Abstract
Tegumentary leishmaniasis is an important public health problem in several countries. The capacity of the Leishmania species, at the initial moments of the infection, to invade and survive inside the host cells involves the interaction of surface molecules that are crucial in determining the evolution of the disease. Using C57BL/6 wild-type and TLR-2−/− mice infected with L. (L.) amazonensis, we demonstrated that TLR-2−/− mice presented eosinophilic granuloma in the ear dermis, different from C57BL/6 wild-type mice that presented a cellular profile characterized mainly by mononuclear cell infiltrates, besides neutrophils and eosinophils, during the two first week of infection. When the parasite load was evaluated, we found that the absence of TLR-2 lead to a significant reduction of the infection in deficient mice, when compared with C57BL/6 mice which were more susceptible to the infection. Using TLR-2 deficient mice, it was possible to show that the absence of this receptor determined the reduction of the parasite load and the recruitment of inflammatory cells during the two first weeks after L. (L.) amazonensis infection.
Keywords: C57BL/6 wild-type, cellular profile, histopathology, Leishmania (L.) amazonensis, TLR-2 deficient mice, Toll-‘like’ receptor-2
Introduction
Leishmaniasis is an anthropozoonosis widely distributed worldwide. As a result of a multiplicity of agents, of insect vectors and animal reservoirs, this disease occurs in different clinical modalities. In South America, Brazil is the country with the highest occurrence of American tegumentary leishmaniasis (ATL), with more than 25,000 cases annually (Ministério da Saúde 2007). Among various parasites of the genus Leishmania, L. (L.) amazonensis is the causative agent of cutaneous leishmaniasis and cutaneous diffuse leishmaniasis (Almeida et al. 1996), characterized by the appearance of chronic lesions and disseminated through the skin, being a rare and disabling disease and with difficult treatment. The severity and the clinical form of the illness are directly related with the parasite as well as with the genetic and immunological factors of the host (Kane & Mosser 2000). According to the genotype of the mouse, L. (L.) major infection leads to the development of polarized Th1 or Th2 responses: where BALB/c mice represent a susceptibility model with a Th2 response that results in increased injury and the number of parasites, and C57BL/6 mice represent a resistant model, with a Th1 response with inhibition of parasite proliferation and healing of the lesion (McMahon-Pratt & Alexander 2004). In contrast, in the L. (L.) amazonensis infection the host can display an intermediate phenotype, where there is a balance between the Th1 and Th2 responses leading to the susceptibility of most mice strains (Ji et al. 2003).
So, the initial moments of the infection are crucial to determine the evolution of this disease (De Almeida et al. 2003). Skin is the main organ involved in the infection, where the resident and inflammatory cells play a crucial role in the initial immune response to the pathogens through the release of cytokines, chemokines and growth factors (Williams & Kupper 1996; Fuhlbrigge & Kupper 2004).
The capacity of the Leishmania species to invade and survive in the host cells involves complex mechanisms, with the participation of parasite and surface components of host cells. The TLR has been described as a family of Pattern Recognition Receptors (PRR), used by several cell types in the recognition, internalization and processing of antigens, acting as a molecule in the central link between innate and adaptive immune responses (Medzhitov & Janeway 2000; Akira & Hemmi 2003). Several studies have demonstrated the role of TLR in immunity against parasites through the recognition of molecules such as lysophosphatidylserine of S. mansoni (van der Kleij. 2002), anchor of GPI and GIPL of T. cruzi (Campos et al. 2001; Ouaissi et al. 2002).
Recent data have shown the involvement of TLR-2 by macrophages and NK cells in the recognition of the LPG of Leishmania spp. (Becker et al. 2003; De Veer et al. 2003). On the other hand, TLR-4 deficient mice infected by L. (L.) major presented an increase in the synthesis of IL-10 and the expression of the receptor for IL-4, besides the increased activity of arginase promoting parasite proliferation (Kropf et al. 2004a,b;). Several questions about the initial response to infection caused by L. (L.) amazonensis should be elucidated. Thus, in this work we studied the influence of TLR-2 in cellular recruitment and parasite load during the initial stages of L. (L.) amazonensis infection.
Materials and methods
Mice
Female C57BL/6 mice were obtained from the Animal Facility (CECAL) of the Fundação Oswaldo Cruz (CECAL/FIOCRUZ). Toll-Like Receptor 2 deficient mice (TLR-2−/−) in a homogeneous C57BL/6 background (Takeuchi et al. 1999) were kindly donated by Dr. Shizuo Akira (Osaka University, Japan). Animals were bred and maintained under standard conditions at the breeding unit of the Fundação Oswaldo Cruz, Brazil. The animals were used according to the rules set out by the Ethics Committee of FIOCRUZ for use of animals under the Protocol No p024705.
Parasite culture
Promastigote forms of Leishmania (Leishmania) amazonensis of MHOM/BR/77/LTB0016 strain, provided by Dr. Gabriel Grimaldi of the Center for Reference Laboratory of Leishmaniasis, Department of Immunology – IOC/FIOCRUZ, RJ, were used in all experiments. Parasites were incubated at 25 °C in BHI (Brain Heart Infusion) supplemented with 10% foetal bovine serum (FBS) and used in the stationary phase of growth until the third in vitro passage.
