS signalling could activate PI-3K/Akt signalling in endothelial progenitor cells (Zheng et al. 2007); therefore, we hypothesized that the SDF-1α/CXCR4 could activate PI-3K/Akt signalling in VSMCs.
Although hyperglycaemia may directly cause many vascular diabetic complications, our current knowledge of the molecular mechanisms of gene regulation by glucose in VSMCs is incomplete and the underlying mechanisms of high glucose-induced atherosclerosis are not yet fully elucidated. The aim of this study was to investigate the role of SDF-1α/CXCR4 axis in the high glucose-induced proliferation and chemotaxis of VSMCs.
Method
Animal and reagents
Sprague–Dawley (SD) rats were obtained from the experimental animal centre of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, P.R. China). Rabbit anti-rat SDF-1α, Akt, phospho-Akt (Ser 473), CXCR4, SM22α, and PCNA; mouse-anti rat osteopontin (OPN) and goat-anti rat myocardin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); LY294002 was from Calbiochem (La Jolla, CA, USA). Streptozotocin, d-glucose, d-mannitol and the CXCR4 antagonist, AMD3100, were from Sigma Aldrich (Sigma Aldrich, St Louis, MO, USA). Recombinant rat SDF-1α was from PeproTech (PeproTech Inc., Rocky Hill, NJ, USA) and SDF-1α neutralization antibody (goat-anti mouse and rat) was from Torrey Pines Biolabs (Torrey Pines Biolabs, East Orange, NJ, USA). All procedures were performed in accordance with the Guidelines of the Hubei Council of Animal Care and approved by the Animal Use Subcommittee at the Huazhong University of Science and Technology, P.R.China.
Establishing rat model of type I diabetes
SD rats (180–220 g) were made diabetic by a single injection of streptozotocin (55 mg/kg, intraperitoneally) according to Zhao and Ramana's reports (Zhao et al. 2000; Ramana et al. 2004). Blood glucose was monitored for up to 3 weeks, and only the rats with blood glucose >16.6 mM were used for further study. At the end of 3 weeks after injection of streptozotocin, thoracic arterial samples were obtained. For protein homogenates and total RNAs, the intima and outer and inner tissue layers were removed carefully from samples, the tunica media was quickly immersed in liquid nitrogen for later use; for paraffin-embedded sections, samples were fixed in neutral formalin.
Cell culture
Primary VSMCs were isolated from SD rat aortic arteries and identified as previously described (Majack & Clowes 1984; Fujiwara et al. 1994). VSMCs were maintained in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA, USA) containing 5.5 mM glucose and 10% FBS (Gibco). Cells were used at passage 4–6 for the experiments. DMEM containing 25 mM d-glucose was used as a high glucose condition (HG) in all experiments, while basal DMEM containing 5.5 mM glucose (normal glucose, NG) and DMEM containing 25 mM d-mannitol were used as the NG control and an osmotic control respectively. In all experiments, cells were serum-starved for 24 h with DMEM containing 0.5% FBS and then subjected to the treatments for 48 h.
VSMC proliferation assay
VSMCs were seeded in six-well plates (Corning, Lowell, MA, USA) (1 × 104/well). After recovery overnight in an incubator at 37 °C, 5% CO2, the cells were then serum-starved for 24 h, exposed to the indicated treatments for 48 h and counted using a haemocytometer.
VSMC chemataxis assay
Chemotaxis of VSMCs was assessed as previously described (Kuang et al. 2008). Briefly, 200 μl of basal or treated DMEM was placed in the lower chamber of a 24-well transwell plate (Corning). A polycarbonate membrane with 8-μm pores separated the upper and lower chambers. Preconfluent VSMCs treated in the same way were then suspended in DMEM/0.5% FBS to a concentration of 4 × 105 cells/ml. Then, 200 μl of the cell suspension was added to the upper compartment. After 8 h at 37 °C in 5% CO2, the chamber was disassembled. The membrane was removed and scraped to remove non-migrating VSMCs from the upper surface. The membrane was then fixed and stained. The numbers of VSMCs that had migrated to the lower surface of the membrane were counted in ten random high-power fields (HPFs) by light microscopy and the chemotactic index (CI) was calculated to express stimulated migration. Each assay was performed in triplicate wells.
Determination of SDF-1α protein by ELISA
The amount of SDF-1α protein secreted into the medium by cultured VSMCs and in protein homogenates of VSMCs and hyperglycaemic rat thoracic arties was determined by ELISA (R&D systems, Minneapolis, MN, USA). The polystyrene microplate (96 wells) was coated with a rabbit polyclonal anti-SDF-1α antibody, and recombinant rat SDF-1α was used as the standard.
Small interfering RNA knockdown experiments
An siRNA targeting rat SDF-1α, described by Menon et al. (2007) was used to knockdown high-glucose-induced SDF-1α in VSMCs. The VSMCs plated in six-well plates were transfected with siRNAs using Lipofectamine 2000 transfection reagent in accordance with the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). The siRNA sequences were as follows: sense, 5′-GCA GUG AUU ACU UCA AGG Utt-3′; antisense, 5′-ACC UUG AAG UAA UCA CUG Ctt-3′. A negative control siRNA (scrambled sequence) (catalogue number 4611; Ambion Inc., Austin, TX, USA) was used as a control for nonspecific gene silencing. After transfecting the VSMCs with siRNA for 8 h, the medium was changed into HG-enriched DMEM and incubated for 48 h. The cells were then harvested for Western blotting and RT-PCR analysis.
