Abstract
Tissue engineering is a promising technique for cartilage repair, but to optimize novel scaffolds before clinical trials, it is necessary to determine their characteristics for binding and release of growth factors. Toward this goal, a novel, porous collagen–glycosaminoglycan scaffold was loaded with a range of concentrations of insulin-like growth factor-1 (IGF-1) to evaluate its potential as a controlled delivery device. The kinetics of IGF-1 adsorption and release from the scaffold was demonstrated using radiolabeled IGF-1. Adsorption was rapid, and was approximately proportional to the loading concentration. Ionic bonding contributed to this interaction. IGF-1 release was studied over 14 days to compare the release profiles from different loading groups. Two distinct phases occurred: first, a burst release of up to 44% was noted within the first 24 h; then, a slow, sustained release (13%–16%) was observed from day 1 to 14. When the burst release was subtracted, the relative percentage of remaining IGF-1 released was similar for all loading groups and broadly followed t½ kinetics until approximately day 6. Scaffold cross-linking using dehydrothermal treatment did not affect IGF-1 adsorption or release. Bioactivity of released IGF-1 was confirmed by seeding scaffolds (preadsorbed with unlabeled IGF-1) with human osteoarthritic chondrocytes and demonstrating increased proteoglycan production in vitro.
Introduction
Current orthopedic techniques used to repair articular cartilage injuries produce variable and unpredictable outcomes.1 Recent research has focused on the field of tissue engineering to enhance this repair process.2 Tissue engineering employs the combination of a scaffold, cells, and growth factors to direct tissue regeneration. Successful advances in this field include the use of a porous collagen–glycosaminoglycan (GAG) scaffold for skin regeneration and a tubular form of this matrix for peripheral nerve regeneration.3,4 Collagen has also proved effective in oesophagus and trachea tissue repair,5,6 whereas a collagen scaffold combined with bone morphogenic protein-2 has been approved by the FDA for clinical bone repair.
The scaffold's role is to act as an artificial extracellular matrix (ECM); therefore, it must be highly porous and possess sufficient surface area and mechanical strength to carry out this function.1,7,8 Scaffold microstructure, which can be characterized by porosity, mean pore size, interconnectivity, and specific surface area, significantly affects cell adhesion and proliferation.9 Ideally, the scaffold should also mimic cell–ECM interactions and provide adequate signals to cells via the ECM and growth factors to induce or maintain a desired state of cell differentiation to aid tissue regeneration.10 Growth factors are soluble proteins that stimulate cell proliferation and differentiation and can be used to aid cell migration, increase cell numbers, and increase matrix production.2,8,11 Injected growth factors have very short half-lives in vivo as they are rapidly dispersed by diffusion or digested by enzymes; hence, they require a delivery device to protect the growth factor from proteolysis until it is released.1,8,12 To this end, the scaffold could be used to reversibly bind growth factors, confine their release to the defect location to limit any possible side effects, and ensure that their bioactivity is maintained when released.13
The present study describes the in vitro evaluation of a novel collagen–GAG scaffold as a potential growth factor delivery device for articular cartilage repair. Native articular cartilage is a highly ordered matrix composed mainly of water, collagen, and proteoglycans, which is maintained by cartilage cells called chondrocytes. Proteoglycans consist of proteins attached to hyaluronic acid that carry covalently bound GAG chains. GAGs attract and bind water molecules, generating the high osmotic activity and swelling pressure essential to maintaining cartilage biomechanics.14 Chondroitin sulfate (CS) is the main GAG found in articular cartilage; therefore, this GAG was attached to the scaffold to create a similar microenvironment. Chondroitin sulfate is polyanionic and thus readily interacts with proteins in the ECM and binds effector molecules such as growth factors and cytokines that influence cell metabolism.15–17 It has also been reported to promote chondrocyte adhesion via enhancing the attachment of chondronectin to collagen.18 Previous studies have shown that the covalent attachment of CS to collagen matrices stimulated significantly higher chondrocyte proliferation and cartilage formation compared to collagen-only matrices both in vitro and in vivo.16,19
The collagen–GAG scaffold in this study was cross-linked via a chemical carbodiimide method using 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC) and N-hydroxysuccinimide (NHS) and a physical method called dehydrothermal treatment (DHT). Carbodiimide (EDC/NHS) chemistry activates the carboxylic acid groups of glumatic and aspartic acids to react with free amine groups present on lysine and hydroxylysine residues to form covalent zero length amide bonds between the collagen molecules.20 Cross-links are also created between CS carboxylic acid groups and collagen-free amine groups. DHT heats collagen (>90°C) under vacuum, which removes water molecules to form intermolecular cross-links. These condensation reactions form covalent cross-links between different amino acid side chains and between collagen molecules and CS.21
Scaffolds that are cross-linked using EDC/NHS possess a higher cross-link density, higher compressive stiffness, and a greater resistance to enzymatic degradation than non-cross-linked or DHT cross-linked matrices.22 Previously, cross-linking had been shown to be at a maximum when a molar ratio of EDC:NHS:COOH 5:2:1 was used.23 The degree of cross-linking is inversely proportional to the amount of free amine groups. Non treated collagen has 32 free amine groups per 1000 amino acid residues (100% free amine groups).24 Pieper et al. reported that similar collagen–GAG scaffolds retained 68% of free amine groups when cross-linked by DHT and EDC/NHS compared to EDC/NHS treatment, which retained 63%.24 DHT partially denatures collagen by breaking the hydrogen bonding needed to maintain the collagen triple-helix structure.24,25 However, although DHT appears to have little effect on scaffold structure, it is not known if it affects the binding and release of insulin-like growth factor-1 (IGF-1) from this type of scaffold, and therefore we compared scaffolds cross-linked via DHT and EDC/NHS (+DHT) with scaffolds cross-linked by EDC/NHS only (−DHT).
IGF-1 is an anabolic growth factor that is very important in cartilage development and homeostasis.25–27 IGF-1 increases the amount of proteoglycan and type II collagen synthesized by chondrocytes, and promotes chondrogenesis in bone-marrow-derived stem cells such as mesenchymal stem cells.26,28 In addition, IGF-1 also protects the ECM from interleukin-1 and tumor necrosis factor α–mediated degradation during cartilage injury; hence, this growth factor was selected as the growth factor of choice for the present study.29–31
Many studies have demonstrated the efficacy of IGF-1 for articular cartilage repair,26,27,32–34 but none have examined the behavior of a relevant cell type (i.e., chondrocytes from osteoarthritic donors) seeded within an IGF-1-loaded collagen–GAG scaffold. Fortier et al. demonstrated that 10–100 ng/mL IGF-1 enhanced proteoglycan and type II collagen synthesis by chondrocytes seeded in fibrin matrices and that the cells maintained their phenotype in vitro.33 In vivo, IGF-1-loaded fibrin matrices have been used to repair full-thickness articular cartilage defects in horses.26 These loaded matrices enhanced the formation of hyaline-like repair tissue and had better adhesion to the subchondral bone compared to the repair tissue formed in empty defects. Tuncel et al. demonstrated that collagen sponges loaded with 5 μg IGF-1 enhanced the tissue response and produced significantly better gross, histological, and histochemical neocartilage compared to the fibrocartilage tissue that was produced by the collagen sponge controls in a rabbit osteochondral defect model.27 Hence, one of the aims of our study was to ensure that the IGF-1 released from our collagen–GAG scaffolds would be at therapeutic levels and enhance matrix production by human chondrocytes.
