Abstract
Background
Seneca Valley virus (NTX-010) is a non-recombinant, replication competent RNA virus that is undergoing phase 1 clinical trials in adults for tumors with neuroendocrine characteristics. Here we have evaluated the antitumor activity of NTX-010 administered systemically.
Procedures
In vitro NTX-010 was tested against 23 cell lines exposed for 96 hours at 1 × 10−4 to 104 viral particles (vp)/cell. In vivo NTX-010 was administered intravenously once at 3 × 1012 vp/kg. Three measures of antitumor activity were used: 1) an objective response measure modeled after the clinical setting; 2) a treated to control (T/C) tumor volume measure; and 3) a time to event (4-fold increase in tumor volume for solid tumor models), measure based on the median event-free survival (EFS) of treated and control animals for each xenograft.
Results
In vitro NTX-010 demonstrated a marked cytotoxic effect in a subset of the cell lines from the neuroblastoma, Ewing sarcoma, and rhabdomyosarcoma panels. In vivo the most consistent activity was observed for the rhabdomyosarcoma and the neuroblastoma panels, with all four of the alveolar rhabdomyosarcoma xenografts and 4 of 5 neuroblastoma xenografts achieving CR or maintained CR. Objective responses were also observed in the rhabdoid tumor, Wilms tumor, and glioblastoma panels.
Conclusions
NTX-010 demonstrated a high level of activity both in vitro and in vivo. Further analysis of existing testing and molecular characterization data may help define the biological characteristics of cancer cells that are associated with response to NTX-010.
Keywords: Preclinical Testing, Developmental Therapeutics, Seneca Valley virus (NTX-010)
INTRODUCTION
Oncolytic viruses have been extensively tested as cancer therapies in preclinical models (reviewed in [1–3]) and have undergone clinical testing since the 1950’s [4]. Clinical trials of adenoviruses, adenovirus associated virus (AAV), and RNA viruses such as mumps, Newcastle disease virus, measles virus, vesicular stomatitis virus and reovirus have been reported [4]. Adenovirus has been genetically modified in multiple ways to achieve selectivity for replication in cancerous tissue [5], while RNA viruses such as reovirus appear to have selectivity based on their replication and oncolytic effect in cells with an activated RAS signaling pathway [6,7]. Common to the life cycles of RNA viruses is the synthesis of double stranded RNA that potently stimulates PKR, a protein kinase that phosphorylates eIF2α to inhibit protein synthesis and promote apoptosis [7,8]. As tumors are frequently deficient in their PKR signaling pathway, there is selective proliferation of some RNA viruses in tumor cells, resulting in tumor cell death.
Seneca Valley Virus (NTX-010) is a newly discovered, naturally occurring picornavirus being developed as an oncolytic virus for human cancers. NTX-010 is to be placed in a new genus as a new species within the picornavirus family and is most closely related to the cardioviruses [9,10]. It has a single-stranded positive-sense RNA genome that encodes a single polyprotein that is processed by proteases to make specific viral proteins [9]. Normal exposure to NTX-010 appears to not be prevalent in the human population [10]. NTX-010 is homologous to and serologically related to 12 viruses isolated from pig specimens in the United States, and it is nonpathogenic in humans and animal species [10]. NTX-010 can be delivered systemically to treat metastatic disease, as it is not inhibited by any component of human blood and because the virion is small (25–30 nm) relative to other viruses, such as adenovirus, allowing greater distribution to tumor than other candidate oncolytic viruses [10].
In a cell line screen of NTX-010, approximately half of cancer cells with one or more neuroendocrine properties were permissive and allowed selective infection [10]. Notably, the most sensitive cell line, IMR-32, was derived from a childhood neuroblastoma. In contrast 3 of 80 non-endocrine cells were permissive to virus replication. The majority of non-permissive cancer cell lines do not bind and/or internalize NTX-010, suggesting that binding and entry through a productive internalization pathway is the primary determinant of viral tropism for neuroendocrine tumor cells [11]. Neuroblastoma, Ewing sarcoma, as well as medulloblastoma and alveolar rhabdomyosarcoma, demonstrate neuroendocrine markers. Here we report initial testing results evaluating NTX-010 against the in vitro and in vivo panels of tumors used in the Pediatric Preclinical Testing Program (PPTP).
MATERIALS AND METHODS
In vitro testing
NTX-010 was evaluated against the the 23 cell lines in the PPTP in vitro panel using 96 hour exposures and log dilutions from 104 virus particles per cell (vp/cell) to 10−4 vp/cell with replicates of 6 for each virus particle concentration tested. In vitro testing was performed using DIMSCAN, a semiautomatic fluorescence-based digital image microscopy system that quantifies viable (using fluorescein diacetate [FDA]) cell numbers in tissue culture multiwell plates [12].
In vivo tumor growth inhibition studies
CB17SC-M scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [13–16]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Ten mice were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] were determined as previously described [17] and responses were determined using three activity measures as previously described [17]. Models of acute lymphoblastic leukemia were not evaluated in this study. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Virus formulation and administration
All experiments reported were approved by the institutional biological safety committee for each consortium member, and were performed within adherence to institutional guidelines. NTX-010, supplied by Neotropix Inc. as frozen stocks (4.4 × 1013 viral particles (vp)/ml), was tested against the PPTP in vivo panel of xenografts using a single dose of 3 × 1012 vp per kg administered intravenously via the tail vein. Tumor volumes (mean ± SD) for control and treatment groups at the time of NTX-010 administration were 0.354 ± 0.177 and 0.350 ± 0.182 cm3, respectively.
