Abstract
Serotonin 1B (5-HT1B) heteroreceptors on nucleus accumbens shell (NAcSh) projection neurons have been shown to enhance the voluntary consumption of alcohol by rats, presumably by modulating the activity of the mesolimbic reward pathway. The present study examined whether increasing 5-HT1B receptors expressed on NAcSh projection neurons via viral mediated gene transfer enhances ethanol consumption during the initiation or maintenance phase of drinking and alters the temporal pattern of drinking behavior. Animals received stereotaxic injections of viral vectors expressing either 5-HT1B receptor and green fluorescent protein or green fluorescent protein alone. Home cages equipped with a three-bottle (water, 6%, and 12% ethanol) lickometer system recorded animals’ drinking behavior continuously, capturing either initiation or maintenance drinking behavior patterns. Overexpression of 5-HT1B receptors during initiation increased consumption of 12% ethanol during both forced access and free choice consumption. There was a shift in drinking pattern for 6% ethanol with an increase in number of drinking bouts per day, although the total number of drinking bouts for 12% ethanol was not different. Finally, increased 5-HT1B expression induced more bouts with very high frequency licking from the ethanol bottle sippers. During the maintenance phase of drinking, there were no differences between groups in total volume of ethanol consumed; however, there was a shift toward drinking bouts of longer duration, especially for 12% ethanol. This suggests that during maintenance drinking, increased 5-HT1B receptors facilitate longer drinking bouts of more modest volumes. Taken together, these results indicate that 5-HT1B receptors expressed on NAcSh projection neurons facilitate ethanol drinking, with different effects during initiation and maintenance of ethanol drinking behavior.
Keywords: ethanol, maintenance, initiation, serotonin, viral-mediated gene expression
Introduction
Alcohol is a legal drug that is widely consumed in social contexts, yet one in 12 Americans (17.6 million) is either dependant on or abuses alcohol (Grant et al., 2004). In the United States, car accidents, medical complications, and lost earnings due to illness or premature death associated with alcohol cost almost 200 million dollars per year (Harwood, 2000). Previous reports suggest that environmental, genetic, and neuroplastic factors contribute to alcohol abuse and reinstatement of alcohol seeking behavior (Alen et al.,2008; Enoch, 2006; Hansson et al.,2006; Hansson et al.,2008; Prakash et al.,2008; Reich et al.,1998; Schroeder et al.,2008). The serotonergic system is thought to play an important role in the rewarding and reinforcing properties of alcohol consumption. For example, the nucleus accumbens (NAc) and ventral tegmental area (VTA) are key nodes in the brain’s reward circuitry (Leshner and Koob, 1999), and they receive dense projections containing serotonin (5-HT) and express several 5-HT receptors that have been implicated in reward mechanisms (Muller and Huston, 2006). Genetic studies have also found associations between the serotonergic system and alcoholism including the identification of gene polymorphisms for serotonin transporter (Mokrovic et al., 2008; Pinto et al., 2008) and the 5-HT receptor subtype 1B (5-HT1B) (Fehr et al., 2000; Hasegawa et al., 2002; Lappalainen et al., 1998), but not all reports are in agreement (Gorwood et al., 2002). Additionally, alcoholics have decreased 5-HT neurotransmission (Berglund et al., 2006), and tryptophan depletion (which acutely reduces 5-HT synthesis and transmission) induces the urge to drink in alcoholics (Pierucci-Lagha et al., 2004). Thus, there is a putative genetic and physiological link between 5-HT neurotransmission and alcoholism.
Non-human models offer additional insight suggesting that serotonergic function affects the drive for ethanol consumption. For example, ethanol consumption causes increased 5-HT release in the shell region of the NAc (NAcSh), a response which habituates in control animals but not in ethanol-preferring rats (De Montis et al., 2004), suggesting that ethanol-induced neural feedback mechanisms are impaired in this population. Furthermore, ethanol-preferring rats have decreased 5-HT transporter immunoreactivity in the NAc and some regions of the dorsal raphe nucleus (Casu et al., 2004), suggesting decreased 5-HT innervation. Finally, ethanol preferring strains of rats and mice have decreased availability of 5-HT in the brain (Badawy, 1999; McBride et al., 1989), and 5-HT reuptake inhibitors, which increase the availability of 5-HT, decrease ethanol self administration (McBride et al., 1989). Together, these observations suggest that alterations in 5-HT transmission contribute to the drive to consume ethanol.
Several 5-HT receptors have been implicated in alcoholism, including 5-HT1B receptors. These receptors are located on axon terminals where they inhibit the release of neurotransmitter; they function both as autoreceptors (inhibiting 5-HT release from serotonergic terminals) and heteroreceptors (inhibiting the release of various other neurotransmitters in a wide range of neuron types). Human imaging studies suggest that alcoholics have increased 5-HT1B receptors in the ventral striatum, which includes the NAc (Hu et al., 2010). In rats, administration of 5-HT1B agonists decreases ethanol consumption (Tomkins and O'Neill, 2000; Wilson et al., 2000), and antagonists reverse some, but not all, ethanol-related behaviors (Maurel et al., 1998; Tomkins and O'Neill, 2000; Wilson et al., 2000). Work with 5-HT1B receptor knockout mice has shown that constitutive absence of this receptor causes decreased ethanol intake (Crabbe et al., 1996; Risinger et al., 1996; Risinger et al., 1999). However, other studies with these mice have yielded opposite results (Bouwknecht et al., 2000); notably, the absence of this receptor during brain development has been proposed to produce some of the behavioral phenotypes of 5-HT1B knockout mice (Castanon et al., 2000).