Immunohistochemical staining
After hyperglycaemic rats were sacrificed, the thoracic arterial slices were prepared for immunohistochemical staining by using rabbit polyclonal antibodies (1:100) against rat SDF-1α, CXCR4, SM22α, OPN and PCNA. Endogenous peroxidase was blocked by 0.3% H2O2, sections were incubated with primary antibodies overnight at 4 °C, non-immune IgGs were used as negative control and antigenic sites were localized using an SP kit (Zymed, San Francisco, CA, USA).
Immunofluorescent staining
Indirect immunofluorescence was performed on VSMCs. After incubation overnight with the primary antibodies (CXCR4 and SDF-1α; 1:100), the antigenic sites were localized using FITC or TRITC-conjugated goat anti-rabbit IgG. Images of the antigenic sites were captured by a laser scanning confocal microscope (FV500; Olympus, Tokyo, Japan).
Protein isolation and immunoblot analysis
Cells and the thoracic arterial samples were homogenized in cell lysis buffer and RIPA buffer respectively. Protein expression was measured by semiquantitative immunoblots. The membranes were probed with primary antibodies (CXCR4, 1:1000, p-Akt, 1:500, Akt 1:500, OPN 1:1000, myocardin 1:1000, PCNA 1:1000 and β-actin 1:2000) in TBST plus 3% skimmed milk overnight at 4 °C. Horseradish peroxidase-coupled secondary antibodies were diluted at 1:2000 and incubated for 1 h at room temperature. Bands were visualized using enhanced chemiluminescence reagents (Thermo Fisher, Rockford, IL, USA) and analysed with a gel imaging system.
RNA extraction and RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen). Briefly, 2 μg of total RNA was used as a template to generate first-strand cDNA by random priming using the Promega RT System. The pairs of primers as follows were used: SDF-1α forward: 5′-CCC TGC CGA TTC TTT GAG-3′, SDF-1α reverse: 5′-TGG GCT GTT GTG CTT ACT TG-3′; CXCR4 forward: 5′-CAG AAG AAG CTG AGG AGC ATG ACA-3′, CXCR4 reverse: 5′-CTG ATG AAG GCC AGG ATG AGA ACA-3′; SM22α forward: 5′-AGC CAG TGA AGG TGC CTG AGA AC-3′, SM22α reverse: 5′-TGC CCA AAG CCA TTA GAG TCC TC-3′; OPN forward: 5′-CTC TGA AGA AAC GGA TGA CT-3′, OPN reverse: 5′-CTG GGA TGA CCT TGA TAG CC-3′; PCNA forward: 5′-CTT GGA ATC CCA GAA CAG G-3′, PCNA reverse: 5′-AGA CAG TGG AGT GGC TTT T-3′; myocardin forward: 5′-GGG TCT GAA CAC TCT TTG C-3′, myocardin reverse: 5′-ATT ACC GTG GAG GCT TGG A-3′; β-actin forward: 5′-CGT TGA CAT CCG TAA AGA-3′, β-actin reverse: 5′-AGC CAC CAA TCC ACA CAG-3′. Duplicate real-time quantitative PCR was performed to analyse SDF-1α and CXCR4 mRNA expression by monitoring the increase in fluorescence of the SYBR Green dye using iCycler iQ (Bio-Rad, Hercules, CA, USA) in accordance with the manufacturer's instructions. The relative abundance of mRNA was determined from the CT values and was plotted as fold-change compared with control. The remaining genes were amplified by conventional PCR and assessed by semi-quantification; the products of PCR were separated by 1.5% agarose gel electrophoresis and visualized under UV using gel documentation system (Bio-Rad Gel Doc1000; Bio-Rad).
Statistical analysis
All data are expressed as mean ± SEM. For analysis of differences between two groups, Student's t-test was performed. For multiple groups, anova was carried out followed by Student–Newman–Keuls test. The level of statistical significance was set at P < 0.05.
Results
Effects of HG on SDF-1α and CXCR4 expression in VSMCs
We first established a rat model of type I diabetes by administration of streptozotocin. The results of immunohistochemistry staining indicated that the SDF-1α and CXCR4 in arteries were obviously up-regulated under hyperglycaemic conditions (Figure 1a,d), which further confirmed by the results of ELISA (Figure 1b), western blotting (Figure 1e) and qRT-PCR (Figure 1c,f). As SDF-1α and CXCR4 have not been characterized in primary VSMCs, we determined the cell type-specific expression of SDF-1α and CXCR4 in VSMCs cultured in NG and HG medium. The results of immunofluorescence, ELISA, western blotting and qRT-PCR confirmed that the expression of SDF-1α and CXCR4 was significantly enhanced in HG-treated VSMCs (Figure 2). These results suggested that high glucose enhanced the SDF-1α and CXCR4 expression ex vivo and in vitro.
Figure 1.
