Abstract
Symptomatic heart failure is a complex clinical syndrome with a poor prognosis. Many efforts have been made to develop new therapeutic strategies to improve prognosis associated with heart failure. In this context, different stem cell populations for cardiac regenerative therapy have been examined recently. Here we discuss the potential strategies for using stem cells in cardiac regenerative therapy and the barriers that remain before an effective cell-based cardiac regenerative therapy can be employed clinically.
Introduction
Heart failure (HF) is a complex clinical syndrome resulting from structural or functional cardiac disorders, which impairs the ability of the ventricle to fill with or eject blood [1]. Leading manifestations are dyspnea and fluid retention.
In the United States, 4.9 million patients suffer from heart failure [2]. Approximately 80% of patients hospitalized with HF are 65 years and older [3].
A major cause of heart failure is coronary artery disease with myocardial infarction leading to a substantial loss of cardiomyocytes corresponding to the supply area of the affected vessel. Myocardial infarction and subsequent ventricular remodeling results not only in a decreased systolic function but also in an overall ventricular dilation, mitral valve dysfunction and the formation of a fibrous scar tissue or an aneurysm [4]. The facts that heart failure affects predominantly the elderly and that myocardial infarction involves not only a loss of cardiomyocytes but also post-infarct remodeling suggest that multiple non-cellular effects such as hemodynamic load, ventricular dimensions, and aging related myocardial damages should all be considered in the development of potential strategies for a cardiac regenerative therapy.
Approaches to cardiac regenerative therapy
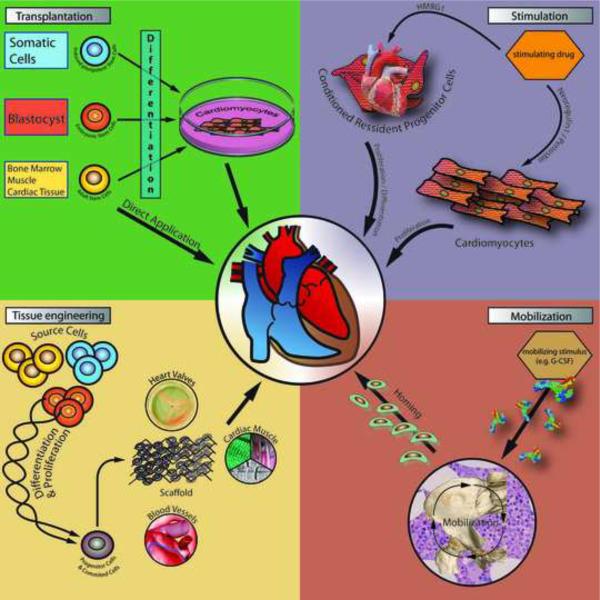
The need for an effective therapy for heart failure is underlined by the particularly poor prognosis of patients with this disease [4]. Given this fact, the promise that stem or progenitor cells may be useful for cardiac regenerative therapy has driven much of the basic and clinical research in the past decade. Currently, there are a variety of stem cell-based approaches being used for cardiac regenerative therapy [5, 6] (Figure 1). In this review, we address the most popular approaches that have been investigated in recent years.
Fig. 1.
The figure shows the mechanism of the most popular stem cell-based approaches for cardiac regenerative therapy.
Transplantation of Adult and Embryonic Stem Cells
The most direct, and conceptually straight-forward, mean of deploying stem or progenitor cells for cardiac regenerative therapy is to directly inject these cells into the injured heart. The cells that are destined for transplantation could be either injected immediately after harvest or expanded in vitro and subsequently differentiated into cardiac progenitor cells or cardiomyocytes before transplantation [7]. In addition, different methods of introducing stem/progenitor cells into the heart have also been actively examined. Depending on the clinical context, adult stem cells derived from circulation or from bone marrow harvest have been injected into coronary arteries or directly deposited within the myocardial wall using a catheter based or surgical approach.
We distinguish the three major cell types for transplantation based on the origin of these cells – adult stem cells, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs).