RESULTS
NTX-010 in vitro testing
NTX-010 was evaluated against the the 23 cell lines in the PPTP in vitro panel using 96 hour exposures. Three of 4 neuroblastoma, 2 of 4 rhabdomyosarcoma, and 1 of 4 Ewing sarcoma cell lines had IC50 value less than 1 virus particle per cell (vp/cell) (Table I). Viral exposures causing 50% reduction in cell number from controls are shown in Figure 1A. At the highest concentration tested (1×104 vp/cell), 9 of the 23 cell lines showed > 90% inhibition compared to control cells, with all of these cell lines being in either the rhabdomyosarcoma, Ewing sarcoma, or neuroblastoma panels, Figure 1B. Representative dose-response data for sensitive and insensitive cell lines are shown (Figures 1C and D). NTX-010 demonstrated no cytotoxic effect against cell lines of lymphoid or myeloid origin, and was not tested in vivo against the ALL panel.
Table I.
Activity of NTX-010 against Cell Lines in the PPTP in Vitro Panel
| Cell Line | Histology | IC50 (vp/cell) |
T/C at 1 vp/cell (% of control) |
T/C at 1E+04 vp/cell (%of control) |
|---|---|---|---|---|
| RD | Rhabdomyosarcoma | >1.0E+04 | 99.43 | 97.58 |
| Rh41 | Rhabdomyosarcoma | 2.29E-02 | 1.74 | 0.47 |
| Rh18 | Rhabdomyosarcoma | 9.66E-04 | 1.13 | 0.54 |
| Rh30 | Rhabdomyosarcoma | 1.75E+01 | 89.56 | 8.08 |
| BT-12 | Rhabdoid | >1.0E+04 | 95.63 | 100.00 |
| CHLA-266 | Rhabdoid | >1.0E+04 | 100.00 | 55.79 |
| TC-71 | Ewing sarcoma | 1.57E+01 | 95.17 | 0.00 |
| CHLA-9 | Ewing sarcoma | <1.0E-04 | 0.01 | 0.00 |
| CHLA-10 | Ewing sarcoma | 7.73E-01 | 56.20 | 0.02 |
| CHLA-258 | Ewing sarcoma | >1.0E+04 | 100.00 | 59.15 |
| GBM2 | Glioblastoma | >1.0E+04 | 100.00 | 33.84 |
| NB-1643 | Neuroblastoma | <1.0E-04 | 0.02 | 0.01 |
| NB-EBd | Neuroblastoma | 2.46E-03 | 4.89 | 1.50 |
| CHLA-90 | Neuroblastoma | >1.0E+04 | 98.92 | 61.26 |
| CHLA-136 | Neuroblastoma | <1.0E-04 | 1.31 | 0.44 |
| NALM-6 | ALL | >1.0E+04 | 81.66 | 83.20 |
| COG-LL-317 | ALL | >1.0E+04 | 100.00 | 100.00 |
| RS4;11 | ALL | >1.0E+04 | 91.71 | 83.37 |
| MOLT-4 | ALL | >1.0E+04 | 100.00 | 96.59 |
| CCRF-CEM | ALL | >1.0E+04 | 100.00 | 85.73 |
| Kasumi-1 | AML | >1.0E+04 | 100.00 | 82.78 |
| Karpas-299 | ALCL | >1.0E+04 | 93.42 | 83.51 |
| Ramos-RA1 | NHL | >1.0E+04 | 100.00 | 89.11 |
Figure 1.