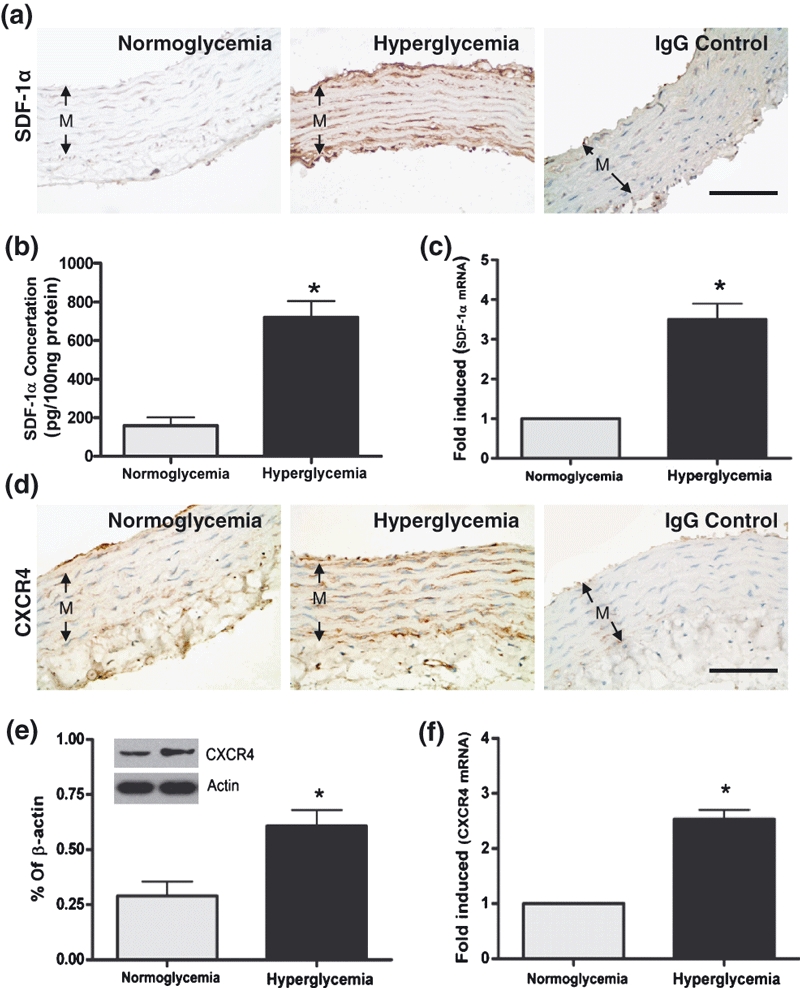
Expression of SDF-1α in the tunica media (M) of the thoracic arterial samples from the control (normoglycaemia) and type I diabetes (hyperglycaemia) rats by immunohistochemical staining (a), ELISA (b) and Q-PCR(c). Expression of CXCR4 in the tunica media (M) of the thoracic arterial samples from the control (normoglycaemia) and T1D (hyperglycaemia) rats by immunohistochemical staining (d), western blot (e) and qRT-PCR (f). For the immunohistochemical staining, non-immune IgGs were used as negative control. n = 10 per groups. Bars = 100 μm. Results were depicted as mean ± SEM. *P < 0.05.
Figure 2.
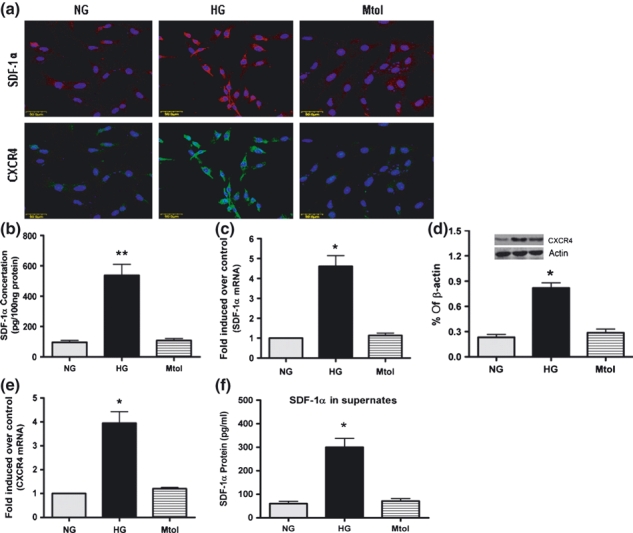
Expression of SDF-1α and CXCR4 in VSMCs cultured in normal glucose (NG), high glucose (HG) and osmotic control (Mtol) medium. (a) Immunofluorescent detection of SDF-1α and CXCR4 expression in VSMCs. TRITC- and FITC-conjugated anti-rabbit IgGs were used respectively, and cells were counterstained with Hoechst 33258. Expression of SDF-1α in VSMC was assessed by ELISA (cell lysates, b; cell supernates, f) and qRT-PCR (c). Expression of CXCR4 in VSMCs was determined by western blotting (d) and qRT-PCR (e). Bars = 50 μm. Results are mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 when vs. NG or Mtol.
HG-upregulated CXCR4 through the expression of SDF-1α
To explore whether HG-induced CXCR4 expression in VSMCs was dependent on up-regulation of SDF-1α, we pretreated the VSMCs with a neutralizing antibody (1 μg/ml) or siRNA against SDF-1α. The results revealed that pretreatment with the SDF-1α antibody or siRNA attenuated HG-upregulated CXCR4 expression; interestingly, when exogenous recombinant SDF-1α (100 ng/ml) was added, the decrease of CXCR4 expression by administration of siRNA against SDF-1α was rescued (Figure 3), these results suggested that the HG-induced CXCR4 expression in VSMCs was at least partly dependent on the present of SDF-1α, and that the SDF-1α/CXCR4 axis is active in VSMCs.
