Abstract
A combination of NMR, kinetic, and computational methods are used to examine reactions of lithium diethylamide in tetrahydrofuran (THF) with n-dodecyl bromide and n-octyl benzenesulfonate. The alkyl bromide undergoes competitive SN2 substitution and E2 elimination in proportions independent of all concentrations except for a minor medium effect. Rate studies show that both reactions occur via trisolvated-monomer-based transition structures. The alkyl benzenesulfonate undergoes competitive SN2 substitution (minor) and N-sulfonation (major) with N-sulfonation promoted at low THF concentrations. The SN2 substitution is shown to proceed via a disolvated monomer suggested computationally to involve a cyclic transition structure. The dominant N-sulfonation follows a disolvated-dimer-based transition structure suggested computationally to be a bicyclo[3.1.1] form. The differing THF and lithium diethylamide orders for the two reactions explain the observed concentration-dependent chemoselectivities.
Introduction
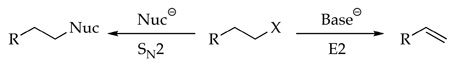
Many may remember being confounded by the substitution-elimination dichotomy presented in our first course on organic chemistry (eq 1).1 It was difficult to grasp why a given electrophile-nucleophile-solvent combination causes the prevalence of substitution over elimination (or vice versa), despite support from an enormous body of empirical observations. In our opinion, the confusion stems from the incomplete picture of how solvation and aggregation influence nucleophilicity and basicity. The nomenclature based on “ion pairing” prevalent in the older literature is too inflexible to describe underlying aggregation effects. Similarly, using terms such as “polarity” to explain solvent-dependent reactivities and selectivities is inadequate to describe inherently molecular solvation events. Amid the few studies designed to untangle the coordination chemistry underlying substitutions and eliminations,2 the efforts of Streitwieser and coworkers are prominent.3
 |
(1) |
Understanding the SN2-E2 dichotomy is more than an aging academic problem. One is struck, for example, by the profound importance of C-N bond formation in pharmaceutical syntheses and the role played by SN2 substitutions.4 Given the scope of the applications and their scales,5 even incremental improvements in simple N-alkylations of mono- and dialkylamines could prove significant.
We describe herein reactions of lithium diethylamide (Et2NLi) in tetrahydrofuran (THF) with an n-alkyl bromide (eq 2) and an n-alkyl sulfonate (eq 3). The competing N-substitution, elimination, and N-sulfonation (O-desulfonation) pathways are traced to specific solvation and aggregation events.6
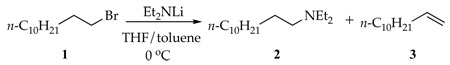 |
(2) |
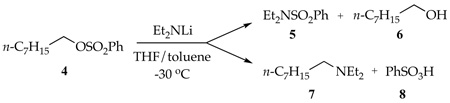 |
(3) |
Results
Concentration-Dependent Selectivities
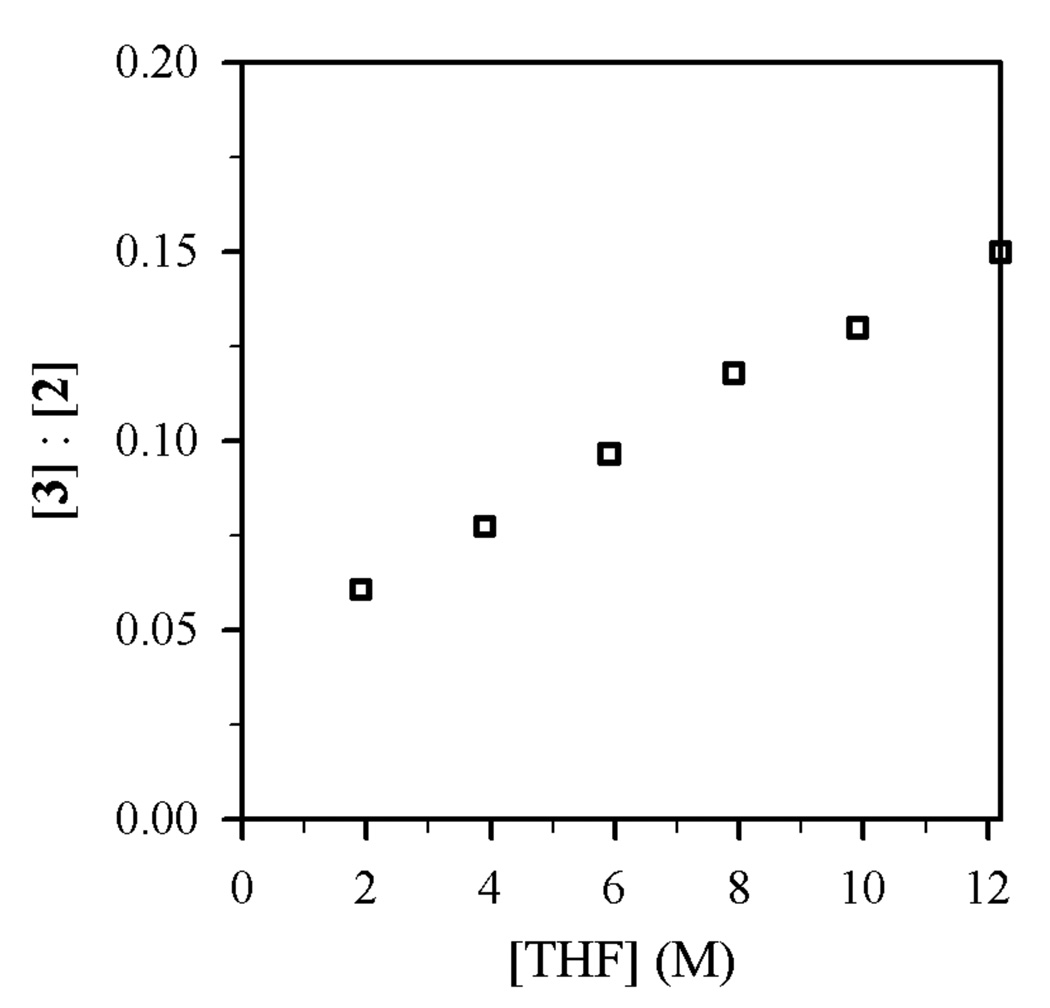
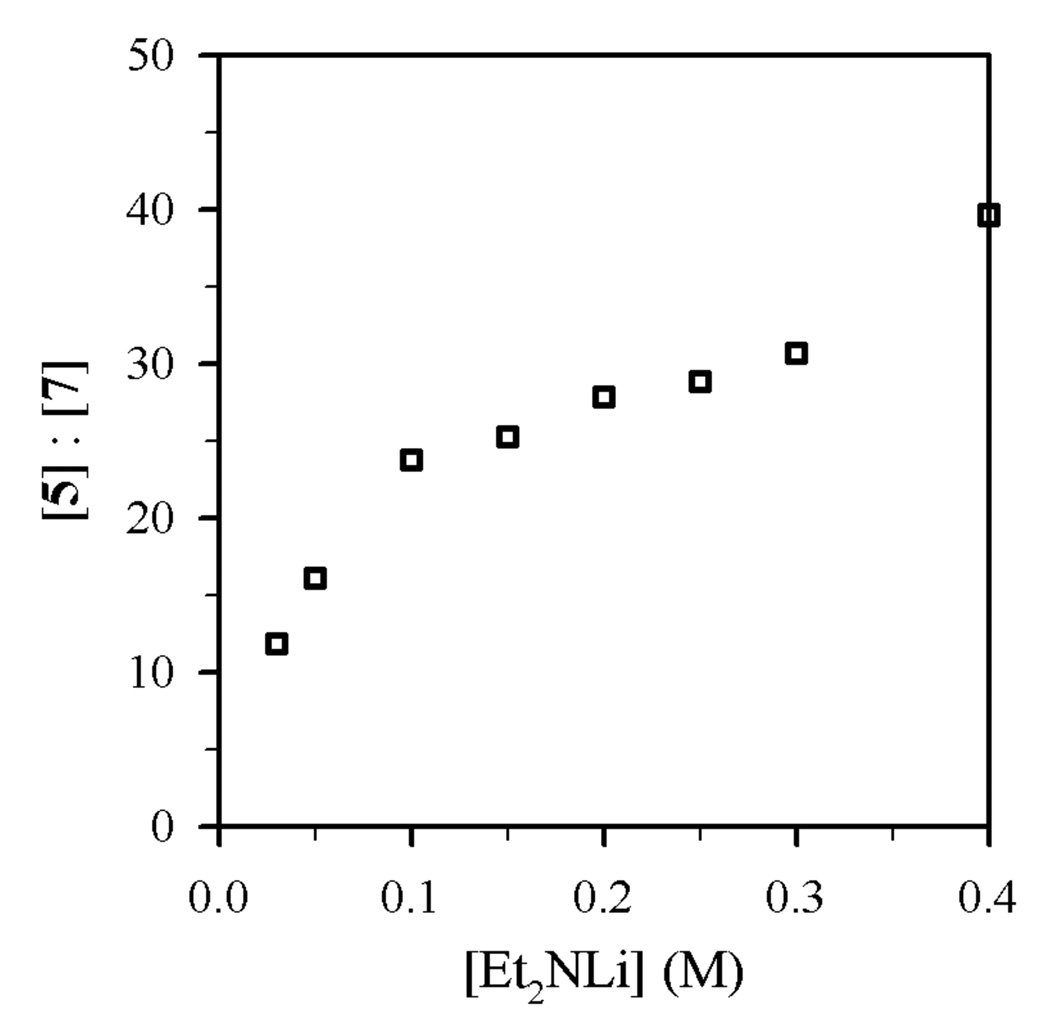
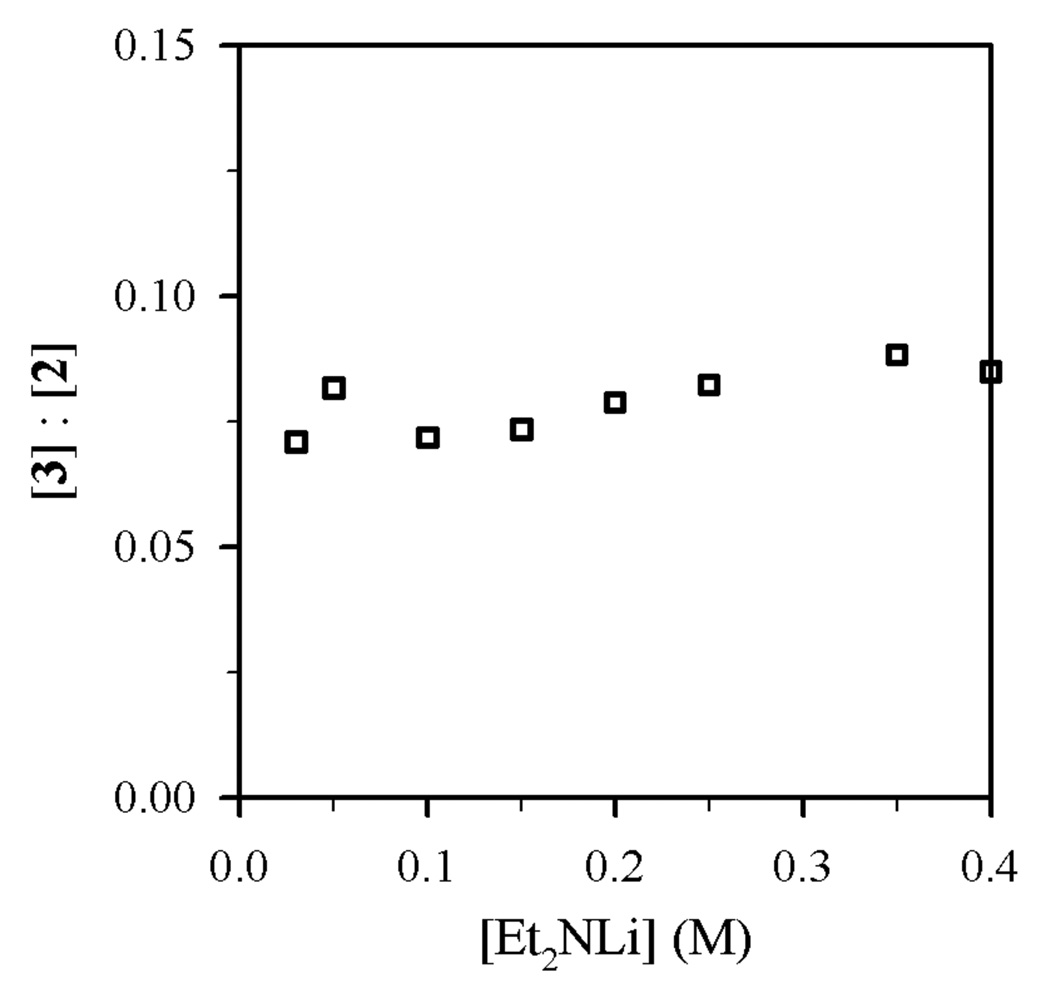
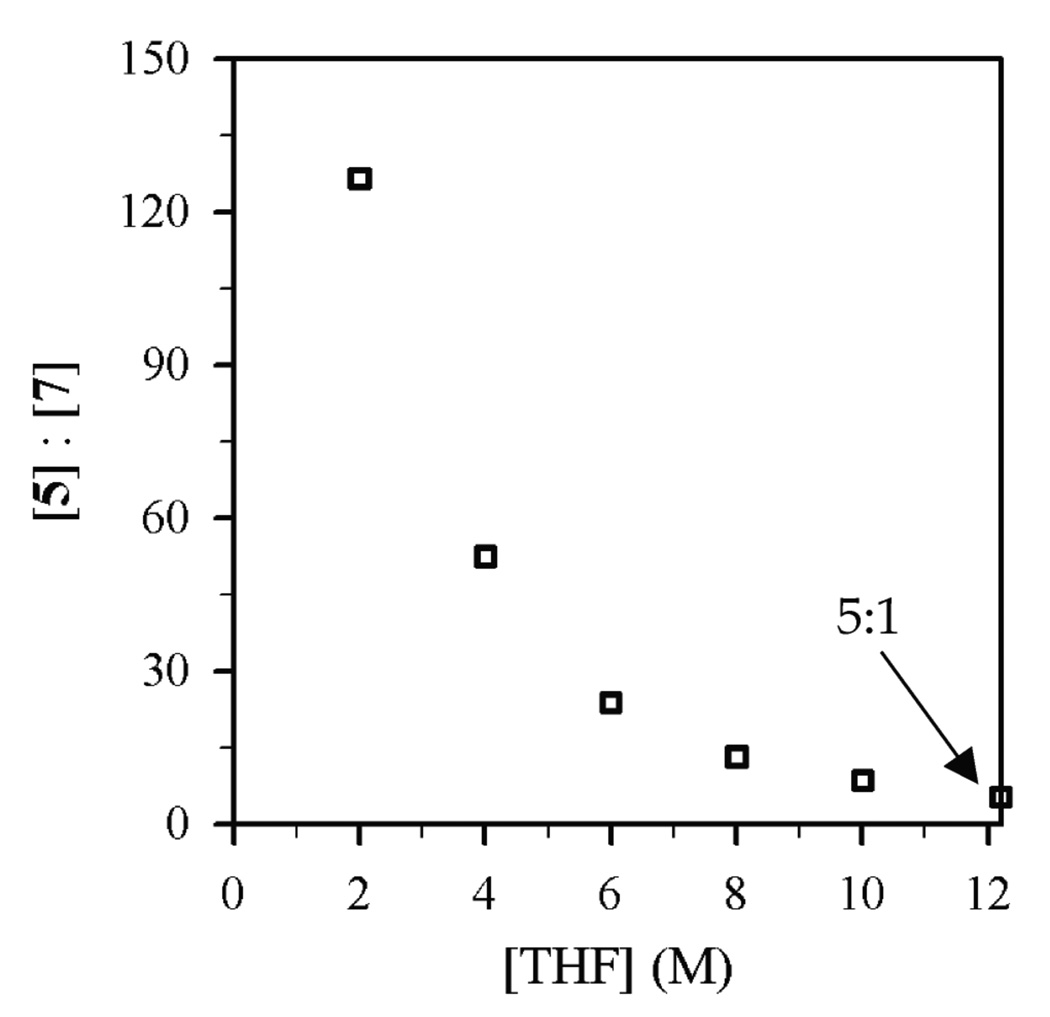