In addition to developmental adaptations, the cellular diversity of 5-HT1B expression in multiple neuron types throughout brain further complicates the role of these receptors in reward mechanisms. 5-HT1B receptors involved in the mesolimbic reward circuit illustrate this problem. For example, 5-HT1B receptors in the NAcSh are localized on glutamatergic afferents (Muramatsu et al., 1998), on serotonergic projections from the dorsal raphe (Sari, 2004), and on collaterals of GABAergic medium spiny neurons that primarily project to the VTA where they synapse on dopaminergic neurons (Bruinvels et al., 1994; Johnson et al., 1992). By inhibiting GABA release in the VTA, these latter 5-HT1B heteroreceptors disinhibit dopaminergic neurons (David et al., 2004; O'Dell and Parsons, 2004; Yan et al., 2004) that play a role in reward mechanisms associated with addictive behaviors involving alcohol and other drugs (Balfour, 2009; Meyer et al., 2009; Moaddab et al., 2009). Thus, 5-HT1B receptors are present in key brain regions that modulate alcohol reward but are expressed in multiple cell types that may produce different effects on reward processes. We are focusing on 5-HT1B receptors expressed in medium spiny neurons since they are expressed at high levels in ventral striatum (Bruinvels et al., 1994) and are thought to be major regulators of dopaminergic neurons residing in the VTA that provide reciprocal innervation to the NAcSh (Barot et al., 2007; Morikawa et al., 2000; Neumaier et al., 2002). Previous work specifically addressing the role of 5-HT1B receptors in the reward circuit in the effects of ethanol has shown that intraperitoneal injection of 1–2 g/kg of ethanol results in increased dopamine levels in both the NAcSh and VTA. Local VTA administration of a 5-HT1B agonist facilitated, while an antagonist attenuated, ethanol’s effects on DA release in the NAc (Yan et al., 2005), consistent with the hypothesis that the 5-HT1B receptors on NAcSh terminals in the VTA modulate ethanol-induced DA increases.
Increasing expression of 5-HT1B receptors is a useful experimental strategy to sort out the roles of different populations of 5-HT1B receptors because they can be precisely targeted to a specific group of neurons, even if the resulting receptors are transported to these neurons’ axon terminals elsewhere. Previously we used viral mediated gene transfer to overexpress 5-HT1B receptors in NAcSh projection neurons; this increased the consumption of 12% ethanol (Hoplight et al., 2006). In alcohol-preferring rats, drinking patterns over time vary between animals, suggesting that multiple factors drive ethanol consumption behavior (Bell et al., 2006). Therefore, we studied the patterns of ethanol intake over time as well as total volume consumed each day in order to develop and better understand animal models of alcoholism. Given the links between 5-HT, alcoholism, and the 5-HT1B receptor, the current study hypothesizes that overexpression of 5-HT1B receptor mRNA in NAcSh projection neurons will alter both the motivational aspects and temporal pattern of ethanol drinking during both the initiation and maintenance phases of ethanol consumption.
Materials and Methods
Subjects
Male Long Evans rats (Harlan Laboratories, Inc; 225–250g) were used in all experiments. Animals were individually housed in a temperature and humidity controlled vivarium, on a 12-12h light-dark cycle, with lights-on at 0200. This allowed us to work with the animals close to the end of the light cycle, minimizing disruptions during the rats’ resting period. Food and water were available ad libitum, except as specified below. Animals acclimated to our facility for seven days prior to any experimental procedures. All procedures were approved by the Institutional Animal Care and Use Committee. This research was conducted according to the requirements of all applicable local, national, and international standards for the care and use of laboratory animals (Institute of Laboratory Animal Research, 1996).
Viral vector injection
Viral vectors were prepared as previously described (Clark et al., 2004). Rats were bilaterally stereotaxically injected with 2µl/side of a neuron-specific, replication deficient herpes simplex virus (HSV) that drives expression of 5-HT1B receptor and green fluorescent protein from separate transcriptional cassettes (pHSV-HA1B/GFP) or green fluorescent protein alone (pHSV-GFP). Transgene expression peaks at approximately 3–4 days and dissipates approximately ten days post injection (Barot et al., 2007; Clark et al., 2004; Ferguson et al., 2008). Injections were targeted to the medial shell of the NAc (AP +1.7 mm, ML ± 0.8 mm, DV −6.8) (Paxinos, 1986). About 95% of striatal neurons are medium spiny neurons, and most but not all of the HSV infected neurons are of this class. Injection accuracy was assessed in a blinded fashion, by three separate observers. Location of green fluorescent protein or injection tract marks visualized with cresyl violet staining were used to determine hits or misses. To be considered a hit, both sides of the brain needed to have a majority of green fluorescent protein or have tract marks that terminated in the NAcSh region. Surgical hit rates were as follows: initiation: 76%, maintenance: 69%, tastant: 75%, and locomotion: 80%.