Figure 3.
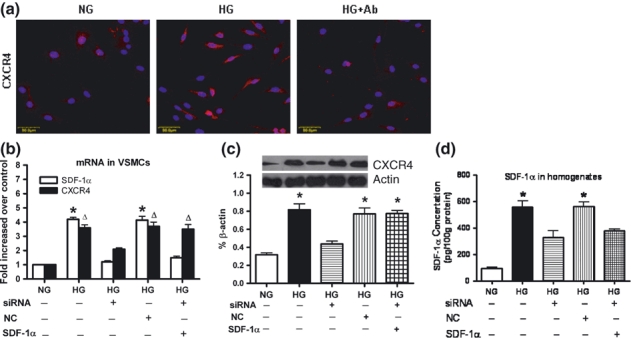
Activation of SDF-1α up-regulated CXCR4 in VSMCs. (a) Neutralizing antibody of SDF-1α abolished high glucose (HG)-induced CXCR4 expression in VSMCs, as assessed by immunofluorescence with TRITC-conjugated anti-rabbit IgG and counterstaining with Hoechst 33258. Bars = 50 μm. siRNA targeting SDF-1α attenuated HG-induced SDF-1 and CXCR4 expression in VSMCs, while administration of recombinant SDF-1a (100 ng/ml) rescued the SDF-1α siRNA-induced decrease of CXCR4 expression, as assessed by qRT-PCR (b) and Western blotting (c). ELISA results demonstrated that siRNA attenuated HG-induced SDF-1 expression in cell homogenates (d). Results are mean ± SEM of three independent experiments. *P < 0.05, ΔP < 0.05 when vs. NG or siRNA + HG. NC: negative control siRNA.
HG up-regulated Akt phosphorylation by activating the SDF-1α/CXCR4 axis
The results of western blotting revealed that in the NG medium, the cells expressed basal levels of total Akt and p-Akt, while HG significantly up-regulated p-Akt expression (Figure 4a,b). The administration of LY294002 (20 μM), an inhibitor of PI-3K, attenuated HG-induced up-regulation of p-Akt (Figure 4a,b), suggesting that HG activated the PI-3K/Akt signalling pathway. Pretreating VSMCs with AMD3100 (10 μM), a selective and highly specific CXCR4 antagonist (Hatse et al. 2002), markedly down-regulated Akt phosphorylation in HG-treated VSMCs. Similarly, pretreatment with the SDF-1α neutralizing antibody (1 μg/ml) also attenuated Akt phosphorylation (Figure 4c,d). When exogenous SDF-1α (100 ng/ml) was added, p-Akt expression in VSMCs was much higher than that in no SDF-1α-treated VSMCs (Figure 4e,f), and ELISA results (Figure 2f) revealed that HG simulated VSMCs to secret SDF-1α into the medium. Taken together, these findings indicated that the activation of PI-3K/Akt signalling in HG-treated VSMCs was partly as a result of the activation of SDF-1α/CXCR4 axis.
Figure 4.
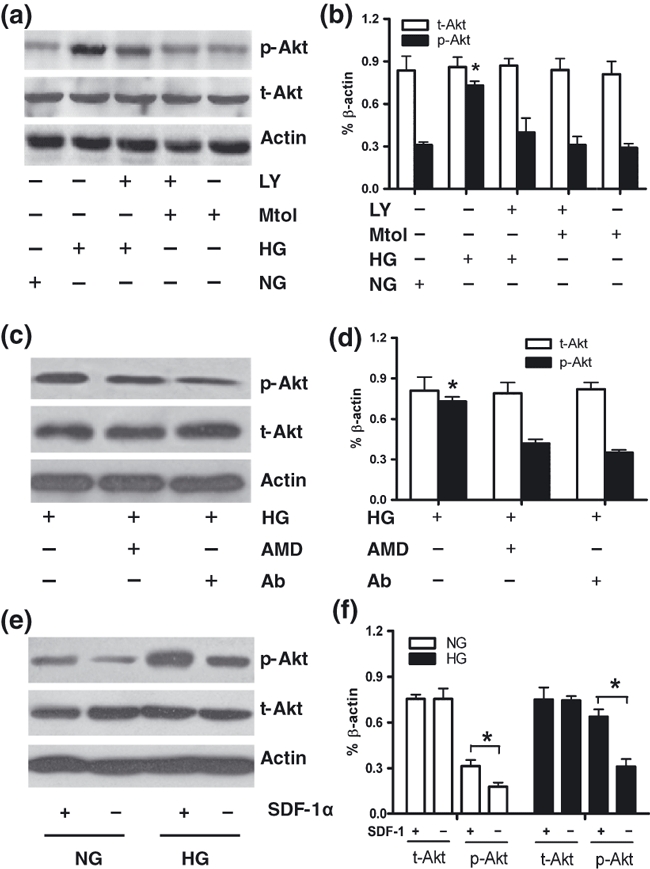
The expression of total Akt (t-Akt) and phosphorylated Akt (p-Akt) in VSMCs was analysed by Western blotting. (a and b) VSMCs were cultured in normal glucose (NG) or high glucose (HG) medium supplemented with or without the PI-3K inhibitor LY294002 (LY, 20 μM). Mannitol (Mtol) was used as an osmotic control. (c and d) VSMCs were maintained in HG medium supplemented with or without an antagonist of CXCR4, AMD3100 (AMD, 10 μM), or a neutralizing antibody against SDF-1α (Ab, 1 μg/ml). (e and f) western blot analysis showed that p-Akt expression was upregulated in recombinant SDF-1α-treated VSMCs either in NG or HG condition. Results are mean ± SEM of three independent experiments. *P < 0.05 (f) and *P < 0.05 when vs. other associated groups (b, d).