Intradermal inoculation and lesion measurement
Mice were sedated by an intraperitoneal injection with Compaz® (Cristália, São Paulo, SP, Brasil) (Diazepam 5 mg/ml) at a dosage of 5 mg/kg and Fentanyl® (Janssen-Cilag, São Paulo, SP, Brasil) (Fentanyl citrate 78.5 μg/10 ml) at a dosage of 0.02 mg/kg. A total of 250,000 metacyclic promastigotes of L. (L.) amazonensis were inoculated intradermally into the ears of the animals in 10 μl of PBS. A group of mice of each strain was inoculated only with 10 μl of PBS as control. The lesion developments were measured with a calliper (Schnelltaster, HC Kröplin, GMBH, Hessen, Germany) and ear thicknesses given in millimetres. After 1, 7 and 15 days of intradermal inoculation, mice were killed in a CO2 chamber and their ears were collected. Each experiment was carried out three times and the same results were obtained.
Histological Analysis
Ears of control and infected animals were washed in PBS and fixed in 10% buffered formalin. After fixation, samples were routinely processed for paraffin embedding in an Automatic Tissue Processor (Leica TP1020, Wetzlar, Germany). Five micrometre thick sections were obtained in a Rotary Microtome (Micron HM 360, Walldorf, Germany). The sections were stained with haematoxylin-eosin, differentiated into 1% hydrochloric alcohol, stained with alcoholic eosin 1%, assembled with Entelan and analysed in a light microscope (Zeiss Axioplan 2; Zeiss Inc., Thornwood, NY, USA).
Transmission Electron Microscopy
Ears were removed after 1, 7 and 15 days of infection, fixed in 2.5% glutaraldehyde in the buffer cacodylate sodium 0.1 M, pH 7.2 with 3.5% sucrose and postfixed with 1% of osmium tetroxide (OsO4) for 1 h at 4 °C. Then, they were dehydrated in an acetone series and embedded in resin PolyBed 812. After polymerization, semi-thin sections (0.5 μm) were stained with toluidine blue and eosin and observed under a light microscope (Zeiss Axioplan 2). The quantification of cellular profile was made in five semi-thin sections with an area of 60 mm2 per ear in an average of 3–5 mice/group. After the choice of areas, the ultra-thin sections were prepared (ultramicrotome Reichert OmU3), collected on copper grids of 300 mesh, contrasted with 5% uranyl acetate and lead citrate and observed by a transmission electron microscope (Zeiss EM10C) of the Oswald Cruz Institute electron microscopy Platform. Data were obtained from three independent experiments.
Statistical analysis
The significance of the results was calculated by a non-parametric one-way analysis of variance test (Kruskal–Wallis) and a P-value of <0.05 was considered significant.
Results
Evolution of the dermal lesion in TLR-2−/−
Macroscopic analysis of C57BL/6 wild-type (WT) and TLR-2−/− mice showed increased vascularization of the inoculation site after the first week of L. (L.) amazonensis promastigote infection. After the second week of infection, C57BL/6 WT mice presented lesions with little ulceration, while in TLR-2−/− mice the formation of a small nodule at the inoculation site was observed (Figure 1). The evaluations of the thickness of the inoculation site, after different times of infection, are represented in the Figure 2.
Figure 1.
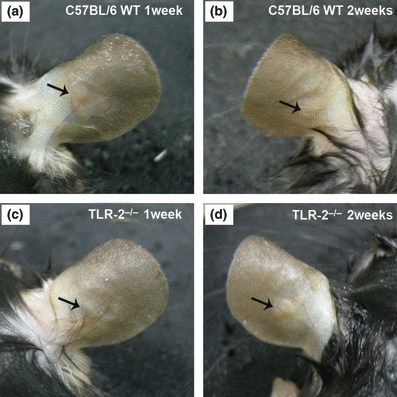
Ear lesions in C57BL/6 WT and TLR-2−/− mice 1 and 2 weeks following intradermal inoculation of 2.5 × 105 Leishmania (L.) amazonensis promastigotes. After the first week, C57BL/6 WT (a) and TLR-2−/− mice (c) present increased vascularization of the inoculation site. After the second week of infection, C57BL/6 WT mice (b) presented lesions with little ulceration, while in TLR-2−/− mice (d) the formation of small non-ulcerative nodular lesions were observed.
Figure 2.
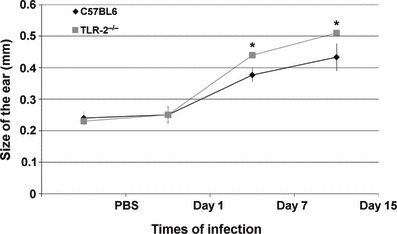
Diameter of induration following intradermal inoculation of 2.5 × 105 Leishmania (L.) amazonensis promastigotes in C57BL/6 WT (♦) and TLR-2−/− mice ( ). Values represent the mean induration (millimetres) ±1 SD (10 mice/group). Kruskal–Wallis statistical test and a *P < 0.05 was considered significant.
). Values represent the mean induration (millimetres) ±1 SD (10 mice/group). Kruskal–Wallis statistical test and a *P < 0.05 was considered significant.