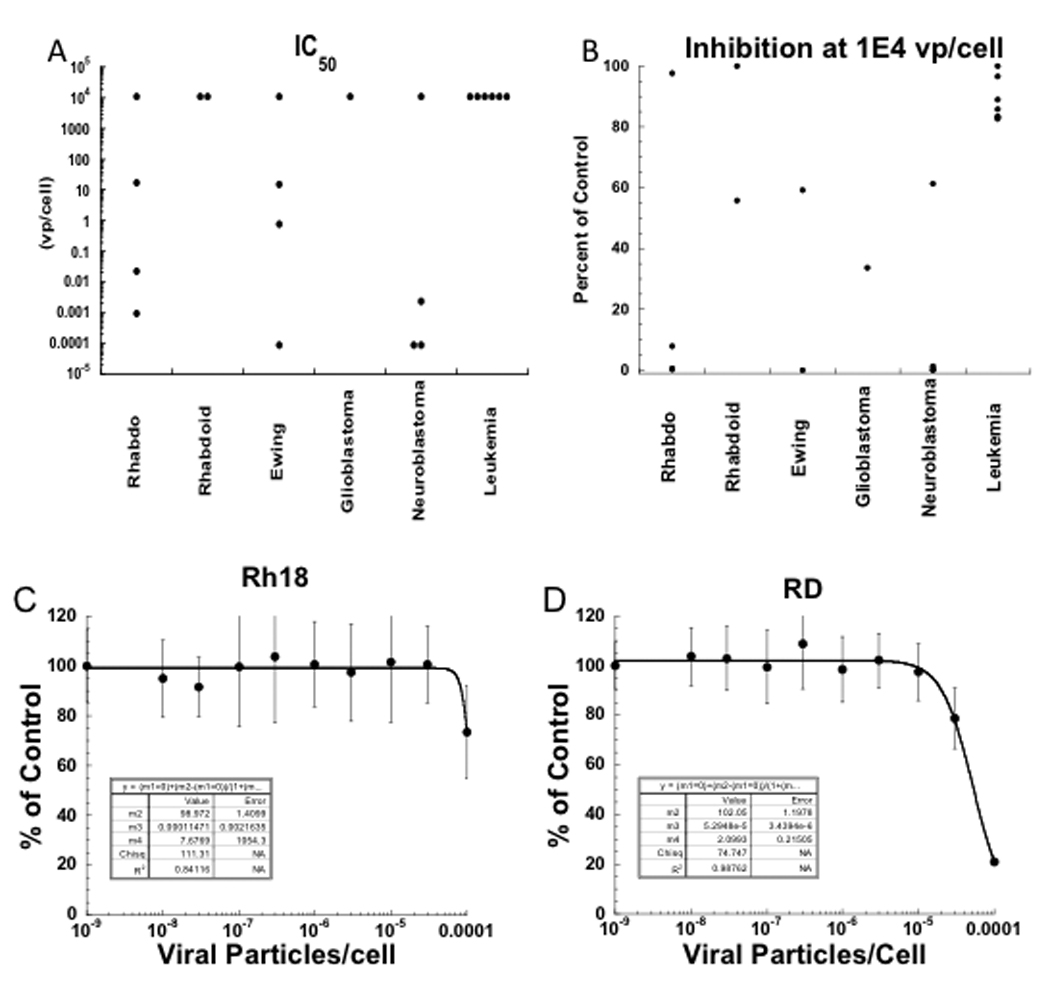
NTX-010 in vitro activity. (A) graphically represents the relative potency of NTX-010 against each cell line with each point representing the IC50 of an individual line.
(B) shows the cell number (% control) at the highest NTX-010 concentration tested (1×104 vp/cell). Each of the 9 cell lines that show more than 90% inhibition to NTX-010 at this concentration are in either the rhabdomyosarcoma, Ewing sarcoma, or neuroblastoma panels. (C) Representative dose-response data for sensitive (Rh18) and (D) insensitive (RD) cell lines exposed to NTX-010.
NTX-010 in vivo testing
NTX-010 was tested against the PPTP in vivo panel of xenografts using a single dose of 3 × 1012 viral particle per kg administered intravenously. Nine of 711 mice died during the study (1.3%), with 6 of 354 in the control arms (1.7%) and 3 of 357 in the NTX-010 treatment arms (0.8%). A complete summary of results is provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and median times to event, tumor growth delay, as well as numbers of responses and T/C values. All 36 solid tumor xenograft models were evaluable for activity. NTX-010 induced significant differences in EFS distribution compared to controls in 24 of 36 evaluable solid tumor xenografts tested as shown in Table II. Among these 24 models, 15 achieved EFS T/C values meeting the criteria for intermediate activity for the time to event activity measure (EFS T/C > 2), including 5 of 5 neuroblastoma xenografts 4 of 5 rhabdomyosarcoma xenografts 2 of 3 rhabdoid tumor xenografts, and 2 of 4 glioblastoma xenografts. Six models achieved high activity judged by all three criteria (3 neuroblastomas, 1 rhabdomyosarcoma, 1 Wilms tumor, and 1 rhabdoid tumor). By contrast, no osteosarcoma or medulloblastoma xenografts had EFS T/C values > 2, and only 1 of 5 Ewing sarcoma xenografts reached this mark.
Table II.
