Abstract
The bidirectional paradigm of tolerance involving reciprocal host vs. graft and graft vs. host reactions was examined after Lewis (LEW) → Brown Norway (BN) transplantation of different whole organs (liver, intestine, heart, and kidney) or of 2.5×108 LEW leukocytes obtained from bone marrow, spleen, lymph nodes, and thymus. The experiments were performed without immunosuppression or under 14 daily doses of postoperative tacrolimus, which were continued in weekly doses to 100 days in a “continuous treatment” subgroup, and to 27 days in a short treatment group. Without immunosuppression, all organs and cell suspensions failed to engraft or were acutely rejected. GVHD (usually fatal) was always caused when either the long or short treatment was used for recipients of intestinal grafts and cell suspensions of spleen and lymph nodes. In contrast, both immunosuppressive protocols allowed engraftment of bone marrow cells, liver, heart, and kidney without clinical GVHD, whereas thymus cell suspensions and small doses of whole blood neither engrafted nor caused GVHD. At 100 days, now drug-free for 73 days, the liver, bone marrow, and heart recipients were tolerant in that they accepted all challenge LEW heart and/or liver grafts for 100 more days despite in vitro evidence of donor-specific reactivity (split tolerance). At 200 days, histopathologic studies of the challenge livers were normal no matter what the priming graft. However, the still-beating challenge hearts had a spectrum from normal to severe chronic rejection that defined the tolerogenicity of the original primary grafts: liver best → bone marrow next → heart least. Both the GVHD propensity and tolerogenicity in these experiments were closely associated with recipient tissue chimerism 30 and 100 days after the experiments began. The tissue chimerism was invariably multilineage, but the GVHD outcome was associated with T cell over-representation. These observations provide guidelines that should be considered in devising leukocyte augmentation protocols for human whole organ recipients. The results are discussed in relation to the historical tolerance studies of Billingham, Brent, and Medawar; Good; Monaco; and Calne.
The persistence of microchimerism in human whole organ recipients years or decades after transplantation (1, 2) reflects the migration long before of bone marrow-derived donor leukocytes from the allografts (3, 4). We have postulated that these immunocompetent donor cells represent one limb of initially antagonistic but ultimately attenuated or abrogated host-versus-graft (HVG,* rejection) and graft-versus-host (GVH) reactions (1-5) (Fig. 1). We describe here a study in rats of the HVG and GVH components of this two-way immunologic paradigm. The clinical and histopathologic expression of the two arms with and without immunosuppression was correlated with the quantity and quality of recipient tissue chimerism and with the development of donor-specific tolerance following transplantation from Lewis (LEW) donors to Brown Norway (BN) recipients of different organs (intestine, liver, heart, kidney) and of different free leukocyte suspensions (bone marrow, lymph nodes, spleen, thymus, and blood).
Figure 1.
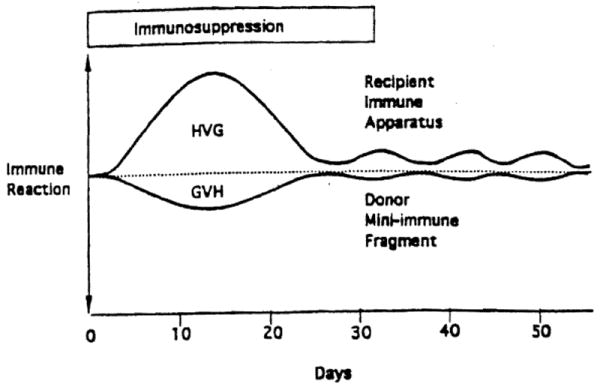
Dualistic immune reactions of host-versus-graft (HVG) and graft-versus-host (GVH) in the two-way paradigm of transplantation immunology. Following the acute reaction, the evolution of tolerance of each leukocyte population to the other is seen as a low-grade stimulatory state that may wax and wane rather than a deletional one.
Although the results leave numerous questions unanswered about basic mechanisms, they cast light on 4 issues that are relevant to planning of clinical tolerance induction protocols: (1) The relative risk of producing clinical GVHD with the tranplantation of different organs and with infusion of a standardized dose of leukocytes obtained from various lymphoid organs, (2) the relative tolerogenicity of the parenchymal organs and the leukocyte suspensions, (3) correlation of 1 and 2 with the density and lineage profile of the chimerism in recipient tissues and, (4) the relation to the quantity and lineage composition of chimerism to chronic rejection.
MATERIALS AND METHODS
Animals and transplant procedures
Male Lewis (LEW, RT1l) and Brown Norway (BN, RTln) rats weighing 200–300 g were purchased as donors and recipients, respectively (Harlan Sprague Dawley, Indianapolis, IN), and maintained in conventional animal facilities. The kidney (6), small intestine (7), and liver allografts (8) were placed orthotopically after removal of the corresponding native organ. The heart grafts were vascularized heterotopically in the abdomen (9).
Leukocytes were washed from the bone marrow of tibias and femurs. The preliminary step of cell extraction from the spleen, lymph nodes, and thymus was by compression of fragments of the whole organs through a stainless steel mesh and filtration of the product through a nylon mesh. The cells were processed with RPMI 1640 supplemented with 25 mM Hepes buffer. 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (all from Gibco, Grand Island, NY). Trypan blue exclusion testing always showed >90% viability before intravenous injection into the penile vein of the BN recipients. The cell counts of the suspensions were determined (2.5× 108 per experiment), permitting a uniform cell dose and therefore a meaningful comparison of eventual results. The cell dose was not quantitated in experiments involving 3 ml unaltered donor whole blood infusion. However, spot samples of buffy coats from 3 ml blood had approximately 2.5~3×107 cells, or about 1/10 the dose of the cell suspensions. The leukoprofile of naive LEW as well as BN rats of the cell suspensions of the central lymphoid organs was reported in detail previously (10), and is summarized schematically for the LEW donor strain in Fig. 2. Although there were differences between the other central lymphoid organs, the bone marrow was dramatically different from all because of the large number of immature cells of undetermined lineage.
Figure 2.

Leukocyte profile of cell suspensions from LEW rat hematolymphopoietic organs and blood. Full data have been reported elsewhere (10). The CD4+ and CD8+ phenotypes was characteristic for thymocytes. The monoclonal antibodies used for the CD4+ and CD8+ phenotypes were not lineage-specific but principally identified T cells.
Immunosuppression
Continuous therapy
The recipients of the whole organs or cell suspensions (Table 1) were given intramuscular injections of 1.0 mg/kg/day tacrolimus (dissolved in HCO-60 and D mannitol; Fujisawa Pharmaceutical, Osaka, Japan) for 14 days starting on the day of transplantation, and weekly thereafter until 100 days in animals surviving this long.
Table 1.
Effect on survival and GVHD incidence of no, short course, and continuous treatment with tacrolimus following LEW→BN organ and cell transplantion
| Graft | No treatment |
Short course tacrolimusa |
Continuous tacrolimusb |
|||
|---|---|---|---|---|---|---|
| Survival (days) | Median (days) | Survival (days) | Median (days) | Survival (days) | Median (days) | |
| Organc | ||||||
| Heart | 7, 8, 8, 8, 8, 8, 10, 11 | 8.0 | >100 (×9) | >100 | >100 (×6) | >100 |
| Kidney | 6, 7, 8, 9 | 7.5 | — | — | >100 (×4) | >100 |
| Liver | 23, 23, 23, 25, 28, 29, 29, 30, 32, 27 | 28.5 | >100 (×6) | >100 | >100 (×4) | >100 |
| Small bowel | 11, 12, 13 | 12.0 | 35, 43, 49 | 43.0 | 35, 42, 44, 47, 49, 54 | 45.5 |
| Cellsd | ||||||
| Bone marrow | >100 (×5) | >100 | >100 (×8) | >100 | >100 (×6) | >100 |
| Lymph nodes | >100 (×2) | >100 | 43, 46, 46, 47, 47, 51, 53, 54 | 47.0 | 36, 48, 50, 63, 71, >100 | 56.5 |
| Spleen | >100 (×2) | >100 | 51, 53, 53, 60, >100 (×3) | 60.0 | 62, 72, 74, 77, 78, >100 | 75.5 |
| Thymus | — | — | >100 (×8) | >100 | >100 (×3) | >100 |
| Whole blood | >100 (×3) | >100 | >100 (×8) | >100 | >100 (×3) | >100 |
1.0 mg/kg/day for 14 days (days 0 to 13) and on days 20 and 27.
1.0 mg/kg/day for 14 days (days 0 to 13), followed by weekly injection of 1.0 mg/kg.
Animal (synonymous with graft) survival and occurrence of host GVHD (italics) after LEW → BN transplantation of different organs to untreated recipients (left) and to recipients given an abbreviated (middle) or continuous (right) course of tacrolimus.
Animal survival and GVHD (italics) after i.v. infusion of different cell suspensions, using the same treatment as above.
Short-course therapy
For tolerance induction experiments, the same 2-week daily schedule of tacrolimus was begun in the BN rats on the day of the LEW organ or cell transplantation (day 0, Table 1), and supplemented with single injections on days 20 and 27. At 100 days, 73 days after the last of the 16 doses, the surviving BN recipients were tested for donor-specific nonreactivity (tolerance) by challenging them in the absence of drug treatment with a LEW heart or liver or with a third-party (ACI) heart.
Experimental end points
Whole Organ graft survival
Liver, intestine, and kidney graft survival was considered synonymous with recipient death or sacrifice before then because of moribund state. Heterotopic heart graft survival ended with cessation of a palpable heartbeat, at which time the animals were sacrificed.