Ethanol access and equipment
For initial ethanol exposure in both experiments, a forced-choice access paradigm was used. Upon initial ethanol exposure, water bottles were removed from the cage and animals were given access to two bottles: one with 6% ethanol and one with 12% ethanol (w/v). Both bottles were weighed and refilled, and bottle order was changed daily. There are several different methods available to increase drinking behavior in outbred rat strains; we selected forced choice for this study so as to avoid a different set of potential confounds such as learning effects associated with sucrose fading or noncontingent administration for vapor chambers, for example. Therefore, forced-choice access followed by free-choice access was the chosen paradigm for two reasons: 1) to maximize subsequent voluntary ethanol consumption in order to draw out more robust differences in drinking behavior and 2) to minimize the dependence of learned behavior on this experiment. Additionally, recent work suggests that plasticity during the forced access period may contribute to subsequent drug behavior (Timberlake et al., 2009).
Ethanol administration occurred in home cages equipped with lickometers throughout the entire experiment. Custom constructed lickometer equipment (Lafayette Instruments; West Lafayette, IN) consisted of a standard polycarbonate cage (48cm L × 25cm W × 20cm H), with a specialized lid and steel panel that extended into the cage. Three 50 ml bottles were housed behind the panel and sipper tubes passed through three cutouts on the panel. The metallic sipper tubes were coupled to a relay that recorded the number of licks, which were then downloaded onto a computer. Animals completed the circuit by standing on the metal platform and drinking from one of the bottles. Activity was monitored with modified Animal Wheel Monitoring software (Lafayette Instruments, West Lafayette, IN). The total number of licks from each bottle was recorded every five minutes. Differences during forced access were not anticipated; therefore, drinking behavior was recorded only during free-access to ethanol.
Experiment 1: Initiation
The purpose of this experiment was to determine the effects of increased 5-HT1B expression on NAcSh projection neurons on both the acquisition of drinking behavior and the preference of ethanol concentration. On day 1, animals received viral vector injections (Figure 1A; pHSV-GFP n=12; pHSV-HA1B/GFP n=15). On the night following surgery, they had ad libitum access to tap water. On days 2– 4, animals had ad libitum access to two bottles of liquid: 6% ethanol and 12% ethanol. On days 5–11, animals had ad libitum access to three bottles of liquid: tap water, 6% ethanol, and 12% ethanol Drinking behavior, body weight, and food intake were recorded.
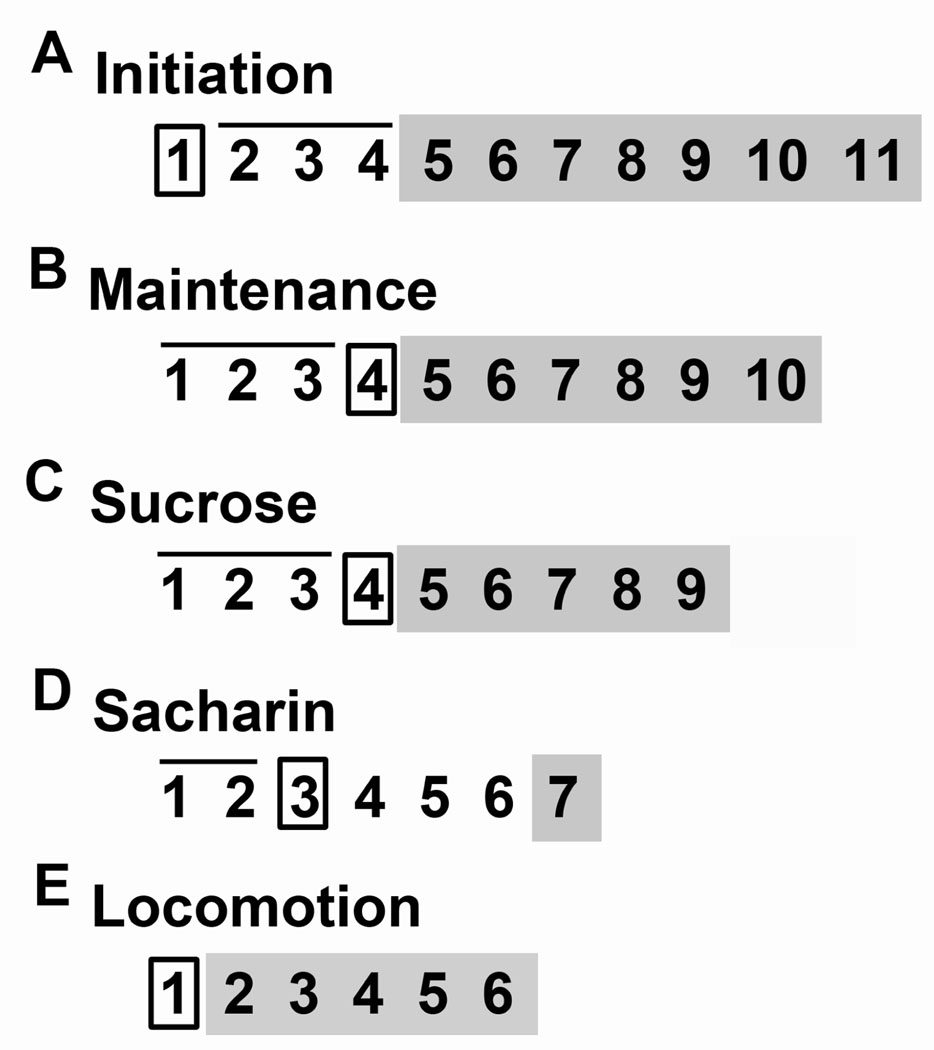
Figure 1.