Adult stem cells can be found in different organs in the adult. They have been reported to transdifferentiate into different cell types (e.g. cardiomyocytes, smooth muscle cells, endothelial cells) in vitro and may retain the ability for self-renewal. The earliest studies on cell-based cardiac regeneration have employed skeletal myoblasts. These cells, which normally mediate regeneration of skeletal muscle, were initially shown to exhibit great success in regenerating the heart in animal models. The transplantation of myoblasts into infarcted hearts led to an improvement in cardiac function [8] however these cells were shown not to transdifferentiate into cardiomyocytes [9]. Nevertheless, the improvement of cardiac function after myoblast transplantation in animals eventually leads to the initiation of clinical trials using myoblasts for human therapy. The MAGIC trial, a randomized, placebo controlled and double blinded study, which uses myoblast transplantation during coronary artery bypass grafting, failed to show a significant improvement of left ventricular ejection fraction (LVEF) 6 months after myoblast transplantation [10]. Beside this disappointing clinical outcome regarding cardiac function, transplanted myoblasts were shown to induce cardiac arrhythmias that required all patients to be implanted with a intracardiac defibrillator [11].
Shortly after the initial myoblast studies, investigator focused on cells derived from a different source – the bone marrow. In 2001, Orlic and colleagues [12] reported that bone marrow stem (BMS) cells were able to regenerate the heart. In this rodent model, lineage negative mouse BMS cells were constitutively expressing the enhanced green fluorescent protein (eGFP) which serve as the tracer for cell identification. These cells were then injected into the peri-ischemic region of the heart of wild type mice, shortly after coronary ligation. They reported that newly formed myocardium occupied 68% of the infarcted portion of the ventricle 9 days after transplantation. Subsequently other investigators found potential confounding issues with this original study and were unable to support the conclusions reached [13–15]. Furthermore, other studies have reported that the described cardiac transdifferentiation from bone marrow cells may have resulted from cell fusion [16]. It is now becoming clear that BMS cell transplantation do not lead to significant cardiomyocyte transdifferentiation. Nevertheless, a growing list of clinical studies has now been performed to address whether BMS cell treatment may be beneficial in patients with recent or prior history of myocardial infarction. Table 1 gives a summary of these prospective, randomized, controlled, and double-blinded studies using BMS cells for cardiac repair. Overall, these studies found little to no improvement in the LVEF of patients with a history of myocardial infarction. A Meta analysis of nearly 1000 patients from multiple trials showed a modest LVEF gain of 3.66% without evidence for mortality benefit [17]. Given the disappointing results from the BMS cell studies, investigators have recently turned toward the employment of resident cardiac stem cells (CSC) for cardiac regenerative therapy. Three different adult cardiac stem cell populations have been studied recently using different surface markers. A clonogenic, multipotent, self-renewing population of c-kit+ Lin− cells were initially described by Beltrami et al [18] in the adult mouse and rat hearts. After transplantation into the border zone of an infarcted heart these cells reduced up to 22% of the infarct size. Four years later, the same investigators reported the identification of c-kit+ cells within the human adult heart, which differentiated into new cardiomyocytes after transplantation into rat or mouse hearts [19]. In addition to c-kit, Sca1+ cells have been found within the adult mouse heart [20]. These cells have also been reported to improve cardiac function after transplantation into the infarcted heart [21]. Furthermore, an Abcg2 expressing - Hoechst dye effluxing (e.g. Side Population) cells from the adult heart have also been reported to give rise to mature sarcomerized cardiomyocytes in vitro [22] within co-culture with neonatal cardiomyocytes they were show to generate spontaneous action potential profiles resembling ventricular cardiomyocyte [23]. So far, no resident cardiac stem cell population has been tested in clinical trials. The presence of these apparently distinct cardiac stem cell populations in the heart, an organ with little regenerative capacity, suggests that these putatively distinct populations are likely to have greater overlap with one another than previously suspected [24]. Further studies will clarify the biological relationships between these cell populations.
Table 1.