Activity for NTX-010 against the PPTP in Vivo Panel
| Xenograft Line |
Histology | KM Estimate of Median Time to Event |
P-value | EFS T/C |
Median Final RTV |
Tumor Volume T/C |
P-value | T/C Activity |
EFS Activity |
Response Activity |
|---|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | >EP | <0.001 | >2.4 | 3.7 | 0.58 | 0.002 | Low | Int | Int |
| KT-14 | Rhabdoid | >EP | <0.001 | >2.1 | 0 | 0.05 | <0.001 | High | High | High |
| KT-12 | Rhabdoid | 11.3 | 0.012 | 1.3 | >4 | 0.79 | 0.136 | Low | Low | Low |
| KT-10 | Wilms | 12.2 | <0.001 | 1.7 | >4 | 0.51 | <0.001 | Low | Low | Int |
| KT-11 | Wilms | 11.7 | 0.002 | 1.2 | >4 | 0.66 | 0.005 | Low | Low | Low |
| KT-13 | Wilms | >EP | <0.001 | >2.0 | 0.6 | 0.14 | <0.001 | High | High | High |
| SK-NEP-1 | Ewing | 15.5 | <0.001 | 2.5 | >4 | 0.4 | <0.001 | Int | Int | Int |
| EW5 | Ewing | 10.4 | 0.015 | 1.4 | >4 | 0.78 | 0.075 | Low | Low | Low |
| EW8 | Ewing | 10 | 0.248 | 1.3 | >4 | 0.88 | 0.604 | Low | Low | Low |
| TC-71 | Ewing | 13.1 | 0.944 | 1.1 | >4 | 0.59 | 0.035 | Low | Low | Low |
| CHLA258 | Ewing | 8.8 | 0.104 | 0.9 | >4 | 1.25 | 0.19 | Low | Low | Low |
| Rh10 | ALV RMS | >EP | <0.001 | >2.0 | 0 | 0.05 | <0.001 | High | NE | High |
| Rh28 | ALV RMS | >EP | <0.001 | >2.5 | 0 | 0.04 | <0.001 | High | High | High |
| Rh30 | ALV RMS | >EP | <0.001 | >4.0 | 0.2 | 0.33 | 0.002 | Int | High | High |
| Rh30R | ALV RMS | >EP | <0.001 | >3.2 | 0.2 | 0.18 | <0.001 | Int | High | High |
| Rh18 | EMBRMS | 13 | 0.019 | 1.9 | >4 | 0.67 | 0.063 | Low | Low | Int |
| BT-28 | Medulloblastoma | 6.3 | 0.234 | 1.1 | >4 | 0.66 | 0.351 | Low | Low | Low |
| BT-45 | Medulloblastoma | 11 | 0.894 | 0.7 | >4 | 1.14 | 0.604 | Low | Low | Low |
| BT-50 | Medulloblastoma | >EP | 0.571 | >1.2 | 4 | 0.78 | 0.604 | Low | NE | Low |
| BT-41 | Ependymoma | 28.3 | 0.006 | 1.5 | >4 | 0.39 | 0.002 | Int | Low | Low |
| BT-44 | Ependymoma | 22 | 0.841 | 1 | >4 | 0.92 | 0.739 | Low | Low | Low |
| GBM2 | Glioblastoma | 25.8 | <0.001 | 1.8 | >4 | 0.41 | <0.001 | Int | Low | Int |
| BT-39 | Glioblastoma | 13 | 0.757 | 1 | >4 | 0.96 | 0.853 | Low | Low | Low |
| D645 | Glioblastoma | >EP | <0.001 | >5.7 | 0.4 | 0.28 | <0.001 | Int | High | High |
| D456 | Glioblastoma | >EP | <0.001 | >2.9 | 3.6 | 0.26 | 0.004 | Int | Int | High |
| NB-SD | Neuroblastoma | >EP | <0.001 | >4.1 | 3.2 | 0.15 | <0.001 | High | Int | High |
| NB-1771 | Neuroblastoma | >EP | <0.001 | >7.2 | 0 | 0.04 | <0.001 | High | High | High |
| NB-1691 | Neuroblastoma | >EP | <0.001 | >4.1 | 0 | 0.1 | <0.001 | High | High | High |
| NB-EBc1 | Neuroblastoma | 17 | <0.001 | 2.7 | >4 | 0.23 | 0.002 | Int | Int | Int |
| NB-1643 | Neuroblastoma | >EP | <0.001 | >7.3 | 0 | 0.08 | <0.001 | High | High | High |
| OS-1 | Osteosarcoma | 21.7 | 0.02 | 1.2 | >4 | 0.74 | 0.011 | Low | Low | Low |
| OS-2 | Osteosarcoma | 18.4 | 0.225 | 1 | >4 | 1.15 | 0.052 | Low | Low | Low |
| OS-17 | Osteosarcoma | 15.8 | 0.251 | 1 | >4 | 1.02 | 0.631 | Low | Low | Low |
| OS-9 | Osteosarcoma | 19.1 | 0.144 | 1 | >4 | 0.93 | 0.165 | Low | Low | Low |
| OS-33 | Osteosarcoma | 23.5 | <0.001 | 1.3 | >4 | 0.63 | 0.002 | Low | Low | Low |
| OS-31 | Osteosarcoma | 22.1 | 0.152 | 1.1 | >4 | 0.74 | 0.063 | Low | Low | Low |
KM: Kaplan-Meier estimate.
Objective responses were observed in the neuroblastoma, rhabdomyosarcoma, rhabdoid tumor, Wilms tumor, and glioblastoma panels. The most consistent activity was observed for the rhabdomyosarcoma and the neuroblastoma panels, with all four of the alveolar rhabdomyosarcoma xenografts achieving either complete response (CR) or maintained CR and with 4 of 5 neuroblastoma xenografts achieving CR or maintained CR. Objective responses were not observed for the Ewing sarcoma, medulloblastoma, and osteosarcoma panels. The objective response results are shown using ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Material and Methods and the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease), and with bars to the right representing regression and bars to the left representing progressive disease (Figure 2). Responses of individual neuroblastoma xenografts (Figure 3) and rhabdomyosarcoma xenografts (Figure 4) are presented.
Figure 2.