Using protocols and conditions described below, the selectivities of N-alkylation, elimination, and N-sulfonation versus Et2NLi and THF concentrations were measured and are depicted graphically in Figures 1–4. The notable feature is that n-alkyl bromide 1 affords ratios of 2 and 3 displaying a minor THF dependence (Figures 1 and 2), whereas the relative proportions of N-sulfonation (5) and N-alkylation (7) show both a dependence on the Et2NLi concentration and a striking THF concentration dependence (Figures 3 and 4). The product ratios allow us to deconvolute the mechanistic contributions to each pathway.
Figure 1.
Plot of [3]:[2] vs [THF] in toluene cosolvent for the reaction of 0.004 M 1-bromododecane (1) with Et2NLi (0.10 M) at 0 °C.
Figure 4.
Plot of [5]:[7] vs [Et2NLi] in THF (6.0 M) and toluene cosolvent for the reaction of 0.004 M 1-octyl benzenesulfonate (4) with Et2NLi at −30 °C.
Figure 2.
Plot of [3]:[2] vs [Et2NLi] in THF (3.9 M) and toluene cosolvent for the reaction of 0.004 M 1-bromododecane (1) with Et2NLi at 0 °C.
Figure 3.
Plot of [5]:[7] vs [THF] in toluene cosolvent for the reaction of 0.004 M 1-octyl benzenesulfonate (4) with Et2NLi (0.10 M) at −30 °C.
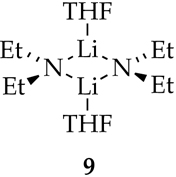
Structure of Lithium Diethylamide
Previous 6Li and 15N NMR spectroscopic investigations have shown that [6Li,15N]Et2NLi is a dimer in THF (9).7 Computational studies suggest that dimer 9 is disolvated (see Supporting Information). At low THF concentrations (<2.0 M), minor amounts of 3- and 4-rung ladders are observed.7,8
General Protocols
Pseudo-first-order rate constants (kobsd) were determined using excess Et2NLi (0.030–0.40 M) and limiting substrate concentrations (0.004 M). THF was restricted to >2.0 M to avoid the larger aggregates observed at low THF concentrations.7,8 The disappearance of the substrate (1 or 4), and the formation of products were monitored relative to an internal n-decane standard using gas chromatographic (GC) analysis of quenched aliquots; they displayed clean first-order decays. Measured values of kobsd are independent of the initial concentrations of the substrate (±10%), consistent with first-order dependencies on the substrates. The product ratios allow kobsd to be partitioned into the rate constants for the parallel pathways as described below. Results from the rate studies are summarized in Table 1. Additional data are archived in Supporting Information.
Table I.
| Entry | Substrate | Product(s) | THF order | Et2NLi order |
kH/kDd | kH/kDe |
|---|---|---|---|---|---|---|
| 1 | 1 | 2+3 | 2.0 ± 0.1a | 0.54 ± 0.03b | 1.1 ± 0.1 | 1.22 ± 0.05 |
| 2 | 2 | 2.0 ± 0.1a | 0.52 ± 0.03b | 1.1 ± 0.1 | 1.12 ± 0.05 | |
| 3 | 3 | 2.5 ± 0.1a | 0.57 ± 0.03b | 1.1 ± 0.1 | 3.02 ± 0.04 | |
| 4 | 4 | 5+7 | 0a | 0.98 ± 0.05c | ||
| 5 | 5 | 0a | 0.99 ± 0.04c | |||
| 6 | 7 | 1.29 ± 0.05a | 0.59 ± 0.04c |
[Et2NLi] = 0.10 M.
[THF] = 3.9 M in toluene cosolvent.
[THF] = 6.0 M in toluene cosolvent.
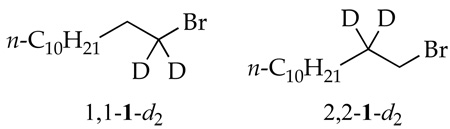
Measured using 1 and 1,1-1-d2.
Measured using 1 and 2,2-1-d2.