Previous studies have demonstrated that a steady or daily growth factor action would be advantageous35–38 and that 10 ng/mL IGF-1 is sufficient to stimulate the proliferative and metabolic activity of chondrocytes cultured in vitro,39 while proteoglycan production is at a maximum with 100 ng/mL IGF-1.40 The addition of IGF-1 to explants and monolayer cultures at concentrations of 10–200 ng/mL increased collagen type II and DNA synthesis and inhibited proteoglycan degradation,41 whereas 200 ng/mL also inhibited chondrocyte apoptosis in vitro.42 The optimal dose of IGF-1 required to enhance proteoglycan synthesis in osteoarthritic cartilage was 30 ng IGF-1/mL.43 Previous research also indicated that in vitro chondrocytes do not respond any better to 1000 ng IGF-1/mL than 100 ng IGF-1/mL.44 Considering this evidence, our collagen–GAG scaffold was loaded with very high levels of IGF-1 to accomplish an initial therapeutic drug release >50 ng IGF-1/mL/day.
IGF-1 delivery devices have been widely investiga-ted,26–28,35,40,41,45 but we consider the collagen–GAG scaffold used in this study to be more closely related to the articular cartilage that it is designed to replace. For example, Laffargue et al. loaded IGF-1 onto porous tricalcium phosphate cylinders via adsorption in vitro and reported significant IGF-1 release up to day 4 followed by much slower release up to day 20.45 At the end of this experiment up to 60% of the adsorbed IGF-1 remained on these matrices, indicating a strong binding interaction.45 Protein adsorption on to collagen membranes is mainly due to hydrogen bonding and ionic bonding between the collagen and the diffusing protein, and adsorption is proportional to both the number of sites available for binding and the protein concentration.46 Previously, the uptake of IGF-1 into normal human cartilage was studied, and binding experiments indicated that the growth factor binds to proteoglycans.47 IGF-1 (isoelectric point = 8.5) is electropositive at neutral pH,2 and thus it may adsorb onto the collagen–GAG scaffold via electrostatic binding to the negatively charged CS groups.47
This study has focused on evaluating a porous collagen–GAG scaffold as a reservoir of IGF-1. The time of incubation and the ionic strength of the incubation solution were varied to maximize adsorption and to investigate the binding interaction. Adsorption and elution of IGF-1 were compared for scaffolds loaded with different IGF-1 concentrations and scaffolds cross-linked under different conditions. The aim of this work was to understand the kinetics of IGF-1 binding and release under in vitro conditions and to use these results to design cell culture experiments to assess whether the IGF-1 release profile from this system provides a therapeutic dose before the scaffold is used in vivo or for clinical trials. Our ultimate goal is to manipulate the composition of the scaffold to optimize growth factor binding and release while maintaining structural integrity to produce a commercial product for osteochondral repair.
Materials and Methods
Materials
Type I insoluble collagen prepared from bovine skin (Devro Plc), and chondroitin-6-sulfate (Bioiberica) were used to prepare collagen–GAG scaffolds. Human recombinant IGF-1 was purchased from R&D Systems, and human recombinant 125I-IGF-1 (925 kBq, 81.4 TBq/mmol) labeled using a lactoperoxidase procedure was purchased from Perkin Elmer, Inc.
Preparation of collagen–GAG scaffolds and cross-linking
Collagen was dissolved in aqueous HCl solution (0.001 M) to give a final concentration of 0.9 wt% collagen. Chondroitin-6-sulfate (GAG) was added (0.08 wt%) and this slurry was blended using a high-energy blender (Turax [VWR International]) with cooling on ice. The blended slurry was poured into glass molds and freeze-dried (Virtis Advantage XL) using a previously described protocol.3,9,48 The slurry was solidified by using a constant cooling rate of 0.01°/min and a final freezing temperature of − 12°C.
Cross-linking of collagen–GAG scaffolds
Method 1 (+DHT): The discs were cross-linked via DHT at 105°C for 24 h as previously described.3 The discs were then chemically cross-linked using EDC/NHS (Sigma-Aldrich) using EDC/NHS/COOH at a molar ratio of 5:2:1.23 Method 2 (−DHT): The discs were cross-linked using the chemical EDC/NHS method only.
All samples were prepared using a 5 mm sterile punch. Each scaffold disc (5 mm diameter × 2 mm thick) prepared weighed 1.5 ± 0.1 mg irrespective of cross-linking method.
Imaging of collagen–GAG scaffolds
Sections of the scaffolds cross-linked using each method (described in section Preparation of collagen–GAG scaffolds and cross-linking) were sputter coated with Au and imaged under a scanning electron microscope (JEOL 820), using an accelerating voltage of 10 kV.
Scaffold porosity
The scaffold pores imaged by the scanning electron microscope were measured using image analysis (QCapture Pro 6.0), and 35 pores were measured and averaged for each type of cross-linked scaffold. Scaffold porosity was determined by using X-ray microtomography (Tomo NT 1072; source voltage of 24 kV, pixel size 7.01 μm, 1 s exposure time, 0.45° rotation step, and averaging over eight frames). Sequential cross sections were imaged and 2D reconstructions were analyzed using Image J software.
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds over time
Each collagen–GAG scaffold was placed into a plastic tube (LP4 tubes; Sarstedt Ltd.), and an aqueous solution (0.5 mL, 10 μg/mL) of IGF-1 was dropped onto each dry scaffold. These solutions were placed under vacuum for 10 min to remove any air trapped in the scaffold pores, and the tubes resealed, placed on a shaker (VWR International rocking platform), and incubated at 37°C. Scaffolds were removed at 20 min and 1, 4, 8, 24, and 48 h and centrifuged in filter tubes (Whatman Centrex centrifuge filter tubes) at 1000 rpm for 3 min to remove solution trapped within the scaffold pores. However, after centrifugation ∼0.6% of the incubation volume (<3 μL) remained and it was not possible to remove further liquid without drying the scaffold, which would inactivate the growth factor. This trapped liquid was constant for all samples and as it was not possible to measure its contribution to IGF-1 adsorption, it has to be treated as adsorbed IGF-1 as it is held within the matrix. The solution removed during centrifugation is hereinafter termed the “absorbed solution.” The residual incubation solution, the absorbed solution, and the dry scaffold radioactivity were counted using a gamma counter (LKB Wallac 1261 multigamma counter).