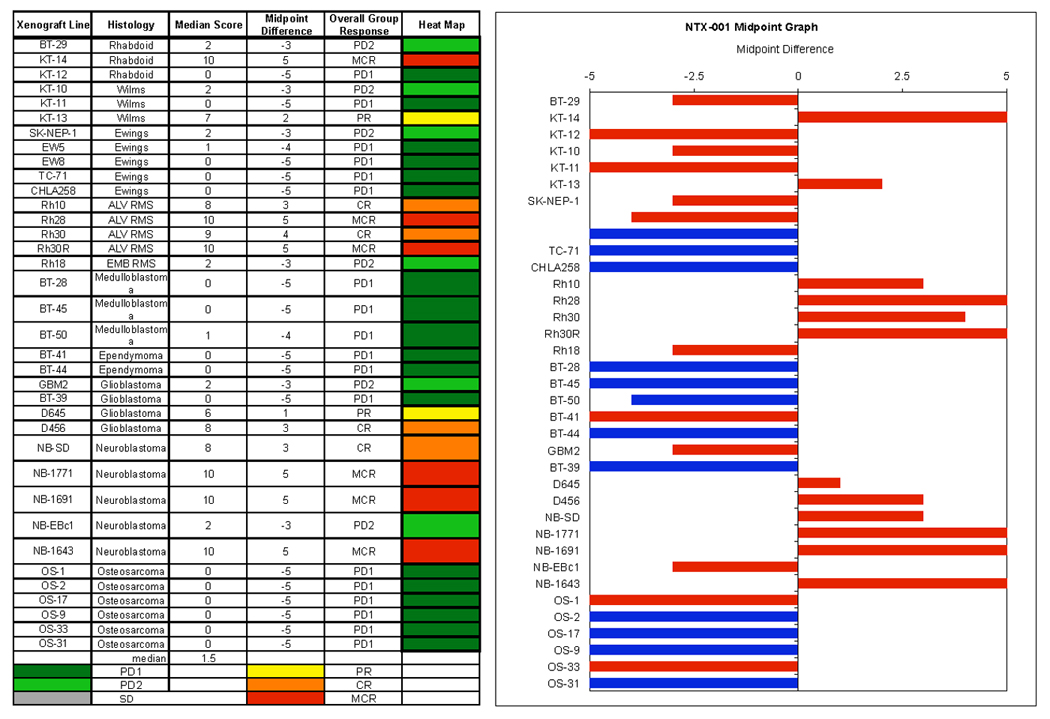
NTX-010 in vivo objective response activity. Left: The colored ‘heat map’ depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥ 2 but < 6, and low activity by a score of < 2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive (objective responses), and to the left are tumor models that are less sensitive (non-objective response). Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Figure 3.
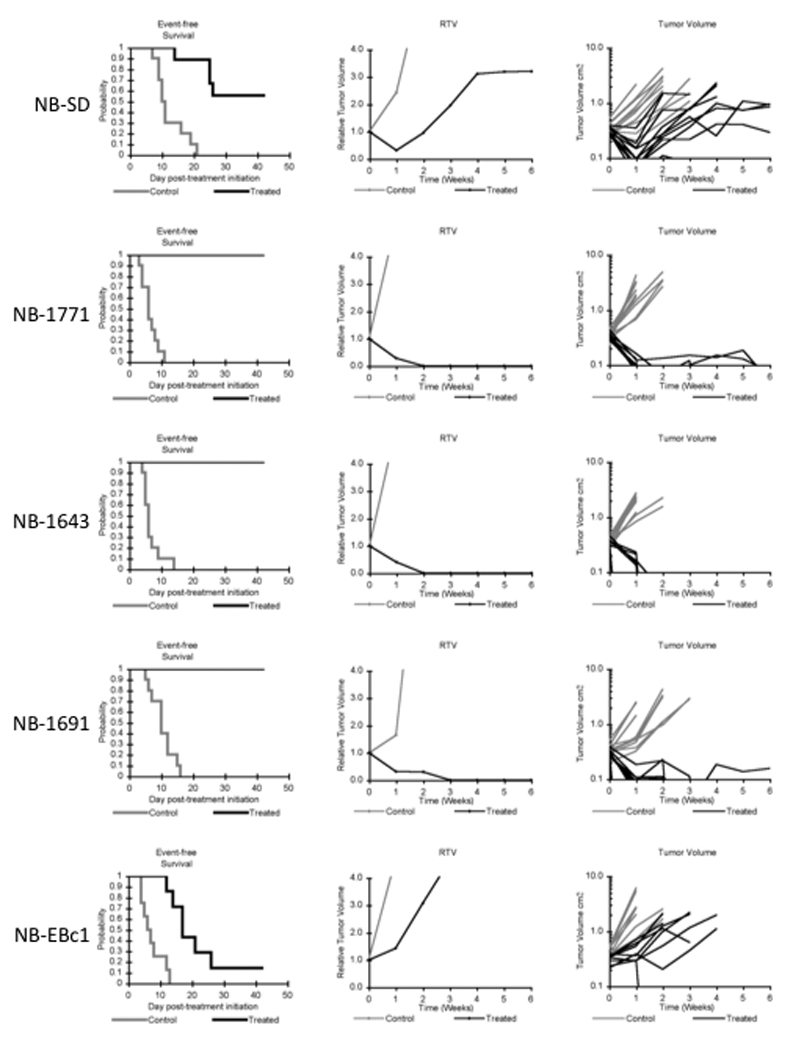
NTX-010 activity against neuroblastoma xenografts. Kaplan-Meier curves for EFS, median relative tumor volume graphs, and individual tumor volume graphs are shown for each line. (A) NB-SD (B) NB-1771, (C) NB-1643, (D) NB-1691, (E) NB-EBc1. Controls (gray lines); Treated (black lines).