N-Alkylation and Elimination of 1-Bromododecane
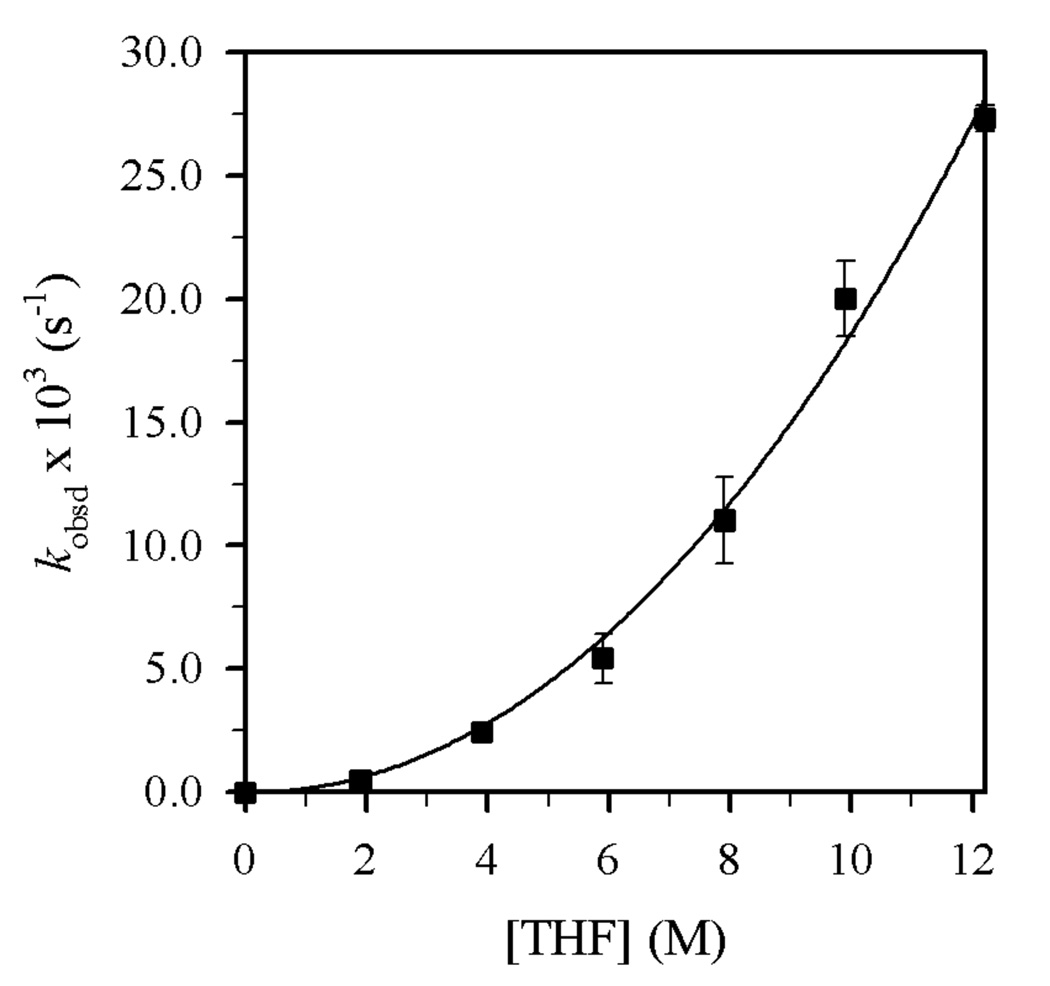
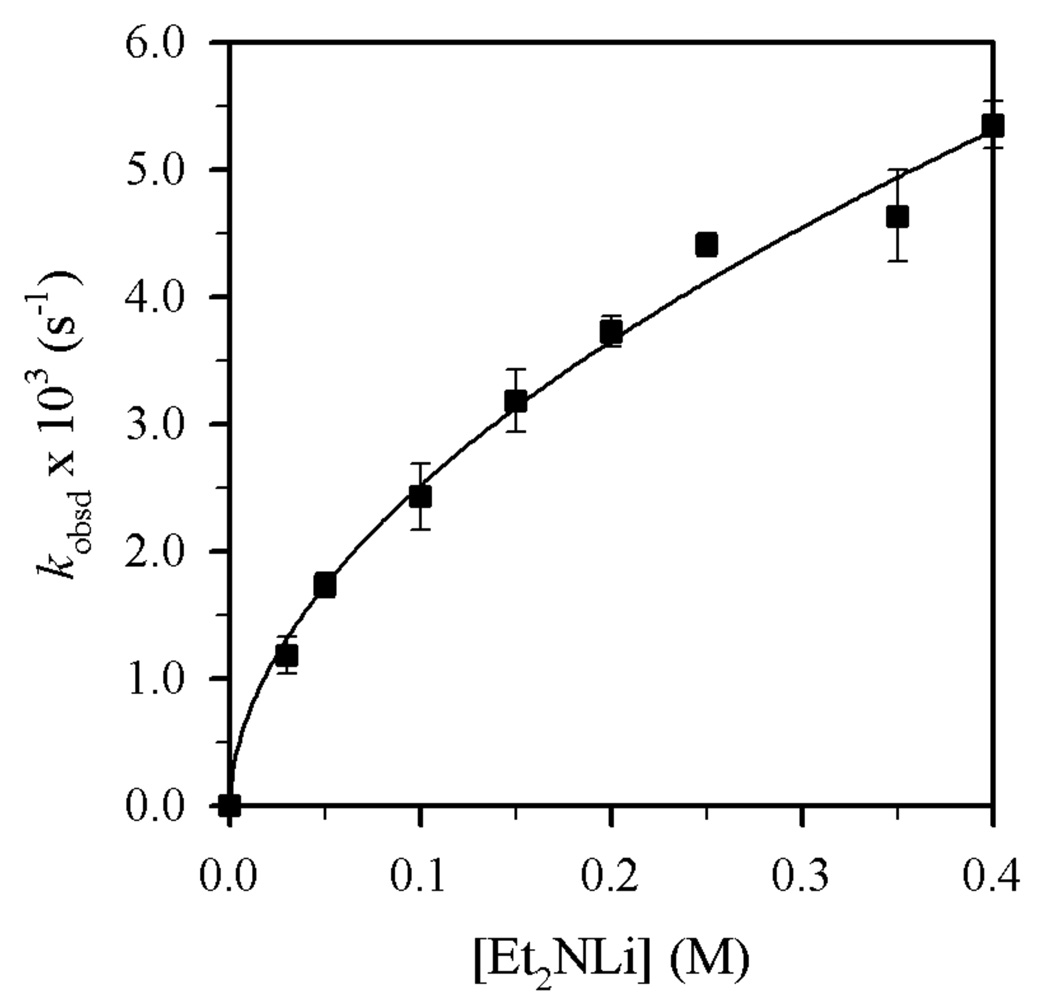
Reaction of Et2NLi with 1-bromododecane (1) in THF/toluene yields N,N-diethyldodecylamine (2)9 and 1-dodecene (3) as shown in eq 2 and Figure 5. n-Dodecane that resulted from reduction10 was also detected, but the concentrations were erratic and very low (<2%).11 Plots of kobsd versus THF concentration (Figure 6) and kobsd versus Et2NLi concentration (Figure 7) furnish orders of 2.0 ± 0.1 and 0.54 ± 0.03, respectively. Replacing toluene cosolvent with 2,2,4,4-tetramethyltetrahydrofuran revealed no measurable cosolvent dependence, arguing against long-range medium effects as the source of second-order THF dependence.6b,12
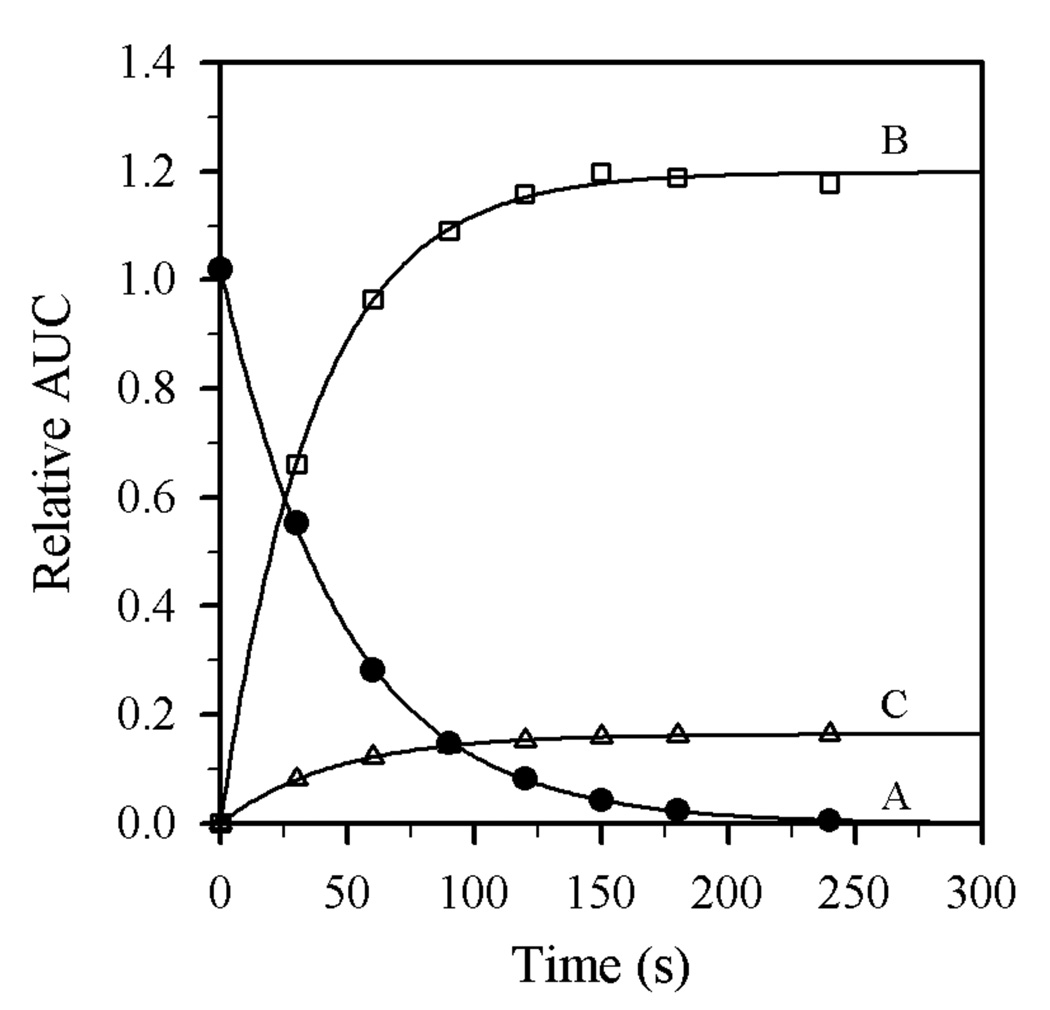
Figure 5.
Representative plot of the time-dependent decay of 1 (curve A) and formation of 2 (curve B) and 3 (curve C) relative to an n-decane internal standard (relative area under the curve, AUC) for sequentially quenched samples of a reaction mixture containing Et2NLi (0.10 M), THF (9.90 M), 1 (0.004 M), and toluene cosolvent at 0 °C. The curves depict least squares fit to: (A) y = ae−bx (a = 1.022 ± 0.005, b = kobsd = (2.11 ± 0.02) × 10−2); (B) y = {a(1−e−bx)} (a = 1.199 ± 0.006, b = kalk = (2.71 ± 0.06) × 10−2); (C) y = {a(1−e−bx)} (a = 1.648 ± 0.003) × 10−1, b = kelim = (2.25 ± 0.02) × 10−2)
Figure 6.
Plot of kobsd vs [THF] in toluene cosolvent for the reaction of 1 (0.004 M) with Et2NLi (0.10 M) at 0 °C. The curve depicts an unweighted least-squares fit to kobsd = k[THF]n (k = (1.6 ± 0.4) × 10−4, n = 2.0 ± 0.1).
Figure 7.
Plot of kobsd vs [Et2NLi] in THF (3.9 M) and toluene cosolvent for the reaction of 1 (0.004 M) with Et2NLi at 0 °C. The curve depicts an unweighted least-squares fit to kobsd = k[Et2NLi]n (k = (8.7 ± 0.4) × 10−3, n = 0.54 ± 0.03).