HG-potentiated VSMC proliferation and chemotaxis via activation of the SDF-1α/CXCR4/PI-3K/Akt pathway
Studies have documented that the SDF-1α/CXCR4 signalling pathway stimulates proliferation and migration in multiple types of cells (Shichinohe et al. 2007; Nemenoff et al. 2008; Schober 2008; Opatz et al. 2009); therefore, we further examined the effects of exogenous SDF-1α on the proliferation and chemotaxis of VSMCs. Our results showed that SDF-1α dose-dependently stimulated proliferation and chemotaxis in VSMCs (Figure 5a). Pretreatment of VSMCs with AMD3100 (10 μM) attenuated these stimulatory effects of SDF-1α (Figure 5b), suggesting that the SDF-1α/CXCR4 axis is involved in the proliferation and chemotaxis of VSMCs.
Figure 5.
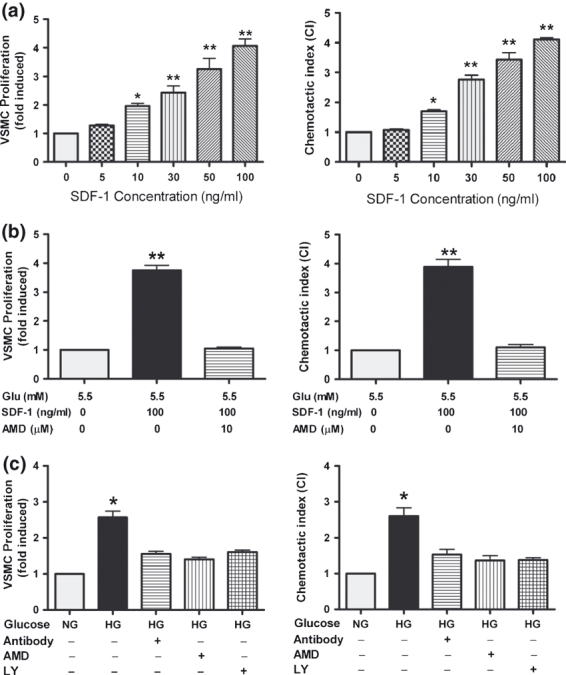
High glucose (HG) induced proliferation and chemotaxis of VSMCs. VSMCs were cultured in normal glucose (NG) medium containing 0, 5, 10, 30, 50 or 100 ng/ml of exogenous rat SDF-1α (a) or in NG medium supplemented with/without exogenous rat SDF-1α (100 ng/ml) and the CXCR4 antagonist, AMD3100 (AMD, 10 μM) (b) or in medium containing NG or HG supplemented with or without LY294002 (LY, 20 μM), AMD3100 (AMD, 10 μM), or neutralizing antibody against SDF-1α (Ab, 1 μg/ml) (c). Results are mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 (in A, vs. control only).
We then explored the role of HG on the proliferation and chemotaxis of VSMCs. HG significantly promoted the proliferation and chemotaxis of VSMCs. Treating VSMCs with AMD3100 (10 μM), SDF-1α neutralizing antibody (1 μg/ml) or LY294002 (20 μM) abolished HG-potentiated proliferation and migration (Figure 5c). These results showed that high glucose may induce the increased abilities in proliferation and chemotaxis of VSMCs at least partly by activating the SDF-1α/CXCR4/PI-3K/Akt signalling pathway.
HG-promoted phonotypic switching in VSMCs
As modulation of VSMCs growth properties occurs under certain microenvironment (Hultgardh-Nilsson et al. 1997; Xu et al. 1997; Lagna et al. 2007; Chadjichristos et al. 2008), the expression of the contractile and synthetic phenotype marker of VSMCs under high glucose were then detected. Three weeks of hyperglycaemia led VCMCs to down-regulate the expression of SM22α and up-regulate the expression of OPN; furthermore, hyperglycaemia promoted VSMCs to express PCNA, a marker for cell proliferation (Figure 6a). Results of in vitro also revealed that treating VSMCs with HG led to up-expression of OPN, PCNA and down-expression of SM22α, which were confirmed by RT-PCR and western blotting analysis (Figure 6b–e). Besides, myocardin (MYOCD), a nuclear transcription factor co-activated with serum reaction factor (SRF) in VSMCs differentiation (Chen et al. 2002; Lagna et al. 2007), was also decreased significantly in HG-treated VSMCs (Figure 6b–e). Interestingly, blockade of SDF-1/CXCR4 signalling with AMD3100 (10 μm) or SDF-1α neutralizing antibody (1 μg/ml) resulted in a significant downregulation of PCNA, but no significant changes in SM22α, OPN and myocardin mRNA in HG-treated VSMCs (Figure 6f,g).