GVHD and rejection
The rats were weighed at least twice a week, and observed for skin rashes, hair loss, diarrhea, and other clinical findings. All animals had histopathologic examination of tissues after death or sacrifice. Conventional criteria were used to diagnose GVHD and rejection.
Tolerance
The mixed lymphocyte response (MLR) was determined in BN rats 100 days after priming with bone marrow, thymus cells, and whole blood under a short course of tacrolimus. The response of lymphocytes from the primed animals was compared with that of naive BN lymphocytes, using irradiated naive LEW lymphocytes or appropriate third-party and syngeneic control cells as stimulators (10). Operational tolerance was then determined directly by the survival after transplantation of LEW or ACI (third-party) hearts (groups 6, 8, 10, Table 2) or of LEW livers (group 14).
Table 2.
LEW heart or liver survival in drug-free BN rats previously transplanted with different whole organs or leukocyte suspensions with or without an induction course of tacrolimus
| Group | Organ/cell pretreatment (day 0) | Tacrolimusa | n | Survival of challenge organ (days) | Median (days) | Pathology of challenge graft b |
Chimerism |
||
|---|---|---|---|---|---|---|---|---|---|
| Cellular infiltrate | Obliterative arteriopathy | 30 days | 100 days | ||||||
| Heart as challenge organ (day 100) | |||||||||
| 1 | None | − | 8 | 7, 8, 8, 8, 8, 8, 10, 11 | 8.0 | + + | +S | — | — |
| 2 | None | + | 5 | 9, 9, 9, 11, 13 | 9.0 | + + | +S | — | — |
| 3 | Heart | + | 6 | >100 (×6) | >100 | + + | + + + | ± | − |
| 4 | Liver | + | 3 | >100 (×3) | >100 | − | − | + + | ± |
| 5 | Bone marrow | − | 3 | 4, 5, 6 | 5.0 | + + | +S | − | − |
| 6 | Bone marrow | + | 5 | >100 (×5) | >100 | +/+ + | +/+ + | + | ± |
| 7 | Spleen | + | 2 | 14, 71 | 42.5 | NTc | NT | + + + | NT |
| 8 | Thymus | + | 6 | 6, 6, 6, 7, 10, 13 | 6.5 | + + + | +S | − | − |
| 9 | Blood | − | 2 | 5, 6 | 5.5 | + + | +S | NT | NT |
| 10 | Blood | + | 6 | 7, 8, 10, 13, 13, 15 | 11.5 | + + + | +S | − | − |
| Liver as challenge organ (day 100) | |||||||||
| 11 | None | − | 10 | 23, 23, 23, 25, 28, 29, 29, 30, 32, 37 | 28.5 | + + | +S | — | — |
| 12 | None | + | 3 | 20, 24, 31 | 24.0 | + + | +S | — | — |
| 13 | Heart | + | 2 | >100 (×2) | >100 | ± | ± | ± | − |
| 14 | Bone marrow | + | 4 | >100 (×4) | >100 | ± | − | + | ± |
1.0 mg/kg/day for 14 days on days 0 to 13, followed by two weekly injections on days 20 and 27.
(+S) = inflammatory arteritis.
NT, not tested.
When priming had been done with LEW heart grafts, the recipients were challenged at 100 days with a second heart (group 3, Table 2) or a liver (group 13). Recipients primed with livers were challenged with a heart (group 4). Experiments after priming with spleens were unsatisfactory (see Results).
In additional nonsurvival experiments used for histopathologic studies. BN animals primed under the same immunosuppression with LEW bone marrow were sacrificed 1,3,5, and 7 days (n=2 each) after transplantation of a challenge liver. The organ allografts and spleens were examined histopathologically for signS of rejection and evidence of proliferation, respectively.
Control experiments
The same protocols were followed in experiments that omitted either the priming transplant procedure, the tacrolimus treatment, or both (Tables 1 and 2). Previously reported controls were not repeated, showing that priming with syngeneic bone marrow had no effect on either GVHD or the outcome of subsequent transplantation (3).
Pathologic studies
A complete autopsy was carried out on all rats. Tissues were fixed in formalin for paraffin embedding and routine H&E staining. Samples also were snap-frozen in liquid nitrogen for immunophenotypic analysis. Donor LEW cells were identified in BN recipients by using L-21-6, a monoclonal antibody that recognizes class II MHC antigens of most rat stains, except BN (3, 10-12) (gift from Dr. Yuichi Iwaki, Professor of Pathology, University of Pittsburgh). The number of donor class II MHC positive cells present in recipients lymph nodes and spleen was estimated in a semiquantitative fashion according to the following scale: (−) donor cells (on whole-mount section of lymph node and spleen) not detected; (±) rare, ≤5 cells; (+) occasional, ≥5 ≤10; (+ +) moderate, ≥10 ≤550; (+ + +) many donor cells ≥50.
In conjunction with L-21-6, previously reported double-labeling immunofluorescence (4) and immunoperoxidase (12) techniques were used to determine the phenotype of surviving class II MHC–positive donor cells. The reagent panel contained monoclonal antibodies against all of the principal leukocyte subsets (panel available on request).
Flow cytometric analysis
Donor and recipient hematolymphoid cells from recipient BN lymph nodes were examined after preparation of single-cell suspensions as described above and from recipient peripheral blood after lysis of red blood cells (red cell lysing buffer, Sigma, St. Louis, MO). LEW or BN cells were identified with affinity-purified biotinylated rat monoclonal antibodies (McAb) 163 and 42, (gifts from Dr. Heinz Kunz, Professor of Pathology, University of Pittsburgh) that react with MHC class I RTIA1 and RTIAn antigens, respectively (l3). Phycoerythrin-conjugated streptavidin (Pharmigen, San Diego, CA) was used as a secondary antibody. Lineage phenotype was determined with the same panel of monoclonal antibodies used for immunohistochemistry. Samples were analysed on an Epics flow cytometer (Coulter Corporation, Hialeah, FL).
RESULTS
Rejection
No treatment
Completion of organ rejection, defined as the day of animal death or sacrifice occurred at medians of 7.5, 8, 12, and 28.5 days after kidney, heart, intestine, and liver transplantation, respectively (Table 1, upper left). All of the organs had conventional histopathologic findings of rejection.
Infusion of the cell suspensions or blood caused no mortality (Table 1, bottom left). When the animals were spot-checked at 30 days and sacrificed at 100 days, no donor cells could be found in any recipient tissues. These were assumed to have been rejected.
Short-course and continuous tacrolimus
With either regimen of immunosuppression, all liver and heart recipients and their grafts survived 100 days (Table 1, upper middle and right). The livers were essentially normal using both treatment regimens. However, the hearts treated with the short course had developed obliterative arteriopathy with lymphyocytic infiltrates 73 days after drug distoninuance: these abnormalities were not present under continuous therapy. Contniuously treated kidney recipients also had essentially normal allografts at the end of 100 days. All intestinal recipients died at about 6 weeks postoperatively whether given a short or continuous course of immunosuppression (Table 1, upper middle and right). Histopathologic stigmas of rejection were either not present or minimal.
Rejection of cell suspensions or blood leukocytes could not be monitored decisively. However, avoidance of rejection of lymph node leukocytes and splenocytes at 30 days and time of death under both regimens of immunosuppression was evidenced by the copious presence of these donor cells in the tissues of all animals; 22 of these 27 rats died before 100 days (Table 1, bottom middle and right). After bone marrow infusion, donor cells also could invariably be found, but in contrast none of these animals died (survival 14/14). Infusion of thymus suspensions and whole blood under immunosuppression also was without mortality (Table 1, bottom middle and right). However, donor leukocytes could never be found in the tissues, implying their rejection or failed initial engraftment. The differences in outcome with the suspensions of lymph node, spleen, bone marrow, and thymus cells could not be explained by different cell doses (2.5×108 in all).
GVHD
No treatment
Intestinal recipients developed transient skin rashes as previously reported (10, 14, 15) that quickly receded as the bowel and presumably the donor leukocytes were rejected. No evidence of GVHD Was detected with any of the other organs (Table 1, upper left) or with the cell suspensions (lower left).
Short-course and continuous tacrolimus
Similar to previous report with the LEW → BN strain combination (10, 14, 15), intestinal recipients developed clinically obvious and histopahtologically confirmed GVHD that was equally lethal whether immunosuppression was stopped after 27 days (n=3) or continued (n=6) (Table 1, upper middle and right). Liver recipients were healthy with both treatment regimens, including absence of clinical GVHD despite the presence in some animals of a mononuclear cell/T cell–rich infiltrate of donor cells in the epidermis. Kidney and heart recipients (Table 1, top middle and right) were clinically and histopathologically free of GVHD.
Rats given thymus cell suspensions and whole blood never developed GVHD, which was explained by the absence of donor cells in the tissues of these animals. Bone marrow suspensions, however, resulted in obvious donor cell engraftment under both treatment schedules. The animals were ostensibly healthy despite the presence of dendritic-shaped donor cells in the dermis (without epidermal infiltration) that were most evident in animals treated continuously for 100 days. The same dose of splenocytes and lymph node leukocytes always caused GVHD, and this was the cause of the usual fatal outcome (Table 1, bottom middle and right).
Correlation with chimerism after short-course tacrolimus
GVHD was associated with the density of chrimerism as well as its T cell constituency (Tables 3 and 4). Thirty days after the primary allotransplantations and 3 days after discontinuance of tacrolimus, striking chimerism was detected immunocytochemically with the L-21-6 (class II+) antibody in the tissues of recipients of small bowel, splenocytes, and lymph node leukocytes. Double-labeling showed that these included T cells (alpha-beta TCR+), B cells (IgM+), dendritic cells (OX62+), macrophages (ED2+), and natural killer cells (NK 3.2.3 +). Flow cytometry of peripheral blood or recipient lymph node suspensions using anti LEW MHC class I antibody (McAb 163) showed that 4–7.5% of the cells were donor (Table 3). In the small bowel recipients, more than 60% were T cells (alpha-beta TCR+) of the W 3/25+ subset, but donor B cells (OX33+) could also be detected.