Evolution of the cellular profile of dermal lesion in TLR-2 −/−
Analysis of the control mice ears showed the presence of resident cells of the dermis, whereas in PBS inoculated mice only a mild inflammatory infiltrate was observed (data not shown). On the first day of infection the presence of congested blood vessels with marginalization and diapedesis of inflammatory cells (Figure 3a,d) were seen in both C57BL/6 WT and TLR-2−/− mice. In the dermal ear, inflammatory infiltrates composed of neutrophils, macrophages, degranulated mast cells were observed in both C57BL/6 WT (Figures 4a and 5a) and TLR-2−/− mice (Figures 4d and 5d).
Figure 3.
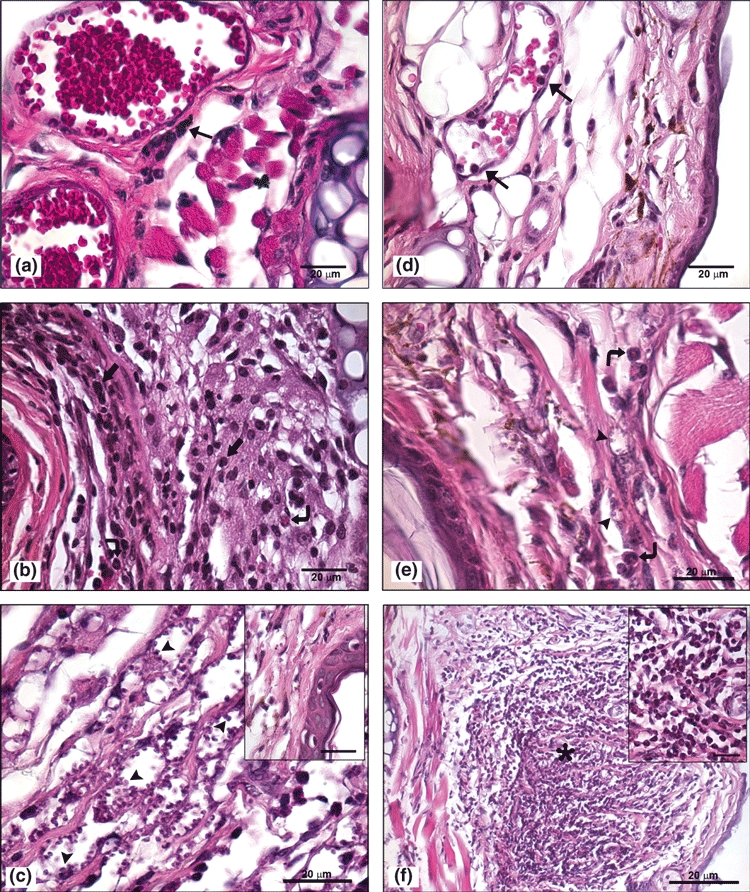
(a–f) – Histological analysis of sections from the ear region of C57BL/6 WT mice and TLR-2−/− mice after 1, 7 and 15 days of Leishmania (L.) amazonensis infection stained with haematoxylin-eosin (bar = 20μm). On the first day of infection the presence of congested blood vessels with marginalization and diapedesis of inflammatory cells ( ) in C57BL/6 WT (a) and TLR-2−/− mice (d) were seen. After first week of infection, the C57BL/6 WT mice showed mononuclear (
) in C57BL/6 WT (a) and TLR-2−/− mice (d) were seen. After first week of infection, the C57BL/6 WT mice showed mononuclear ( ) and polymorphonuclear (
) and polymorphonuclear ( ) cell infiltrations in ear dermis (b) and second week after infection the presence of epidermal alterations such as exocytosis (inset) and dermis with an increase of intracellular amastigotes (
) cell infiltrations in ear dermis (b) and second week after infection the presence of epidermal alterations such as exocytosis (inset) and dermis with an increase of intracellular amastigotes ( ) (c). In TLR-2−/− mice during the first week of infection some polymorphonuclear cells (
) (c). In TLR-2−/− mice during the first week of infection some polymorphonuclear cells ( ) were seen (e). After the second week of infection organized granulomas (
) were seen (e). After the second week of infection organized granulomas ( ) formed by eosinophils and without parasites (inset) were observed (f). The experiment is representative of three separate experiments.
) formed by eosinophils and without parasites (inset) were observed (f). The experiment is representative of three separate experiments.
Figure 4.