Figure 4.
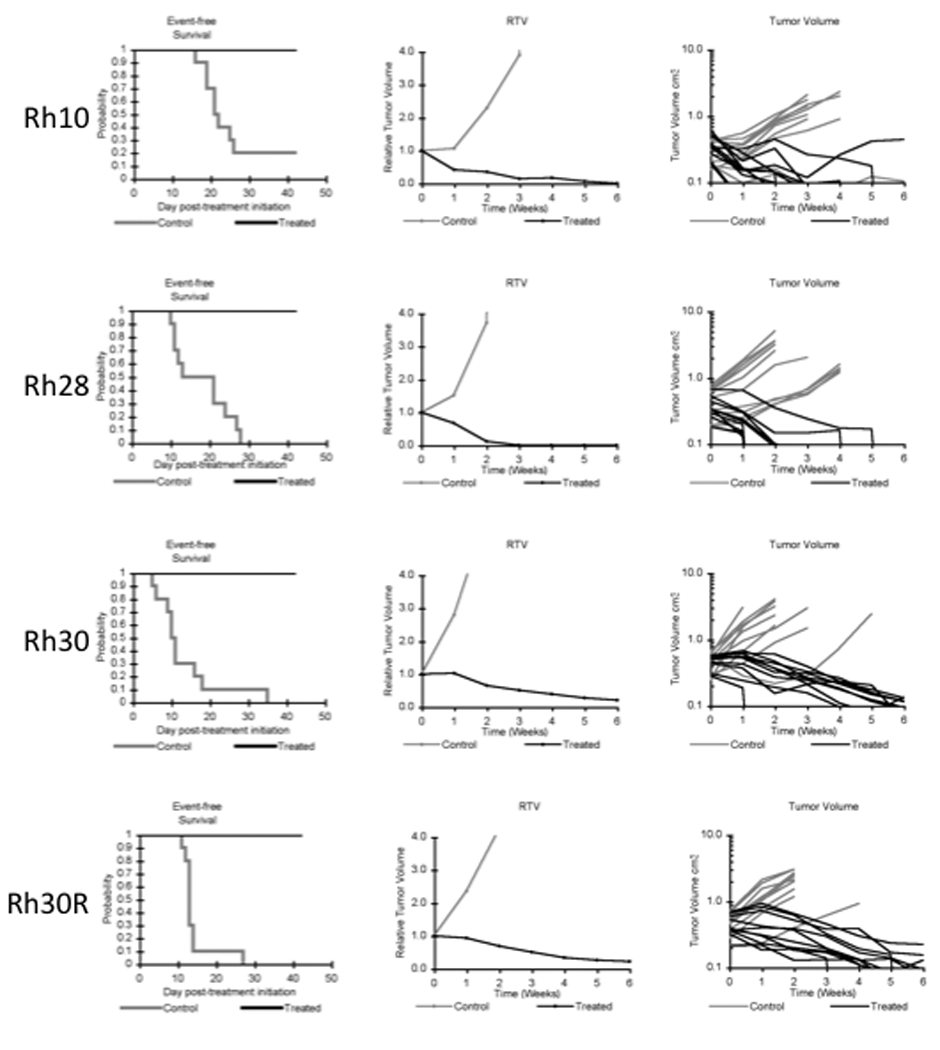
NTX-010 activity against rhabdomyosarcoma xenografts. Kaplan- Kaplan-Meier curves for EFS, median relative tumor volume graphs, and individual tumor volume graphs are shown for rhabdomyosarcoma lines: (A) Rh-10 (B) Rh28, (C) Rh30, (D) Rh30R. Controls (gray lines); Treated (black lines).
Expression of neuroendocrine markers
Although the cellular receptor for NTX-010 is unknown, the virus appears to replicate selectively in cells having neuroendocrine characteristics. The expression of neuroendocrine markers [neural cell adhesion molecule 1 (NCAM1, CD56), synaptophysin, chromogranin A, and neuronal cell adhesion molecule (NRCAM)] in the PPTP cell line and xenograft panels is presented in Figure 5. The xenografts and cell lines of the rhabdomyosarcoma and neuroblastoma panels show consistent high level expression of NCAM1 and NCRAM.
Figure 5.