Table 3.
Chimerism in BN recipients 30 and 100 days after transplantation of LEW organs or leukocyte suspensions under a short course of tacrolimusa
| 30 Days |
100 Days |
|||||
|---|---|---|---|---|---|---|
| Flow cytometry (class I)b | Immunohistochemistry (class II)c | Lineage | Immunocytochemistry (class II)e | Tolerance | GVHD | |
| Small bowel | 4.0±2.3%d | + + + | T > Me | — | No | 100% |
| Lymph nodes | 4.1±1.8% | + + + | T > M | — | No | 100% |
| Spleen | 7.5±0.2% | + + + | T > M | — | Minimalf | 100% |
| Liver | <1.0% | + + | M = T | ± | Yes | 0 |
| Bone marrow | 0 | + | M > T | ± | Yes | 0 |
| Heart | 0 | ± | — | − | Partialg | 0 |
| Kidney | NT | ± | — | − | NT | 0 |
| Thymus | NT | − | — | − | No | 0 |
| Blood | NT | − | — | − | No | 0 |
1.0 mg/kg/day on days 0 to 13; 20, and 27.
McAb 163, blood samples, or recipient lymph node suspensions.
L-21-6Ab, spleen, and cervical lymph nodes.
Mean ± SD.
NT: not tested; M: multilineage; T: T lymphocyte.
Delayed acute rejection.
Chronic rejection.
Table 4.
Location and number of L-21-6–positive cells in different tissues of recipient 30 days after small intestine, liver, and bone marrow transplantation
| Tissue | Donor cells after transplantation numbera / location |
|||||
|---|---|---|---|---|---|---|
| Small intestine | Liver | Bone marrow | ||||
| Spleen | + | Red pulp, PALSc | + | PALS, red pulp | + | PALS |
| Thymus | −/+ | Distorted architecture, small medulla | + | Medulla | + + | Medulla |
| Lymph nodes | + + + | Cortical, T cell–rich | + + | Mixed cortical and paracortical | + | Paracortical, dendritic cell–rich |
| Skin | + + + | Dermal-epidermal junction | −/+ | Deep dermis | −/+ | Deep dermis |
| Liver | − | — | NA | — | + | Portal tracts, rare sinusoids |
Donor cells on whole-mount section.
(−/+) rare; (+) 5< <10; (+ +) 10< <50; (+ + +) >50.
PALS, periarteriolar lymphoid shealth.
In bone marrow and liver recipients, none of which developed GVHD, too few donor cells were present at 30 days to permit flow cytometry. However, immunochemical labeling with the L-21-6 class II antibody and double-labeling with lineage phenotype markers permitted quantitative and qualitative estimates of the chimerism (Tables 3 and 4). The spleen and cervical lymph nodes from liver recipients contained 10–50 donor cells per whole mount section, with a clear dominance of T cells. All lineages also were present in the tissues of the less densely chimeric bone marrow recipients (5–10 cells/whole-mount section), but without a dominant lineage. The donor cells in kidney and heart recipients were too rare to permit lineage analysis. No donor cells could be found after 30 days in animals conditioned with thymus cell suspensions or whole blood.
At 100 days after primary allotransplantation, 73 days after the last drug dose, donor class II (L-21-6+) cells had become sparse in the liver and bone marrow recipients (estimated <0.01%). However, T, B, and dendritic cell lineages still could be detected. No definite L-21-6+ cells were found in the kidney and heart recipients by the cytostaining techniques. Recipients of thymus cells and whole blood were negative for chimerism (Table 3).
Tolerance induction
Organ-induced
Although lethal GVHD after small bowel transplantation precluded the demonstration of tolerance by transplantation of a challenge donor organ, none of the priming allografts had evidence of rejection at the time of death, 8 to 22 days after discontinuance of immunosuppression, suggesting self tolerance had been induced by the intestine.
In BN recipients primed with LEW heart (n = 6) and liver (n=3) grafts under the short course of tacrolimus, tolerance to challenge LEW hearts was convincingly demonstrated. All of the challenge hearts transplanted at 100 days survived for >100 additional days (groups 3 and 4, Table 2), compared with the median survival of 8 days in naive control recipients (group 1, Table 2). The hearts preceded by livers appeared to beat more vigorously than those preceded by hearts, an impression of superiority that was confirmed by histopatholgic study (see below). ACI challenge hearts (third-party controls) at 100 days were normally rejected.
Conversely, a priming heart was tolerogenic for a subsequently transplanted liver (group 13, Table 2).
Lymphopoietic cell–induced
Thymus cell suspensions (group 8) and whole blood (group 10) under short-course tacrolimus were not tolerogenic for hearts, and actually reduced survival to below the 8 days recorded in naive LEW → BN control experiments. Only 2 experiments could be attempted at 100 days in splenocyte primed animals. Both rats had slowly resolving GVHD after discontinuance of tacrolimus at 27 days. Survival of the 2 challenge LEW heart allografts were prolonged to 14 and 71 days before the organs were rejected (group 7, Table 2).
In contrast, LEW bone marrow cell suspensions infused under the same treatment conditions allowed >100-day survival of all LEW challenge hearts (group 6, Table 2) and livers (group 14). The effect was donor-specific in that 3 of 3 BN rats primed with LEW bone marrow rejected third-party ACI hearts in 6 days.
The loss of antidonor alloreactivity in the intact animals was not reflected in the MLR results. Lymphocytes from the tolerant BN rats responded equivalently to naive LEW and ACI stimulator cells. This pattern of response was essentially the same as that of lymphocytes obtained from naive BN rats from nontolerant BN rats reconditioned with thymus cells or blood transfusion who rejected their heart grafts in the usual time (Table 5).
Table 5.
MLR against BN (syngeneic), LEW (donor), and ACI (third-party) in naive and bone marrow–, whole blood–, or thymocyte-primed BN ratsa
| BN responderb | Stimulator |
||
|---|---|---|---|
| BN (syngeneic) | LEW (donor) | ACI (third-party) | |
| Naive | 235±78 | 36.558±1844 | 26.979±6276 |
| Bone marrow | 703±184 | 21.122±5176 | 24.450±12.367 |
| Blood | 965±449 | 30.983±5215 | 36.020±7877 |
| Thymus | 911±55 | 15.651±4454 | 17.842±4181 |
All values are mean ± SD (cpm) of triplicate wells.
BN animals received bone marrow, thmocytes (250× l06) or 3 ml whole blood from LEW (day 0) and were treated with tacrolimus (1.0 mg/kg/day on days 0 to 13 and 20, and 27). Cervical lymph nodes were obtained on day 100 for analysis.
Alloreactivity was also demonstrated in the operationally tolerant bone marrow primed animals challenged with liver allografts at 100 days and sacrificed for histopathologic studies 1–7 days later. Between 3 and 7 days posttransplantation a vigorous but spontaneously resolving alloresponse was reflected in the LEW liver allografts by a transient heavy mononuclear infiltration that coincided with obvious proliferation in the host spleen.
Correlation with chimerism
Tolerance to challenge organs was not accomplished in any cohort in which chimerism was not demonstrable 30 days after the priming allotransplantation. In spite of the poor chimerism produced by priming hearts, all of the livers were accepted at 100 days and were normal 100 days later. Challenge livers were of equally good quality after transplantation to the recipients primed with bone marrow who had better and more persistent chimerism. The perfection of these results precluded a distinction between cardiac and hepatic tolerogenicity, when the liver was used as the challenge organ.
In contrast, the heart as a challenge organ provided a discriminating test of tolerogenicity. Although all of the hearts transplanted to liver, bone marrow, and heart–primed recipients beat for the l00-day period of subsequent observation, the rank order of tolerogenicity was readily determined by histopathologic grading. The priming liver gave the best protection, bone marrow next, and the heart least (Table 2). These scores paralleled the density of chimerism (Table 3). Cardiac allografts in liver-primed recipients were essentially normal, whereas those in the heart-primed cohort had advanced findings of chronic rejection (Fig. 3) including obliterative arteriopathy and multiple subendocardial, perivascular, and interstitial lymphocytic infiltrates similar to the “Quilty” lesions seen in human cardiac allografts (16). The challenge hearts in bone marrow primed recipients had a mild and patchy version of these lesions.
Figure 3.
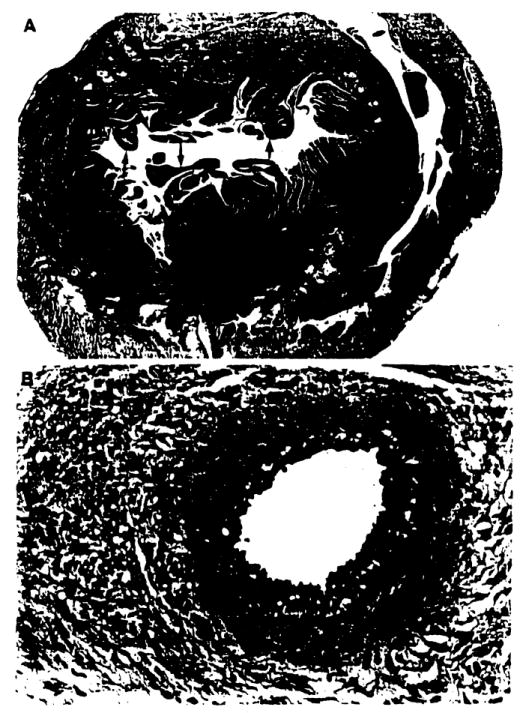
Chronic rejection in LEW challenge heart 100 days after transplantation to a tolerant recipient that had been primed with another LEW heart 100 days before transplantation. The recipient was treated with a short course of tacrolimus after the priming transplantation, and had been drug-free for 73 days at the time of challenge engraftment. (A) Cross-section of challenge heart allograft at sacrifice. Arrows = subendocardial lymphocyte aggregates (Quilty lesions). (H&E stain, original magnification approximately ×20.) (B) Occlusive arterial lesions of chronic rejection. Note minimal cellular intiltrate. (H&E stain, original magnification approximately, ×200.)