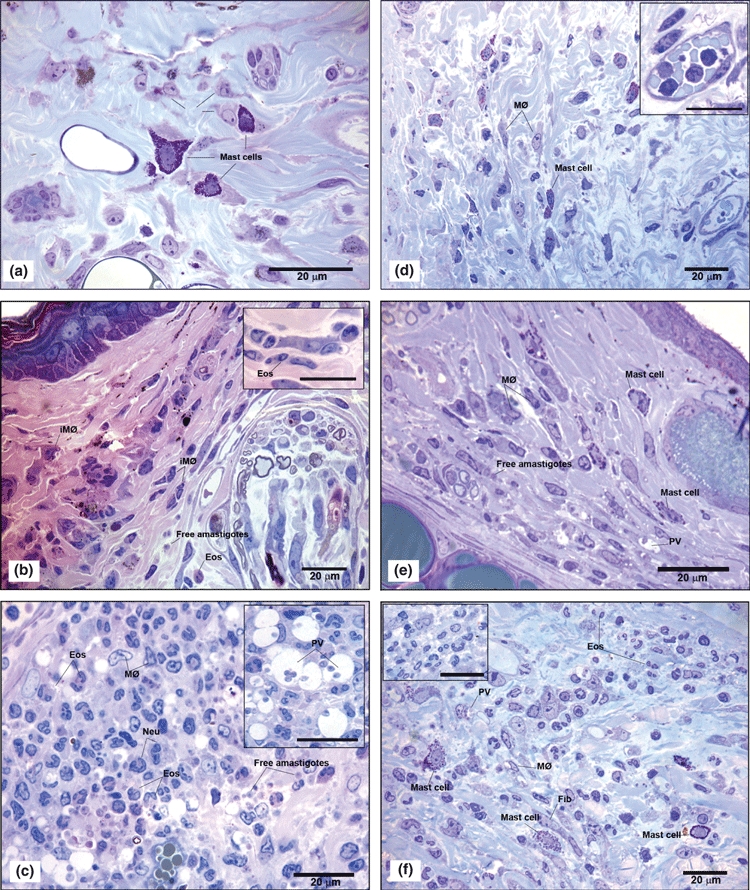
(a–f) – Semi-thin sections from the ear lesion of C57BL/6 WT mice and TLR-2−/− mice after 1, 7 and 15 days of Leishmania (L.) amazonensis infection, stained with toluidine blue and eosin (bar = 20μm). On the first day of infection inflammatory infiltrates composed of neutrophils (inset), macrophages (MØ), degranulated mast cells in C57BL/6 WT (a) and TLR-2−/− mice (d) were observed. In the first week of infection, in C57BL/6 WT mice (b) showed immature macrophages (iMØ) and eosinophils (Eos) composing the inflammatory infiltrate (inset) and free parasites in dermal ear (b). In the second week (c) an increase of inflammatory infiltrate predominantly composed of macrophages (MØ), as well neutrophils (Neu) and eosinophil (Eos) populations were seen. In addition to the presence of free amastigotes and a large amount of macrophages (MØ) containing amastigotes within large parasitophorous vacuoles (PV) (inset) and numerous free amastigotes in the matrix were observed. In TLR-2−/− mice, during the first week of infection (e), mast cells were observed between parasitized macrophages (MØ) and some free amastigotes in the matrix. In the second week of infection (f), organized granulomas formed predominantly of eosinophils (Eos), a large amount of macrophages (MØ), some mast cells and fibroblasts were seen. Also, few macrophages (MØ) with amastigotes in parasitophorous vacuoles (PV) were found. The experiment is representative of three separate experiments.
Figure 5.
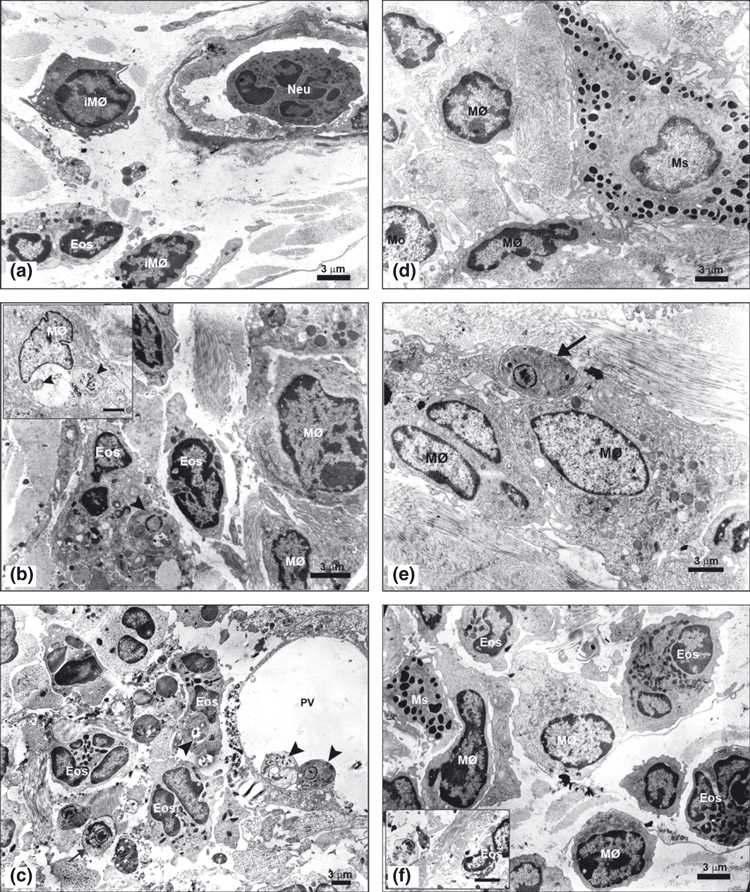
(a–f) – Ultrastructural analysis of the ultra-thin sections from the ear lesion of the C57BL/6 WT and TLR-2−/− mice after 1, 7 and 15 days of Leishmania (L.) amazonensis infection contrasted with 5% uranyl acetate and lead citrate (bar = 3μm). Electron micrography of the first day infection (a) showed neutrophils (Neu) adhered to the endothelium of blood vessel, immature macrophages (iMØ) and eosinophils (Eos) in dermal ear of the C57BL/6 WT. After first week of the infection (b), eosinophils (Eos) containing one amastigotes within parasitophorous vacuoles close ( ) and immature macrophages characterized by few organelles and a nucleous with electron-dense chromatin were seen. Also, mature macrophages (MØ) containing amastigotes within large parasitophorous vacuoles were observed (inset). After the second week (c) mature macrophages (MØ) presented amastigotes (
) and immature macrophages characterized by few organelles and a nucleous with electron-dense chromatin were seen. Also, mature macrophages (MØ) containing amastigotes within large parasitophorous vacuoles were observed (inset). After the second week (c) mature macrophages (MØ) presented amastigotes ( ) attached at the large parasitophorous vacuoles membrane (PV), many eosinophils (Eos) parasitized contained only one amastigote within parasitophorous vacuoles close and free amastigotes (
) attached at the large parasitophorous vacuoles membrane (PV), many eosinophils (Eos) parasitized contained only one amastigote within parasitophorous vacuoles close and free amastigotes ( ) in extracellular matrix were found. Furthermore, several biconvex granules, proceeding from eosinophils, were observed free in the extracellular matrix. In the first day of TLR-2−/− mice infection (d) were found mast cells (Ms) with electron-dense granules characteristic of this cell, distributed in the cytoplasm and macrophages (MØ). In the first week (e), presented some free amastigotes (