Schematic of experimental designs. Boxes indicate surgical injection days; bars above indicate training days; shaded areas indicate testing days. (A) This experiment assessed the effects of 5-HT1B receptor overexpression on the initiation of ethanol drinking. Viral vectors were injected on day 1. Animals had forced ethanol exposure (6% and 12%) on days 2–4 and free-choice drinking (water, 6%, and 12%) on days 5–11. (B) This experiment determined the effect of 5-HT1B receptor overexpression on maintenance of ethanol drinking. Animals had forced ethanol exposure (6% and 12%) on days 1–3. Viral vectors were injected on day 4. Days 5–10 were free choice drinking (water, 6%, and 12%). (C) This experiment assessed the effects of 5-HT1B receptor overexpression on sucrose solution intake. Animals were drink trained on days 1–3. Viral vectors were injected on day 4. Drinking preference was tested on days 5–9. (D) This experiment assessed the effects of 5-HT1B receptor overexpression on saccharin solution intake. Animals were trained on days 1 and 2. Virus was injected on day 3. Animals rested on days 4–6. Drinking preference was tested on day 7. (E) This experiment assessed 5-HT1B receptor overexpression on baseline locomotion. Viral vectors were injected on day 1 and locomotor behavior was recorded on days 2–5.
Experiment 2: Maintenance
The purpose of this experiment was to determine the effects of increased 5-HT1B expression on NAcSh projection neurons during the maintenance of drinking behavior, as well as ethanol concentration preference. To establish stable drinking, animals had forced ethanol exposure for days 1–3 (Figure 1B; pHSV-GFP n=5; pHSV-HA1B/GFP n=8). On day 4, animals were randomly assigned to groups and viral vector was injected. On the night following surgery, they had ad libitum access to tap water. On days 5–10, animals had ad libitum access to three bottles of liquid: tap water, 6% ethanol, and 12% ethanol. Drinking behavior, body weight, and food intake were recorded.
Experiment 3. Tastant Effects
The purpose of these experiments was to determine whether increased expression of 5-HT1B receptors via viral expression affects taste perception. Animals were not liquid deprived at any time during these experiments and all liquids were available ad libitum. Sucrose. This experiment will determine whether 5-HT1B expression affects preference for sucrose. On day 1, animals were exposed to a 50mL bottle of 5% sucrose solution for 24 hours to acclimate them to the presence of bottles in the cage and the taste of the solution. All animals drank the full 50mL. On days 2 and 3, animals were given access to two bottles for one hour; one contained tap water and the other contained 5% sucrose. They were then stereotaxically injected with pHSV-HA1B/GFP (n=8) or pHSV-GFP (n=9) on day 4. On days 5–9, preference for water vs. 5% sucrose was tested daily using the same procedure. All of the 1 hour sessions were performed at 1100 daily. Saccharin. This experiment will determine whether 5-HT1B-expression affects preference for saccharin solution. On days 1 and 2, animals were exposed to 0.1% saccharin for 1 hour at 1100. On day 3, they were stereotaxically injected with pHSV-HA1B/GFP (n=5) or pHSV-GFP (n=7). They rested from days 4–6, and were tested on day 7 with a two bottle choice test comparing their intake of water vs. 0.1% saccharin for 1 hour.
Experiment 4. Locomotor Activity
The purpose of this experiment was to determine whether augmentation of 5-HT1B receptor function via viral expression affects locomotor behavior. Animals were stereotaxically injected with pHSV-HA1B/GFP (n=5) or pHSV-GFP (n=3) and allowed one day of recovery. Then, homecages were placed in infrared locomotion detection frames (San Diego Instruments, San Diego, CA) that recorded levels of locomotion via beam break counts. Behavior was recorded continuously for four days. Animals had ad libitum access to food and water at all times.
Brain Processing
At the end of each experiment, animals were sacrificed via CO2 inhalation and subsequent decapitation. Brains were extracted and post-fixed in 4% paraformaldehyde at 4°C overnight and then stored in phosphate-buffered saline at 4°C until sectioning at 40µm using a vibratome. Injection placement was confirmed via visualization of green fluorescence or cresyl staining in cases when virus was no longer present (see viral vector injection above).
Analysis and Statistics
Cumulative intake of ethanol was used to quantify ethanol exposure over time. To satisfy the normality assumption of general linear model analyses involving continuous dependent variables, we logarithmically transformed volumetric outcomes prior to statistical evaluations (base e). Loge–transformed volumetric outcomes were analyzed with a model that simultaneously included the between group factor VIRAL GROUP (pHSV-HA1B/GFP or pHSV-GFP) and the repeated measure DAY using generalized estimating equations (GEE) (Liang, 1986). GEE is more appropriate than repeated measures ANOVA when the analysis includes correlated longitudinal responses measured at multiple time-points (Fitzmaurice, 2004). GEE analysis estimated the effect of GROUP as a difference of logarithms defined as loge (volume consumed by the pHSV-HA1B/GFP group) minus loge(volume consumed by the pHSV-GFP group), and the 95% confidence limits of the difference. Back transforming these estimates yielded ethanol intake ratios, each defined as the original-scale ethanol volume consumed by the pHSV-HA1B/GFP group divided by the original-scale volume consumed by the pHSV-GFP group.