To separate contributions from the two pathways one simply notes that kobsd = kalk + kelim and [2]/[3] = kalk/kelim such that kalk and kelim correspond to the pseudo-first-order rate constants for N-alkylation and elimination, respectively. The task was simple because the product ratios were nearly independent of all concentrations (see Figures 1 and 2); the rate laws for substitution and elimination are identical. (A slight preference for the formation of 3 at elevated THF concentrations is reflected by the slightly higher order; Table 1, entry 3.) Thus, the idealized rate law13 is described by eq 4. The product ratios are sensitive to isotopic substitution. The measured isotope effects using 1,1-1-d2 and 2,2-1-d2 (Table 1) are consistent with an SN2 substitution14 and E2 elimination.15 GC-MS analyses also confirmed β-rather than α-eliminations.16 The stereochemistries of N-alkylation and elimination were not addressed experimentally.17
| (4) |
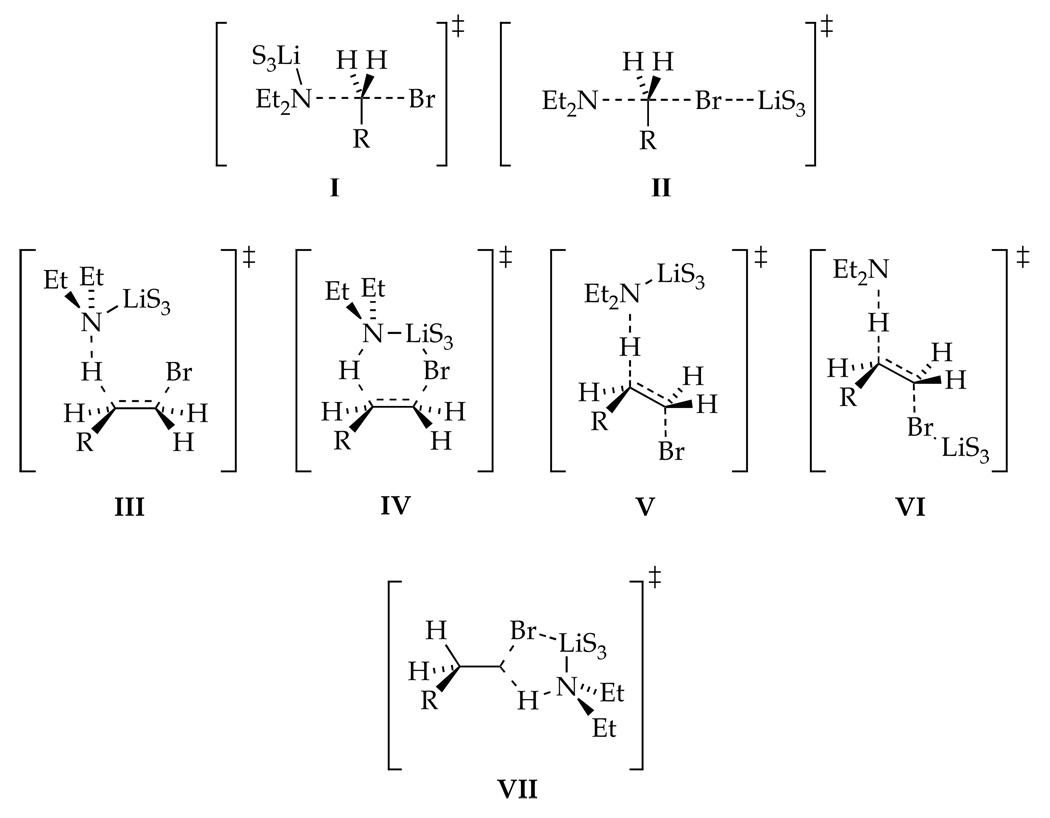
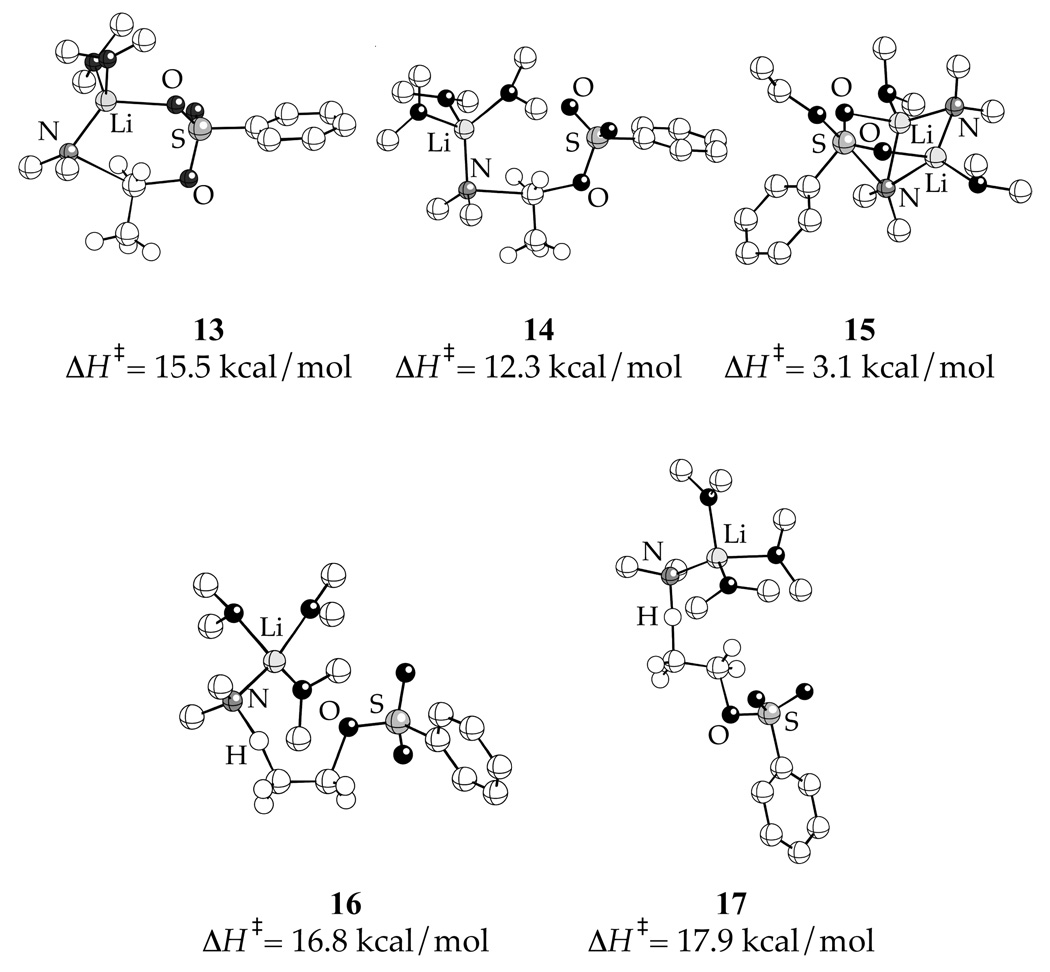
A variety of seemingly plausible transition structures for substitution and elimination are shown in Chart 1. Density functional theory (DFT) calculations using the SVP basis set for Br and 6-31G* for the rest of the atoms18 afforded enthalpies of activation (ΔH‡, kcal/mol) that include thermal corrections at 298.15 K. 1-Bromododecane, Et2NLi, and THF were modeled using EtBr, Me2NLi and Me2O, respectively, to restrict the number of conformers. Calculated activation free energies were ridiculously high even with MP2/6-31G*//B3LYP/6-31G* single-point calculations. Enthalpies of activation are reported according to eq 5. Although absolute energies are not terribly informative, the relative values and calculated geometries are.
Chart 1.
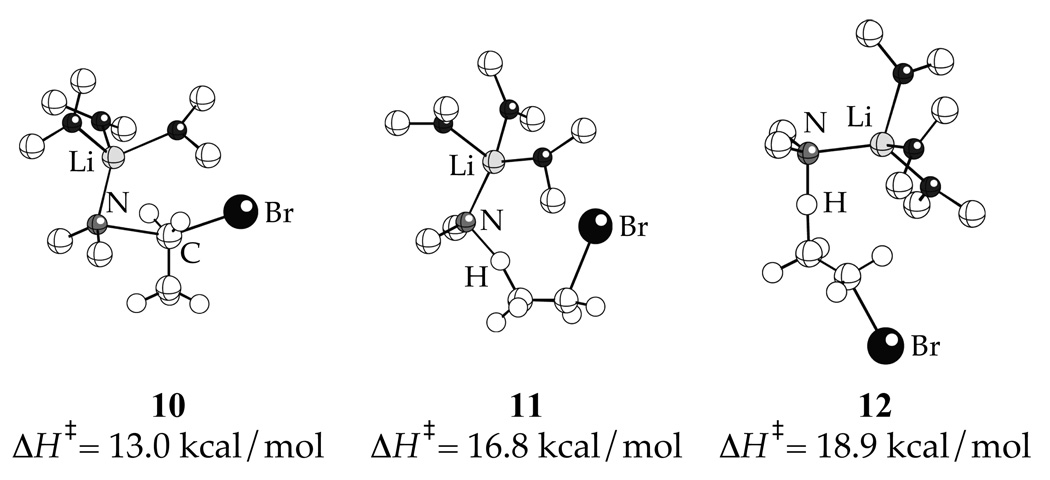
The results of the DFT computations (B3LYP/6-31G(d)) are illustrated in Chart 2. Optimization of isomer I resulted in legitimate transition structure 10 displaying an N-Li interaction and a highly bent N-C-Br bond angle (155°).19 In contrast, we failed to locate structures akin to II.20 Optimizations of β-eliminations III–VI afforded only 11 and 12 (types III and V), both containing N-Li contacts. No structures of type IV or VI displaying Br-Li contacts could be found.21 Efforts to find structure VII corresponding to a hypothetical (unobserved) α-elimination failed, possibly because the trisolvation implicated by the rate studies precludes a Br-Li interaction.22 The relative enthalpies of transition structures 10, 11 and 12 indicate that the nucleophilic substitution (10) is enthalpically favored.
| (5) |
Chart 2.
N-Alkylation and N-Sulfonation of n-Octyl benzenesulfonate
The reaction of 1-octyl benzenesulfonate (4) with 0.10 M Et2NLi in THF/toluene mixtures at −30 °C affords products derived from N-sulfonation (5 and 6) and N-alkylation (7 and 8) to the exclusion of 1-octene expected from elimination (eq 3). Figure 3 shows the THF dependence on the ratio of substitution and elimination (5/7). In contrast to the reaction of 1-bromododecane, the selectivity is highly sensitive to the proportion of THF. By monitoring the 5/7 ratio (vide supra), kobsd can be deconvoluted to give the rate constants for the N-sulfonation (ksulf) and N-alkylation (kalk).