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds at different IGF-1 loading concentrations
An aqueous solution (0.5 mL) of IGF-1 (50, 100, and 200 μg/mL) was dropped onto each collagen–GAG scaffold. These solutions were then placed under vacuum and incubated at 37°C as described above. After 24 h the scaffolds were removed and centrifuged as above. The residual incubation solution, the absorbed solution, and the dry scaffold radioactivity were counted, and the sum of this radioactivity was used to calculate the amount of IGF-1 adsorbed onto the dry scaffold.
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds at different ionic strengths
An aqueous solution (0.5 mL, 10 μg/mL) of IGF-1 was dropped onto each collagen–GAG scaffold. Ionic strength was increased by adding NaCl to phosphate-buffered saline (PBS) to give 0.1, 0.5, 1, and 2 M NaCl IGF-1 incubation solutions. These solutions were then placed under vacuum and incubated at 37°C as described above. The scaffolds were removed after 24 h and centrifuged as above. The residual incubation solution, the absorbed solution, and the dry scaffold radioactivity were counted.
Elution of 125I-IGF-1 from collagen–GAG scaffolds
Each collagen–GAG scaffold incorporating IGF-1 (refer to the previous section) was placed in 1 mL of PBS. These solutions were put under vacuum for 15 min to remove any air trapped in the scaffold pores. The tubes were resealed, placed on a shaker, and incubated at 37°C. A 990 μL sample of each solution was taken after 1, 3, 5, and 24 h and 2, 3, 4, 5, 6, 7, and 14 days and counted using a gamma counter. After sampling the same volume (990 μL), PBS was replaced to maintain the elution volume.
IGF-1 bioactivity assay
To test the bioactivity of eluted IGF-1, human primary chondrocytes were derived from articular cartilage samples taken from patients undergoing total joint replacement with full ethics consent. Empty scaffolds and scaffolds loaded with unlabeled IGF-1 (50 μg/mL) using the method described in “Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds over time” section were seeded with 1 × 105 human chondrocytes and cultured for 14 days in Dulbecco's modified Eagle's medium (Gibco Cat. No. 21063-029) containing 10% fetal calf serum. The total amount of proteoglycan synthesized by the cells was measured using the 1,9-dimethyl-methylene blue (DMMB) assay.49
Data analysis
All error bars represent the standard error of the mean and each experiment was carried out for at least N = 3. The amount of IGF-1 (ng/mL) released was determined by measuring the presence of 125I-IGF-1 in the release buffer, and a ratio of hot:cold growth factor, 125I-IGF-1: IGF-1, of 1:10000 was used for all calculations. The relationship between IGF-1 burst release (day 1) and IGF-1 loading concentration was analyzed by linear regression. The total amount of proteoglycan produced by chondrocytes grown in collagen–GAG scaffolds with and without pretreatment with IGF-1 was compared using the two-tailed Student's t-test. The adsorption and elution of IGF-1 from scaffolds cross-linked using different methods was also compared using the two-tailed Student's t-test. In all cases the results were considered statistically significant when p < 0.05.
Results
Collagen–GAG scaffold microstructure
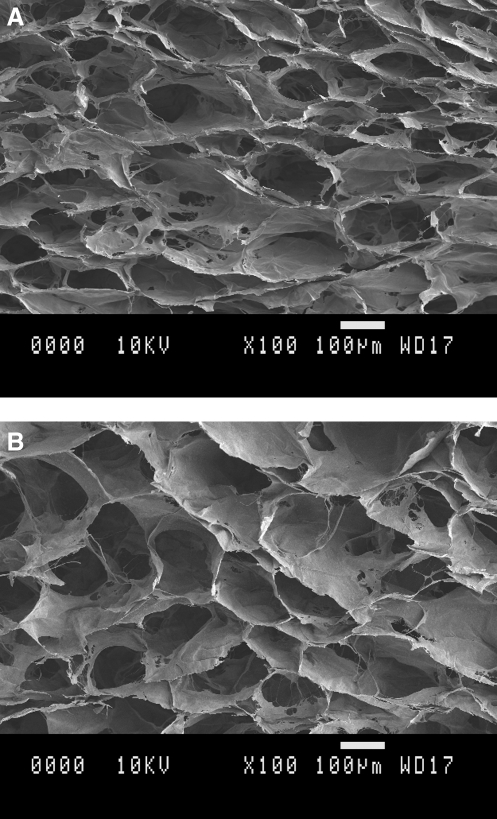
All scaffolds were prepared using the same freeze-drying method and hence possessed similar highly interconnected porous networks (Fig. 1). Scaffolds cross-linked using EDC/NHS and DHT (+DHT) had an average pore size of 203 ± 33 μm, and scaffolds cross-linked using EDC/NHS (−DHT) had an average pore size of 216 ± 39 μm. Scaffold porosity was determined to be 84% ± 4% by X-ray microtomography (method described in Scaffold porosity section) for both cross-linking treatments.
FIG. 1.
Scanning electron microscopy images of the pore structure of cross-linked collagen–glycosaminoglycan (GAG) scaffolds. (A) With dehydrothermal treatment scaffolds. (B) Without dehydrothermal treatment scaffolds. All scale bars: 100 μm.
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds over time
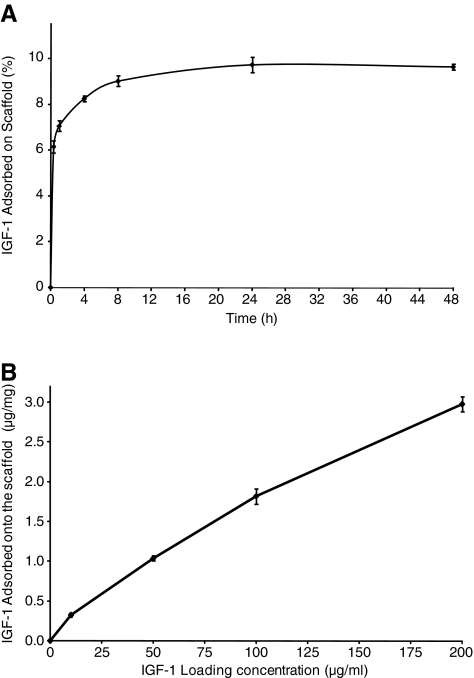
Scaffolds loaded with 10 μg/mL IGF-1 were incubated at 37°C for a range of different incubation times to study the kinetics of IGF-1 adsorption (Fig. 2A). The maximum adsorption reached was ∼10% (0.32 μg/mg). The percent IGF-1 adsorbed onto the scaffold increased rapidly up to 8 h, the adsorption curve reached a plateau between 8 and 24 h, and no further significant adsorption was observed between 24 and 48 h. These results indicate that 24 h incubation is sufficient to achieve the maximal amount of IGF-1 adsorption onto the collagen–GAG scaffold. Consequently, all further scaffolds in this study were incubated in IGF-1 solutions for 24 h at 37°C.