Control experiments
Omission of either the short course of immunosuppression (Table 2, groups 5, 9) or the allograft (groups 2, 12) from the primary stage of the experiment eliminated the tolerogenic effect at the time of the challenge transplantation. Preliminary infusion of priming bone marrow cells or whole blood without tacrolimus appeared to cause more rapid rejection of the subsequent heart allografts (groups 5, 9, Table 2).
DISCUSSION
Interest in the infusion of donor antigen or live cells to facilitate acceptance of organ allografts originated with the demonstration by Billingham, Brent, and Medawar (17, 18) that chimerism and acquired tolerance (proved with skin transplantation) could be produced by infusion of adult mouse splenocytes into immunologically immature (defenseless) recipients during gestation or neonatally. After Billingham and Brent (19) and, independently, Simonsen (20) showed that the engrafted splenocytes posed a risk of GVHD, Billingham and Brent (21) reported that cell suspensions of all the central lymphoid organs had the same range of tolerogenicity except for the weakly effective thymocytes. However, of the potent tolerogens (bone marrow, spleen, lymph node cells, and buffy coat), bone marrow was the least likely to cause GVHD in strain combinations predisposed to this complication (21). Main and Prehn (22) successfully simulated the neonatal tolerance in adult mice, using supralethal irradiation followed by reconstitution with donor bone marrow. This prototype strategy was used for the first successful clinical bone marrow transplantations in 1968 (23-25) and was governed, as in the rodent models, by the need for good histocompatibility matching to avoid lethal GVHD.
The extension of this strategy to prepare patients for organ allografts seemed obvious (26), as summarized by Rapaport et al. (27). However, the momentum carrying organ and bone marrow transplantation on a common pathway was lost between 1959 and 1962 when the combination of total-body irradiation and bone marrow replacement proved to be uniformly lethal as a step to organ transplantation in large outbred animals (28, 29). More important, the incentive to continue such efforts was eroded when 6 human kidney allografts, one in Boston (30) and 5 in Paris (31,32), functioned for extended periods (>1 year) after their transplantation between January 1959 and early 1962 following sublethal total-body irradiation without bone marrow. The case for a bone marrow component declined further when extended canine (33) and human (34, 35) kidney transplant survival was accomplished solely with drugs. Further human experience was particularly influential because the successes using combined azathioprine-prednisone therapy (36) exceeded manyfold what had been accomplished in dogs. The clonal deletion hypothesis that had been accepted as the basis of the classic tolerance models did not provide a tenable explanation for the success of whole organ transplantation (37-43).
In the rodent irradiation chimera preparations and with clinical bone marrow transplantation, the primary objective of cytoablation was immunosuppression. Eventually the belief took root (discussed recently [44, 45]) that an additional critically important effect of cytoablation or cytoreduction was to “make space” in the recipient microenvironment for the infused donor cells. However, this assumption was not supported by some of the earliest therapeutically relevant experiments showing that neither “space” nor even immunosuppression was required in adult mice for production of chimerism and tolerance under specific circumstances that were largely determined by histocompatibility variables. In 1959, Mariani, Martinez, Smith, and Good (46) reported that adult splenocytes could induce chimerism and tolerance to skin grafts across the sex-linked Eichwald-Silmser histocompatibility difference in immunologically competent mature unconditioned syngeneic mice. Brent and Gowland (47) described the same thing in selected allogeneic mouse strain combinations, using frequent inoculations of very large numbers of cells.
In addition, Martinez, Shapiro, and Good (48) demonstrated reciprocal tolerance induction of members of mixed circulatory parabiotic mouse pairs—more or less easily when the joined animals had weak and strong histocompatibility differences, respectively. The parabiotic animals were mixed chimeras, mimicking the effects of placental cross-circulation described by Owen in freemartin cattle (49), that included the acceptance of reciprocal skin grafts (50). The findings of Martinez, Shapiro, and Good (48) defined a principle that presaged the GVHD resistance of mixed chimerism in the total-lymphoid irradiation models of Slavin and Strober (51, 52) and the experiments of Ildstad and Sachs (53-56). A more recent analogy to the archival parabiotic experiments has been provided by the mouse orthotopic hepatic transplant model of Qian et al. (4) in which the liver allograft, which is spontaneously accepted with most strain combinations, has had the uncanny resemblance of a tolerogenic parabiotic partner to its chimeric and reciprocally tolerogenic recipient.
Although leukocyte chimerism seemingly had been proved by 1960 to be unnecessary for whole organ transplantation, the adjuvant use of white cells was never far from the consciousness of transplant surgeons. In a 1964 text, based on a series of successful renal transplantations, attention was drawn to “… current research in many laboratories which is directed toward achieving enhancement by inoculating the recipient with [donor] spleen, liver, or peripheral white cells” (38). The unifying idea that donor leukocytes within tissue grafts could do the same thing was advanced as early as 1970 by Monaco and Wood who wrote: “Various organs may contain variable numbers of mobilizable lymphoid cells which may constitute a significant antigenic innoculum. It is possible that treatment with ALS may facilitate induction of tolerance by the contained lymphoid cells, thus permitting the withdrawal of ALS without the onset of rejection” (57).
The literature of the last 30 years describing attempts to induce tolerance in organ recipients—using live or dead cells of all kinds and antigen extracts—is too vast and confusing to discuss here. However, the independent lines of inquiry by Monaco and Calne into the meaning of organ transplant tolerance deserve specific comment because they are directly relevant to our studies of low-level chimerism reported herein and elsewhere. While only suspecting that infused donor bone marrow leukocytes could suvive in small numbers for long periods and serve as the “veto cells” (58-60) the tolerogenicity of which had been defined by Miller (61-63), Monaco, Wood, and their colleagues have contended since 1966 that tissue and organ recipients could benefit from the antigen load of adjuvant live donor leukocytes. In most of their animal experiments, cryopreserved cells were given a few days to 3 weeks after the primary allograft, under immunosuppression with ALS or ALG during the intervening interval. Tolerance was demonstrated intially with skin grafting in mouse F1 offspring → parent experiments that precluded a GVHD risk (64) and then in non-F1 models (57, 65-67). After testing the bone marrow-ALS strategy for kidney transplantation in dogs (67, 68), they extended it to a human cadaveric renal case (69) under conventional cocktail immunosuppression (including ALG) that defined what has been called the Monaco model.
In subhuman primate variations on the Monaco bone marrow model, Thomas et al. were able to produce tolerance to kidney allografts (70), observed nests of donor leukocytes on the surface of the transplanted kidneys (71), and found evidence of veto cells (72) similar to those seen by Maki in mice (58, 59). Extensive formal trials in renal (73-75) and liver transplantation (76) were recently reported from the University of Alabama and England, respectively. No adverse effects were attributable to the bone marrow, and in the renal trials there may have been a clinical benefit. Barber et al. (74) detected evidence of donor DNA in the blood of some of their nonmarrow control recipients with polymerase chain reaction (PCR) probes, a finding suspected at the time to be an artefact.
The significance of this recently summarized massive body of work (77), most of it using bone marrow for the innoculum, could not be fully appreciated until 1992 when it was discovered that the bone marrow–derived “passenger leukocytes,” that are an important component of all organs, migrated after human organ transplantation and survived ubiquitously in recipient tissues for years or decades (1,2, 78-80). With the new information, it was realized that, except for its delayed timing, the strategy of the “Monaco models” was an iatrogenic amplification of a natural posttransplant event, culminating in microchimerism. In addition, the linkage was evident between organ allograft acceptance, the tolerance of clinical bone marrow transplantation, and the originally described acquired tolerance of Billingham, Brent, and Medawar. All were variations of the same principle.
We have proposed that the interaction, each with the other, of the 2 coexisting cell populations after either isolated or leukocyte-augmented organ transplantation is the fundamental explanation of organ allograft acceptance and of transplantation tolerance generally (1-5, 78-82). A similar reciprocal reaction hypothesis to explain acquired tolerance after splenocyte and bone marrow transplantation was advanced by Simonsen 35 years ago (83, 84) and supported by Michie, Zeiss, and Woodruff (85). The idea faded when it could not be proved. However, the rapidity of its abandonment may have reflected opposition to the implication that transplantation tolerance was an active process, not the thymic clonal deletion that had become the hardening concensus explanation in the early 1960s for transplantation tolerance. In addition, the substrate for a two-way immune interaction in the context of organ (as opposed to bone marrow or splenocyte) transplantation was not recognized to be present until the discovery of spontaneous chimerism 30 years later.