Figure 6.
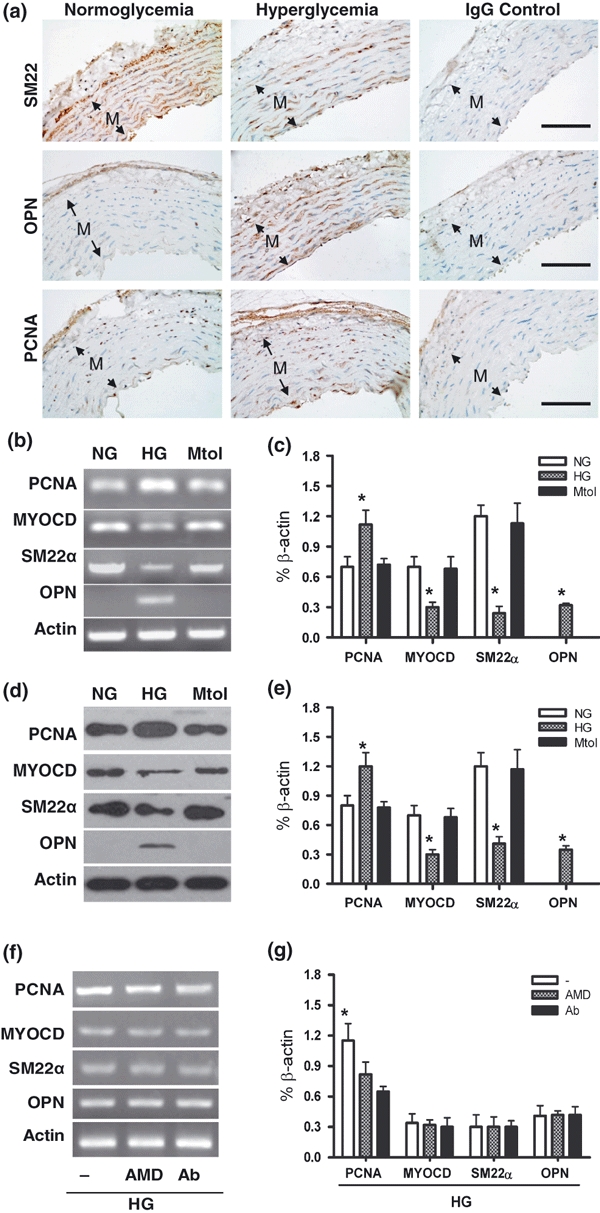
Expression of phenotypic markers in VSMCs. (a) SM22α, OPN and PCNA expressed in thoracic arterial samples from the control (normoglycaemia) and hyperglycaemic rats by immunohistochemical staining. Non-immune IgGs were used as negative control. n = 10 per groups. Bars = 100 μm. mRNA of SM22α, OPN, PCNA and myocardin were assessed by semiquantitative RT-PCR on VSMCs in NG or HG (b) and then the mRNAs expression was normalized to β-actin (c). Protein of SM22α, OPN, PCNA and myocardin were assessed by western blotting on VSMCs in NG or HG (d) and then the protein expression was normalized to β-actin (e). Expression of SM22α, OPN, PCNA and myocardin mRNA was examined in SDF-1α/CXCR4 signalling blockaded VSMCs exposed to HG by administration of AMD3100 (AMD, 10 μM) or SDF-1α neutralizing antibody (Ab, 1 μg/ml) (f and g). Results are mean ± SEM of four independent experiments. *P < 0.05 vs. two associated groups.
Discussion
The interaction between chemoattractants and receptors seems to initiate the signal transduction leading to VSMC chemotaxis (Abedi & Zachary 1995). It is likely that VSMCs are target cells for therapeutic intervention based on chemokines (Schober 2008) and the interaction between chemoattractants and receptors may influence a variety of normal and pathological processes that involve VSMCs, such as atherosclerosis and arterial injury. SDF-1α signalling has a wide range of effects on CXCR4-expressing cells depending on the cell type ranging from cell growth to adhesion, chemotaxis and migration (Yusuf et al. 2006). And the activation of SDF-1α/CXCR4 axis by VSMCs in an autocrine manner had been proposed (Schecter et al. 2003; Nemenoff et al. 2008). In the present study, we found that exposing VSMCs to HG conditions up-regulated SDF-1α and CXCR4 ex vivo and in vitro, and thus stimulated the SDF-1α/CXCR4 axis, and our results also revealed that HG-induced expression of CXCR4 in VSMCs was at least partly because of the presence of SDF-1α, which is similar to the findings reported by Kukreja et al., in which SDF-1α regulated CXCR4 expression in prostate cancer PC-3 cells via MEK/ERK signalling cascade and NF-kappaB activation (Kukreja et al. 2005).