FIG. 2.
(A) In vitro adsorption of insulin-like growth factor-1 (IGF-1) onto the collagen–GAG scaffold. Shown is the percent of IGF-1 adsorbed for a loading concentration of 10 μg/mL as a function of incubation time at 37°C. (B) Amount of IGF-1 adsorbed on collagen–GAG scaffolds as a function of IGF-1 loading concentration.
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds at different IGF-1 loading concentrations
Scaffolds were incubated in a range of solutions of different IGF-1 concentrations to assess the effect of loading concentration on growth factor adsorption. Relatively high concentrations were selected in an attempt to find the concentration at which the scaffold reached saturation. Scaffolds incubated in 10 μg/mL IGF-1 for 24 h adsorbed 0.3 μg/mg IGF-1 (Fig. 2B). Scaffolds incubated in 50 μg/mL IGF-1 adsorbed 1 μg/mg IGF-1 compared with the adsorption of 1.8 μg/mg and 3.0 μg/mg IGF-1 per scaffold as the loading concentration was increased to 100 μg/mL and 200 μg/mL, respectively. These results demonstrate that the amount of IGF-1 adsorbed (μg/mg) was approximately proportional to the loading concentration in the concentration range studied. For scaffolds loaded with 200 μg/mL IGF-1, the amount of adsorption did not increase linearly with the loading concentration.
Adsorption of 125I-IGF-1 onto collagen–GAG scaffolds at different ionic strengths
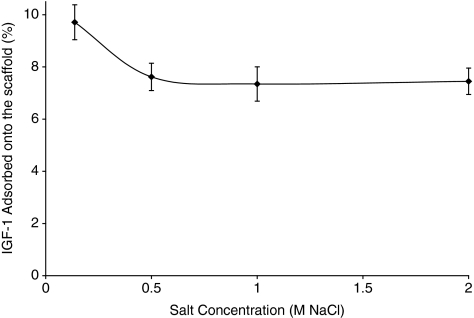
The interaction between IGF-1 and collagen–GAG scaffolds at different ionic strengths was investigated by incubating scaffolds in IGF-1 solutions containing 0.14, 0.5, 1, and 2 M NaCl. The percent IGF-1 adsorbed was measured and compared to determine whether ionic strength affected IGF-1 adsorption (Fig. 3). The amount of IGF-1 adsorbed decreased by 22% as the salt concentration was increased from 0.14 to 0.5 M NaCl; however, no further decrease was observed at higher salt concentrations.
FIG. 3.
The effect of buffer ionic strength on the in vitro adsorption of IGF-1 on collagen–GAG scaffolds, showing the percentage of IGF-1 adsorbed after 24 h incubation.
Elution of 125I-IGF-1 from collagen–GAG scaffolds
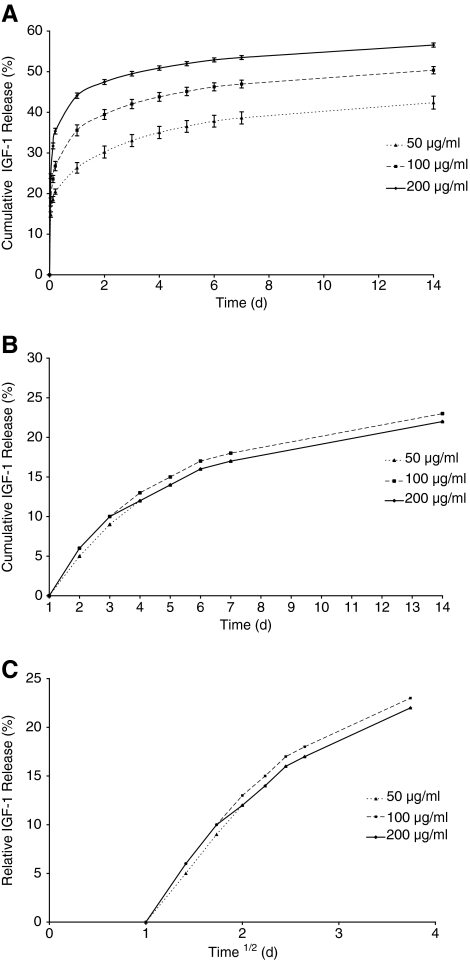
The in vitro elution profile of IGF-1 from these loaded scaffolds (50, 100, and 200 μg/mL) was measured, and Figure 4A shows the cumulative release of IGF-1 over a period of 14 days. The release profile shows that there are two distinct stages: an initial burst release followed by a slow sustained release. The burst release occurred within the first 24 h with 26%–44% IGF-1 being released depending on the initial loading concentration. IGF-1 release over the first day dominates the elution profile.
FIG. 4.
Elution profiles of 125I-IGF-1 from collagen–GAG scaffolds. (A) Percent cumulative release of IGF-1 during 14 days of elution. (B) The data replotted (relative cumulative %) after the subtraction of the release on day 1. (C) The relative percentage of IGF-1 released versus t½.
The correlation of day 1 release with loading concentration was analyzed by linear regression, and this relationship was found to be significant (p < 0.0001).
Initially, the data were normalized to the release from t = 0 (Fig. 4A), but hereafter the analysis renormalizes these data to study the release from day 1 to 14 (Fig. 4B) to remove the influence of the day 1 burst release on the subsequent data. Therefore, 100% release on this scale would correspond to the total release of all of the IGF-1 remaining at day 1. Figure 4B shows that the data now lie on a common curve. If the same analysis is carried out by subtracting the total release from time points earlier than 1 day, the data do not lie on a common curve. When the data from Figure 4B were replotted as percentage release versus t½ (Fig. 4C) a straight line was obtained up to about day 6.
Dose of IGF-1 released from collagen–GAG scaffolds
Table 1 shows the concentration of IGF-1 in the buffer solution, which illustrates the amount of IGF-1 released per day for each loading group. After 1 day 410–1967 ng/mL IGF-1 was released depending on the loading group. After this burst release, the relative percent of further IGF-1 release was independent of the IGF-1 loading concentration (Fig. 4B). However, the actual amount of IGF-1 released was significantly different between the groups proportional to the initial IGF-1 loading concentration. Up to day 7 a further 192–419 ng/mL IGF-1 was released for each loading group.
Table 1.
The Amount of Insulin-Like Growth Factor-1 Released Per Day Between Days 1 and 7 for Each Loading Group
| |
IGF-1 release per day (ng/mL) |
||
|---|---|---|---|
| |
IGF-1 loading concentration (μg/mL) |
||
| Time (day) | 50 | 100 | 200 |
| 1 | 410 | 974 | 1967 |
| 2 | 61 | 106 | 150 |
| 3 | 44 | 71 | 91 |
| 4 | 31 | 48 | 62 |
| 5 | 23 | 36 | 49 |
| 6 | 20 | 31 | 41 |
| 7 | 13 | 19 | 26 |
IGF-1, insulin-like growth factor-1.
