The new immunosuppressant FK 506 shows great promise as a powerful means of inducing long-term allograft acceptance in a clinical setting.1–5 This drug has been shown to be several hundred times more potent than CyA in its immunosuppressive effects.1, 6 In vitro and in vivo studies have also shown synergism between the actions of FK 506 and CyA.3,4,7 The exact basis of this synergism has not yet been elucidated: however, it is known that both drugs inhibit T cell response at the level of IL-2 production and recognition.1,6,8,9
Since these agents may have similar pharmacologic actions, we explored the possibility that they might share similar toxicities. One well-known side effect of CyA administration is nephrotoxicity, which can occur in acute or chronic forms.10–12 The acute form of nephrotoxicity can be exacerbated by concomitant warm ischemia in the case of renal transplantation.13
The purpose of this study was to analyze the effect of FK 506 on the ischemic kidney in a rat model. We hypothesized that the drug would show effects similar to that seen with CyA. Further, we considered the possibility that the coadministration of CyA and FK 506 to the acutely ischemic kidney could have additive toxicity. Such synergistic toxicity would have implications for the use of this combination of drugs in the early management of renal transplant patients.
MATERIALS AND METHODS
Animals
Male inbred Lewis rats (RT11) weighing 235–255 g were purchased from Harlan Sprague-Dawley, Indianapolis, IN.
Pharmacologic Agents
FK 506 crystalline powder was supplied by Fujisawa Pharmaceutical Company, Osaka, Japan. It was dissolved in 9.9% sodium chloride for oral administration.
CyA (Sandoz, Ltd, Basal, Switzerland) was obtained in the commercial formulation (100 mg/ml). It was diluted in olive oil for oral administration.
Surgical Procedure
Animals were anesthetized with pentobarbital, 40 mg/kg intraperitoneally. A midline abdominal incision was made followed by infusion of heparin. 50 IU intravenously. The right perirenal fascia was dissected free, leaving only the pedicle of vessels and ureter. The right renal artery was identified, and was clamped to induce ischemia. The clamp was removed 60 minutes later. Immediately following revascularization of the right kidney, a left nephrectomy was performed.
Experimental Groups
All animals underwent 60 minutes of right renal ischemia followed by left nephrectomy. They were then randomly assigned to the following groups: A (n = 4), no treatment: B (n = 5), FK 506 I mg/kg/d orally; C (n = 5), FK 506 2 mg/kg/d orally; D (n = 4). FK 506 4 mg/kg/d orally: E <n = 5), CyA 5 mg/kg/d orally: F (n = 5), CyA 10 mg/kg/d orally: G (n = 5), CyA 25 mg/kg/d orally: and H (n = 5), FK 506 I mg/kg/d and CyA 10 mg/kg/d orally. All animals were killed on day 10.
Three additional groups underwent the above operation, had renal biopsies performed on day 2 for electron microscopy, and were killed on day 5. These groups were as follows: 1 (n = 5), no treatment: J (n = 5). FK 5064 mg/kg/d orally: and K (n = 5), CyA 25 mg/kg/d orally.
Measurements and Statistical Analysis
Animals were examined and weighed daily. Blood samples for blood urea nitrogen (BUN) and creatinine were collected via tail vein on days 1, 2, 3, 5, 7, and 10. Data were analyzed using one-way analysis of variance followed by pair-wise comparisons using the least significant difference test at a value of P < 0.05. Histologic variables at day 10 were subjectively scored. Ultra-structural and histologic features at days 2 and 5 were subjectively compared.
RESULTS
All animals survived the operative procedure. Group A animals maintained a normal level of activity during the experimental period. Rats in the CyA and FK 506 treatment groups appeared somewhat emaciated, with a moderate degree of hair loss. Postoperative weight loss occurred in all groups. The mean maximum weight loss in the control group (A) was 15%, whereas in the experimental groups it ranged from 23–27%.
Mean levels of serum creatinine are given in Table 1. A graphic representation of the time courses of these mean levels in the high-dose groups is shown in Fig 1. Both CyA and FK 506 were associated with significantly elevated creatinine levels when compared with controls. The highest elevations occurred on the first day and levels dropped progressively thereafter. At 25 mg/kg/d (group G). CyA was associated with the highest initial serum creatinine level. In general, enzyme levels increased as drug dosage increased. No clear-cut differences emerged between low-dose FK 506 compared with low-dose CyA in regard to degree of creatinine elevation (calculations not shown).
Table 1.