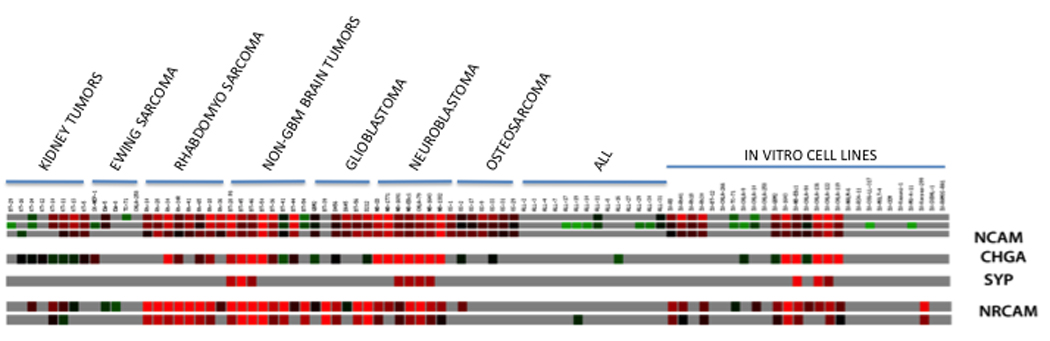
Gene expression (Affymetrix U133 Plus 2.0) in PPTP cell lines and xenografts as visualized using GeneSifter software (VizX Labs, Seattle, WA) for selected genes that serve as markers for cells of neuroendocrine origin. Gray indicates an absent call from Affymetrix quality control. Gene expression analysis methods are as previously described [34]. NCAM = neural cell adhesion molecule 1 (NCAM1, CD56); SYP = synaptophysin; CHGA = chromogranin A; and NRCAM = neuronal cell adhesion molecule
DISCUSSION
The use of viruses to deliver pro-drug activating enzymes or to directly lyse tumor cells has shown promise in preclinical models [18]. For example local injection or intraperitoneal administration of Newcastle disease virus induced regressions of subcutaneous IMR-32 neuroblastoma xenografts [19,20], and administration of reovirus administered either by local intratumoral or systemic administration has potent activity against breast, ovarian, colon and glioblastoma xenografts [7,21–24]. In human trials, local injection of ONYX-015 induced regressions (14%) and stable disease (41%) in head and neck cancer [25] and a low response rate (6%) in hepatobiliary cancer [26]. Objective responses have also been observed with systemically administered Newcastle Disease Virus in adult cancer patients [27]. NTX-010 represents a new therapeutic virus that infects cells having neuroendocrine characteristics. A phase I study of intravenous NTX-010 was conducted in adults with neuroendocrine cancers across 5 log-increment dose cohorts from 107 vp/kg to 1011 vp/kg. No dose-limiting toxicities were observed at any dose level, and the primary adverse events were mild flu like symptoms that persisted for one to two days. [28]. Currently, NTX-010 is being evaluated in a randomized, double-blinded phase 2 study in adults with extensive-stage small cell lung cancer. A phase 1 dose-escalation study in children with relapsed/refractory neuroblastoma, rhabdomyosarcoma, or other rare tumors with neuroendocrine features is also being conducted (http://www.clinicaltrial.gov/ct2/show/NCT01048892).
NTX-010 shows high level activity against selected cell lines and xenografts from the PPTP’s in vitro and in vivo panels. A single dose of NTX-010 induced complete responses in 8 of 10 of the rhabdomyosarcoma and neuroblastoma xenografts evaluated, including all 4 alveolar rhabdomyosarcoma xenografts studied. Of note is the similar sensitivity to NTX-010 in Rh30 xenografts (established at diagnosis) and Rh30R xenografts (established at patient relapse), suggesting NTX-010 has therapeutic utility in both chemosensitive and chemorefractory disease. NTX-010 activity may extend to additional diagnoses, as objective responses were also noted among xenografts in the glioblastoma, rhabdoid, and Wilms tumor panels.
The in vivo results match, in a general way, the in vitro testing results, as the rhabdomyosarcoma and neuroblastoma cell lines were the most sensitive to this lytic virus for both in vitro and in vivo testing. There is limited overlap between the cell lines of the in vitro panel and the xenografts of the in vivo panel (9 lines). For the 9 overlapping cell lines, there is not a tight correlation between the in vitro activity and the in vivo activity of NTX-010. While both of the models that achieved CR or MCR (Rh30 and NB-1643) showed > 90% inhibition at the 1 × 104 vp/cell concentration, only one of them (NB-1643) showed similar activity at the 1 vp/cell concentration. Conversely, three of the five overlapping models that failed to achieve CR or MCR showed high activity at the 1 × 104 vp/cell concentration (Rh18, TC-71, and NB-EBc1), and two additionally showed high activity at the 1 vp/cell concentration (Rh18 and NB-EBc1). The lack of a tight correlation between the in vitro and in vivo activity may result from subtle biological differences in the models when grown as xenografts versus when grown in vitro.
A key question is the biological characteristics of tumor cells that underlie responsiveness to NTX-010. Previous work by Neotropix Inc. has shown that cancer cells with neuroendocrine properties appear to be more responsive to virus than other cancer cells [10]. The gene expression heat map (Figure 5) shows the expression pattern of four genes commonly expressed by cells of neuroendocrine origin: NCAM1, chromogranin A, synaptophysin, and NRCAM. Both rhabdomyosarcoma and neuroblastoma xenografts express NCAM1 and NRCAM, while the neuroblastoma xenografts and cell lines additionally express chromogranin A. By contrast, Ewing sarcoma cell lines and xenografts, show limited expression of these four genes. Consistent with the expression observed for PPTP xenografts, clinical specimens for alveolar rhabdomyosarcoma show high level expression of NCAM1 and NRCAM [29,30], while Ewing sarcoma clinical specimens show low levels of NCAM1 [31]. The PPTP leukemia and lymphoma cell lines and xenografts show little expression of the four neuroendocrine genes selected for analysis. Testing of additional cell lines and xenografts will provide data that will be useful in defining the biological characteristics associated with response to NTX-010.