IGF-1 bioactivity assay
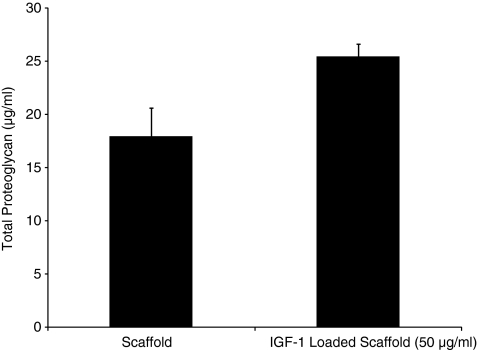
The bioactivity of the released IGF-1 was demonstrated by an increase in the amount of proteoglycan produced by human osteoarthritic chondrocytes seeded in a scaffold pretreated with IGF-1 compared with an untreated scaffold (Fig. 5). This increase was found to be significant when analyzed by the Student's t-test (p < 0.05).
FIG. 5.
Total amount of proteoglycan produced by chondrocytes grown in collagen–GAG scaffolds with and without pretreatment with IGF-1 at day 4.
Adsorption and elution of 125I-IGF-1 from different collagen–GAG scaffolds
Scaffold cross-linking was also varied to determine whether DHT would affect IGF-1 adsorption or release from the scaffold. Scaffolds loaded with 50 μg/mL IGF-1 adsorbed 1 μg/mg IGF-1 independent of cross-linking method. Both scaffolds released 26% IGF-1 on day 1 followed by a further 16%–18% by day 14, confirming that DHT had no significant effect on IGF-1 adsorption or release (p > 0.05).
Discussion
Adsorption of IGF-1
We investigated the interaction between IGF-1 and the collagen–GAG scaffold with the aim of maximizing growth factor binding before in vivo trials with this commercial scaffold. Our kinetic studies showed that adsorption of IGF-1 was rapid and that 24 h was sufficient to reach a maximum. Understanding the binding interaction between IGF-1 and the collagen–GAG scaffold is also fundamental to optimizing adsorption and reducing the initial burst release from this system. In the study of bovine articular cartilage explants, Luyten et al. proposed that since IGF-1 is a cationic, low-molecular-weight protein it binds to and is concentrated in the anionic proteoglycan matrix.50 Moreover, the uptake of IGF-1 by ex vivo human articular cartilage was attributed to reversible binding to anionic proteoglycan molecules.47 Ionic interactions contribute to IGF-1 binding in vivo, and therefore we postulate that ionic interactions between the electropositive IGF-1 and the negatively charged CS groups in our scaffold contribute to the binding. This was confirmed as the amount of IGF-1 adsorbed on to the scaffold decreased by 22% as the salt concentration was increased from 0.14 M (physiological ionic strength) to 0.5 M NaCl (Fig. 3).
Scaffolds were also incubated in a range of solutions with different IGF-1 loading concentrations to assess the effect of loading concentration on growth factor adsorption (Fig. 2B). The amount of IGF-1 adsorbed was approximately proportional to the loading concentration, confirming previous studies using similar scaffolds.45 We found that at low IGF-1 loading concentrations, adsorption increased almost linearly (Fig. 2B). However, at a higher IGF-1 concentration (200 μg/mL) there was a relative decrease in the rate of adsorption although the scaffold was not saturated with this level of loading. This may be due to the IGF-1 initially binding to very tight binding sites at low loading concentrations and binding to much weaker lower affinity binding sites at higher loading concentrations, as found previously in native cartilage.47 Our future studies will study collagen scaffolds containing different proportions of GAG to establish the role of GAGs in growth factor binding in this type of scaffold.
At the lowest loading concentration (10 μg/mL), 10% of the loaded IGF-1 was adsorbed onto the scaffold compared with only 4% adsorption at the highest loading concentration (200 μg/mL) (Fig. 2B). In terms of loading efficiency, these percentages are low, but in terms of IGF-1 adsorption per scaffold weight, the loading efficiency is relatively high (0.3 μg/mg at the lowest loading and 3.0 μg/mg at the highest loading concentration). In a similar study, tricalcium phosphate cylinders loaded with 0.3 μg/mL IGF-1 only adsorbed 0.0005 μg/mg of IGF-1 per scaffold weight,45 whereas 10% of growth factor sorption has also been reported for similar cross-linked collagen scaffolds loaded with 0.84 μg/mL basic fibroblast growth factor.51
Elution of IGF-1
After loading, the scaffolds were placed into PBS and IGF-1 release was studied over 14 days to determine the strength of binding and to compare the release profiles from the different loading groups. After 14 days 43%–58% of the loaded IGF-1 remained on each scaffold depending on the loading group (Fig. 4A). This large amount of residually bound IGF-1 demonstrated that there was a strong binding interaction between IGF-1 and the scaffold and that IGF-1 release depended on the initial loading concentration. Despite the adsorption study indicating that ionic interactions contributed to the binding, high ionic strength did not completely abolish IGF-1 adsorption (Fig. 3), suggesting that other physiological noncovalent interactions could be involved.
IGF-1 elution proceeded via two distinct stages. The majority of IGF-1 release (26%–44% of the loaded IGF-1) occurred in a rapid burst release up to 24 h. This was followed by a slow controlled release in which a further 13%–16% was released between days 1 and 14. Many studies have reported similar burst releases of growth factor from such delivery devices occurring within the first 24 h.45,51–54 For example, Ueda et al. reported that ∼30% of transforming growth factor-β1 incorporated in a collagen scaffold was released into the PBS by simple diffusion within the first hour of an in vitro release test.53 We found that the percent of IGF-1 released after 24 h depended on the initial IGF-1 loading concentration and that therefore reducing the volume or loading concentration of IGF-1 could be used to reduce the burst release from this system. There is no evidence that IGF-1 binds directly to collagen, and therefore the collagenous part of the scaffold is thought to have little effect on IGF-1 binding or release.55 As we have demonstrated that at least part of the IGF-1 binding is through ionic interactions, it is logical to assume that this is via the negatively charged GAG component. Previously, CS has proved important in reducing the release of platelet-derived growth factor from similar chitosan–CS scaffolds.56 Park et al. compared chitosan with chitosan–CS scaffolds and demonstrated that increasing the CS content reduced both the burst release and following release from this system.56 The proportion of GAG in our study was chosen to be optimal in terms of physical characteristics57 and therefore a vital extension to our study will be to alter the proportion of GAG, use a GAG with higher charge or to increase the ionic charge of the scaffold chemically. It is likely that the unreleased IGF-1 that has not bound through ionic interactions is retained purely through physical entrapment within the scaffold pores. DHT failed to change IGF-1 retention, but it also failed to change the porosity or pore size of the scaffold. We would predict that decreasing the pore size without further decreasing the porosity of the scaffold would increase the binding of the IGF-1 both through increased available surface area of GAG and also physical retention. This could decrease the burst release and may also allow a longer period of IGF-1 release at a therapeutic dose and we will study this in the future.