Licking behavior were normally distributed with equal variance; therefore ANOVA was used for analysis. To assess the licking frequency during drinking bouts, (i.e., the number of licks in each five minute interval), the number of licks were binned into four ranges: 0–600 (low), 601–1200 (medium), 1201–1800 (high), and 1801–2400 (very high). The number of data cells that reflected a drinking response within each range was counted (number of bins) and the results from both ethanol concentrations were combined to reflect the total activity of ethanol consumption. Data were analyzed with two factor ANOVA with VIRAL GROUP and NUMBER OF BINS as factors.
In addition to within interval analysis, we measured the pattern of bout duration of ethanol consumption over extended intervals (i.e., whether animals drank in short intermittent bursts or extended drinking episodes). The number of consecutive non-zero data cells was totaled, beginning with single bouts (one positive data cell flanked by zero data cells) and ending with 10 or more consecutive bouts (ten or more positive data cells in a row flanked by zero data cells). Water, 6%, and 12% ethanol concentrations were analyzed separately, each via two factor ANOVA with VIRAL GROUP and NUMBER OF CONSECUTIVE CELLS as factors.
Locomotor experiment data were divided into non-directed, non-extensive beam breaks called fine motor movement and directed, extensive beam breaks called ambulation. Data (fine motor, ambulation, and total) were analyzed with single factor ANOVA with VIRAL GROUP as the factor. Tastant assessment experiments were analyzed via two-factor repeated-measures ANOVA with VIRAL GROUP and DAY as factors.
Results
Experiment 1: Initiation
Overall drinking behavior. Figure 1A depicts the design for experiment 1, which tested the hypothesis that overexpression of 5-HT1B receptors in NAcSh projection neurons facilitates the initiation of ethanol consumption and alters underlying drinking patterns. A schematic diagram (Paxinos, 1986) that includes the NAcSh target is shown in figure 2A. Figures 2B and C are a representative section from a GFP injected animal; 2B is magnified by a factor of 4X, while 2C is magnified by a factor of 10X. pHSV/GFP signal is expressed in both cell bodies and processes. Table 1 lists individual ethanol consumption across both forced and free choice access days in raw volume of ethanol consumed. Analysis revealed no significant difference between groups in water intake (F 1,26 = 1.78, p=0.19) (data not shown). There were no differences in intake during forced (Wald chi-square = 2.52, p=0.11) or free choice access (Wald chi-square = 0.41, p=0.52) of 6% ethanol. However, there were significant group differences in intake of 12% ethanol during both forced (Wald chi-square = 8.87, p=0.003) and free access (Wald Chi-square = 3.72, p=0.05), with pHSV-HA1B/GFP animals drinking more. The pHSV-HA1B/GFP intake data are graphically presented in Figure 3A as a ratio of the intake by pHSV-GFP controls, revealing that 12% ethanol intake is significantly higher for pHSV-HA1B/GFP animals during both forced and free access phases. Table 2 details the consumption of ethanol by day, by conversion into grams per kilogram.
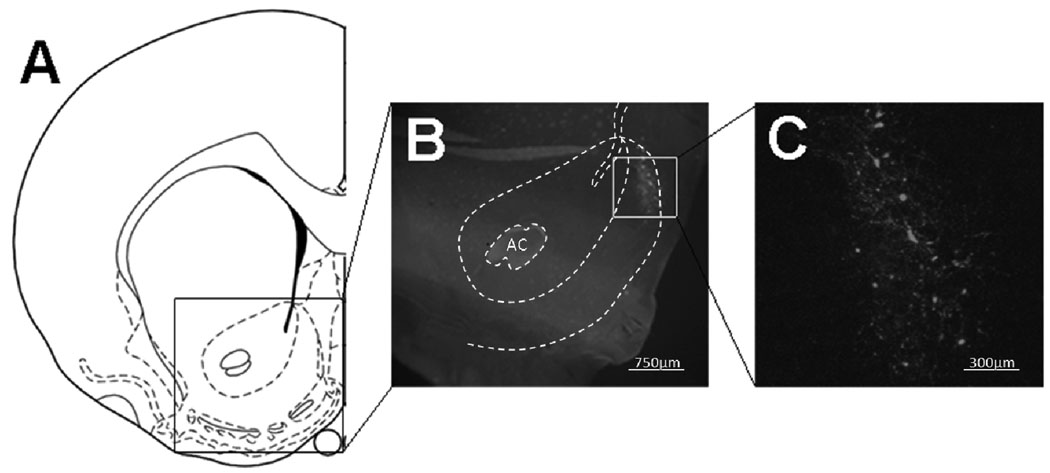
Figure 2.
NAcSh injection site. (A) A schematic diagram that includes the NAcSh target is shown in figure 2A. Figures 2B and C are a representative section showing GFP fluorescence in the NAcSh; 2B is magnified by a factor of 4X, while 2C is magnified by a factor of 10X. pHSV/GFP signal is present in both cell bodies and processes.
Table 1.
Raw volume of 6% and 12% ethanol consumed by pHSV-GFP and pHSV-HA1B/GFP animals during initiation drinking.
| Forced Access |
Free Choice Access |
|||
|---|---|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP | pHSV-GFP | pHSV-HA1B/GFP | |
| 6% EtOH (g) | 25.6 ± 1.50 | 22.72 ± 1.96 | 3.91 ± 1.84 | 2.92 ± 1.01 |
| 12% EtOH (g) | 1.10 ± 0.20 | 2.94 ± 1.00* | 0.87 ± 0.13 | 2.44 ± 1.25* |
Fluid volumes are in raw grams and represent the average amount consumed per day. During both forced and free-access to ethanol, pHSV-HA1B/GFP animals consumed more 12% ethanol than pHSV-GFP animals. Data are presented as mean ± SEM.