Serum Creatinine Levels of Various Groups Over a 10-Day Period Following Induction of Renal Ischemia
| Group (mg/kg/d) | Day 0 | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | Day 10 |
|---|---|---|---|---|---|---|---|
| A (control) | 1.0 ± .09 | 2.2 ± .29 | 1.8 ± .26 | 1.3 ± .14 | 1.1 ± .08 | 1.0 ± .09 | 1.0 ± .06 |
| B (FK506 1) | 1.0 ± .02 | 2.9 ± .20 | 2.1 ± .21 | 1.7 ± .14 | 1.7 ± .13 | 1.3 ± .05 | 1.1 ± .03 |
| C (FK 506 2) | 1.0 ± .04 | 3.0 ± .28 | 2.8 ± .28 | 2.1 ± .17 | 1.3 ± .14 | 1.1 ± .03 | 1.1 ± .03 |
| D (FK 506 4) | 1.2 ± .06 | 3.2 ± .31 | 2.8 ± .42 | 2.3 ± .33 | 2.1 ± .23 | 1.3 ± .09 | 1.2 ± .03 |
| E (CyA 5) | 1.2 ± .05 | 2.4 ± .08 | 1.9 ± .11 | 1.7 ± .10 | 1.5 ± .10 | 1.3 ± .06 | 1.1 ± .03 |
| F (CyA 10) | 1.2 ± .05 | 2.8 ± .31 | 2.3 ± .24 | 2.0 ± .29 | 1.7 ± .10 | 1.6 ± .07 | 1.2 ± .06 |
| G (CyA 25) | 1.2 ± .05 | 4.9 ± .26 | 4.8 ± .46 | 4.2 ± .11 | 2.2 ± .24 | 1.4 ± .22 | 1.0 ± .08 |
| H (FK 506 1 + CyA 10) | 1.3 ± .00 | 3.8 ± .13 | 2.7 ± .16 | 2.5 ± .14 | 1.7 ± .14 | 1.0 ± .04 | 0.9 ± .06 |
Note: Values are sxpressed as mean ± SEM.
Fig 1.
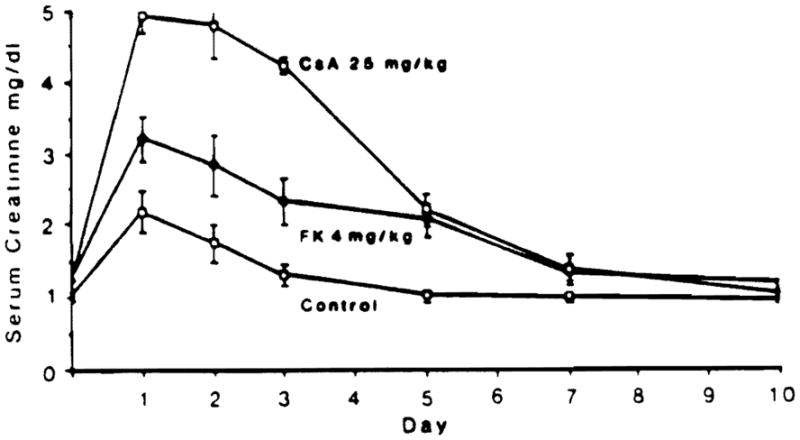
Graphic representation of serum creatinine levels in high-dose FK 506- and CyA-treated animals. Results are expressed as mean ± SEM.
The early rise in serum creatinine that occurred in animals given combination FK 506 1 mg/kg/d and CyA 10 mg/kg/d (group H) was greater than that seen when the drugs were given alone (groups B and F). However, the levels dropped down to normal in all groups by day 10.
In same general trends were observed in analysis of BUN levels (data not shown).
Histologic Analysis
Tissue for ultrastructural analysis was available from groups I, J, and K on day 2; both light and ultrastructural studies were performed on these groups on day 5. Tissue for light microscopy was available from the remainder of animals killed on day 10.
Control tissue from day 2 showed a moderate degree of cytoplasmic vacuolization, located predominantly near the luminal surface. Although this may have arisen in some areas from dilated endoplasmic reticulum, it appears to also have been related to numerous small microvesicles that were observed to arise from the cell membrane directly below the microvillous border. Cytoplasmic blebs also arose from the cell membranes and were extruded into the tubular lumens.
Animals treated with 25 mg/kg/d CyA showed a marked increase in cytoplasmic vacuolization, with involvement of both the adluminal and basal aspects of cytoplasm by this process. In addition, there was frequent coalescence of vacuoles to form large, space-occupying structures that appeared to have rent the cytoplasm apart.
FK 506-treated animals showed slightly more cytoplasmic vacuolization than did controls, but noticeably less than CyA animals. In the FK 506 group, the majority of cytoplasmic vacuoles were located in the luminal half of the cell, and coalescence was seen only rarely as compared with CyA animals.
The difference among groups was also apparent at the level of thick (1 μm) plastic-embedded sections.