) in extracellular matrix were found. Furthermore, several biconvex granules, proceeding from eosinophils, were observed free in the extracellular matrix. In the first day of TLR-2−/− mice infection (d) were found mast cells (Ms) with electron-dense granules characteristic of this cell, distributed in the cytoplasm and macrophages (MØ). In the first week (e), presented some free amastigotes ( ) in the extracellular matrix showed entire plasma membrane and nucleous with chromatin attached to the nuclear envelope and central nucleolus being phagocytized by mature macrophages (MØ). And, in the second week (f), mature macrophages (MØ), eosinophils (Eos) and mast cells (Ms) in ear dermis were observed. In this time, rare free amastigotes in the extracellular matrix presented cytoplasm rarefied and disruption at the plasma membrane and nuclear envelope, indicating cell death of these parasites (inset). The experiment is representative of three separate experiments.
) in the extracellular matrix showed entire plasma membrane and nucleous with chromatin attached to the nuclear envelope and central nucleolus being phagocytized by mature macrophages (MØ). And, in the second week (f), mature macrophages (MØ), eosinophils (Eos) and mast cells (Ms) in ear dermis were observed. In this time, rare free amastigotes in the extracellular matrix presented cytoplasm rarefied and disruption at the plasma membrane and nuclear envelope, indicating cell death of these parasites (inset). The experiment is representative of three separate experiments.
In the first week of infection, the analysis of the inoculation site in the C57BL/6 WT mice showed inflammatory infiltrates composed of mononuclear and polymorphonuclear cells in the dermal ear (Figure 3b). In this infiltrate, many eosinophils and neutrophils with a preponderance of monocytes, immature and mature macrophages were observed. Moreover, in these mice large amounts of parasitized cells and free parasites in the extracellular matrix were observed (Figure 4b). Ultrastructural analysis showed the presence of eosinophils containing generally one amastigote in parasitophorous vacuoles close in the dermal ear. Also, Immature macrophages characterized by cytoplasm with few organelles and nucleus with electron-dense chromatin and macrophages containing several amastigotes within large parasitophorous vacuoles were observed (Figure 5b). Moreover, in the TLR-2−/− mice a reduced infiltrate of inflammatory cells composed mostly of immature and mature macrophages and large amount of mast cells and fibroblasts were observed (Figure 3e). Also a small amount of parasitized cells such as macrophages, fibroblasts and rare free amastigotes in extracellular matrix (Figures 4e and 5e) were seen.
During the second week of infection there was a significant increase in the influx of inflammatory cell infiltrates in the dermal ear in both mice strains, thus the cellular profile for the initial response to infection by L. (L.) amazonensis could be defined. In the C57BL/6 WT mice, we observed the presence of disorganized granuloma predominantly composed of macrophages, as well as the presence of neutrophils and eosinophils (Figures 3c and 4c). Also, a large amount of macrophages containing amastigotes in large parasitophorous vacuoles and eosinophils with only one amastigote in the parasitophorous vacuoles close were observed. Furthermore, free amastigotes and biconvex eosinophils granules in extracellular matrix were seen (Figure 5c). In these mice the number of parasitized cells was significantly higher when compared with the number of free amastigotes in extracellular matrix (Figure 6). In the ear dermis of TLR-2−/− mice there were organized granulomas (Figure 3f) formed predominantly of eosinophils and a large amount of macrophages and mast cells (Figures 4f and 5f). Differently to what was found in WT mice, the TLR-2−/− mice showed a low parasite load, few eosinophils and parasitized macrophages and rare amastigotes free in extracellular matrix (Figures 4f and 6). An important observation was the presence of eosinophils near the infected macrophages in both mice strains.