= p<0.05 between groups.
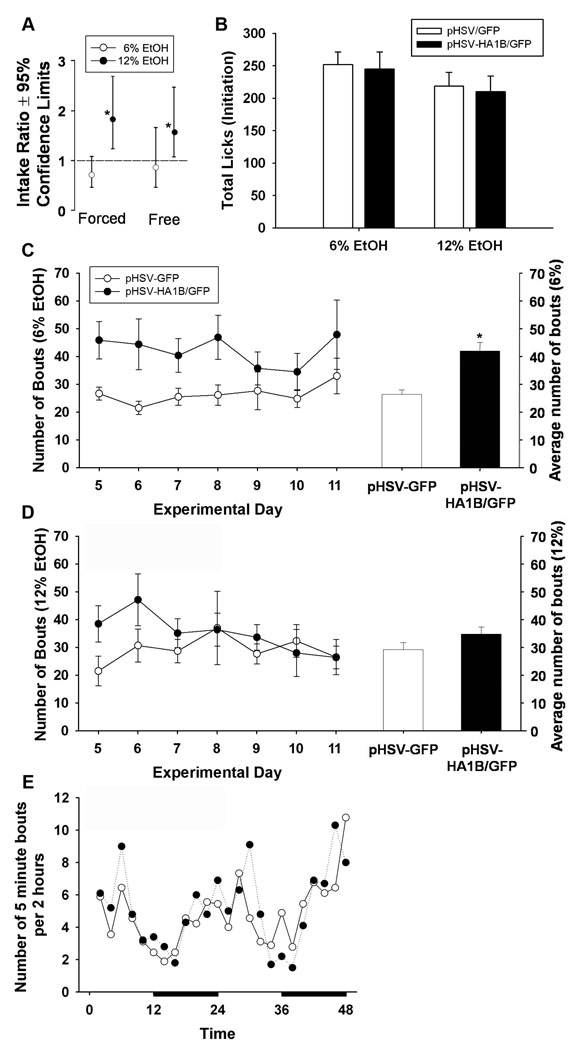
Figure 3.
Consumption of ethanol and drinking patterns during initiation drinking. (A) Drinking volume data from generalized estimating equations were back transformed to yield ethanol intake ratios (See analysis and statistics section), which revealed that pHSV-HA1B/GFP animals drank almost twice as much 12% ethanol during both forced and free access drinking, compared to pHSV-GFP animals. There were no significant differences between groups in total average licks per day on ethanol sippers (B). The number of bouts per day, as well as overall average bouts for 6% are depicted in C. pHSV-HA1B/GFP animals had significantly increased bouts for 6% ethanol, compared to pHSV-GFP animals. (D) There were no differences in bouts for 12% ethanol. (E) Representative 48-hour period of circadian activity shows the number of bouts on 6% and 12% ethanol sippers (combined) per 2 hours. Dark bars denote lights-off period. Data are presented as mean ± SEM. * = p<0.05, between groups.
Table 2.
Ethanol consumption by day during initiation drinking (g/kg).
| Total EtOH (g/kg) |
||
|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP | |
| Day 2 | 3.28 ± 0.23 | 4.95 ± 0.41 |
| Day 3 | 5.09 ± 0.29 | 5.44 ± 0.47 |
| Day 4 | 4.77 ± 0.28 | 4.69 ± 0.35 |
| Day 5 | 0.89 ± 0.26 | 1.73 ± 0.75 |
| Day 6 | 0.88 ± 0.26 | 1.42 ± 0.68 |
| Day 7 | 0.78 ± 0.39 | 1.3 ± 0.62 |
| Day 8 | 0.80 ± 0.33 | 1.45 ± 0.69 |
| Day 9 | 0.96 ± 0.38 | 1.25 ± 0.54 |
| Day 10 | 1.06 ± 0.33 | 1.44 ± 0.69 |
| Day 11 | 0.86 ± 0.36 | 1.14 ± 0.55 |
Data are represented as grams of ethanol consumed per kg of body weight. Day 1 was the stereotaxic injection day; the dotted line separates forced from choice ethanol exposure. Data are presented as mean ± SEM.
We also examined the microarchitecture of drinking behavior over time. Figure 3B shows the average number of licks (per day) after viral gene transfer. There was no main effect of treatment (F 1,20 = 0.81, p=0.38), suggesting no difference in overall licking activity per day. However, overexpression of 5-HT1B receptors increased the number of drinking bouts per day for 6% ethanol (F 1,12 = 4.99, p<0.05; Figure 3C), but not for 12% ethanol (F 1,12 = 1.02, p=0.33; Figure 3D). Figure 3E shows the circadian pattern of drinking over a representative 48 hour period. Data points represent the number of bouts on both 6% and 12% sippers per 2 hour period. Circadian patterns did not significantly differ between groups (p=0.27).