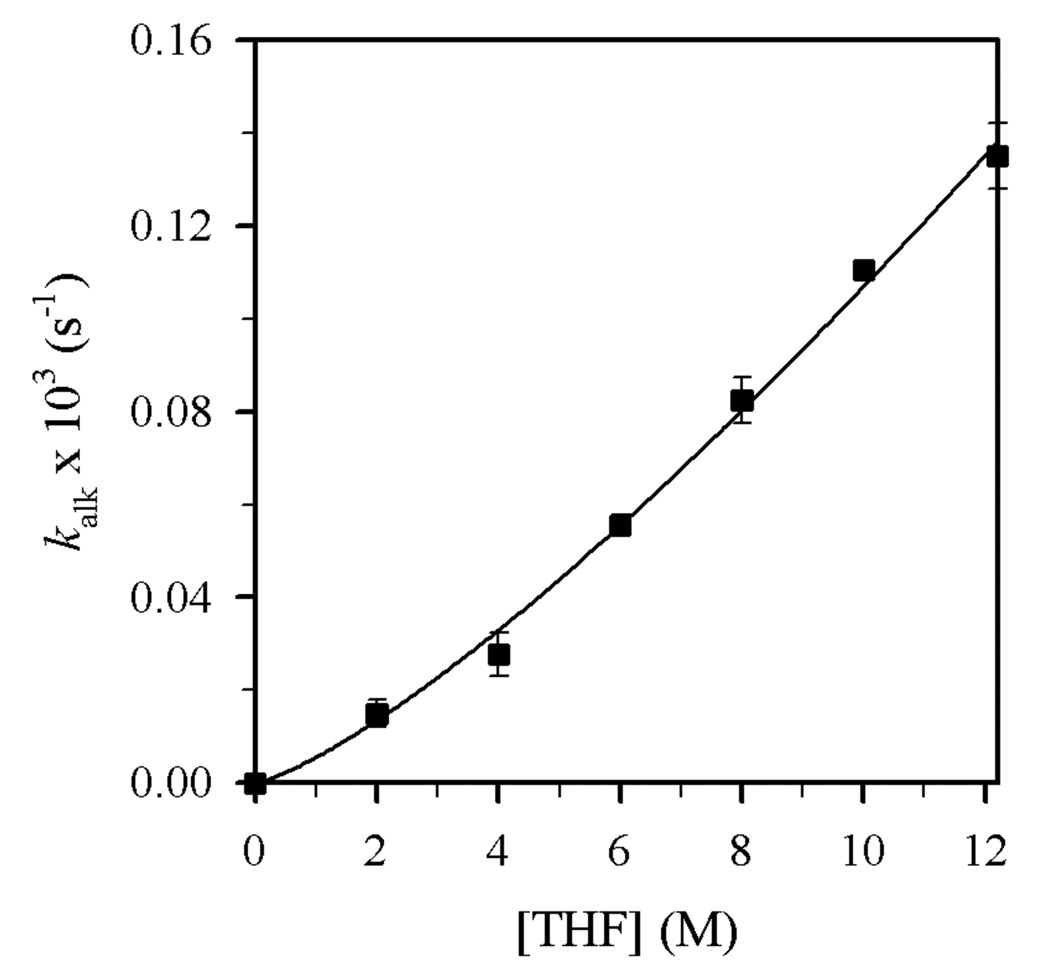
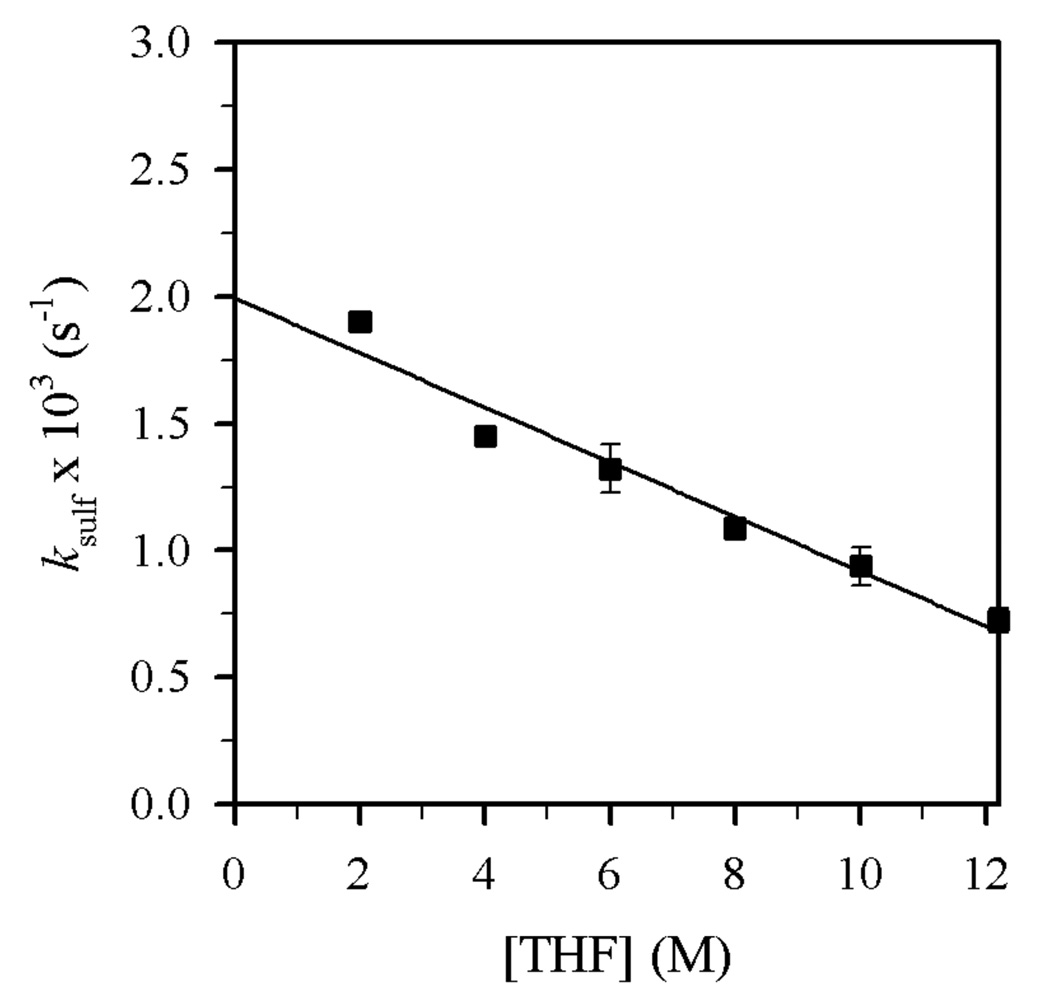
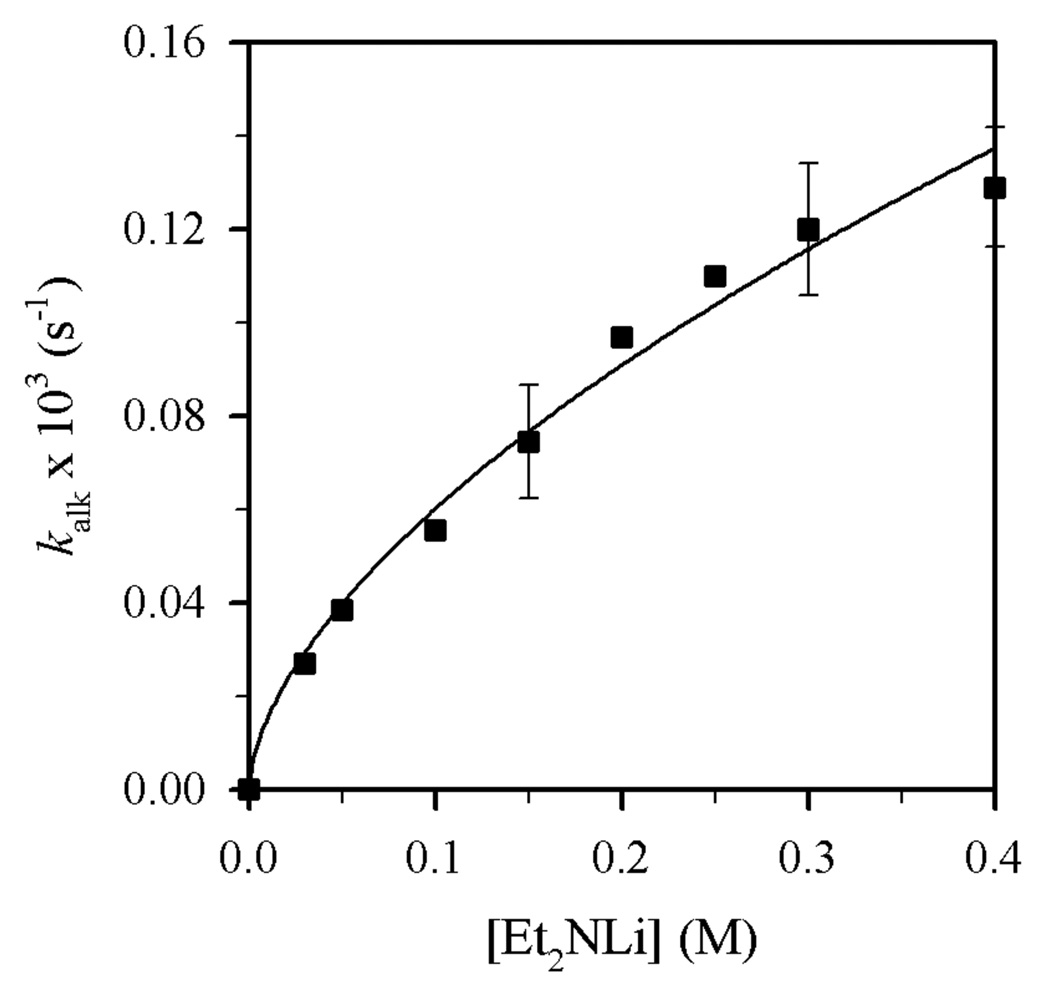
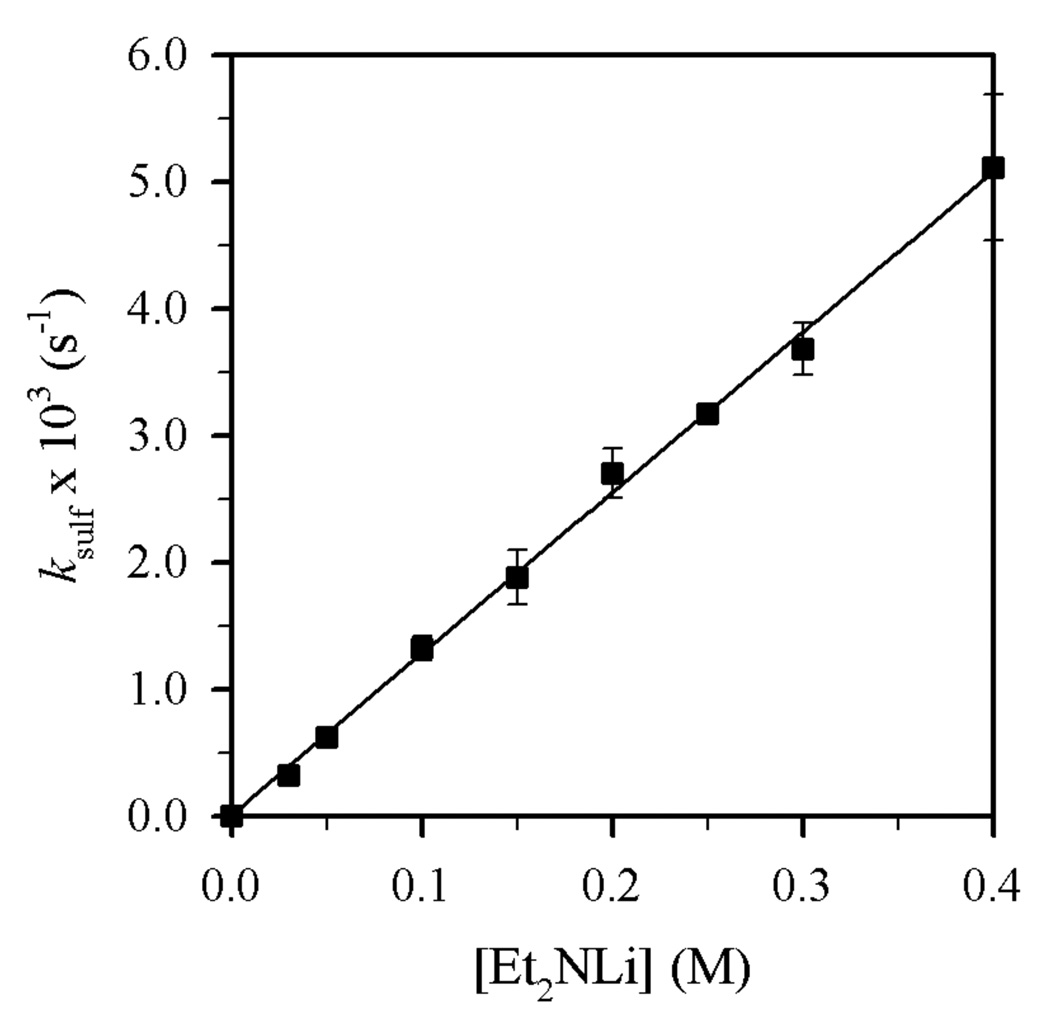
Plots of kalk and ksulf versus THF concentration (Figures 8 and 9) reveal first- and zeroth-order dependencies, respectively. The linear and slightly inverse THF concentration dependence observed for ksulf is consistent with secondary shell (medium) effects accompanying the increasing THF concentration.6b,12 Figures 10 and 11 illustrate the dependence of kalk and ksulf on the Et2NLi concentration (0.03–0.40 M) in 6.0 M THF. The fractional order (kalk ∝ [Et2NLi]0.59 ± 0.04) and first order (ksulf ∝ [Et2NLi]0.99 ± 0.04) are consistent with monomer- and dimer-based pathways, respectively. The data support the idealized rate law in eq 6 and the generic mechanisms in eqs 7 and 8.
| (6) |
| (7) |
| (8) |
Figure 8.