The fully allogeneic LEW → BN rat strain combination used in the experiments reported here had unusual advantages for examination of the HVG and graft-versus-host (GVH) components of the “2-way paradigm” and of the effect of expanding the GVH limb. First, the distinction of donor from recipient leukocytes in tissues and blood could be made with precision because of the availability of the L-21–6 monoclonal antibody that densely stains class II+ cells of almost all rat strains, including LEW, but not those from BN rats (3, 10-12). In addition, the BN rat is highly susceptible to GVHD (10, 15), allowing this usually “invisible” limb of the two-way immune reaction to be readily exposed for investigation. Finally, the HVG (rejection) limb in both strain directions is weak enough to allow the induction of tolerance to at least one kind of whole organ allograft of each strain after transplantation to recipients of the other strain, using either a short course of induction immunosuppression (3, 11, 86, 87) or, in the case of BN → LEW liver replacement, no treatment at all (7, 88).
The outcome of the LEW-BN experiments herein reported provided strong support for the two-way paradigm (82) as well as general guidelines for its therapeutic exploitation. The first question asked was one of safety. As previously reported in the LEW → BN recipient (10, 14, 15), the risk of clinical GVHD from intestinal transplantation under either an abbreviated or continuous course of tacrolimus was overwhelming, similar to that in the parent → offspring F1 hybrid (defenseless recipient) experiments of Monchik and Russell (89). In contrast, GVHD was never seen after liver, kidney, or heart transplantation. Cell suspensions of splenocytes and lymph node leukocytes behaved like the intestine, invariably causing GVHD that was usually fatal, while the same dose of easily engrafted bone marrow never did. Thymus leukocytes and the much smaller doses of blood leukocytes did not engraft.
The discontinuance of immunosuppression after 4 weeks in the recipients of intestine, or in animals given lymph node and spleen suspensions, did not ameliorate the lethal course of the GVHD, which was highly associated with the florid persistence of donor leukocytes in the recipient tissues. Under the same treatment conditions, the donor cells dwindled but were still easily detectable at 100 days in the liver and bone marrow recipients. The chimeric cells could no longer be detected at 100 days in animals given hearts. These observations confirmed those in earlier reported experiments in which liver transplant–induced chimerism was documented out to 300 days (3), whereas the chimerism induced by hearts had already reached a very low but still detectable level by the end of the first 30 postoperative days (90). More sensitive probes were not available to determine trace chimerism in the heart recipients of the present report at the time of challenge transplantation at 100 days.
Tolerogenicity of the various cell and organ grafts could not be conclusively determined in the presence of clinical GVHD. However, the liver, bone marrow, and heart defined in that order of completeness a spectrum of ultimately drug-free self tolerance, as well as tolerance to subsequently transplanted donor strain organs. The tolerance was strongly associated with tissue microchimerism, which was poorest after priming with hearts. Although the cardiac allografts were unquestionably tolerogenic, the development in them of chronic rejection after drug discontinuance and the same findings in the subsequent challenge hearts showed how incomplete the tolerance was. However, even this suboptimal immunologic status allowed not only acceptance but long-term rejection free maintenance of challenge livers, presumably because of the heavy boost of donor-strain leukocytes brought in by the test liver. This assumption has support from elegant mouse experiments by Smith et al. (91) in which the allograft combination was bone marrow and skin. The resulting chimerism in the mouse skin grafts originated from both donor sources.
The liver, bone marrow, and heart were tolerogenic in that order of potency under the circumstances of our experiments, but the inability to quantitate the leukocyte dose of whole organ grafts precluded sweeping conclusions about the role of either dose or quality of the passenger leukocyte lineages. Because Kupffer cells alone are 15-20% as numerous as hepatocytes in the liver and contribute about 2.5% of the liver’s cellular protein (compared with 15% by the hepatocytes) (92), the load of white cells contained in a 5 g liver used for a 250 g recipient would be huge (an estimated 100 mg) compared with that in a cardiac allograft, and substantially more by weight than that used in the bone marrow experiments.
When the dose factor was controlled, as was possible in a comparison among the 4 cell suspensions, the results strongly supported the long-held contention of Wood and Monaco (57, 67, 93) that bone marrow would be a potently tolerogenic cell suspension for clinical tolerance induction in whole organ recipients. The present experiments also showed that the bone marrow is the most free of GVHD, being incomparably safer than spleen or lymph node cells. Acquisition of this kind of information depends upon testing in GVHD-prone models like the one used for our experiments. A GVHD/tolerogenicity spectrum of different cell sources very similar to that in our rat studies was observed 35 years ago by Billingham and Brent (21) using several mouse strain combinations in their neonatal tolerance model. By implication, the striking differences in the GVHD/tolerance outcome with the various leukocyte suspensions was a function of their lineage profile. The numerous immature cells of undetermined lineage in LEW bone marrow (Fig. 2) resembled those studied by Lu et al. (94) in the mouse liver, and shown by them to include precursor dendritic cells that we (1-5, 95, 96) and others (97) have postulated to present donor antigen in a tolerogenic context, and to be critical for peripheral engraftment and persistence of tolerance maintaining microchimeric populations. Lu and Thomson et al. have shown that such cells are exported from the transplanted liver and establish ubiquitous cellular oases consisting of precursor (and presumably stem) cells of mixed donor and recipient phenotype (98, 99). Such observations as well as the results of the present study have reduced the distinction of bone marrow versus liver leukocyte source to a largely semantic one.
In chimeric recipient tissues, over-representation of donor T lymphocytes was associated with the undesirable result of GVHD. However, because the benign chimerism following the highly tolerogenic liver and bone marrow allotransplantations also had a generous T cell component, we suspect that the engraftment as well as the tolerogenic processes are complex, beyond the independent capability of any single lineage. The ineffectiveness of blood was undoubtedly due to the small dose of leukocytes infused (estimated 2.5–3×107), which was only 1/10 that of the cell suspensions. The importance of doseage with all of the cell suspensions was demonstrated by Billingham and Silvers (100) who confirmed Billingham and Brent’S (18) original observations that blood leukocytes in sufficient quantity are easily engrafted, and can be tolerogenic or cause GVHD. The same thing with highly purified blood leukocytes was emphasized by DeFazio et a1. (101), who also showed the ability of these cells to sensitize (102) as noted in our nonimmunosuppressed rats. However, the perplexing inability to transplant thymus cell suspensions suggested that even the initial step of engraftment is dependent on an appropriate multilineage mix from which some essential ingredient was missing in the T cell-dominated thymic leukocyte suspension. The difficulty of engrafting adult thymocytes was first described in mice (21, 100), but not with all strain combinations (103).
Beyond its relevance to Monaco’s research, the two-way paradigm allows reexamination of the literature on the inherent tolerogenicity of whole organs, much of which can be traced back to the 1969 report by Calne et al. (104). It was already well known by then that canine liver allografts could self-induce tolerance during a 4-month postoperative course of azathioprine (105), and that this occurred even more frequently in untreated outbred pigs (106-110), many of which passed through spontaneously resolving rejection crises (109, 111, 112). First in pigs (104) and then in rodents (88, 113, 114), Calne, Zimmermann, and Kamada—and subsequently others (115, 116)—showed that the tolerization extended to other donor organs transplanted at the same time or later. Caine’s hypothesis that soluble MHC class I antigen secreted by the hepatocytes was responsible (104, 113, 117-120) was weakened when Corry et al. (121) and Russell et al. (122) showed that mouse heart and kidney allografts were also tolerogenic, but with weaker MHC disparities. The results reported herein leave little doubt that organ tolerogenicity is not liver-specific, but rather an extreme example of a phenomenon based on donor leukocyte chimerism that is common to all tissues and organs.
How the miniscule population (including stem cells) of chimeric donor leukocytes is able to be integrated and survive within the dominant recipient immune system has not been resolved despite detailed study (4, 94-96, 98, 99, 123-126). In 1992, Calne (127) raised the possibility that a “special type of self-limiting Kupffer cell graft versus host reaction causes T cell decloning of the recipient” and asked “could a similar effect be produced with isolated Kupffer cells or other phagocytes? To be active, must they reside in live sinusoids, or could they perform as well elsewhere?” In an additional modification of the original Cambridge hypothesis that further accommodated the recent chimerism discoveries, but still in the context of a liver-specific phenomenon (128, 129), Calne et al. have continued to assign a role to soluble hepatocyte-secreted class I antigen as a critical cofactor with out which engraftment and persistence of the donor leukcytes cannot occur. There has been little direct experimental support for this position in whole animals. Although an immunosuppressive effect of serum was ascribed by Kamada et al. (113) to soluble class I antigens, purified antigens in the subsequent studies have had minimal (119) or no immunosuppressive or tolerogenic action (130, 131). Finally, results from studies in mice (4) including those with “knocked-out” class I genes (132) have further eroded the hypothesis. Nevertheless, a role of soluble class I antigens in tolerance cannot easily be dismissed, largely because of results from in vitro studies (133) suggesting the modulation by soluble antigen of cell-mediated cytotoxicity.
In spite of these reservations, Calne’s ideas remain collectively powerful as well as relevant to all organ allografts if growth factors rather than soluble class I antigens are envisioned to be facilitators of chimerism. This concept could explain many enigmatic observations, such as the greater ease noted by Liegeois et al. (134) of engrafting bone marrow in mice in conjunction with donor skin compared with bone marrow alone, a collaboration termed by them “reciprocal graft enhancement.” Takahashi et al. (59) have shown an increased tolerogenicity of bone marrow that was cultured and IL-2 and IL-3 prior to administration. Many growth factors are cytokines, including most of those discovered during research on liver regeneration (135). Although the greatest sources of growth factors are leukocytes of various lineages (136-138), granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) are only two examples of diverse-action candidates known to be secreted by hepatocytes (139, 140) as well as by parenchymal cells of other organs.