randomized, double blinded, controlled clinical trials for stem cell transplantation
| Trial | No. of patients | Treatment | Follow up (months) | Follow up (method) | Change in LVEF (%) (Treatment vs. control) | p-value |
|---|---|---|---|---|---|---|
| MAGIC [10] | 97 | myoblasts | 6 | ECHO | 3.4(−0.3; 12.4)/5.2(−4.4; 11) vs. 4.4(0.2; 7.3) | NS |
| REPAIR-AMI [57] | 204 | BMC | 4 | LV-Angio | 5.5±7.3 vs. 3.0±6.5 | <0.01 |
| Janssens [58] | 67 | BMC | 4 | MRI | 3.4±6.9 vs. 2.2±7.3 | NS |
| BOOST* [59, 60] | 60 | BMC | 6/60 | MRI | 6.7±6.5 vs. 0.7±8.1/−2.5±11.9 vs. −3.3±9.5 | 0.003/NS |
| Chen [61] | 69 | BMMC | 6 | LV-Angio | 18 vs 6 | 0.01 |
BMC= bone marrow stem cells
BMMC = bone marrow mesenchymal stem cells
LVEF = left ventricular ejection fraction
LV-Angio = left ventricular angiography
MRI = magnetic resonance imagin
NS = not significant
= not double blinded
Beyond adult stem cells, the use of embryonic stem (ES) cells in cardiac regenerative therapy has gained increasing attention in recent years. ES cells are derived from the inner cell mass of preimplantational blastocytes. There are a variety of published protocols available to differentiate ES cells into cardiac progenitor cells or cardiomyocytes. One major challenge in the use of these ES cell-derived cells for transplantation is the risk of teratomas formation at the transplantation site and beyond [24]. Currently, investigators are actively seeking ways to enrich ES cell derived cardiomyocytes [25] or cardiac progenitor cells [26] by identifying appropriate surface markers or by transgenic approaches whereby a reporter gene is driven by cardiac progenitor or cardiomyocyte-specific promoter (e.g. α myosin heavy chain). In animal models, the transplantation of ES cell derived cardiac progenitor cells or cardiomyocytes into infarcted hearts has led to improvement in cardiac function [27–29]. However, it is now clear that ES cell derived cardiomyocytes continues to exhibit fetal or, at best, neonatal phenotypes such that there is very little like hood that the small amount of ES cell-derived cardiomyocytes engrated in the infarcted heart is responsible for the functional improvement observed after transplantation [30]. Furthermore, the immunological barrier that hinders the long-term survival of transplanted graft will continue to be an issue with the use of ES cells. Indeed, in the study by van Laake et al, the observed functional benefit was transient and became absent at 12 weeks after transplantation [31]. These challenges in the use of ES cells for regenerative therapy have led investigators to turn to other cell sources that may be more compatible immunologically and easier to obtain than human ES cells.
The newest kid on the pluripotent stem cell block is induced pluripotent stem cells (iPSCs). This revolutionary technology to generate iPSCs from somatic cells was first reported by Takahashi and Yamanaka in 2006 [32]. By the transduction of four key transcription factors (Klf4, Oct4, Sox2 and c-Myc) in embryonic fibroblasts, these investigators were able to turn these apparently well differentiated cells into pluripotent ES cell-like cells. These cells have the advantage of unlimited self-renewal and are capable of three germ layer differentiation in vitro as well as in vivo contribution to chimeric mice. Furthermore, these cells presumably bypass the contentious ethical issues of embryo destruction as well as immunological rejection. Nevertheless significant obstacles will need to be overcome before iPSC derived cardiac progenitor cells/cardiomyocytes could be used for regenerative therapy. These obstacles include 1. the dependence on the use of genome integrating viruses for the reprogramming of somatic cells will need to be eliminated, 2. the biological differences between iPSCs and ES cells and their differentiated progenies will need to be clarified, 3. the logistics of deriving somatic tissue from each patient and the labor involved in making custom iPSCs for each patient followed by differentiation will unlikely be feasible for treating patients during the critical window of therapy, shortly after myocardial infarction but before ventricular remodeling sets in at 6 to 12 weeks [33]. Given these issues, one possible outcome from the pluripotent cell reprogramming work is the development of technology to directly program cardiac progenitor cells or cardiomyocytes from somatic cells. In fact, this strategy was used to successful generate pancreatic beta cells [34] from pancreatic exocrine cells or functional neurons from skin fibroblasts [35].
Mobilization of Bone Marrow-derived Cells into the Circulation
Circulating stem cell mobilization for cardiac regenerative therapy was centered on the premise that enhanced mobilization of BMS cells into the blood stream by administration of different cytokines may enhance cardiac injury healing, increase vasculogenesis, and/or directly transdifferentiate into cardiomyocytes when these cells accumulate in the area of myocardial infarction. Preclinical data were able to show a reduction in infarct size, improvement in LVEF as well as survival after cytokine treatment (G-CSF, SCF, EPO and PTH) in a myocardial infarction rodent model [36–38]. Encouraged by these positive animal study results coupled with a presumed relatively low health risk for patients, clinical studies using cytokine treatment in patients with acute myocardial infarction were conducted over the past decade (Table 2). Overall, the reported effects on cardiac function following cytokine treatment after myocardial infarction have been disappointing. A meta-analysis conducted by Zohlhöfer et al. [39] on 445 patients who received G-CSF as stem cell mobilizing treatment showed that G-CSF neither enhance the improvement of LVEF nor leads to a reduction in infarct size compared to the control group.