Cytoplasmic vacuolization persisted in all groups at day 5. At this time, more electron-dense material was seen in a number of vacuoles, in either an amorphous or myelin configuration. CyA and FK 506, but not control animals, also showed marked heterogeneity in the size of mitochondria, including enlarged forms (megamitochondria). Many vacuoles appeared to be distorting and possibly disrupting mitochondria. At the 1 μm level, FK 506 animals appeared to have more tubular cytoplasmic vacuolization than at day 2. However, this did not approach the degree of cell disruption observed in CyA animals at day 2. By light microscopy, both FK 506 and control animals showed a moderate degree of tubular cytoplasmic basophilia in areas. This was not as prominent in CyA animals, in whom the tubular cells had a more eosinophilic and generally swollen appearance.
At day 10, experimental animals showed increased tubular basophilia, tubular dilation, and evidence of individual degenerated tubular cells compared with controls. No clear-cut differences could be discerned among the various experimental groups.
DISCUSSION
FK 506 is the forerunner of a new class of immunosuppressants that combines high efficacy with a low incidence of side effects, in both clinical and animal studies to date (Starzl, this issue). However, since the mode of therapeutic action appears similar to that of CyA, we were prompted to ask whether or not FK 506 would show any evidence of similar toxicity under specific experimental conditions. We chose to examine this question in the setting of renal ischemia, since it is known that CyA plus ischemia act synergistically to cause acute renal damage.
Our findings indicate that FK 506 does cause acute tubular alterations in a rat model of renal ischemia. The ultrastructural observations of cytoplasmic vacuolization and megamitochondria are similar to those changes described for CyA-induced ischemia renal damage.
CyA at 25 mg/kg/d was associated with higher elevations of BUN and creatinine at 1 and 2 days than was FK 506 at 4 mg/kg/d. This coincided with more severe ultrastructural changes in the renal tubular cells of CyA-treated animals at this time. At lower doses, there was no discernible difference between the creatinine levels of experimental groups treated with either drug. Serum enzyme values in all groups descended toward baseline by day 10, consistent with a reversible toxic reaction.
The combination of CyA, 10 mg/kg/d, and FK 506, 1 mg/kg/d, resulted in higher early serum BUN and creatinine values than did treatment with either agent alone at these doses. Thus, there is at least an additive toxicity of the two agents under these conditions.
These observations, if translated literally, have interesting implications in the clinical situation. First, the doses of FK 506 used in this study are several 100-fold more immunosuppressive in humans than are the doses of CyA. Thus, although the mechanism of action on the ischemic kidney appears similar, the study suggests that such acute nephrotoxicity might be subclinical to non-existent in FK 506-treated patients, except under extreme circumstances. Second, the additive effects of FK 506 and CyA on ischemic kidneys imply that induction of immunosuppression with FK 506 alone may be easier than with combination FK 506 and CyA. Third, this study suggests that switching a patient from CyA to FK 506 could be associated with an acute reversible renal toxicity, which would subside as CyA was eliminated from the system. Finally, the results raise the hope that FK 506 may also be found to be less nephrotoxic than CyA in the chronic setting. Long-term studies are indicated to explore this possibility.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MO.
References
- 1.Ochiai T, Sakamoto K, Nagata M, et al. Transplant Proc. 1988;20(suppl 1):209. [PubMed] [Google Scholar]
- 2.Murase N, Todo S, Lee P-H, et al. Transplant Proc. 1987;19(suppl 6):71. [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Murase Y, Ueda L, et al. Transplant Proc. 1988;20(suppl 1):215. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Demetris A, Ueda Y, et al. Surgery. 1989;106:444. [PubMed] [Google Scholar]
- 5.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeevi A, Duquesnoy RJ, Eiras G, et al. Surg Res Commun. 1987;1:315. [PMC free article] [PubMed] [Google Scholar]
- 7.Zeevi A, Duquesnoy RJ, Eiras G, et al. Transplant Proc. 1987;19(suppl 6):40. [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima K, Sakamoto K, Ochiai T, et al. Transplantation. 1988;45:1147. doi: 10.1097/00007890-198806000-00033. [DOI] [PubMed] [Google Scholar]
- 9.Chabannes D, LeMauff B, Hallet MM, et al. Transplantation. 1988;46(suppl):97S. doi: 10.1097/00007890-198808001-00018. [DOI] [PubMed] [Google Scholar]
- 10.Klintmalm GBG, Iwatsuki S, Starzl TE. Transplantation. 1981;32:488. doi: 10.1097/00007890-198112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryffel B, Foxwell BM, Gee A, et al. Transplantation. 1988;46(suppl):90S. doi: 10.1097/00007890-198808001-00017. [DOI] [PubMed] [Google Scholar]
- 12.Dische FE, Neuberger K, Keating J, et al. Lab Invest. 1988;58:395. [PubMed] [Google Scholar]
- 13.Khauli RB, Strzelecki T, Kumar S, et al. Transplant Proc. 1987;19:1395. [PubMed] [Google Scholar]