Figure 6.
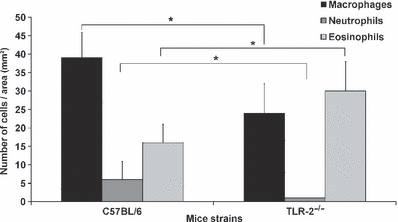
Cellular profile presented in the ear lesion of the C57BL/6 WT and TLR-2−/− mice after the second week of infection with metacyclic promastigotes of Leishmania (L.) amazonensis. The quantification was performed in five semi-thin sections with an area of 60 mm2 per ear. Each bar represents mean ± SEM from three experiments (3–5 mice/group). Kruskal–Wallis statistical test and a *P < 0.05 was considered significant.
A quantitative evaluation of the cellular profile and the parasite load in the second week of L. (L.) amazonensis infection in C57BL/6 and TLR-2−/− mice is represented in Figures 6 and 7 using the Kruskal–Wallis statistical test and a P-value of <0.05 was considered significant.
Figure 7.
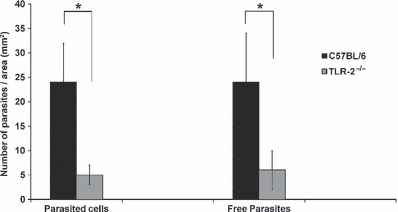
Parasite load in the ear lesion of the C57BL/6 WT and TLR-2−/− mice intradermally inoculated with 250,000 metacyclic promastigotes of Leishmania (L.) amazonensis after the second week of infection. The quantification was performed in five semi-thin sections with an area of 60 mm2 per ear. Each bar represents mean ± SEM from three experiments (3–5 mice/group). Kruskal–Wallis statistical test and a *P < 0.05 was considered significant.
Discussion
The first moments of infection by Leishmania are crucial to drive the progress of the disease. There is evidence that the phenotype of the leishmaniasis can be determined in the first hours after infection, starting with the recognition of PAMPs of the parasite by PRRs present on the surface of host cells, such as TLRs (Launois et al. 1995; Sacks & Noben-Trauth 2002). The involvement of TLRs in the initial response to infection with Leishmania have been described in recent years, through various in vivo and in vitro studies (Hawn et al. 2002; De Veer et al. 2003; Debus et al. 2003; De Trez et al. 2004).
So, to evaluate the involvement of TLR-2 during in vivo L. (L.) amazonensis infection TLR-2 deficient mice were used. The ear dermis was chosen as the inoculation site because, in addition to being a common transmission site in rodent reservoirs, it offers the advantage that all the dynamic events occur at the infection site, facilitating the study of the initial inflammatory response (Belkaid et al. 1996, 1998).
The control group of mice, inoculated only with PBS, showed a small inflammatory infiltrate composed of neutrophils, increase of vascularization and the presence of oedema at the inoculation site. This initial inflammatory response, generated by mechanical disruption of cells in the epidermis and dermis was consistent with the results presented by Grimaldi and Moriearty (1981). Nonetheless, the histological analysis of inoculation site after the first day of L. (L.) amazonensis infection showed that the inflammatory infiltrate in the ear dermis was higher when compared with mice inoculated only with PBS. This fact is related to the presence of free parasites in the extracellular matrix of the ear dermis leading to activation of resident cells, through recognition of parasite surface molecules by receptors present in these cells (De Almeida et al. 2003).
Since the first days of infection, we observed the neutrophils infiltration at the inoculation site in both mice strains, being able to occur because of the disruption of the skin caused by either the insect vector or the needle. This mechanical injury induces the recruitment of these cells to participate in the process of tissue repair, even in the absence of the parasite (Peters et al. 2008).
The initial recruitment of neutrophils to the inoculation site is supported by several studies that demonstrate the role of these cells in the first line of defence against infection by protozoa of the Leishmania genus, acting directly on the endocytosis and destruction of the parasite by proteolytic enzymes, production of reactive oxygen intermediates, inflammatory mediators and cellular recruitment to the infection site (Chang. 1981; Pimenta et al. 1987; Awasthi et al. 2004).
C57BL/6 WT mice showed significant increase of immature and mature macrophages at the infection site in first week of infection. This high concentration of macrophages at the inoculation site may be corroborated by Soong et al. (1996, 1997) who showed that mice infected with L. (L.) amazonensis are able to recruit a large quantity of immature macrophages, but they are unable to eliminate the parasite facilitating the spread of infection. Thus, the increased number of immature and mature macrophages found in C57BL/6 mice during the first week of infection may be related to the maturation of recruited monocytes in the early stages of the infection. These observations could explain the presence of many macrophages parasitized with numerous amastigotes within large parasitophorous vacuoles and the presence of free amastigotes in the tissue from the disruption of these cells.