Table 2.
randomized, double blinded, controlled clinical trials for stem cell mobilisation
| Trial | No. of patients | Treatment | Follow up (months) | Follow up (method) | Change in LVEF (%) (Treatment vs. control) | p-value |
|---|---|---|---|---|---|---|
| REVIVAL-2 [62] | 114 | G-CSF | 6 | MRI | 0.5±3.8 vs. 2.0±4.9 | NS |
| STEMMI [63] | 78 | G-CSF | 6 | MRI | 8.5 vs. 8 | NS |
| G-CSF-STEMI [64] | 44 | G-CSF | 3 | MRI | 6.2±9.0 vs. 5.3±9.8 | NS |
| Ellis [65] | 18 | G-CSF | 1 | ECHO | 4.5±11.3/5.2±6.7 vs. 8.0±9.9 | NS |
| HEBE III*[66] | EPO | 1,5 | PVR | ongoing | -- |
not double blinded;
LVEF = left ventricular ejection fraction;
G-CSF = granulocyte colonie stimulating factor;
EPO = Erythropoietin;
MRI = magnetic resonance imaging;
ECHO = echocardiography;
PVR = planar radionuclide ventriculography
NS = not significant
In summary, mobilization of BMS cells showed no impact on cardiac function in patients with myocardial infarction and the role of BMS cells in cardiac injury repair require further investigation.
Stimulation of Endogenous Stem/Progenitor Cells
There is growing evidence that a limited capacity for cardiomyogenesis exists in the postnatal mammalian heart. Bergmann and colleagues [40] reported a low but measurable rate of new cardiomyocyte generation that declines exponentially with age. Taking advantage of the rise in atmospheric carbon-14 (C-14) level due to nuclear bomb tests during the cold-war era, which led to a rise in the level of C-14 integration into cellular DNA, they found an annual cardiomyocyte turnover of 1% at the age of 25, which decreases to 0.45% at the age of 75. The fact, that new cardiomyocyte generation occurs within the adult heart suggest that stimulation of a defined cell population with pharmacological agents may enhance this cardiomyocyte renewal process (Figure 1). While the mechanism of benefit has not been fully elucidated, high-mobility group box 1 (HMGB1) has been reported by Limana and collegues [41] to increase adult cardiac c-kit+ stem cell proliferation and improve cardiac function. Furthermore, they reported an enhanced infarcted wall thickness two and four weeks after infarction in the HMGB1 treated group compared to the control group. Similarly, Kohno et al. [42] reported an increased wall thickness of non-infarcted segments and a decreased wall thickness in infarcted segments after systemic blockade of HMGB1 by subcutaneous delivered antibodies in a rat myocardial infarction model. Further studies will be needed to determine whether these reported improvement in cardiomyocyte generation are a direct consequence of neocardiomyogenesis from resident cardiac stem cells or due to other effects such as post-injury cardiomyocyte protection as reported by Bock-Marquette et al [43].
As an alternative to cardiac stem/progenitor cell regulation, the stimulation of cell cycle re-entry of differentiated cardiomyocytes have been shown to induce the generation of new cardiomyocytes in the adult heart. Studies employing periostin or neuregulin1b (NRG1b) have provided a proof-of-principal that these strategies may be therapeutically viable. As reported by Bersell and colleagues [44] NRG1b interacts with its tyrosin kinase receptor ERB4 to induce specifically the proliferation of mononucleated but not binucleated cardiomyocytes. They further show that systemic injection of NRG1 in mice after myocardial infarction led to a reduction in infarct size and to an improvement of the LVEF compared to control treatment. Similar results were also obtained in studies where epicardial Gelfoam-loaded with periostin was administered [45]. It is anticipated that future large animal studies will help to clarify whether these benefits in rodent models can translate into similar benefits in species such as sheep, pig, or primates that are biologically more representative of the responses observed in humans.