Plot of kalk vs [THF] in toluene cosolvent for the N-alkylation of 4 (0.004 M) with Et2NLi (0.10 M) at −30 °C. The curve depicts an unweighted least-squares fit to kalk = k[THF]n (k = (5.5 ± 0.7) × 10−6, n = 1.29 ± 0.05).
Figure 9.
Plot of ksulf vs [THF] in toluene cosolvent for the N-sulfonation of 4 (0.004 M) with Et2NLi (0.10 M) at −30 °C. The curve depicts an unweighted least-squares fit to ksulf = c[THF] ± k′ (c = (−1.07 ± 0.08) × 10−4, k′ = (1.99 ± 0.06) × 10−3).
Figure 10.
Plot of kalk vs [Et2NLi] in THF (6.0 M) and toluene cosolvent for the N-alkylation of 4 (0.004 M) with Et2NLi at −30 °C. The curve depicts an unweighted least-squares fit to kalk = k[Et2NLi]n (k = (2.4 ± 0.1) × 10−4, n = 0.59 ± 0.04).
Figure 11.
Plot of ksulf vs [Et2NLi] in THF (6.0 M) and toluene cosolvent for the N-sulfonation of 4 (0.004 M) with Et2NLi at −30 °C. The curve depicts an unweighted least-squares fit to ksulf = k[Et2NLi]n (k = (1.26 ± 0.07) × 10−2, n = 0.99 ± 0.04).
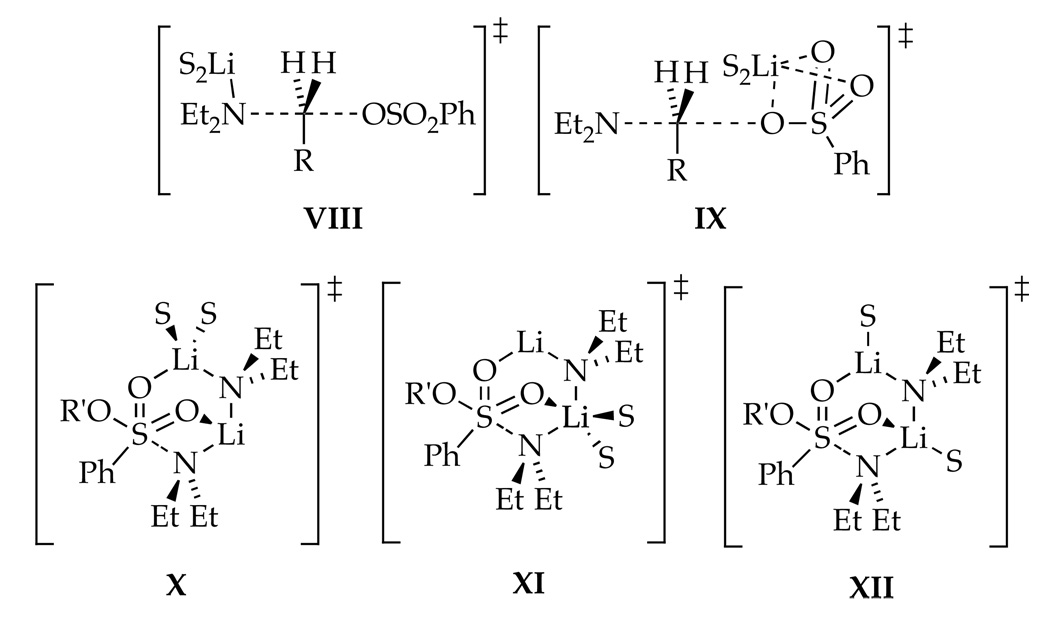
Rate data indicate that N-alkylation occurs via disolvated Et2NLi monomer, possibly with a minor contribution from trisolvated monomer, whereas N-sulfonation takes place via disolvated Et2NLi dimer. The generic transition structures in Chart 3 seem plausible, yet a much smaller subset proved computationally viable (Chart 4). Attempts to optimize parent geometries VIII and IX for the nucleophilic substitution (using PhSO2OEt as a model) converged on hybrid isomer 13, which displays a 6-membered ring and a tetracoordinate lithium23 that interacts with the sulfonyl leaving group.24 Transition structure 13 corresponds to a 6-endo-tet closure, which is considered to be geometrically implausible in many settings.25 Transition structure 14, a trisolvated analog of 13, lacks an S=O-Li contact.
Chart 3.
Chart 4.
Searches for disolvated-dimer-based transition structures for N-sulfonation afforded only structure 15 (type XII), solvated at the external and internal Li atoms. (Optimization of proximally solvated forms (types X and XI) led to desolvation.26,27) Transition structure 15 displays a bicyclo[3.1.1] ring system with coordination of each S=O moiety to one lithium atom of the Me2NLi dimer. The sulfur atom adopts trigonal bipyramidal hybridization with the attacking nitrogen and the leaving RO group in apical positions. IRC calculations support a stepwise addition-elimination.28 The computations qualitatively support a preference for N-sulfonation over N-alkylation.
Cursory searches of hypothetical (unobserved) monomer-based β-eliminations afford 16 and 17. Activation enthalpies (and free energies) suggest that eliminations will not compete with alkylation and sulfonation.
Discussion
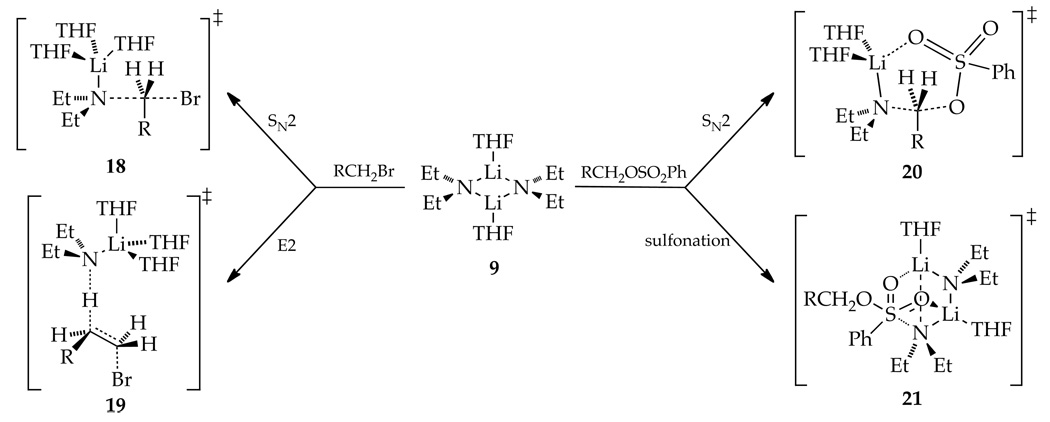
We introduced this paper with the assertion that nucleophilic substitutions and eliminations can be confounding because of a limited understanding of how aggregation and solvation—two inherently molecular phenomena—influence the mechanisms. A combination of kinetic and computational methods was used to study reactions of Et2NLi in THF with n-alkyl bromide 1 and n-alkyl benzenesulfonate 4 (eqs 2 and 3). The resulting mechanistic scenario summarized in Scheme 1 is discussed in the context of several longstanding issues.
Scheme 1.
SN2-E2 Dichotomy
We intended to study the mechanistic basis underlying competing substitutions and eliminations. Such an analysis of sulfonate 4 was precluded by its failure to undergo detectable elimination that is somewhat surprising given the pronounced Brönsted basicity of Et2NLi.29 Focussing on n-alkyl bromide 1, we found that both substitution and elimination proceed via isomeric trisolvated-monomer-based pathways. One of the most obvious and practical consequences is that concentration changes provide no means of controlling selectivity (Figures 1 and 2). The drifting selectivity with increasing THF concentration shown in Figure 1 derives from secondary shell solvation effects; vide infra.
SN2 Substitutions: RBr versus ROSO2Ph
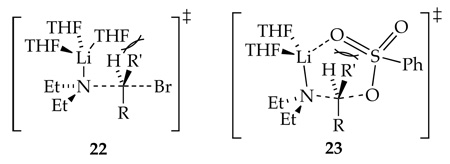
Inspection of transition structure 18 (or the structurally simpler computed analog 10) reveals pronounced steric interactions between the CH2-Br moiety and the THF ligands. Now imagine an analogous substitution of a secondary alkyl bromide via transition structure 22. The vast literature suggests that it would be markedly slower (possibly orders of magnitude). It appears, however, that solvent-substrate interactions are pronounced and that focusing on Et2N-RBr interactions may be misleading. The literature also suggests that highly ionizing conditions can markedly promote the SN2 substitution,21c,30 which would logically stem from both the increasing charge on the nucleophile as well as dissociation of the lithium cation and, with it, the solvent-substrate contacts. This scenario is very unlikely to occur, however, for Et2NLi even under highly ionizing conditions. The conclusion is a recurring theme: steric demands of solvation are an important determinant of aggregate structure and reactivity.31
Comparing the mechanism for SN2 substitutions of n-alkyl bromide 1 and benzenesulfonate 4 reveals that the sulfonate ester undergoes substitution via a disolvated rather than a trisolvated monomer. Computational studies show that transition structure 20 (Scheme 1), in which a THF ligand has been replaced by chelation of the sulfonate, is quite plausible with a 25° distortion of the N-C-O angle from the optimal 180°, a distortion comparable to that observed for the alkyl bromide.32 Those who use Baldwin's ring closure rules categorically may find transition structure 20 disquieting. Let us return to the hypothetical displacements and consider the displacement of a secondary alkyl sulfonate ester. The additional alkyl moiety (R') in cyclic transition structure 23 would likely render the reaction untenable because of acute interactions between the sulfonyl moiety and the alkyl group of the sulfonate ester. The reaction would be forced to proceed through a noncyclic form, which is suggested by the rate studies to be less viable. The interactions within the sulfonate ester moiety are quite prominent whereas those with the Et2N moiety almost seem to be of secondary importance. Although this description is certainly an oversimplification, most conventional discussions of SN2 displacements of sulfonate esters do not consider interactions between the sulfonyl moiety and the alkyl substituents as potentially dominant.33
SN2 Substitution versus N-Sulfonation
The reaction of Et2NLi with sulfonate 4 in THF affords products of substitution and N-sulfonation. The name N-sulfonation, however, is a lithium amide-centric view. It would be equally valid to call it O-desulfonation. Such desulfonations are consequential side reactions during displacements of tosylates and related sulfonate esters.34 In contrast to the substitution-elimination selectivity observed for n-alkyl bromide 1, the alkylation-sulfonation selectivity is sensitive to both THF and Et2NLi concentrations (Figures 3 and 4). The dominant sulfonation (120:1) becomes less so (<5:1) at low Et2NLi and high THF concentrations. The concentration dependencies derive from differential solvation and aggregation numbers in transition structures 20 and 21. The sulfonation appears to benefit from multidentate contacts with lithium as well as from conservation of the Et2NLi dimer structure. IRC calculations revealed a two-step (addition-elimination) mechanism.