In the cultured VSMCs exposed to HG, the activation of some signalling mediators such as ERK, MAPK, Rho/Rho-kinase, IGF-1 and NF-κB has been reported (Igarashi et al. 1999; Campbell et al. 2003, 2004; Maile et al. 2007; Akiyama et al. 2008; Cifarelli et al. 2008; Yang et al. 2008). In the present study, the administration of the CXCR4 antagonist AMD3100, an inhibitor of PI-3K, LY294002, or the SDF-1α neutralizing antibody attenuated HG-induced Akt phosphorylation, which then affected VSMC proliferation and migration. This suggests that the activation of the SDF-1α/CXCR4/PI-3K/Akt signalling pathway plays a critical role in HG-potentiated cell proliferation and migration. This study is the first to demonstrate an association between SDF-1α/CXCR4 signalling and PI-3K/Akt signalling in VSMCs. However, recent studies showed that SDF-1α may be the downstream of Akt (Lee et al. 2006; Greijer et al. 2008), and multiple signalling pathways were activated after VSMCs were exposed to high glucose (Huang & Sheibani 2008), the association between SDF-1α/CXCR4/PI-3K/Akt and others signalling seems to be worth further investigation.
Cellular responses to HG are numerous and varied. It is clear that glucose can induce oxidative/nitrosative stress in many cell types (Ding et al. 2004; Connell et al. 2007), which may partially interpret the pro-atherogenic vascular remodelling (Pandolfi & De Filippis 2007). In this study, HG-induced decrease in SM22α expression and increase in OPN and PCNA expression were identified ex vivo and in vitro. SM22α and OPN are deemed as a marker for contractile and synthetic phenotype in mature VSMCs respectively, and OPN promotes the development of atherosclerosis (Kawamura et al. 2004). Mori et al. reported that the upregulation of OPN induced by high glucose may mediate via PKC-dependent pathway in VSMCs (Mori et al. 2002). Our results demonstrated that exposing VSMCs to high-glucose conditions led to the phenotypic switching, which is consistent with the report of Pandolfi et al. (2003). Besides, during the development of atherosclerosis, VSMCs undergo partial dedifferentiation, conducing them to acquire a more motile and proliferative phenotype (Ross 1993). The present results showed that treating VSMCs with HG led to down-expression of myocardin, a critical serum response factor coactor in the regulation of VSMC differentiation (Du et al. 2003). Furthermore, myocardin family of transcriptional co-activators are also versatile regulators of cell growth and migration (Pipes et al. 2006). However, administration of AMD3100 and SDF-1α neutralizing antibody led to decrease in PCNA, whereas the mRNA expression of SM22α, OPN and myocardin showed no significant changes. Thus, additional studies are needed to address the relationship between HG exposure and phonotypic switching of VSMCs.
In conclusion, our results support the hypothesis that exposing VSMCs to high glucose potentiates the proliferation and chemotaxis of these cells, most likely because of the activation of SDF-1α/CXCR4/PI-3K/Akt signalling. Our findings underscore the role of elevated glucose levels in VSMC functions, particularly in conditions of diabetic insult on the vasculature system.
Acknowledgments
This study was supported by research grants 30400192, 30770942 (Q. Ao), 30470710 and 30971153 (G. Wang) from the National Natural Science Foundation of China, and NCET-04-0711 from the Program for New Century Excellent Talents in University of China (G. Wang).
References
- Abedi H, Zachary I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc. Res. 1995;30:544–556. [PubMed] [Google Scholar]
- Akiyama N, Naruse K, Kobayashi Y, et al. High glucose-induced upregulation of Rho/Rho-kinase via platelet-derived growth factor receptor-beta increases migration of aortic smooth muscle cells. J. Mol. Cell. Cardiol. 2008;45:326–332. doi: 10.1016/j.yjmcc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Silversides JA, Trimble ER. Glucose-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase-dependent upregulation of the platelet-derived growth factor-beta receptor potentiates vascular smooth muscle cell chemotaxis. Diabetes. 2003;52:519–526. doi: 10.2337/diabetes.52.2.519. [DOI] [PubMed] [Google Scholar]
- Campbell M, Allen WE, Sawyer C, Vanhaesebroeck B, Trimble ER. Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on cross-talk between the PI3K and MAPK signaling pathways. Circ. Res. 2004;95:380–388. doi: 10.1161/01.RES.0000138019.82184.5d. [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Morel S, Derouette JP, et al. Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ. Res. 2008;102:653–660. doi: 10.1161/CIRCRESAHA.107.170472. [DOI] [PubMed] [Google Scholar]
- Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- Cifarelli V, Luppi P, Tse HM, He J, Piganelli J, Trucco M. Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis. 2008;201:248–257. doi: 10.1016/j.atherosclerosis.2007.12.060. [DOI] [PubMed] [Google Scholar]
- Connell P, Walshe T, Ferguson G, Gao W, O’Brien C, Cahill PA. Elevated glucose attenuates agonist- and flow-stimulated endothelial nitric oxide synthase activity in microvascular retinal endothelial cells. Endothelium. 2007;14:17–24. doi: 10.1080/10623320601177213. [DOI] [PubMed] [Google Scholar]
- Ding QF, Hayashi T, Packiasamy AR, et al. The effect of high glucose on NO and O2- through endothelial GTPCH1 and NADPH oxidase. Life Sci. 2004;75:3185–3194. doi: 10.1016/j.lfs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Du KL, Ip HS, Li J, et al. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Hayashi T, Nakai T, Miyabo S. Diltiazem inhibits DNA synthesis and Ca2+ uptake induced by insulin, IGF-I, and PDGF in vascular smooth muscle cells. Cardiovasc. Drugs Ther. 1994;8:861–869. doi: 10.1007/BF00877405. [DOI] [PubMed] [Google Scholar]