Engineerd Cardiac Tissue for Regenerative Therapy
A promising approach to generate functional, living cardiac tissues such as myocardium [46], heart valves [47, 48] or blood vessels [49] is the deployment of tissue engineering technologies. The flexibility of tissue engineering approaches allows for the utilization of both adult and embryonic stem cell sources. In general, the desired cell type is induced to undergo proliferation and or cardiac differentiation in vitro. After sufficient differentiation/proliferation the harvested cell population is then seeded onto a biological or synthetical scaffold followed by an ex-vivo remodeling process in a bioreactor, which try to mimic physiological conditions (Figure 1). After tissue reorganization has occurred in response to the specific condition given, the engineered construct is then surgically implanted into the recipient heart. Since embryonic and neonatal heart tissues are more pliable and expandable, they have been utilized extensively in this field. Zimmermann et al [50] engineered cardiac tissue from rat neonatal heart cells and were able to show an improvement of cardiac function after implantation of these constructs in rats after myocardial infarction. Together with Kit Parker's group at Harvard University and Ken Chien's group at Massachusetts General Hospital, we demonstrated the feasibility of generating muscle thin film from ES cell-derived ventricular cardiac progenitor cells in vitro [51]. While the engineered muscle thin film beats spontaneously at a rate of 20 times per minute, a condition that reflects the embryonic phenotype of these in vitro derived cardiomyocytes, these cells are able to react to pacing stimulation at 0.5 or 1.0 Hz, demonstrating their cardiomyocyte functional properties. One major challenge that must be overcome for engineered heart muscle tissue to reach clinical stages is the critical need for vascularization to allow sufficient nutrition and oxygenation. The diffusion limited thickness of an engineered heart muscle sheet is between 50 and 100μm. Optimized culture conditions in a bioreactor allows for growth of up to 500μm [46]. The development of techniques to incorporate a sufficient microvasculature into the engineered heart muscle is essential for successful translation of heart muscle tissue engineering in cardiac regenerative therapy. In addition to engineering functional myocardium, recent studies have reported the successful construction of living heart valves using adult stem cells [52, 53]. Sutherland et al [54] reported the generation of a BMS cell-derived heart valve from a biodegradable scaffold. The engineered heart valve was implanted in the pulmonary position in sheep. After 8 months, the explanted heart valve showed an extracellular matrix organization consisting of three layers comparable to a native valve. Cebotari and colleagues [55] reported the first promising clinical results using a decellularized homograft valve implanted in the pulmonary position in two pediatric patients (ages 11 and 13). The valves were constructed with pulmonary valves from cadaveric sources and seeded with autologous endothelial progenitor cells to prevent immune-related graft destruction. These studies demonstrate the promise and feasibility of stem cell-based tissue engineering and we anticipate this approach to be widely accepted in the near future.
Barriers to effective cardiac regenerative therapy
While rapid progress has been made in recent years to obtain greater understanding of cardiovascular stem cell biology, significant challenges remain that must be overcome in order to translate these exciting findings into effective cardiac regenerative therapy. We present here the issues that we consider the most critical.
Number of cells
Myocardial infarction, the most common cause for heart failure, usually results in macroscopic loss of cardiomyocytes, often more than a billion cells each time [24]. This degree of cell loss and the ensuing replacement of the necrotic tissue by fibrous and collagen rich scar that contributes to no contractile force generation all lead to a decline in cardiac function. To achieve a meaningful functional recovery, a significant portion of this loss cardiomyocytes must be replaced. Hence, the scalability of stem cells to match the degree of cardiomyocyte loss after a myocardial infarction will require the deployment of creative solutions to solve this problem.
Survival after transplantation
After myocardial infarction the myocardium begins to undergo irreversible changes within 20 minutes of ischemia. The resulting myocardial necrosis leads to an inflammatory response that recruits granulocytes early followed by macrophages infiltration late into the area of myocardial infarction. Over time, this highly inflammatory tissue remodels to form a fibrous scar [24]. To achieve success with cell transplantation, the delivered cell must be able to survive in this highly hostile and inflammatory environment and engraft and expand within a collagen-rich and blood vessel-deficient scar tissue. LaFlamme et al [27] have employed a cocktail of protective factors to facilitate the engraftment and survival of human ES cell-derived cardiomyocytes following their introduction into a post infarct rodent heart. While the result from this study appears promising, the number of cells that have successfully engrafted remains low and further work will be needed to determine whether it is the inflammatory element or the nutrient/oxygen deprivation effects that is responsible for insufficient cell survival.
Integration with existing cardiomyocytes
Assuming the challenges of generating a sufficient number of cardiomyocytes and the survival/engraftment after transplantation issue can be overcome, the next barrier is a functional and electrophysiological integration as well as mechanical coupling of transplanted cells with the existing cardiomyocytes in the heart. Studies involving transgenic over-expression of gap junction proteins such as connexin 43 in transplanted myoblasts have shown successful coupling of transplanted cells with one another as well as with the host myocardium [56]. In this regard, since ES cells and iPS cell-derived cardiomyocytes express connexin 43, these cell types are likely to have the greatest potential to generate an electrically competent cardiomyocyte networks.