If the burst release is discounted, the master curve of percent IGF-1 released versus time (Fig. 4B) indicated that scaffolds from all loading groups released similar relative percentages of IGF-1 during the controlled stage of the release profile. Figure 4C shows that between days 1 and 6 the release is broadly linear with respect to t½, suggesting that this release can be described as Fickian.58,59 This diffusion model assumes that the flux and the concentration of the diffusing material are proportional and that diffusion from the system scales with the square root of time, although the exponent is likely to be affected slightly by the geometry of the 3D porous network.58,59 Beyond 6 days the release rate deviates from t½ kinetics. This is typical of release from such a system where a slower exponential decay is generally seen at later time points. Silk fibroin scaffolds loaded with IGF-1 also showed that although the total cumulative release of IGF-1 depended on the loading concentration, the kinetics of release was independent of IGF-1 loading.60
Various studies have used DHT to cross-link collagen; however, this treatment causes up to 25% collagen denaturation, which would disrupt the collagen triple helical conformation,61 potentially making the scaffold more susceptible to degradation. Therefore, it was important to compare IGF-1 release from scaffolds cross-linked with and without DHT. Our results demonstrated that DHT had no significant effect on the adsorption or release of IGF-1 from our scaffolds, and this may add weight to the hypothesis that IGF-1 binding is influenced by the presence of CS rather than collagen in the scaffold47,50,56 Previous studies demonstrated that increasing the length of DHT treatment had no effect on transforming growth factor-β1 release from a collagen scaffold, but scaffolds cross-linked without DHT were not compared.53 Our future studies will study collagen scaffolds containing different proportions of GAG to establish the role of GAGs in growth factor binding in this type of scaffold.
IGF-1 dosage
Growth factors have the potential to be cytotoxic at high concentrations, but previous studies have shown that neither fibrin matrices loaded with 25 μg IGF-1 implanted into cartilage defects in horses nor intraarticular injections of 2 μg IGF-1 three times per week over 3 weeks in an osteoarthritic canine model induced any toxic or adverse affects.62,63 Thus, the collagen–GAG scaffolds used in this study, which adsorbed 0.5–4.5 μg and released up to 2000 ng/mL IGF-1 as a burst release, are not expected to be cytotoxic in vivo. Moreover, this high burst release may prove advantageous in terms of stimulating a wound-healing response in vivo because the autoinductive properties of IGF-1 are at their greatest 1 day after exogenous exposure.63 It is proposed that a very high initial dose may help to maintain an effective IGF-1 concentration during the repair process. We confirmed that the IGF-1 released in this system maintains its bioactivity as demonstrated by increased proteoglycan production from human osteoarthritic chondrocytes (Fig. 5). There is an initial low level of GAG release from the scaffold itself, which is detectable by DMMB assay (data not shown), but this is a constant for all treatments and controls, and therefore any changes in GAG concentration are as a result of the IGF-1 treatment.
After the initial burst release, scaffolds loaded with 200 μg/mL IGF-1 released ≥50 ng/mL/day until day 5 followed by >20 ng/mL/day days 6–14 (Table 1). In comparison, the scaffolds loaded with 50 μg/mL released >50 ng/mL IGF-1 on day 2 followed by ≥20 ng/mL/day until day 6 and ∼10 ng/mL/day until day 14 (Table 1). This is comparable to physiological levels of IGF-1. Synovial fluid in adult human cartilage is reported to contain 30–50 ng/mL IGF-1, while human serum contains 25 ng/mL IGF-1.64,65 Ideally, the sustained release from the scaffolds would provide more persistent stimuli over time to aid the differentiation of bone marrow stromal cells or other progenitor cells that would be found at a cartilage defect site.54 Although the IGF-1 release profile from the collagen–GAG scaffold was slightly lower than 50 ng IGF-1/mL/day over 14 days for all loading groups, previous work has suggested that concentrations of IGF-1 as low as 10 ng/mL are sufficient to stimulate the metabolic actions of cultured chondrocytes.34 There are reports of in vivo osteochondral repair being enhanced by IGF-1 after 8–12 weeks.27 We have demonstrated a release of a therapeutic dose of IGF-1 for at least a week in vitro and we have also shown a significant increase in proteoglycan production by osteoarthritic cells after 4–14 days release (data not shown), so we believe that this may be sufficient to initiate repair in vivo. However, it is also important to consider the action of proteases in vivo that will gradually break down the collagen–GAG and this may lead to a faster release of IGF-1. Moreover, the free IGF-1 released in this experiment would not all be available to cells in vivo due to the presence of IGF binding proteins (IGFBPs) in synovial fluid.44 IGF-1 must be released from the IGFBPs to bind to the IGF-1 receptor and to modulate cell behavior; thus, IGFBPs may have a large impact on the IGF-1 bioavailability in vivo.
Conclusions
IGF-1 adsorption onto collagen–GAG scaffolds was optimized. Ionic interactions contribute to the binding between IGF-1 and collagen–GAG, but our data also suggest that other physiological noncovalent interactions may also be also important. The amount of IGF-1 adsorbed was approximately proportional to the loading and the elution profile of IGF-1 followed two distinct phases: a burst release dependent on the loading concentration, followed by a slow sustained release. The in vitro release profile of IGF-1 was dominated by this burst release, which occurred during the first day of elution. When the burst release was discounted, the relative percent of remaining IGF-1 released from day 1 to 14 was similar for all loading groups. This sustained release phase broadly follows Fickian diffusion kinetics until approximately day 6. The binding and release of IGF-1 was not affected by the DHT cross-linking method. The IGF-1 released from this system was shown to be bioactive in terms of increased proteoglycan production by human chondrocytes. The scaffold described in this study has several advantages over previously reported growth factor release systems. It can achieve relatively high loading compare to equivalent systems, and the burst release, although high, is still lower that other scaffold types. The burst release from our scaffolds is not high enough to be cytotoxic but is still adequate to allow therapeutic levels of bioactive IGF-1 release. These results demonstrate that this type of collagen–GAG scaffold adsorbs IGF-1 and that its release can be controlled by tailoring the loading concentration, thus making the system suitable for further development in vivo.
Acknowledgments
EPSRC and Orthomimetics Limited, Byron House, Cambridge Business Park, Milton Road, Cambridge, are gratefully acknowledged for a studentship for L.M.M. Dr. Roger Brooks acknowledges funding from the National Institute for Health Research. The authors thank Kevin Taylor for his advice and the use of the gamma counter at the Department of Biochemistry and Endocrinology, Addenbrooke's Hospital, Cambridge.