Using TLR-2−/− mice, we showed that the absence of this receptor leads to an alteration of the cellular profile and an expressive reduction in the susceptibility of these animals to L. (L.) amazonensis infection, which are capable of controlling the parasite load during the first 2 weeks of infection. TLR-2−/− mice presented a lower infiltrate of inflammatory cells at the inoculation site forming organized granulomas mainly made up of eosinophils, unlike the C57BL/6 mice, which had infiltrated more expressive, but no organized granulomas were observed.
The eosinophils constitutively express few TLRs on their surface and the direct activation of eosinophils through TLR-2 is controversial (Sabroe et al. 2002; Nagase et al. 2003; D’Avila et al. 2007; Driss et al. 2009). Although a role for TLR-2 in regulating eosinophils recruitment and activation through direct and indirect mechanisms has been described in bacterial and parasitic infections (D’Avila et al. 2007; Driss et al. 2009), similar to our observations, a lack of impairment or increased recruitment of eosinophils in TLR-2 deficient animals has been reported following ocular filarial infection and also in a ear model of contact dermatitis (Daehnel et al. 2007; Jin et al. 2009). The tissue eosinophilia presented in TLR-2−/− mice is characteristic of a Th2 response, as observed with asthma and helminthic infections (Del Prete. 1992; Mehlotra et al. 1998). However, a Th2 response would be favourable for parasite proliferation, which was not observed in TLR-2 deficient mice. Of note, eosinophils may participate in the process of killing parasites through their ability to phagocytize, mount a respiratory burst and mobilize cytotoxic proteins from specific granules after infection, suggesting an immunomodulatory and in some conditions protective role of eosinophils in infections (Akuthota et al. 2008; Blanchard & Rothenberg 2009).
On the other hand, TLR-2−/− mice showed a significant reduction in the number of neutrophils during the second week of infection when compared with C57BL/6 WT mice. This reduction can be related to the involvement of TLR-2 in the recruitment, activation and apoptosis of these cells (Sabroe et al. 2005; Jablonska et al. 2006).
Furthermore, we observed that these mice showed a low parasite load from the first day of infection when compared with C57BL/6 WT mice. This fact indicates that these receptors have an effective participation in the adhesion and internalization of the parasite in the host cells present in the initial stages of infection. The difference in the parasite load observed in the second week of infection in TLR-2−/− and C57BL/6 WT mice may be related to the association of macrophages with eosinophils present at the inoculation site. This association was described by Grimaldi et al. (1984) who found that the eosinophils could serve as donors of peroxidase for mature macrophages. Moreover, these eosinophils may be acting in direct control of amastigotes free through the release of extracellular peroxidase, can then be adsorbed at plasma membrane of Leishmania by making it more susceptible to death after phagocytosis by macrophages as suggested by Grimaldi et al. (1984) and described by Pimenta et al. (1987).
The presence of mast cells in TLR-2−/− and C57BL/6 WT mice from the first day of infection is supported by data from the literature that show the presence of these cells in the dermis during infections caused by protozoa of the genus Leishmania and their direct participation in the initial immune response through the production of several inflammatory mediators (Bidri et al. 1997; Saha et al. 2004). So, we can suggest that mast cell degranulation, mainly seen in TLR-2−/− mice infected with L. (L.) amazonensis, is helping to reduce the number of free amastigotes in the extracellular matrix.
With these studies, we have demonstrated the importance of TLR-2 in the initial response to L. (L.) amazonensis infection, where the absence of these receptors in initial stages of infection favours the control of the parasite load. Thus, we suggest the study of TLR pathways as an alternative for the development of new medicines for the treatment of infections caused by L. (L.) amazonensis.
Acknowledgments
The authors would like to thank Andrea Henriques Pons for helpful discussions. We thank Generval Luciano Batista, Renata Corrêa Hespanhol and Vanessa de Souza Vaz for their technical assistance.
This work was supported by grants from Instituto Oswaldo Cruz-IOC/FIOCRUZ, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (48.0629/2004-8) and PAPES V-CNPq/FIOCRUZ (403642/2008-6).