- Gao C, Li Y. SDF-1 plays a key role in the repairing and remodeling process on rat allo-orthotopic abdominal aorta grafts. Transplant. Proc. 2007;39:268–272. doi: 10.1016/j.transproceed.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Greijer AE, Delis-van Diemen PM, Fijneman RJ, et al. Presence of HIF-1 and related genes in normal mucosa, adenomas and carcinomas of the colorectum. Virchows Arch. 2008;452:535–544. doi: 10.1007/s00428-008-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- Hayes IM, Jordan NJ, Towers S, et al. Human vascular smooth muscle cells express receptors for CC chemokines. Arterioscler. Thromb. Vasc. Biol. 1998;18:397–403. doi: 10.1161/01.atv.18.3.397. [DOI] [PubMed] [Google Scholar]
- Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am. J. Physiol. Cell Physiol. 2008;295:C1647–C1657. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgardh-Nilsson A, Lovdahl C, Blomgren K, Kallin B, Thyberg J. Expression of phenotype- and proliferation-related genes in rat aortic smooth muscle cells in primary culture. Cardiovasc. Res. 1997;34:418–430. doi: 10.1016/s0008-6363(97)00030-8. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J. Clin. Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Yokote K, Asaumi S, et al. High glucose-induced upregulation of osteopontin is mediated via Rho/Rho kinase pathway in cultured rat aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:276–281. doi: 10.1161/01.ATV.0000112012.33770.2a. [DOI] [PubMed] [Google Scholar]
- Kodali R, Hajjou M, Berman AB, et al. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc. Res. 2006;69:706–715. doi: 10.1016/j.cardiores.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Kuang D, Zhao X, Xiao G, et al. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res. Cardiol. 2008;103:265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65:9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J. Biol. Chem. 2007;282:37244–37255. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Youn SW, Cho HJ, et al. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–159. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- Li S, Sims S, Jiao Y, Chow LH, Pickering JG. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ. Res. 1999;85:338–348. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology. 2007;148:2435–2443. doi: 10.1210/en.2006-1440. [DOI] [PubMed] [Google Scholar]
- Majack RA, Clowes AW. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J. Cell. Physiol. 1984;118:253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- Mori S, Takemoto M, Yokote K, Asaumi S, Saito Y. Hyperglycemia-induced alteration of vascular smooth muscle phenotype. J. Diabetes Complications. 2002;16:65–68. doi: 10.1016/s1056-8727(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Nemenoff RA, Simpson PA, Furgeson SB, et al. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ. Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- Opatz J, Kury P, Schiwy N, et al. SDF-1 stimulates neurite growth on inhibitory CNS myelin. Mol. Cell. Neurosci. 2009;40:293–300. doi: 10.1016/j.mcn.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Pandolfi A, De Filippis EA. Chronic hyperglicemia and nitric oxide bioavailability play a pivotal role in pro-atherogenic vascular modifications. Genes Nutr. 2007;2:195–208. doi: 10.1007/s12263-007-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi A, Grilli A, Cilli C, et al. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J. Cell. Physiol. 2003;196:378–385. doi: 10.1002/jcp.10325. [DOI] [PubMed] [Google Scholar]
- Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sakihama H, Masunaga T, Yamashita K, et al. Stromal cell-derived factor-1 and CXCR4 interaction is critical for development of transplant arteriosclerosis. Circulation. 2004;110:2924–2930. doi: 10.1161/01.CIR.0000146890.93172.6C. [DOI] [PubMed] [Google Scholar]
- Schecter AD, Berman AB, Taubman MB. Chemokine receptors in vascular smooth muscle. Microcirculation. 2003;10:265–272. doi: 10.1038/sj.mn.7800192. [DOI] [PubMed] [Google Scholar]
- Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler. Thromb. Vasc. Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- Schober A, Karshovska E, Zernecke A, Weber C. SDF-1alpha-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc. Med. 2006;16:103–108. doi: 10.1016/j.tcm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Jones GE, Allen WE, et al. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat. Cell Biol. 1999;1:69–71. doi: 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- Xu Y, Stenmark KR, Das M, Walchak SJ, Ruff LJ, Dempsey EC. Pulmonary artery smooth muscle cells from chronically hypoxic neonatal calves retain fetal-like and acquire new growth properties. Am. J. Physiol. 1997;273:L234–L245. doi: 10.1152/ajplung.1997.273.1.L234. [DOI] [PubMed] [Google Scholar]
- Yang WH, Park SY, Nam HW, et al. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc. Natl. Acad. Sci. USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf F, Rehimi R, Morosan-Puopolo G, Dai F, Zhang X, Brand-Saberi B. Inhibitors of CXCR4 affect the migration and fate of CXCR4+ progenitors in the developing limb of chick embryos. Dev. Dyn. 2006;235:3007–3015. doi: 10.1002/dvdy.20951. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Schober A, Bot I, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- Zhao W, Devamanoharan PS, Henein M, Ali AH, Varma SD. Diabetes-induced biochemical changes in rat lens: attenuation of cataractogenesis by pyruvate. Diabetes Obes. Metab. 2000;2:165–174. doi: 10.1046/j.1463-1326.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]