Growth factor therapy is beyond the pilot phase in the bone marrow transplant field for the promotion of alloengraftment (141). Further efforts will be facilitated by the rapidly expanding discovery and availability of recombinant growth factors, most of which have multiple physiologic actions (135). With recognition of the common basis for bone marrow transplantation and organ acceptance (chimerism), therapy with these molecules could become an adjunct to, or even a substitute for, the leukocyte augmentation in whole organ recipients currently under trial (142). For example, we suspect that increased chimerism explains the significant improvement in BN → LEW heart allograft survival described by Foster et al. in animals treated postoperatively with GCSF (143). Conversely, Monaco et al. (144) have described improved tolerance induction using donor bone marrow pretreated with GM-CSF.
The foreoging discussion concerns central issues of chimerism augmentation in clinical tolerance induction trials such as we are conducting (142). However, we have frequently emphasized (1, 2, 5, 82, 142) that chimerism is in no sense a substitute for the immunosuppression upon which the donor leukocyte engraftment and the eventual stability of chimerism depends—especially if the MHC barrier is a difficult one or if the chimeric population is small. In humans, the dividend of stable chimerism and its corollary of drug-free tolerance are expected to take years rather than the days or weeks of our rodent experiments. The operationally tolerant state can not be identified by current tests, including cell-mediated lymphocytotoxicity (CML), any more accurately in humans (80) than in rodents (4, 125). The MLR was always intact in our bone marrow-conditioned rat recipients of the present study, which accepted heart and liver allografts in every experiment.
Previous investigators have used the term “split tolerance” to describe the dichotomy between the in vitro and in vivo results (125, 145). The subtle changes in the in vivo immunologic repertoire of our bone marrow–primed rat recipients that permmitted challenge hearts to survive did not prevent histopathologically verified chronic rejection of the cardiac grafts. However, the rejection of livers was self-resolving, as has been observed many times before with a variety of organs in small and large animals with and without induction immunosuppression—exemplified by the original pig liver studies (104, 109, 111, 112) and most completely in studies of the exceptionally valuable mouse liver transplantation model (4). These events are compatible with the view that the tolerance induction is an inherently active rather than deletional process (3, 5, 58-60, 81, 82).
Finally, the characteristic dwindling of the chimeric cell population following whole organ or leukocyte transplantation deserves special comment. This was accurately described by Liegeois (a former fellow of Monaco), Charriere, and Brennan (146) after bone marrow infusion in mice—to a low level after 5 months, for which they coined the term “microchimerism.” The association of loss of tolerance and chronic rejection with further decline of these cells could be prevented completely in our rat heart recipients, by continuation of tacrolimus. Presumably this was not accomplished merely by retention of an inadequate number of residual chimeric cells, but also by allowing new ones to be generated. The process of peripheral donor leukocyte cell renewal demonstrated by Lu and Thomson et al. (98,99) is the postulated mechanism. No matter what means of tolerance induction is used, or the level of resulting chimerism, the hazard of premature discontinuance of therapy in clinical practice is self-evident.
Footnotes
This work was supported by Research Grants from the Veterans Administration and by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbrevations: BN, Brown-Norway; GVH, graft-versus-host; GVHD, graft-versus-host-disease; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony stimulating factor; HVG, host-versus-graft; LEW, Lewis; MLR, mixed lymphocyte response; McAb, monoclonal antibody.
References
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 3.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 4.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B, Lee S. Microvascular surgical techniques in research, with special reference to renal transplantation in the rat. Surgery. 1965;58:904. [Google Scholar]
- 7.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 8.Kamada N, Calne R. Orthotopic liver transplantation in the rat: technique using cuff for portal vein anastomposia and biliary drainage. Transplantation. 1979;28:47. [PubMed] [Google Scholar]
- 9.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;7:225. [PubMed] [Google Scholar]
- 10.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murase N, Demetria AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 12.Demetris AJ, Qian S, Sun H, et al. Early events in liver allograft rejection: delineation of sites simultaneous intragraft and recipient lymphoid tissue sensitzation. Am J Pathol. 1991;138:609. [PMC free article] [PubMed] [Google Scholar]
- 13.Gill TJ, Kunz HW, Misra DN, Hassett ALC. The major histocompatibility complex of the rat. Transplantation. 1987;43:773. [PubMed] [Google Scholar]
- 14.Murase N, Demetris AJ, Woo J, et al. Lymphocyte traffice and graft-versus-host disease after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246. [PMC free article] [PubMed] [Google Scholar]
- 15.Tanabe M, Murase N, Demetris AJ, et al. The influence of donor and recipient strains in isolated small bowel transplantation rats. Transplant Proc. 1994;26:3733. [PMC free article] [PubMed] [Google Scholar]
- 16.Billingham ME. Some recent advances in cardiac pathology. Hum Pathol. 1979;10:367. doi: 10.1016/s0046-8177(79)80043-x. [DOI] [PubMed] [Google Scholar]
- 17.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 18.Billingham R, Brent L, Medawar P. Quantitative studies on tissue transplantation immunityz: III. Actively acquired tolerance. Philos Trans R Soc Lond (Biol) 1956;239:357. [Google Scholar]
- 19.Billingham R, Brent L. A simple method for inducing tolerance of skin homografts in mice. Transplant Bull. 1957;4:67. [PubMed] [Google Scholar]
- 20.Simonsen M. The impact on the developing embryo and newborn animal of adult homologous cells. Acta Pathol Microbiol Scand. 1957;40:480. [PubMed] [Google Scholar]
- 21.Billingham R, Brent L. Quantitative studies on transplantation immunity: IV. Induction of tolerance in newborn mice and studies on the phenomenon of runt disease. Philos Trans R Soc Lond (Biol) 1959;242:439. [Google Scholar]
- 22.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15:1023. [PubMed] [Google Scholar]
- 23.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 24.Bach FH. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet. 1968;2:1364. doi: 10.1016/s0140-6736(68)92672-x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas ED. Allogeneic marrow grafting—a story of man and dog. In: Terasaki PI, editor. History of transplantation: thirty-five recollections. Vol. 379 Los Angeles: UCLA; 1991. [Google Scholar]
- 26.Mannick JA, Lochte HL, Ashley CA, Thomas ED, Ferrebee JW. A functioning kidney homotransplant in the dog. Surgery. 1959;46:821. [PubMed] [Google Scholar]
- 27.Rapaport FT, Bachvaroff RJ, Mollen N, Hirasawa H, Asano T, Ferrebee JW. Induction of unresponsiveness to major transplantable organs in adult mammals. Ann Surg. 1979;190:461. doi: 10.1097/00000658-197910000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hume DM, Jackson BT, Zukoski CF, Lee HM, Kauffman HM, Egdahl RH. The homotransplantation of kidneys and of fetal liver and spleen after total body irradiation. Ann Surg. 1960;152:354. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Butz GW, Jr, Brock DR, Linman JT, Moss WT. Canine liver homotransplants: the effect of host and graft irradiation. Arch Surg. 1962;85:460. doi: 10.1001/archsurg.1962.01310030108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray JE, Merrill JP, Dammin GJ, et al. Study of transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272. [PubMed] [Google Scholar]
- 31.Hamburger J, Vaysse J, Crosnier J, Auvert J, Lalanne CL, Hopper J., Jr Renal homotransplantation in man after radiation of the recipient. Am J Med. 1962;32:854. doi: 10.1016/0002-9343(62)90032-3. [DOI] [PubMed] [Google Scholar]
- 32.Kuss R, Legrain M, Mathe G, Nedey R, Camey M. Homologous human kidney transplantation: experience with six patients. Postgrad Med J. 1962;38:528. doi: 10.1136/pgmj.38.443.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calne RY. Inhibition of the rejection of renal homografts in dogs with purine analogues. Transplant bull. 1961;28:445. [PubMed] [Google Scholar]
- 34.Goodwin WE, Kaufman JJ, Mims MM, et al. Human renal homotransplantation: I Clinical experiences with six cases of renal homotransplantation. J Urology. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 35.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 36.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homogratt tolerance. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 37.Murray JE, Sheil AGR, Moseley R, Knoght PR, McGavic JD, Dammin GJ. Analysis of mechanism of immunosuppressive drugs in renal homotransplantation. Ann Surg. 1964;160:449. doi: 10.1097/00000658-196409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starzl TE. Experience in renal transplantation. Vol. 164 Philadelphia: Saunders; 1964. Host-graft adaptation. [Google Scholar]
- 39.Medawar PB. Transplantation of tissues and organa: introduction. Br Med Bull. 1965;21:97. [Google Scholar]
- 40.Nossal GJV. Immunologic tolerance. In: Rapaport FT, Dausset J, editors. Human transplantation. Vol. 643 New York: Grune & Stratton; 1968. [Google Scholar]