The Ewing sarcoma results warrant comment. Several of the Ewing sarcoma cell lines showed some degree of responsiveness to NTX-010 in vitro, with one of the 4 cell lines showing near complete growth inhibition at the lowest concentration tested and with an additional 2 cell lines showing near complete inhibition at the highest concentration tested. However, the Ewing sarcoma xenografts studied did not show in vivo responsiveness to this agent. Study of additional models may be helpful in defining the extent of activity for NTX-010 for Ewing sarcoma.
The in vivo results reported share the same potential limitation noted in the report of Reddy and colleagues [10], as the in vivo efficacy data were generated using immune-deficient mice. It is unknown whether immune responses in cancer patients will limit the effectiveness of NTX-010, and whether strategies to limit immune responses to NTX-010 may be necessary to optimize its clinical effectiveness. Preclinical results with other oncolytic viruses in immune competent hosts support the use of virus in combination with chemotherapy agents such as cyclophosphamide to modulate the immune response to the virus [32,33].
In conclusion, NTX-010 shows high in vitro and in vivo activity against selected pediatric preclinical models used in the PPTP. NTX-010 appears particularly promising for neuroblastoma and alveolar rhabdomyosarcoma. Further preclinical work to identify molecular characteristics associated with response will help direct pediatric clinical development of NTX-010.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used NTX-010 supplied by Neotropix, Inc. In addition to the authors this represents work contributed by the following: Sherry Ansher, Catherine A. Billups, Joshua Courtright, Edward Favours, Henry S. Friedman, Nino Keshelava, Debbie Payne-Turner, Charles Stopford, Mayamin Tajbakhsh, Chandra Tucker, Jianrong Wu, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors consider that there are no actual or perceived conflicts of interest.
Reference List
- 1.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18(7):723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 2.O'Shea CC. Viruses - seeking and destroying the tumor program. Oncogene. 2005;24(52):7640–7655. doi: 10.1038/sj.onc.1209047. [DOI] [PubMed] [Google Scholar]
- 3.Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105(9):1169–1172. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9(12):961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- 5.Alemany R. Cancer selective adenoviruses. Molecular aspects of medicine. 2007;28(1):42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Kelly K, Nawrocki S, Mita A, et al. Reovirus-based therapy for cancer. Expert opinion on biological therapy. 2009;9(7):817–830. doi: 10.1517/14712590903002039. [DOI] [PubMed] [Google Scholar]
- 7.Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 8.Strong JE, Coffey MC, Tang D, et al. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. The EMBO journal. 1998;17(12):3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hales LM, Knowles NJ, Reddy PS, et al. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. The Journal of general virology. 2008;89(Pt 5):1265–1275. doi: 10.1099/vir.0.83570-0. [DOI] [PubMed] [Google Scholar]
- 10.Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. Journal of the National Cancer Institute. 2007;99(21):1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy P, Jones B, Idamakanti N, et al. Mechanism of tumor selective replication of Seneca Valley Virus (SVV-001), a novel oncolytic picornavirus for systemic treatment of patients with solid tumors with neuroendocrine features. 98th AACR Annual Meeting; 2007. Abstr #1839. [Google Scholar]
- 12.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 13.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer research. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 14.Graham C, Tucker C, Creech J, et al. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin Cancer Res. 2006;12(1):223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 16.Friedman HS, Colvin OM, Skapek SX, et al. Experimental Chemotherapy of Human Medulloblastoma Cell Lines and Transplantable Xenografts with Bifunctional Alkylating Agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 17.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka R. Alphavirus vectors for cancer gene therapy (review) Int J Oncol. 2004;24(4):919–923. [PubMed] [Google Scholar]
- 19.Phuangsab A, Lorence RM, Reichard KW, et al. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer letters. 2001;172(1):27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 20.Lorence RM, Reichard KW, Katubig BB, et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. Journal of the National Cancer Institute. 1994;86(16):1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63(2):348–353. [PubMed] [Google Scholar]
- 22.Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13(5):641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 23.Hirasawa K, Nishikawa SG, Norman KL, et al. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62(6):1696–1701. [PubMed] [Google Scholar]
- 24.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93(12):903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 25.Nemunaitis J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19(2):289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 26.Makower D, Rozenblit A, Kaufman H, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9(2):693–702. [PubMed] [Google Scholar]
- 27.Lorence RM, Roberts MS, O'Neil JD, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Current cancer drug targets. 2007;7(2):157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 28.Rudin CM, Senzer N, Stephenson J, et al. Phase I study of intravenous Seneca Valley virus (NTX-010), a replication competent oncolytic virus, in patients with neuroendocrine (NE) cancers. J Clinical Oncology. 2009;27(15s) abstract 4629. [Google Scholar]
- 29.Bahrami A, Gown AM, Baird GS, et al. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21(7):795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 30.Lae M, Ahn EH, Mercado GE, et al. Global gene expression profiling of PAX-FKHR fusionpositive alveolar and PAX-FKHR fusion-negative embryonal rhabdomyosarcomas. The Journal of pathology. 2007;212(2):143–151. doi: 10.1002/path.2170. [DOI] [PubMed] [Google Scholar]
- 31.Olsen SH, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol. 2006;19(5):659–668. doi: 10.1038/modpathol.3800569. [DOI] [PubMed] [Google Scholar]
- 32.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Zeng Z, Fu X, et al. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer research. 2007;67(16):7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- 34.Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14(14):4572–4583. doi: 10.1158/1078-0432.CCR-07-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