Table 3 details licking frequency during bouts by showing the number of five minute bins that had lick counts (the number of times the electronic circuit was completed) within four ranges: 0–600 (low), 601–1200 (medium), 1201–1800 (high), and 1801–2400 (very high). There were no significant treatment differences in the 1–600 range (F 1,18 = 0.158, p=0.70), the 601–1200 range (F 1,18 = 0.02, p=0.89), or the 1201–1800 range (F 1,18 = 0.25, p=0.63). However, there was a main effect of viral group on the number of intervals falling in the 1801–2400 range (F 1,18 = 3.54, p<0.05), suggesting that pHSV-HA1B/GFP-treated animals had more drinking bouts with very high frequency lick counts.
Table 3.
Distribution of lick frequencies during free access to ethanol during initiation drinking.
| pHSV-GFP | pHSV-HA1B/GFP | |
|---|---|---|
| 1–600 | 33.4 ± 9.08 | 32.2 ± 7.63 |
| 601–1200 | 2.06 ± 0.16 | 2.11 ± 0.17 |
| 1201–1800 | 0.38 ± 0.07 | 0.47 ± 0.07 |
| 1801–2400 | 0.05 ± 0.20 | 0.52 ± 0.08* |
The number of licks on ethanol sippers during five minute bouts was recorded. Since animals sometimes alternated between 6% and 12% ethanol, these are combined for this analysis. Bouts were then divided into four ranges: low, medium, high, and very high. During initiation drinking, pHSV-HA1B/GFP animals significantly increased very high range licking frequency. Data are presented as mean ± SEM.
= p<0.05 between groups.
The microarchitecture of bout duration is shown in Table 4. We determined bout duration by counting the number of consecutive 5 minute intervals in which an animal consumed ethanol (bounded by intervals with no licking). There were no treatment differences between viral groups in bout duration for 6% (F 1,23 = 1.93, p=0.18) or 12% ethanol (F 1,23 = 0.83, p=0.37), indicating that pHSV-HA1B/GFP-treated animals did not have longer drinking bouts than GFP-only controls.
Table 4.
Duration of drinking bouts during free access initiation drinking.
| 6% |
12% |
|||
|---|---|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP | pHSV-GFP | pHSV-HA1 B/GFP | |
| 1 | 150.36 ± 8.99 | 172.36 ± 13.15 | 151.00 ± 9.93 | 167.36 ± 11.68 |
| 2 | 34.93 ± 3.88 | 40.91 ± 4.74 | 27.21 ± 2.59 | 30.00 ± 4.30 |
| 3 | 3.93 ± 1.10 | 4.82 ± 1.32 | 3.00 ± 0.62 | 2.55 ± 0.81 |
| 4 | 0.79 ± 0.21 | 0.91 ± 0.25 | 0.71 ± 0.19 | 0.64 ± 0.24 |
| 5 | 0.36 ± 0.17 | 0.36 ± 0.20 | 0.43 ± 0.23 | 0.18 ± 0.12 |
| 6 | 0.14 ± 0.14 | 0.00 ± 0.00 | 0.36 ± 0.20 | 0.09 ± 0.09 |
| 7 | 0.00 ± 0.00 | 0.91 ± 0.09 | 0.07 ± 0.07 | 0.09 ± 0.09 |
| 8 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.07 ± 0.07 | 0.00 ± 0.00 |
| 9 | 0.07 ± 0.07 | 0.00 ± 0.00 | 0.07 ± 0.07 | 0.00 ± 0.00 |
| 10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10+ | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
To assess the duration of drinking bouts, consecutive non-zero cells were tabulated, starting with one non-zero cell flanked by zero cells and ending with ten non-zero cells flanked by zero cells. There was no main effect of treatment on bout duration during initiation drinking. Data are presented as mean ± SEM.
Experiment 2: Maintenance
Overall drinking behavior. Experiment 2 (Figure 1B) tested the hypothesis that overexpression of 5-HT1B receptors in NAcSh projection neurons increases consumption of ethanol during the maintenance phase of drinking. Table 5 lists individual ethanol consumption during both forced and free access days in terms of raw volume consumed. There was no main effect of treatment on 6% ethanol, 12% ethanol, or water (F 1,11 = 1.73, p=0.20) (data not shown). Table 6 details grams of ethanol consumed by day by conversion to grams per kilogram of body weight. The pHSV-HA1B/GFP ethanol intake data are graphically presented in Figure 4A as a ratio of pHSV-GFP controls, showing no group differences in 6% ethanol intake during forced (p=0.94) or free access (p=0.24), or 12% ethanol intake during forced (p=0.63) or free access (p=0.74).
Table 5.
Volume of 6% and 12% ethanol consumed by pHSV-GFP and pHSV-HA1B/GFP animals during maintenance drinking.
| Forced Access |
Free Choice Access |
|||
|---|---|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP | pHSV-GFP | pHSV-HA1B/GFP | |
| 6% EtOH (g) |
27.31 ± 3.98 | 26.78 ± 1.58 | 8.65 ± 5.61 | 3.17 ± 1.00 |
| 12% EtOH (g) |
3.39 ± 1.04 | 3.84 ± 1.10 | 1.97 ± 0.37 | 2.61 ± 1.05 |
Fluid volumes are in grams and represent the average amount consumed per day. There were no significant differences between groups in the consumption 6% or 12% ethanol during maintenance. Data are presented as mean ± SEM.
Table 6.