- 41.Starzl TE. Experience in hepatic transplantation. Vol. 226 Philadelphia: Saunders; 1969. Efforts to mitigate or prevent rejection. [Google Scholar]
- 42.Levey R. Immunological tolerance and enhancement: a common mechanism. Transplant Proc. 1971;3:41. [PubMed] [Google Scholar]
- 43.Medawar PB. Tolerance reconsidered—A critical survey. Transplant Proc. 1973;5:7. [PubMed] [Google Scholar]
- 44.Stewart FM, Crittenden RE, Lowry PA, Pearson-White S, Quesenberry PJ. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566. [PubMed] [Google Scholar]
- 45.Harrison DE. Competitive repopulation in unirradiated normal recipients. Blood. 1993;81:2473. [PubMed] [Google Scholar]
- 46.Mariani T, Martinez C, Smith JM, Good RA. Induction of immunological tolerance to male skin isografts in female mice subsequent to neonatal period. Proc Soc Exp Biol Med. 1959;101:596. doi: 10.3181/00379727-101-25030. [DOI] [PubMed] [Google Scholar]
- 47.Brent L, Gowland G. Induction of tolerance of skin homografts in immunologically competent mice. Nature. 1962;196:1298. doi: 10.1038/1961298a0. [DOI] [PubMed] [Google Scholar]
- 48.Martinez C, Shapiro F, Good RA. Essential duration of parabiosis and development of tolerance to skin homografts in mic. Proc Soc Exp Bioi Med. 1960;104:256. doi: 10.3181/00379727-104-25798. [DOI] [PubMed] [Google Scholar]
- 49.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 50.Anderson D, Billingham RE, Lampkin GH, Medawar PB. Tolerance of homografts, twin diagnosis, and the freemartin condition in cattle. Heredity. 1952;6:201. [Google Scholar]
- 51.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slavin S, Reitz B, Bieber CP, Kaplan HS, Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation (TLI): permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978;147:700. doi: 10.1084/jem.147.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 54.Ildstad ST, Wren SM, Sachs DH. In vivo and in vitro characterization of specific hyporeactivity to skin xenografts in mixed xenogeneically reconstituted mice (B10 + F344 rat → B10) J Exp Med. 1984;160:1820. doi: 10.1084/jem.160.6.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ildstad S, Wren SM, Bluestone JA, Barbieri SA, Stephany D, Sacha DH. Effect of selective T cell depletion of host and/or donor bone marrow on lymphopoietic repopulation, tolerance, and graft versus host disease in mixed allogeneic chimeras. J Immunol. 1986;136:28. [PubMed] [Google Scholar]
- 56.Sykes M, Sachs DH. Mixed allogeneIc chimerism as an approach to transplantation tolerance. Immunol Today. 1988;9:23. doi: 10.1016/0167-5699(88)91352-7. [DOI] [PubMed] [Google Scholar]
- 57.Monaco AP, Wood ML. Studies on heterologous antilymphocyte serum in mice: VII. Optimal cellular antigen for induction of immunologic tolerance with antilymphocyte serum. Transplant Proc. 1970;2:489. [PubMed] [Google Scholar]
- 58.Maki T, Gottschalk R, Wood ML, Monaco AP. Specific unresponsiveness to skin allografts in antilymphocyte serum–treated, marrow-injected mice: participation of donor marrow–derived suppressor T cells. J Immunol. 1981;127:1433. [PubMed] [Google Scholar]
- 59.Takahashi T, Mafune K, Maki T. Cloning of self-major histocompatibility complex antigen-specific suppressor cells from adult bone marrow. J Exp Med. 1990;172:901. doi: 10.1084/jem.172.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood ML, Orosz CG, Gottschalk R, Monaco AP. The effect injection of donor bone marrow on the frequency of donor-reactive CTL in antilymphocyte serum–treated, grafted mice. Transplantation. 1992;54:665. doi: 10.1097/00007890-199210000-00020. [DOI] [PubMed] [Google Scholar]
- 61.Muraoka S, Miller RG. Cells in bone marrow and in T cell colonies grown from bone marrow can suppress generation cytotoxic T lymphocytes directed against self antigens. J Exp Med. 1980;152:54. doi: 10.1084/jem.152.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller RG. An immunological suppressor cell inactivating cytotoxic T lymphocyte precursors recognizing it. Nature. 1980;152:54. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- 63.Martin DR, Miller RG. In vivo administration of histoincompatible lymphocytes leads to rapid functional deletion of cytotoxic T lymphocyte precursors. J Exp Med. 1989;170:679. doi: 10.1084/jem.170.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monaco AP, Wood ML, Russell PS. Studies on heterologous antilymphocyte serum in mice: III. Immunologic tolerance and chimerism produced across the H-2 locus with adult thymectomy and antilymphocyte serum. Ann NY Acad Sci. 1966;129:190. [Google Scholar]
- 65.Wood ML, Monaco AP, Gozzo JJ, Liegeois A. Use of homozygous allogeneic bone marrow for induction of tolerance with antilymphocyte serum: dose and timing. Transplant Proc. 1971;3:676. [PubMed] [Google Scholar]
- 66.Wood ML, Monaco AP. The effect of timing of skin grafts on subsequent survival in ALS-treated, marrow-infused mice. Transplantation. 1977;23:78. doi: 10.1097/00007890-197701000-00014. [DOI] [PubMed] [Google Scholar]
- 67.Monaco AP. Post transplantation donor-specific bone marrow transfusion in polyclonal ALS-treated patients: the optimal cellular antigen for induction of unresponsiveness to organ allografts. Transplant Proc. 1988;20:1207. [PubMed] [Google Scholar]
- 68.Caridis T, Liegeois A, Barrett I, Monaco AP. Enhanced survival of canine renal allografts of ALS-treated dogs given bone marrow. Transplant Proc. 1973;5:671. [PubMed] [Google Scholar]
- 69.Monaco AP, Clark AW, Wood ML, Sahyoun AI, Codish SD, Brown RW. Possible active enhancement of a human cadaver renal allograft with antilymphocyte serum (ALS) and donor bone marrow: case report of an initial attempt. Surgery. 1976;79:384. [PubMed] [Google Scholar]
- 70.Thomas J, Carver M, Foil B, Haisch C, Thomas F. Renal allograft tolerance induced with ATG and donor bone marrow in outbred rhesus monkeys. Transplantation. 1983;36:104. [PubMed] [Google Scholar]
- 71.Thomas J, Carver M, Cunningham P, Park K, Gonder J, Thomas F. Promotion of incompatible allograft acceptance in Rhesus monkeys given post-transplant antithymocyte globulin and donor bone marrow. Transplantation. 1987;43:332. doi: 10.1097/00007890-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Thomas JM, Carver FM, Kasten-Jolly J, et al. Further studies of veto activity in Rhesus monkey bone marrow in relation to allograft tolerance and chimerism. Transplantation. 1994;57:101. doi: 10.1097/00007890-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 73.Barber WH, Diethelm AG, Laskow DA, Deierhoi MH, Julian BA, Curtis JJ. Use of cryopreserved donor bone marrow in cadaver kidney allograft recipients. Transplantation. 1989;47:66. doi: 10.1097/00007890-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Barber WH, Mankin JA, Laskow DA, et al. Long-tenn results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 75.McDaniel DO, Naftilan J, Hulvey K, et al. Peripheral blood chimerism in renal allograft recipients transfused with donor bone marrow. Transplantation. 1994;57:852. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 76.Rolles K, Burrough AK, Davidson BR, Karatapanis S, Prentice HG, Hamon MD. Donor-specific bone marrow infusion after orthotopic liver transplantation. Lancet. 1994;343:263. doi: 10.1016/s0140-6736(94)91113-4. [DOI] [PubMed] [Google Scholar]
- 77.Monaco AP, Wood ML, Maki T, Gozzo J. The use of donor-specific bone marrow to induce specific unresponsiveness (tolerance) to tissue allografts. In: Ildstad ST, editor. Chimerism and tolerance. Vol. 99 R.G. Landes Company; Austin, Texas: 1994. [Google Scholar]
- 78.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Starzl TE, Demetris AJ, Trocco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and Type I Gaucher’s disease. N Engl J Med. 1993;328:745. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Starzl TE, Demetris AJ, Trocco M, et al. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and “tolerance” after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, editor. Rejection and tolerance. Vol. 25. Dordrecht, The Netherlands: Kluwer; 1994. p. 325. [Google Scholar]
- 82.Starzl TE, Demetris AJ. Transplantation milestones viewed with one- and two-way paradigms of tolerance. JAMA. 1995;273:876. doi: 10.1001/jama.273.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simonsen M. On the acquisition of tolerance by adult cells. Ann NY Acad of Science. 1960;87:382. doi: 10.1111/j.1749-6632.1960.tb23207.x. [DOI] [PubMed] [Google Scholar]
- 84.Simonsen M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349. [PubMed] [Google Scholar]
- 85.Michie D, Woodruff MFA, Zeiss IM. An investigation of immunological tolerance based on chimera analysis. Immunology. 1961;4:413. [PMC free article] [PubMed] [Google Scholar]
- 86.Morris RE, Wang J, Blum JR, et al. Immunosuppressive effects of the morpholinoethyl ester of mycophenolic acid (RS-61443) in rat and nonhuman primate recipients of heart allografts. Transplant Proc. 1991;23(suppl2):19. [PubMed] [Google Scholar]
- 87.Williams JW, Xiao F, Foster PF, et al. Immunosuppressive effects of leflunomide in a cardiac allograft model. Transplant Proc. 1993;25:745. [PubMed] [Google Scholar]
- 88.Zimmermann FA, Davies HS, Knoll PP, Gocke JM, Schmidt T. Orthotopic liver allografts in the rat. Transplantation. 1984;37:406. doi: 10.1097/00007890-198404000-00019. [DOI] [PubMed] [Google Scholar]
- 89.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 90.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells in grafts and host lymphoid and non-lymphoid tissues after liver and heart allotransplantation under short term immunosuppression. Lancet. 1992;339:1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith JP, Kasten-Jolly J, Field LJ, Thomas JM. Assessment of donor bone marrow cell-derived chimerism in transplantation tolerance using transgenic mice. Transplantation. 1994;58:324. [PubMed] [Google Scholar]