Conclusion
In recent years, a variety of strategies employing adult and embryonic stem cells for cardiac regenerative therapy have been examined in animal as well as patient studies. Currently there is no clear indication to which of these various strategies are most likely to reach clinic in the near future. While some strategies such as circulating stem cell mobilization will likely be out of favor given the disappointing clinical study results thus far. Other strategies, such as BMS transplantation or mesenchymal stem cell transplantation will likely reveal whether the mechanism of cell transplantation is paracrine factor mediated (i.e. short term), or from direct contribution by transplanted cells into vasculogenesis or ventricular remodeling. With the growing interest in IPS cells as the cell source for therapy and disease modeling, we will likely see an exponential growth in studies that incorporate these and other pluripotent stem cell population.
Acknowledgement
M. Krane was supported by a grant from the German Research Foundation (KR3770/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association . Heart Disease and Stroke Statistics–2005 Update. American Heart Association; Dallas, TX: 2005. [Google Scholar]
- 3.Masoudi FA, et al. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 4.Jessup M, et al. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 5.Karra R, et al. Multipotent stem cells in cardiac regenerative therapy. Regen Med. 2008;3:189–198. doi: 10.2217/17460751.3.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YH, et al. Cardiovascular Stem Cells in Regenerative Medicine: Ready for Prime Time? Drug Discov Today Ther Strateg. 2008;5:201–207. doi: 10.1016/j.ddstr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi BA, et al. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor DA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 9.Reinecke H, et al. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 10.Menasche P, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 11.Menasche P. Stem cell therapy for heart failure: are arrhythmias a real safety concern? Circulation. 2009;119:2735–40. doi: 10.1161/CIRCULATIONAHA.108.812693. [DOI] [PubMed] [Google Scholar]
- 12.Orlic D. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 13.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 14.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 15.Nygren JM, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Latif A, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.Bearzi C, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuura K, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin CM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Pfister O, et al. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res. 2008;103:825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laflamme MA, et al. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 25.Doss MX, et al. Global transcriptome analysis of murine embryonic stem cell-derived cardiomyocytes. Genome Biol. 2007;8:R56. doi: 10.1186/gb-2007-8-4-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 28.Caspi O, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 29.Klug MG, et al. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 31.van Laake LW, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, et al. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Hansson EM, et al. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlic D, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaruba MM, et al. Parathyroid hormone treatment after myocardial infarction promotes cardiac repair by enhanced neovascularization and cell survival. Cardiovasc Res. 2008;77:722–731. doi: 10.1093/cvr/cvm080. [DOI] [PubMed] [Google Scholar]
- 38.Westenbrink BD, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 39.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 40.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limana F, et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 42.Kohno T, et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81:565–573. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 43.Bock-Marquette I, et al. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 44.Bersell K, et al. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn B, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 46.Eschenhagen T, et al. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 47.Mendelson K. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–1819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krane M, et al. Bioengineering von Herzklappen. Kardiologie up2date. 2008;4:276–280. [Google Scholar]
- 49.Isenberg BC, et al. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann WH, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 51.Domian IJ, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoerstrup SP, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106:I143–I150. [PubMed] [Google Scholar]
- 53.Schmidt D, et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114:I125–I131. doi: 10.1161/CIRCULATIONAHA.105.001040. [DOI] [PubMed] [Google Scholar]
- 54.Sutherland FW, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 111:2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 55.Cebotari S, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114:I132–I137. doi: 10.1161/CIRCULATIONAHA.105.001065. [DOI] [PubMed] [Google Scholar]
- 56.Roell W, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 57.Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 58.Janssens S, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 59.Meyer GP, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 60.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 61.Chen SL, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 62.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 63.Ripa RS, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 64.Engelmann MG, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 65.Ellis SG, et al. Granulocyte colony stimulating factor in patients with large acute myocardial infarction: results of a pilot dose-escalation randomized trial. Am Heart J. 2006;152:e9–14. doi: 10.1016/j.ahj.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Belonje AM, et al. Effects of erythropoietin after an acute myocardial infarction: rationale and study design of a prospective, randomized, clinical trial (HEBE III) Am Heart J. 2008;155:817–822. doi: 10.1016/j.ahj.2007.12.036. [DOI] [PubMed] [Google Scholar]