Disclosure Statement
No competing financial interests exist.
References
- 1.Coutts R.D. Healey R.M. Ostrander R. Sah R.L. Goomer R. Amiel D. Matrices for cartilage repair. Clin Orthop Relat Res. 2001;391(Suppl):271. doi: 10.1097/00003086-200110001-00025. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M.B. Chen E.H. Lynch S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14:403. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Yannas I.V. Lee E. Orgill D.P. Skrabut E.M. Murphy G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yannas I.V. Biologically active analogues of the extracellular matrix: artificial skin and nerves. Angew Chem Int Ed Engl. 1990;29:20. [Google Scholar]
- 5.Yamamoto Y. Nakamura T. Shimizu Y. Matsumoto K. Takimoto Y. Kiyotani T. Sekine T. Ueda H. Liu Y. Tamura N. Intrathoracic esophageal replacement in the dog with the use of an artificial esophagus composed of a collagen sponge with double-layered silicone tube. J Thorac Cardiovasc Surg. 1999;118:276. doi: 10.1016/S0022-5223(99)70218-7. [DOI] [PubMed] [Google Scholar]
- 6.Sekine T. Nakamura T. Matsumoto K. Liu Y. Ueda H. Tamura N. Shimizu Y. Carinal reconstruction with a Y-shaped collagen-conjugated prosthesis. J Thorac Cardiovasc Surg. 2000;119:1162. doi: 10.1067/mtc.2000.106652. [DOI] [PubMed] [Google Scholar]
- 7.Frenkel S.R. Di Cesare P.E. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 8.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien F.J. Harley B.A. Yannas I.V. Gibson L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Yannas I.V. Tissue Regeneration Templates Based on Collagen-Glycosaminoglycan Copolymers. Biopolymers II. 1995;122:219. [Google Scholar]
- 11.van der Kraan P.M. Buma P. van Kuppevelt T. van den Berg W.B. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage. 2002;10:631. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y. Tabata Y. Experimental regeneration by DDS technology of bio-signaling molecules. J Dermatol Sci. 2007;47:189. doi: 10.1016/j.jdermsci.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Tessmar J.K. Gopferich A.M. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:274. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Wirth C.J. Rudert M. Techniques of cartilage growth enhancement: a review of the literature. Arthroscopy. 1996;12:300. doi: 10.1016/s0749-8063(96)90062-6. [DOI] [PubMed] [Google Scholar]
- 15.Pieper J.S. van Wachem P.B. van Luyn M.J.A. Brouwer L.A. Hafmans T. Veerkamp J.H. van Kuppevelt T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000;21:1689. doi: 10.1016/s0142-9612(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 16.van Susante J.L.C. Pieper J. Buma P. van Kuppevelt T.H. van Beuningen H. van der Kraan P.M. Veerkamp J.H. van den Berg W.B. Veth R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials. 2001;22:2359. doi: 10.1016/s0142-9612(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 17.Cao H. Xu S.Y. EDC/NHS-crosslinked type II collagen-chondroitin sulphate scaffold: characterisation and in vitro evaluation. J Mater Sci Mater Med. 2008;19:567. doi: 10.1007/s10856-007-3281-5. [DOI] [PubMed] [Google Scholar]
- 18.Cui F.Z. Li Y. Ge J. Self-assembly of mineralized collagen composites. Mater Sci Eng R Rep. 2007;57:1. [Google Scholar]
- 19.Buma P. Pieper J.S. van Tienen T. van Susante J.L.C. van der Kraan P.M. Veerkamp J.H. van den Berg W.B. Veth R.P.H. van Kuppevelt T.H. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects—a study in rabbits. Biomaterials. 2003;24:3255. doi: 10.1016/s0142-9612(03)00143-1. [DOI] [PubMed] [Google Scholar]
- 20.Timkovich R. Detection of the stable addition of carbodiimide to proteins. Anal Biochem. 1977;79:135. doi: 10.1016/0003-2697(77)90387-6. [DOI] [PubMed] [Google Scholar]
- 21.Weadock K. Olson R.M. Silver F.H. Evaluation of collagen crosslinking techniques. Biomater Med Devices Artif Organs. 1984;11:293. doi: 10.3109/10731198309118815. [DOI] [PubMed] [Google Scholar]
- 22.Pek Y.S. Spector M. Yannas I.V. Gibson L.J. Degradation of a collagen-chondroitin-6-sulfate matrix by collagenase and by chondroitinase. Biomaterials. 2004;25:473. doi: 10.1016/s0142-9612(03)00541-6. [DOI] [PubMed] [Google Scholar]
- 23.Olde Damink L.H.H.O. Dijkstra P.J. van Luyn M.J.A. van Wachem P.B. Nieuwenhuis P. Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 24.Pieper J.S. Oosterhof A. Dijkstra P.J. Veerkamp J.H. van Kuppevelt T.H. Preparation and characterisation of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials. 1999;20:847. doi: 10.1016/s0142-9612(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 25.Wright N.T. Humphrey J.D. Denaturation of collagen via heating: an irreversible rate process. Annu Rev Biomed Eng. 2002;4:109. doi: 10.1146/annurev.bioeng.4.101001.131546. [DOI] [PubMed] [Google Scholar]
- 26.Nixon A.J. Fortier L.A. Williams J. Mohammed H. Enhanced repair of extensive articular defects by Insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 27.Tuncel M. Halici M. Canoz O. Turk C.Y. Oner M. Ozturk F. Kabak S. Role of insulin-like growth factor in repair response in immature cartilage. Knee. 2005;12:113. doi: 10.1016/j.knee.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Worster A.A. Brower-Toland B.D. Fortier L.A. Bent S.J. Williams J. Nixon A.J. Chondrogenic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-1 in a 3D matrix. J Orthop Res. 2001;19:738. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 29.Tyler J.A. Insulin-like growth factor-1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. J Biochem. 1989;260:543. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fosang A.J. Tyler J.A. Hardingham T.E. Effects of interleukin-1 and insulin like growth factor-1 on the release of proteoglycan components and hyaluronan from pig articular cartilage in explant culture. Matrix. 1991;11:17. doi: 10.1016/s0934-8832(11)80223-4. [DOI] [PubMed] [Google Scholar]
- 31.Frisbie D.D. Nixon A.J. Insulin-like growth factor 1 and corticosteroid modulation of chondrocyte metabolic and mitogenic activities in interleukin 1-conditioned equine cartilage. Am J Vet Res. 1997;58:524. [PubMed] [Google Scholar]
- 32.Elisseeff J. McIntosh W. Fu K. Blunk B. Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 33.Fortier L.A. Lust G. Mohammed H.O. Nixon A.J. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res. 1999;17:467. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- 34.Holland T.A. Tabata Y. Mikos A.G. Dual growth factor delivery from degradable oligo(poly(ethyleneglycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Lindahl A. Isgaard J. Isaksson O.G.P. Growth hormone in vivo potentiates the stimulatory effect of insulin-like growth factor-1 in vitro on colony formation of epiphyseal chondrocytes isolated from hypophysectomized rats. J Endocrinol. 1987;121:1070. doi: 10.1210/endo-121-3-1070. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson A. Carlsson B. Tsgaard J. Isaksson O.G.P. Rymo L. Regulation by GH of insulin-like growth factor-I mRNA expression in rat epiphyseal growth plate as studied with in-situ hybridization. J Endocrinol. 1990;125:67. doi: 10.1677/joe.0.1250067. [DOI] [PubMed] [Google Scholar]