Primary Shell versus Secondary Shell Solvation
Both N-alkylation and β-elimination of 1-bromododecane show approximate second-order THF dependencies, which we attribute to monomer-based pathways in THF/toluene mixtures. The THF order for the elimination pathway is actually 2.5 ± 0.1 (Table 1, entry 3). One consequence is that the selectivity shows a preference for elimination at elevated THF concentrations (Figure 1). By using 2,2,5,5-tetrahydrofuran, a cosolvent with a polarity akin to that of THF but no capacity to coordinate competitively to lithium, the THF order for the β-elimination drops to 2.1 ± 0.1.35 Thus, there is a medium effect of marginal practical consequence. The N-sulfonation using sulfonate 4 shows an analogous medium effect, except that a slight rate reduction occurs at elevated THF concentrations. Similar secondary shell effects contributing to solvent-dependent rates have been documented previously. 6,36 Moreover, they are known to cause both modest accelerations and decelerations, depending on the specific reaction. Although it may be tempting to focus on how and why the medium influences reaction rates, we find that the medium effects are surprisingly minor given that lithium amides are often viewed as highly polar species. The chemistry of lithium amides in particular, and probably organolithium reagents in general, is dominated by ligands in the primary coordination shell.
Conclusion
Reaction of Et2NLi with an n-alkyl bromide reveals competing SN2 substitution and E2 elimination via trisolvated lithium amide monomers in both instances. Within this sliver of the enormous field of substitution and elimination, the relative reaction rates and, consequently, the chemoselectivity are insensitive to solvent and lithium amide concentrations. Analogous reaction of Et2NLi with an n-alkyl arylsulfonate affords low levels of substitution and substantial N-sulfonation to the exclusion of elimination. Because the N-alkylation proceeds via disolvated monomers and the N-sulfonation via disolvated dimers, the selectivity is controllable by adjusting concentrations, although the N-sulfonation remains dominant under all conditions. Whereas primary shell solvation is of profound importance, secondary-shell solvation (medium effects) has marginally detectable influence on rates and selectivities. We are reminded that to understand organolithium reaction mechanism is to understand the coordination chemistry of lithium, not vague notions of polarity and ionicity.
Experimental Section
Reagents and Solvents
THF and toluene were distilled from blue or purple solutions containing sodium benzophenone ketyl. The toluene still contained 1% tetraglyme to dissolve the ketyl. [6Li]Et2NLi and [6Li,15N]Et2NLi were prepared as insoluble white solids by metalating Et2NH and [15N]Et2NH (respectively) with [6Li]n-BuLi in pentane.37 Recrystallization from hexane/diethyl ether as the etherate and subsequent evacuation afforded solvent-free Et2NLi.7 Air- and moisture-sensitive materials were manipulated under argon or nitrogen using standard glove box, vacuum line, and syringe techniques. Solutions of n-BuLi and Et2NLi were titrated for active base using a literature method.38
Kinetics
For a kinetic run corresponding to a single rate constant, a stock solution of Et2NLi (0.03–0.4 M) in a THF-toluene solution was prepared. A series of oven-dried, nitrogen-flushed 5 mL serum vials (10 per rate constant) fitted with stir bars were charged with the Et2NLi stock solution and brought to the desired temperature (± 0.2 °C) using a constant-temperature bath fitted with a thermometer. The substrate (1 or 4) was added as a 0.08 M stock solution in hexane containing decane (0.08 M) as a GC standard. The vessels were periodically quenched with 1:1 H2O-THF at intervals chosen to ensure an adequate sampling of each of the first three half-lives. The quenched aliquots were extracted into Et2O and the extracts analyzed using GC. The reactions were monitored by following the decrease of substrates 1 or 4 and the formation of products 2 and 3 or 5 and 7 (eqs 2 and 3) relative to the internal decane standard. Following the formation of the corresponding products afforded equivalent rate constants within ± 10%. Rate constants were determined using non-linear least-squares fits. The reported errors correspond to one standard deviation. The observed rate constants were shown to be reproducible within ± 10%.
Supplementary Material
Supporting Information: NMR, rate, and computational data, experimental protocols, and complete list of authors for ref 18 (38 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Institutes of Health (GM39764) for direct support of this work.
References and Footnotes
- 1.(a) Smith M, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 6th ed. Chapters 10. Wiley-Interscience; 2007. p. 17. [Google Scholar]; (b) Carey FA, Sundberg RJ. Advanced Organic Chemistry. 5th ed. Chapters 4. Springer; 2007. p. 5. [Google Scholar]; (c) Saunders WH, Jr, Cockerill AF. Mechanisms of Elimination Reactions. Vol. II. New York: John Wiley & Sons; 1973. pp. 60–68. [Google Scholar]; (d) Baciocchi E. In: The Chemistry of Halides, Pseudo Halides and Azides, Supplement D. Patai S, Rappoport Z, editors. Chichester, U. K: Wiley; 1983. [Google Scholar]
- 2.(a) Reichardt C. Solvents and Solvent Effects in Organic Chemistry. 3rd ed. Chapter 5. Weinheim: VCH; 2003. [Google Scholar]; (b) Hiraoka M. Crown Compounds: Their Characteristics and Application. Amsterdam: Elsevier; 1982. [Google Scholar]; (c) Atwood JL, Steed JW, editors. Encyclopedia of Supramolecular Chemistry. CRC Press; 2004. pp. 940–949. [Google Scholar]
- 3. Wang DZ, Streitwieser A. J. Org. Chem. 2003;68:8936. doi: 10.1021/jo034543d. Harder S, Streitwieser A, Petty JT, Schleyer PvR. J. Am. Chem. Soc. 1995;117:3253. Streitwieser A, Choy GSC, Abu-Hasanayn F. J. Am. Chem. Soc. 1997;119:5013. Streitwieser A., Jr Proc. Natl. Acad. Sci. 1985;82:8288–8290. doi: 10.1073/pnas.82.24.8288. See also ref 19. Streitwieser A, Jayasree EG. J. Org. Chem. 2007;72:1785. doi: 10.1021/jo0625709.
- 4.(i) For a survey of chemical syntheses within the process chemistry R&D departments of GlaxoSmithKline, AstraZeneca and Pfizer, see: Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. doi: 10.1039/b602413k. Dugger RW, Ragan JA, Ripin DHB. Org. Process Res. Dev. 2005;9:253. (ii) For examples of N-alkylation of lithium dialkylamides, see: Smith JK, Bergbreiter DE, Newcomb M. J. Org. Chem. 1985;50:4549. Sard H, Duffley RP, Razdan RK. Synth. Comm. 1983;13:813. (iii) For representative reactions of lithium dialkylamides with sulfonates, see: Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew. Chem. Int. Ed. 1998;37:2700. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. Andersen KK, Gowda G, Jewell L, McGraw P, Phillips BT. J. Org. Chem. 1982;47:1884.
- 5.Butters M, Catterick D, Craig A, Curzons A, Dale D, Gillmore A, Green SP, Marziano I, Sherlock J-P, White W. Chem. Rev. 2006;106:3002. doi: 10.1021/cr050982w. [DOI] [PubMed] [Google Scholar]
- 6.(a) DePue JS, Collum DB. J. Am. Chem. Soc. 1988;110:5518. [Google Scholar]; (b) DePue JS, Collum DB. J. Am. Chem. Soc. 1988;110:5524. [Google Scholar]
- 7. Rutherford JL, Collum DB. J. Am. Chem. Soc. 1999;121:10198. For recent computational studies of Et2NLi aggregation and solvation states, see: Pratt LM. THEOCHEM. 2007;811:191.
- 8.(a) Gardiner MG, Raston CL. Inorg. Chem. 1996;35:4162. doi: 10.1021/ic9510425. [DOI] [PubMed] [Google Scholar]; (b) Gardiner MG, Raston CL. Inorg. Chem. 1996;35:4047. doi: 10.1021/ic951251p. [DOI] [PubMed] [Google Scholar]; (c) Gardiner MG, Raston CL. Inorg. Chem. 1995;34:4206. [Google Scholar]; (d) Boche G, Langlotz I, Marsch M, Harms K, Nudelman NES. Angew. Chem., Int. Ed. Engl. 1992;31:1205. [Google Scholar]; (e) Barr D, Clegg W, Hodgson SM, Lamming GR, Mulvey RE, Scott AJ, Snaith R, Wright DS. Angew. Chem., Int. Ed. Engl. 1989;28:1241. [Google Scholar]; (f) Armstrong DR, Barr D, Clegg W, Hodgson SM, Mulvey RE, Reed D, Snaith R, Wright DS. J. Am. Chem. Soc. 1989;111:4719. [Google Scholar]
- 9.Ceruti M, Balliano G, Viola F, Cattel L, Gerst N, Shuber F. Eur. J. Med. Chem. 1987;22:199. [Google Scholar]
- 10.(a) Majewski M, Gleave DM. J. Organomet. Chem. 1994;470:1. [Google Scholar]; (b) Newcomb M, Reeder RA. J. Org. Chem. 1980;45:1489. [Google Scholar]; (c) Newcomb M, Burchill MT. J. Am. Chem. Soc. 1984;106:8276. [Google Scholar]; (d) Newcomb M, Burchill MT. J. Am. Chem. Soc. 1984;106:2450. [Google Scholar]
- 11.We do note, however, that the highest levels of n-dodecane loosely correlated with higher THF concentrations.
- 12.Galiano-Roth AS, Collum DB. J. Am. Chem. Soc. 1989;111:6772. [Google Scholar]
- 13.We define the idealized rate law as that obtained by rounding the observed reaction orders to the nearest rational order.
- 14.(a) Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. Chapter 11. University Science; 2006. [Google Scholar]; (b) Fang Y-R, Westaway KC. Can. J. Chem. 1991;69:1017. [Google Scholar]; (c) Westaway KC, Lai Z-G. Can. J. Chem. 1989;67:345. [Google Scholar]
- 15.(a) Remenar JF, Collum DB. J. Am. Chem. Soc. 1998;120:4081. [Google Scholar]; (b) Remenar JF, Collum DB. J. Am. Chem. Soc. 1997;119:5573. [Google Scholar]
- 16.Dehydrobrominations of 2,2-1-d2-dodecane afforded exclusively 1-d1-dodecene, whereas dehydrobrominations of 1,1-1-d2-dodecane yielded 1-d2-dodecene.
- 17.(a) Bock PL, Whitesides GM. J. Am. Chem. Soc. 1974;96:2826. [Google Scholar]; (b) Whitesides GM, Fischer WF, Jr, San Filippo J, Jr, Bashe RW, House HO. J. Am. Chem. Soc. 1969;91:4871. [Google Scholar]
- 18.All calculations were executed using Gaussian 03, revision B.04; Gaussian, Inc.: Pittsburgh, PA, 2003. See Supporting Information for the full list of authors. The combination of the Ahlrichs all-electron SVP basis set for second-row atoms and 6-31G* for the rest is denoted as 631A and has been previously applied to mechanistic studies on organolithium-mediated reactions: Nakamura E, Yamanaka M, Yoshikai N, Mori S. Angew. Chem., Int. Ed. 2001;40:1935. doi: 10.1002/1521-3773(20010518)40:10<1935::aid-anie1935>3.0.co;2-7. Mori J, Nakamura E, Morokuma K. J. Am. Chem. Soc. 2000;122:7294. and references therein.