- 92.Kuiper J, Brouwer A, Knook DL, van Berkel TJC. Kupffer and sinusoidal endothelial cells. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, editors. The liver and biology and Pathobiology. 3. New York: Raven; 1994. p. 791. [Google Scholar]
- 93.Wood ML, Monaco AP, Gottschalk R. Characterization of spleen cells capable of inducing unresponsiveness in ALS-treated mice. Transplantation. 1991;51:208. doi: 10.1097/00007890-199101000-00034. [DOI] [PubMed] [Google Scholar]
- 94.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony–stimulating factor and their maturational development in the presence of type-l collagen. J Exp Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomson AW, Lu L, Subbotin V, et al. Propagation of dendritic cell progenitors from mouse liver and their in vivo migration to T-dependent areas of allogeneic lymphoid tissue. Transplant Proc. 1995;26:4084. [PubMed] [Google Scholar]
- 96.Thomson AW, Lu L, Subbotin VM, et al. In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Transplantation. 1995;59:544. doi: 10.1097/00007890-199502270-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steinman RM, Inaba K, Austyn JM. Donor-derived chimerism in recipients of organ transplants. Hepatology. 1993;17:1153. [PubMed] [Google Scholar]
- 98.Lu L, Rudert WA, Fu F, et al. Propagstion of cells expressing donor phenotype (MHC class I, II and Y-chromosome) from the bone marrow of murine liver allograft recipients in response to GM-CSF in vitro. Transplant Proc. 1995;27:191. [PMC free article] [PubMed] [Google Scholar]
- 99.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995:182. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Billingham RE, Silvers WK. Quantitative studies on the ability of cells of different origins to induce tolerance of skin homografts and cause runt disease in neonatal mice. J Exp Zoology. 1961;146:113. doi: 10.1002/jez.1401460202. [DOI] [PubMed] [Google Scholar]
- 101.De Fazio SR, Monaco AP, Gozzo JJ. Prolongation of skin allograft survival in antilymphocyte serum–treated mice by posttransplant administration of peripheral blood lymphocytes. Transplantation. 1989;49:163. doi: 10.1097/00007890-198907000-00043. [DOI] [PubMed] [Google Scholar]
- 102.De Fazio SR, Hartner WC, Monaco AP, Gozzo JJ. Effect of posttransplant administration of peripheral blood lymphocytes in skin-grafted mice treated with antilymphocyte serum or antilymphocyte serum plus bone marrow. Transplantation. 1987;44:70. doi: 10.1097/00007890-198707000-00016. [DOI] [PubMed] [Google Scholar]
- 103.Miller JFAP. Studies on mouse leukaemia: III. The fate of thymus homografts in immunologically tolerant mice. Br J Cancer. 1960;14:244. doi: 10.1038/bjc.1960.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 105.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 106.Cordier G, Garnier H, Clot JP, et al. La greffe de foie orthotopique chez le porc. Mem Acad Chir (Paris) 1966;92:799. [PubMed] [Google Scholar]
- 107.Peacock JH, Terblanche J. Orthotopic homotransplantation of the liver in the pig. In: Read AE, editor. The liver. Vol. 333 London: Butterworth; 1967. [Google Scholar]
- 108.Calne RY, White HJO, Yoffa DE, et al. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967;2:478. doi: 10.1136/bmj.2.5550.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calne RY, White HJO, Yoffa DE, et al. Prolonged survival of liver transplants in the pig. Br Med J. 1967;4:645. doi: 10.1136/bmj.4.5580.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Starzl TE. Rejection in unmodified animals. In: Starzl TE, editor. Experience in hepatic transplantation. Vol. 184 Philadelphia: Saunders; 1969. [Google Scholar]
- 111.Hunt AC. Pathology of liver transplantation in the pig. In: Read AE, editor. The liver. Vol. 337 London: Butterworth; 1967. [Google Scholar]
- 112.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. p. 427. [Google Scholar]
- 113.Kamada N, Brons G, Davies HffS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 114.Kamada N, Davies HffS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 115.Houssin D, Gigou M, Franco D, et al. Specific transplantation tolerance induced by spontaneously tolerated liver allograft in inbred strains of rat. Transplantation. 1980;29:418. doi: 10.1097/00007890-198005000-00015. [DOI] [PubMed] [Google Scholar]
- 116.Engelmann R, Ulrichs K, Thiede A, Muller-Ruchholtz W, Hanelmann A mechanism of tolerance in arterialized rat liver transplantation. Transplant Proc. 1983;15:729. [Google Scholar]
- 117.Davies HS, Kamada N, Roser BJ. Mechanisms of donor-specific unresponsiveness induced by liver grafting. Transplant Proc. 1983;15:831. [PubMed] [Google Scholar]
- 118.Singh PB, Brown RF, Roser BJ. Class I transplantation antigens in solution in body fluids and in the urine. J Exp Med. 1988;168:195. doi: 10.1084/jem.168.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;50:678. doi: 10.1097/00007890-199010000-00029. [DOI] [PubMed] [Google Scholar]
- 120.Davies HffS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 121.Corry RJ, Winn HJ, Russell PS. Primary vascularized allogratts of hearts in mice: the role of H-2D. H-2K and non-H-2 antigens 10 rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 122.Russell PS, Chase CM, Colvin RB, Plate JMD. Kidney transplants in mice: an analysis of the immune status of mice bearing long-term H-2 incompatible transplants. J Exp Med. 1978;147:1449. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sriwatanawongsa V, Davies HffS, Brons IGM, et al. Continued presence of donor leukocytes in recipients of liver grafts. Transplant Proc. 1993;25:371. [PubMed] [Google Scholar]
- 124.Sriwatanawongsa V, Davies HffS, Brons IGM, White DJG, Jamieson NY, Calne RY. Conditions required for donor passenger leukocytes in the induction of tolerance by rat liver grafts. Transplant Proc. 1993;25:2855. [PubMed] [Google Scholar]
- 125.Dahmen U, Qian S, Rao AS, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58:1. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thai NL, Qian RS, Fu F, et al. Mouse liver transplantation tolerance: the role of hepatocytes and non-parenchymal cells. Transplant Proc. 1995;27:509. [PMC free article] [PubMed] [Google Scholar]
- 127.Calne R. New Strategies in tolerance. Med Sci Res. 1991;19:231. [Google Scholar]
- 128.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 129.Calne RY, Watson CJE, Brons IGM, et al. Tolerance of porcine renal allografts induced by donor spleen cells and seven days’ treatment with cyclosporine. Transplantation. 1994;57:1433. [PubMed] [Google Scholar]
- 130.Spencer AC, Fabre JW. Bulk purification of a naturally occurring soluble form of RT1-A class I major histocompatibility complex antigens from DA rat liver, and studies of specific immunosuppression. Transplantation. 1987;44:141. doi: 10.1097/00007890-198707000-00028. [DOI] [PubMed] [Google Scholar]
- 131.Priestley CA, Dalchau R, Sawyer CJ, Fabre JW. A detailed analysis of the potential of water-soluble classical class I MHC molecules for the suppression of kidney allograft rejection and in vitro cytotoxic T cell responses. Transplantation. 1989;48:1031. doi: 10.1097/00007890-198912000-00028. [DOI] [PubMed] [Google Scholar]
- 132.Qian S, Fu F, Li Y, et al. Presensitization by skin grafting from MHC class I or class II deficient mice identifies class I antigens as inducers of allosensitization. Immunology. 1995;85:82. [PMC free article] [PubMed] [Google Scholar]
- 133.Buelow R, Burlingham W, Clayberger C. Immunomodulation by soluble HLA class I. Transplantation. doi: 10.1097/00007890-199503150-00001. in press. [DOI] [PubMed] [Google Scholar]
- 134.Liegeosis A, Escourrou J, Ouvre E, Charriere J. Microchimerism: A stable state of low-ratio proliferation of allogeneic bone marrow. Transplant Proc. 1977;9:273. [PubMed] [Google Scholar]
- 135.Francavilla A, Hagiya M, Porter KA, Polimeno L, Ihara I, Starzl TE. Augmenter of liver regeneration (ALR): its place in the universe of hepatic growth factors. Hepatology. 1994;20:747. [PubMed] [Google Scholar]
- 136.Arai K, Lee F, Kiyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 137.Nicola NA. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- 138.Groopman JE, Molina JM, Scadden DT. Hematopoietic growth factors: biology and clinical applications. N Engl J Med. 1989;321:1449. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- 139.Sakamoto T, Mabuchi A, Kuriya S, et al. Production of granulocyte-macrophage colony stimulating factor by adult murine parenchymal liver cells (hepatocytes) Regional Immuno1. 1990;3:260. [PubMed] [Google Scholar]
- 140.Sakamoto T, Saizawa T, Mabuchi A, Norose Y, Shoji T, Yokomuro K. The liver as a potential hematolymphoid organ examined from modifications occurring in the systemic and intrahepatic hematolymphoid system during liver regeneration after partial hepatectomy. Regional Immunol. 1992;4:1. [PubMed] [Google Scholar]
- 141.Rasko JEJ, Gough NM. Granulocyte-macrophage colony stimulating factor. In: Thomson AW, editor. The cytokine handbook. 2. Vol. 343 London: Academic; 1994. [Google Scholar]
- 142.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foster PF, Mital D, Sankary HN, et al. The use of granulocyte colony stimulating factor (G-CSF) following liver transplantation. Tranplantation. 1995;59 [PubMed] [Google Scholar]
- 144.Monaco AP, Wood ML, Gottschalk R, Seiler FR. Effect of granulocyte-macrophage colony stimulating factor on the induction of unresponsiveness by lymphoid cells. Transplantation. 1991;51:213. doi: 10.1097/00007890-199101000-00035. [DOI] [PubMed] [Google Scholar]
- 145.Kamada N. Experimental liver transplantation. Boca Raton, Fl: CRC; 1988. Systemic tolerance induced by liver transplantation; p. 67. [Google Scholar]
- 146.Liegeois A, Charreire J, Brennan LB. Allograft enhancement induced by bone marrow cells. Surg Forum. 1974;25:297. [PubMed] [Google Scholar]