- 37.Oberbauer A.M. Peng R. Growth hormone and IGF-I stimulate cell function in distinct zones of the rat epiphyseal growth plate. Connect Tissue Res. 1995;31:189. doi: 10.3109/03008209509010810. [DOI] [PubMed] [Google Scholar]
- 38.Trippel S.B. Corvol M.T. Dumontier M.E. Rappaport R. Hung H. Mankin H.J. Effect of somatomedin-C Insulin-like growth factor-1 and growth-hormone on cultured growth plate and articular chondrocytes. Pediatr Res. 1989;25:76. doi: 10.1203/00006450-198901000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Nixon A.J. Fortier L.A. New horizons in articular cartilage repair. AAEP. 2001;47:217. [Google Scholar]
- 40.van Susante J.L.C. Buma P. van Beuningen H.M. van den Berg W.B. Veth R.P.H. Responsiveness of bovine chondrocytes to growth factors in medium with different serum concentrations. J Orthop Res. 2000;18:68. doi: 10.1002/jor.1100180111. [DOI] [PubMed] [Google Scholar]
- 41.Fortier L.A. Nixon A.J. Lust G. Phenotypic expression of equine articular chondrocytes grown in three dimensional cultures supplemented with supraphysiologic concentrations of insulin-like growth factor 1. Am J Vet Res. 2002;63:301. doi: 10.2460/ajvr.2002.63.301. [DOI] [PubMed] [Google Scholar]
- 42.Lo M.Y. Kim H.T. Chondrocyte apoptosis induced by collagen degradation: inhibition by capase inhibitors and IGF-1. J Orthop Res. 2004;22:140. doi: 10.1016/S0736-0266(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 43.Morales T.I. The quantitative and functional relation between insulin-like growth factor (IGF) and IGF-binding proteins during human osteoarthritis. J Orthop Res. 2008;26:465. doi: 10.1002/jor.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeser R.F. Todd M.D. Seely B.L. Prolonged treatment of human osteoarthritic chondrocytes with IGF-1 stimulates proteoglycan synthesis but not proteoglycan accumulation in alginate cultures. J Rheumatol. 2003;30:1566. [PubMed] [Google Scholar]
- 45.Laffargue P. Fialdes P. Frayssinet P. Rtaimate M. Hildebrand H.F. Marchandise X. Adsorption and release of insulin-like growth factor-I on porous tricalcium phosphate implant. J Biomed Mater Res. 2000;49:415. doi: 10.1002/(sici)1097-4636(20000305)49:3<415::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Weadock K.S. Wolff D. Silver F.H. Diffusivity of 125I-labelled macromolecules through collagen: mechanism of diffusion and effect of adsorption. Biomaterials. 1987;8:105. doi: 10.1016/0142-9612(87)90098-6. [DOI] [PubMed] [Google Scholar]
- 47.Schneiderman R. Snir E. Popper O. Hiss J. Stein H. Maroudas A. Insulin-like growth factor-I amd its complexes in normal human articular cartilage: studies of partition and diffusion. Arch Biochem Biophys. 1995;324:159. doi: 10.1006/abbi.1995.9914. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien F.J. Harley B.A. Yannas I.V. Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 49.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantification and discrimination of sulphated glycosaminoglycans by use of dimethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 50.Luyten F.P. Hascall V.C. Nissley S.P. Morales T.I. Reddi A.H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 51.Kanematsu A. Marui A. Yamamoto S. Ozeki M. Hirano Y. Yamamoto M. Ogawa O. Komeda M. Tabata Y. Type I collagen can function as a reservoir of basic fibroblast growth factor. J Control Release. 2004;99:281. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Kanematsu A. Yamamoto S. Ozeki M. Noguchi T. Kanatani I. Ogawa O. Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Ueda H. Hong L. Yamamoto M. Shigeno K. Inoue M. Toba T. Yoshitani M. Nakamura T. Tabata Y. Shimizu Y. Use of collagen sponge incorporating transforming growth factor-beta 1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23:1003. doi: 10.1016/s0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 54.Ziegler J. Mayr-Wohlfart U. Kessler S. Breitig D. Gunther K.P. Adsorption and release properties of growth factors from biodegradable implants. J Biomed Mater Res. 2002;59:422. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 55.Liu B. Weinzimer S.A. Gibson T.B. Mascarenhas D. Cohen P. Type Iα collagen is an IGFBP-3 binding protein. Growth Horm IGF Res. 2003;13:89. doi: 10.1016/s1096-6374(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 56.Park Y.L. Lee Y.M. Lee J.Y. Seol Y.J. Chung C.P. Lee S.J. Controlled release of platelet-derived growth factor-BB from chondroitin sulphate-chitosan sponge for guided bone regeneration. J Control Release. 2000;67:385. doi: 10.1016/s0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 57.Lynn A.K. Design and development of an osteochondral scaffold [Ph.D. thesis] Department of Materials Science and Metallurgy, University of Cambridge; Cambridge, United Kingdom: 2005. [Google Scholar]
- 58.Ritger P.L. Peppas N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23. [PubMed] [Google Scholar]
- 59.Ritger P.L. Peppas N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37. [PubMed] [Google Scholar]
- 60.Ubersax L. Merkle H.P. Meinel L. Insulin-like growth factor-I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008;127:12. doi: 10.1016/j.jconrel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Haugh M.G. Jaasma M.J. O'Brien F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J Biomed Mater Res A. 2009;89:363. doi: 10.1002/jbm.a.31955. [DOI] [PubMed] [Google Scholar]
- 62.Rogachefsky R.A. Dean D.D. Howell D.S. Altman R.D. Treatment of canine osteoarthritis with insulin-like growth factor-I (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993;1:105. doi: 10.1016/s1063-4584(05)80025-1. [DOI] [PubMed] [Google Scholar]
- 63.Nixon A.J. Saxer R.A. Brower-Toland B.D. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-1 autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;1:26. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 64.Schneiderman R. Rosenberg N. Hiss J. Lee P. Liu F. Hintz R.L. Maroudas A. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- 65.Breinan H.A. Martin S.D. Hsu H.P. Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781. doi: 10.1002/jor.1100180516. [DOI] [PubMed] [Google Scholar]