- 19.Recent theoretical studies on SN2 reactions involving lithium-containing species: (i) No solvent: Ren Y, Gai J-G, Xiong Y, Lee K-H, Chu S-Y. J. Phys. Chem. A. 2007;111:6615. doi: 10.1021/jp067495k. Streitwieser A, Jayasree EG, Leung SS-H, Choy GS-C. J. Org. Chem. 2005;70:8486. doi: 10.1021/jo051277q. Pratt LM, Nguyen NV, Ramachandran B. J. Org. Chem. 2005;70:4279. doi: 10.1021/jo0503409. Pomelli CS, Bianucci AM, Crotti P, Favero L. J. Org. Chem. 2004;69:150. doi: 10.1021/jo034587m. Ren Y, Chu SY. J. Comput. Chem. 2004;25:461. doi: 10.1002/jcc.10394. Xiong Y, Zhu H, Ren Y. J. Mol. Struct. 2003;664–665:279. Leung SS-H, Streitwieser A. J. Comput. Chem. 1998;19:1325. (ii) Dielectric solvation models: Streitwieser A, Jayasree EG, Hasanayn F, Leung SS-H. J. Org. Chem. 2008;73:9426. doi: 10.1021/jo8020743. Ren Y, Li M, Wong N-B, Chu S-Y. J. Mol. Model. 2006;12:182. doi: 10.1007/s00894-005-0016-8. Ren Y, Chu SY. J. Phys. Chem. A. 2004;108:7079. Zhu H, Ren Y, Ren J. J. Mol. Struct. 2004;686:65. (iii) Microsolvation models: Streitwieser A, Jayasree EG. J. Org. Chem. 2007;72:1785. doi: 10.1021/jo0625709. Ando K. J. Org. Chem. 2006;71:1837. doi: 10.1021/jo0519662. Ando K. J. Am. Chem. Soc. 2005;127:3964. doi: 10.1021/ja044995n. See also reference 20a
- 20.RBr-Li interactions do not appear to be stabilizing: Zuend SJ, Ramirez A, Lobkovsky E, Collum DB. J. Am. Chem. Soc. 2006;128:5939. doi: 10.1021/ja060363k. Ikuta Y, Tomoda S. Org. Lett. 2004;6:189. doi: 10.1021/ol036021q.
- 21.For additional examples showing that Br leaving groups do not require metal assistance during the alkylation of lithium-based nucleophiles, see: Zuend SJ, Ramirez A, Lobkovsky E, Collum DB. J. Am. Chem. Soc. 2006;128:5939. doi: 10.1021/ja060363k. Ikuta Y, Tomoda S. Org. Lett. 2004;6:189. doi: 10.1021/ol036021q. For a general discussion of lithium-assisted departure of the leaving group, see: Reich HJ, Sanders AW, Fiedler AT, Bevan MJ. J. Am. Chem. Soc. 2002;124:13386. doi: 10.1021/ja026915q.
- 22.(i) Basic nucleophiles tend to generate Nuc-Hα complexes upon unrestricted geometry optimizations: Yi R, Basch H, Hoz S. J. Org. Chem. 2002;67:5891. doi: 10.1021/jo020325t. Buhl M, Schaefer HF. J. Am. Chem. Soc. 1993;115:9143. (ii) Nu-CR3-X angle restriction to collinearity is a common technique to circumvent this limitation in the study of SN2 reactions: Hoz S, Basch H, Wolk JL, Hoz T, Rozental E. J. Am. Chem. Soc. 1999;121:7724.
- 23.Schlosser M. In: Organometallics in Synthesis: A Manual. 2nd Edition. Schlosser M, editor. Chapter 1. Chichester: John Wiley & Sons; 2002. [Google Scholar]
- 24.(i) For reports on R2SO2-Li complexation: Linnert M, Bruhn C, Wagner C, Steinborn D. J. Organomet. Chem. 2006;689:2358. Rhodes CP, Frech R. Macromolecules. 2001;34:2660. Pregel MJ, Dunn EJ, Buncel E. J. Am. Chem. Soc. 1991;113:3545. Eisch JJ, Galle JE. J. Org. Chem. 1980;45:4536. (ii) The nucleophilic substitutions of arylsulfonates with lithium halides occur with inversion of configuration: Braddock DC, Pouwer RH, Burton JW, Broadwith P. J. Org. Chem. 2009;74:6042. doi: 10.1021/jo900991z.
- 25.(a) Beak P. Acc. Chem. Res. 1992;25:215. [Google Scholar]; (b) Baldwin JE. J. Chem. Soc., Chem. Commun. 1976:734. [Google Scholar]; (c) Baldwin JE. Further Perspectives in Organic Chemistry; A Ciba Foundation Symposium. Amsterdam: Elsevier; 1978. p. 736. [Google Scholar]; (d) Johnson CD. Acc. Chem. Res. 1993;26:476. [Google Scholar]
- 26.(a) Fressigné C, Lautrette A, Maddaluno J. J. Org. Chem. 2005;70:7816. doi: 10.1021/jo050524n. [DOI] [PubMed] [Google Scholar]; (b) Pomelli CS, Bianucci AM, Crotti P, Favero L. J. Org. Chem. 2004;69:150. doi: 10.1021/jo034587m. [DOI] [PubMed] [Google Scholar]; (c) Gillies MB, Tønder JE, Tanner D, Norrby P. J. Org. Chem. 2002;67:7378. doi: 10.1021/jo0262107. [DOI] [PubMed] [Google Scholar]; (d) Fressigné C, Maddaluno J, Marquez A, Giessner-Prettre C. J. Org. Chem. 2000;65:8899. doi: 10.1021/jo000648u. [DOI] [PubMed] [Google Scholar]
- 27. Ramirez A, Sun X, Collum DB. J. Am. Chem. Soc. 2006;128:10326. doi: 10.1021/ja062147h. Romesberg FE, Collum DB. J. Am. Chem. Soc. 1995;117:2166. Searches for structures disolvated at the external Li led to severe transannular interactions involving the solvent and the S=O-Li bridge.
- 28.For discussions of SN2 mechanism versus that with a discrete tetrahedral intermediate, see: Erdik E, Eroglu F. Cent. Eur. J. Chem. 2008;6:237. Fox JM, Dmitrenko O, Liao L, Bach RD. J. Org. Chem. 2004;69:7317. doi: 10.1021/jo049494z. Weiner H, Sneen RA. J. Am. Chem. Soc. 1965;87:292. Chang FC. Tetrahedron Lett. 1964;6:305.
- 29.(i) For studies on the acidity of Et2NH in THF, see: Furlong JJP, Lewkowicz ES, Nudelman NS. J. Chem. Soc., Perkin Trans. 1990;2:1461. (ii) For selective substitutions of sulfonates with organolithiums, see: Nelson TD, Rosen JD, Smitrovich JH, Payack J, Craig B, Matty L, Huffman MA, McNamara J. Org. Lett. 2005;7:55. doi: 10.1021/ol047925u. Kusumoto T, Ichikawa S, Asaka K, Sato K-I, Hiyama T. Tetrahedron Lett. 1995;36:1071. (iii) For a recent discussion on β-eliminations of benzenesulfonates, see: Mohrig JR, Alberg DG, Cartwright CH, Pflum MKH, Aldrich JS, Anderson JK, Anderson SR, Fimmen RL, Snover AK. Org. Biomol. Chem. 2008;6:1641. doi: 10.1039/b801592a.
- 30.(a) Humeres E, Nunes RJ, Machado VG, Gasques MDG, Machado C. J. Org. Chem. 2001;66:1163. doi: 10.1021/jo0012501. [DOI] [PubMed] [Google Scholar]; (b) Westaway KC, Lai Z. Can. J. Chem. 1988;66:1263. [Google Scholar]; (c) Reichardt C. Solvents and Solvent Effects in Organic Chemistry. 2nd ed. Chapter 7. Weinheim: VCH; 1988. [Google Scholar]; (d) Arnett EM, Maroldo SG, Schriver GW, Schilling SL, Troughton EB. J. Am. Chem. Soc. 1985;107:2091. [Google Scholar]; (e) Doolitle RE. Org. Prep. Proced. Int. 1980;12:1. [Google Scholar]; (f) Parker AJ, Mayer U, Schmid R, Gutmann V. J. Org. Chem. 1978;43:1843. [Google Scholar]; (g) Sam DJ, Simmons HE. J. Am. Chem. Soc. 1974;96:2252a. [Google Scholar]
- 31.Collum DB, McNeil AJ, Ramirez A. Angew. Chem., Int. Ed. Engl. 2007;46:3002. doi: 10.1002/anie.200603038. [DOI] [PubMed] [Google Scholar]
- 32.For challenges to the reputed linear trajectory of ideal SN2 reactions, see: Sato M, Yamataka H, Komeiji Y, Mochizuki Y, Ishikawa T, Nakano T. J. Am. Chem. Soc. 2008;130:2396. doi: 10.1021/ja710038c. and references cited therein.
- 33.(a) Bentley TW, Bowen CT, Morten DH, Schleyer PvR. J. Am. Chem. Soc. 1981;103:5466. [Google Scholar]; (b) Bentley TW, Bowen CT, Parker W, Watt CIF. J. Am. Chem. Soc. 1979;101:2486. [Google Scholar]; (c) Bentley TW, Bowen CT. J. Chem. Soc., Perkin Trans. 1978;2:557. [Google Scholar]; (d) Mori A, Nagayama M, Mandai H. Bull. Chem. Soc. Jpn. 1971;44:1669. [Google Scholar]
- 34.Studies on nucleophilic displacements at sulfonate sulfur: Holterman HAJ, Engberts BFN. J. Org. Chem. 1977;42:2792. Van de Langkruis GB, Engberts JBFN. J. Org. Chem. 1984;49:4152. Mori A, Nagayama M, Mandai H. Bull. Chem. Soc. Jpn. 1971;44:1669. Weiner H, Sneen RA. Tetrahedron Lett. 1963:1309.
- 35.The dielectric constants of substituted THF are only slightly lower than THF. Harada Y, Salomon M, Petrucci. S. J. Phys. Chem. 1985;89:2006. Carvajal C, Tolle KJ, Smid J, Szwarc M. J. Am. Chem. Soc. 1965;87:5548.
- 36.Related secondary shell effects: Ma Y, Collum DB. J. Am. Chem. Soc. 2007;129:14818. doi: 10.1021/ja074554e. Ma Y, Ramirez A, Singh KJ, Keresztes I, Collum DB. J. Am. Chem. Soc. 2006;128:15399. doi: 10.1021/ja060964b. Bordwell FG, Hughes DL. J. Am. Chem. Soc. 1986;108:7300. doi: 10.1021/ja00279a054.
- 37.(a) Rennels RA, Maliakal AJ, Collum DB. J. Am. Chem. Soc. 1998;120:421. [Google Scholar]; (b) Kottke T, Stalke D. Angew. Chem., Int. Ed. Engl. 1993;32:580. [Google Scholar]
- 38.Kofron WG, Baclawski LM. J. Org. Chem. 1976;41:1879. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information: NMR, rate, and computational data, experimental protocols, and complete list of authors for ref 18 (38 pages). This material is available free of charge via the Internet at http://pubs.acs.org.