References
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin. Exp. Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RP, Barral-Netto M, De Jesus AM, De Freitas LA, Carvalho EM, Barral A. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal, or visceral leishmaniasis in BALB/c mice. Am. J. Trop. Med. Hyg. 1996;54:178–184. doi: 10.4269/ajtmh.1996.54.178. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J. Med. Res. 2004;119:238–258. [PubMed] [Google Scholar]
- Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through Toll-like receptor 2. Mol. Biochem. Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Jouin H, Milon G. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J. Immunol. Methods. 1996;199:5. doi: 10.1016/s0022-1759(96)00117-2. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 1998;188:1941. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidri M, Vouldoukis I, Mossalayi MD, et al. Evidence for direct interaction between mast cells and Leishmania parasites. Parasite Immunol. 1997;19:475–483. doi: 10.1046/j.1365-3024.1997.d01-153.x. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv. Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MA, Almeida IC, Takeuchi O, et al. Activation of toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- Chang KP. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am. J. Trop. Med. Hyg. 1981;30(2):322–333. doi: 10.4269/ajtmh.1981.30.322. [DOI] [PubMed] [Google Scholar]
- Daehnel K, Gillette-Ferguson I, Hise AG, et al. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness) Parasite Immunol. 2007;29:455–465. doi: 10.1111/j.1365-3024.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- D’Avila H, Almeida PE, Roque NR, Castro-Faria-Neto HC, Bozza PT. Toll-like receptor-2-mediated C-C chemokine receptor 3 and eotaxin-driven eosinophil influx induced by Mycobacterium bovis BCG pleurisy. Infect. Immun. 2007;75:1507–1511. doi: 10.1128/IAI.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida MC, Vilhena V, Barral A, Barral-Netto M. Leishmanial infection: analysis of its first steps. A review. Mem. Inst. Oswaldo Cruz. 2003;98:861–870. doi: 10.1590/s0074-02762003000700001. [DOI] [PubMed] [Google Scholar]
- De Trez C, Brait M, Leo O, et al. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect. Immun. 2004;72:824–832. doi: 10.1128/IAI.72.2.824-832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veer MJ, Curtis JM, Baldwin TM, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003;33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- Debus A, Gläsner J, Röllinghoff M, Gessner A. High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 2003;71:7215–7218. doi: 10.1128/IAI.71.12.7215-7218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992;47(5):450–455. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Driss V, Legrand F, Hermann E, et al. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood. 2009;113:3235–3244. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kupper TS. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Jr, Moriearty PL. Kinetics and histopathology of the ear thickness test for delayed hypersensitivity in murine leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo. 1981;23:127–132. [PubMed] [Google Scholar]
- Grimaldi G, Jr, Soares MJ, Moriearty PL. Tissue eosinophilia and Leishmania mexicana mexicana eosinophil interactions in murine cutaneous leishmaniasis. Parasite Immunol. 1984;6:397–408. doi: 10.1111/j.1365-3024.1984.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Hawn TR, Ozinsky A, Underhill DM, Buckner FS, Akira S, Aderem A. Leishmania major activates IL-1α expression in macrophages through a MyD88-dependent pathway. Microbes Infect. 2002;4(8):763–771. doi: 10.1016/s1286-4579(02)01596-4. [DOI] [PubMed] [Google Scholar]
- Jablonska E, Marcinczyk M, Jablonski J. Toll-like receptors types 2 and 6 and the apoptotic process in human neutrophils. Arch. Immunol. Ther. Exp. (Warsz) 2006;54:137–142. doi: 10.1007/s00005-006-0014-2. [DOI] [PubMed] [Google Scholar]
- Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kumar L, Mathias C, et al. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. J. Allergy Clin. Immunol. 2009;123:875–882. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MM, Mosser DM. Leishmania parasites and their ploys to disrupt macrophage activation. Curr. Opin. Hematol. 2000;7:26–31. doi: 10.1097/00062752-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Kropf P, Freudenberg MA, Modolell M, et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 2004a;72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf P, Freudenberg N, Kalis C, et al. Infection of C57BL/10ScCr and C57BL/10ScNCr mice with Leishmania major reveals a role for Toll-like receptor 4 in the control of parasite replication. J. Leukoc. Biol. 2004b;76:48–57. doi: 10.1189/jlb.1003484. [DOI] [PubMed] [Google Scholar]
- Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4 + T cells which are NK1.1−. Eur. J. Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- Mehlotra RK, Hall LR, Higgins AW, et al. Interleukin-12 suppresses filaria-induced pulmonary eosinophilia, deposition of major basic protein and airway hyperresponsiveness. Parasite Immunol. 1998;20:455–462. doi: 10.1046/j.1365-3024.1998.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministério da Saúde - Secretaria de Vigilância em Saúde. Manual de Vigilância da Leishmaniose Tegumentar Americana. Brasília, Brasil: Ministério da Saúde; 2007. [Google Scholar]
- Nagase H, Okugawa S, Ota Y, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- Ouaissi A, Guilvard E, Delneste Y, et al. The Trypanosoma cruzi Tc-52-released protein induces human dendritic cell maturation, signals via toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 2002;168:6366–6374. doi: 10.4049/jimmunol.168.12.6366. [DOI] [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. Erratum in: Science 12; 322 (5908):1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta PFP, Dos Santos MAV, De Souza W. Fine estructure and cytochemistry of the interaction between Leishmania mexicana amazonensis and rat neutrophils and eosinophils. J. Submicrosc. Cytol. 1987;19:387–395. [PubMed] [Google Scholar]
- Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin. Infect. Dis. 2005;41(Suppl 7):S421–S426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major mice. Nat. Rev. Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Saha B, Tonkal AM, Croft S, Roy S. Mast cells at the host-pathogen interface: host-protection versus immune evasion in leishmaniasis. Clin. Exp. Immunol. 2004;137:19–23. doi: 10.1111/j.1365-2249.2004.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong L, Xu JC, Grewal IS, et al. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- Soong L, Chang CH, Sun J, et al. Role of CD4 + T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 1997;158:5374–5383. [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT, Tielens AG, Yazdanbakhsh M. A novel host-parasite lipid cross talk: schistosomal lyso-phosphatidylserine activates Toll-like receptor-2 and effects immune polarization. J. Biol. Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- Williams IR, Kupper TS. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 1996;58:1485–1507. doi: 10.1016/0024-3205(96)00042-2. [DOI] [PubMed] [Google Scholar]