Ethanol consumption by day during maintenance drinking (g/kg).
| Total EtOH (g/kg) |
||
|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP | |
| Day 1 | 7.22 ± 0.74 | 6.31 ± 0.49 |
| Day 2 | 5.19 ± 0.81 | 5.63 ± 0.38 |
| Day 3 | 5.19 ± 0.59 | 5.24 ± 0.33 |
| Day 5 | 3.96 ± 0.62 | 3.40 ± 0.19 |
| Day 6 | 1.76 ± 1.40 | 0.79 ± 1.15 |
| Day 7 | 1.50 ± 0.75 | 0.74 ± 0.17 |
| Day 8 | 1.45 ± 0.90 | 0.85 ± 0.12 |
| Day 9 | 1.97 ± 0.93 | 0.80 ± 0.21 |
| Day 10 |
2.56 ± 0.93 | 1.97 ± 0.26 |
Data are represented as grams of ethanol consumed per kg of body weight. Day 4 was the stereotaxic injection day; the dotted line separates forced from choice ethanol exposure. Data are presented as mean ± SEM.
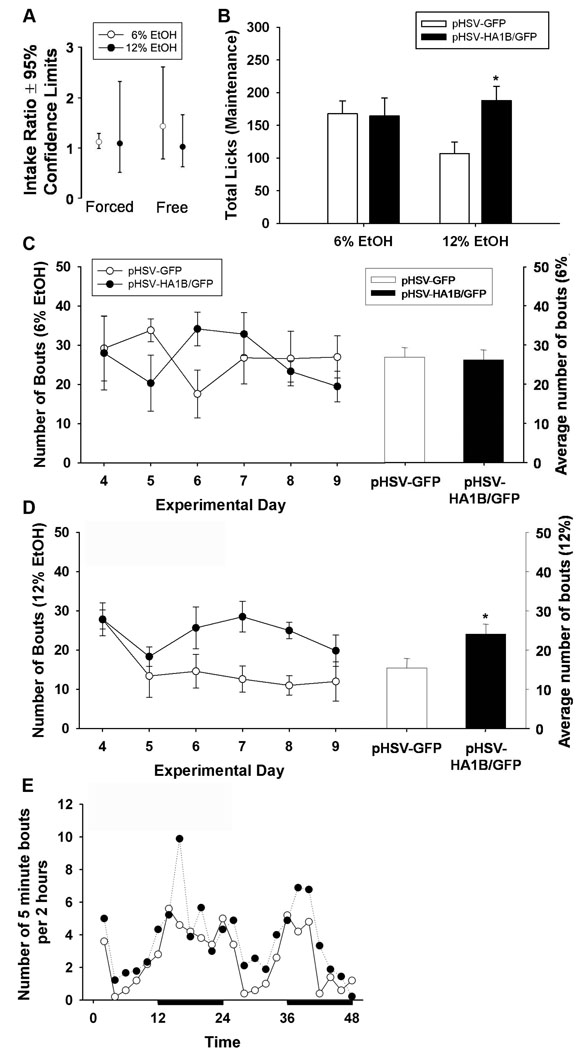
Figure 4.
Consumption of ethanol and drinking patterns during maintenance drinking. (A) Drinking volume data from generalized estimating equations were back transformed to yield ethanol intake ratios (See analysis and statistics section), which revealed no significant differences between groups during either forced or free access to ethanol. (B) There was a main effect of treatment on number of licks, with pHSV-HA1B/GFP animals having increased total licks on the 12% ethanol sipper. (C) There were no differences in number of bouts per day or overall average bouts for 6% ethanol. (D) pHSV-HA1B/GFP animals significantly increased bouts on 12% ethanol sippers, compared to pHSV-GFP animals. (E) Representative 48-hour period of circadian activity shows the number of bouts on 6% and 12% ethanol sippers (combined) per 2 hours. Dark bars denote lights-off period. Data are presented as mean ± SEM. * = p<0.05, between groups.
We also examined the pattern of ethanol drinking over time. Figure 4B shows the average number of licks per day after viral gene transfer. While there was no main effect of viral treatment (F 1,13 = 1.16, p=0.30), there was an interaction between fluid type and viral treatment (F 2,26 = 5.03, p<0.05), with pHSV-HA1B/GFP animals having increased licks on the 12% sipper. The number of bouts with 6% and 12% ethanol is shown in Figures 4C–D. There was no main effect of viral treatment on 6% ethanol (F 1,9 = 0.01, p=0.94), but there was a significant increase in the number of bouts for 12% ethanol (F 1,9 = 4.97, p=0.05) in the pHSV-HA1B/GFP group. Figure 4E shows the circadian pattern of drinking over a representative 48 hour period. Data points represent the number of bouts on both 6% and 12% sippers per 2 hour period. Circadian patterns did not differ between groups (p=0.26).
Table 7 details the microarchitecture of licking frequency during the maintenance of drinking. There were no significant differences in the 1–600 range (F 1,9 = 0.002, p=0.97), the 601–1200 range (F 1,9 = 0.05, p=0.83), the 1201–1800 range (F 1,9 = 3.47, p=0.06), or the 1801–2400 range (F 1,9 = 0.50, p=0.50), suggesting that altered 5-HT1B receptor expression does not play a role in the licking frequency during drinking bouts during the maintenance phase of drinking.
Table 7.
Distribution of lick frequencies during free access to ethanol during maintenance drinking.
| pHSV-GFP | pHSV-HA1B/GFP | |
|---|---|---|
| 1–600 | 29.2 ± 11.0 | 29.4 ± 7.73 |
| 601–1200 | 1.83 ± 0.34 | 2.02 ± 0.23 |
| 1201–1800 | 0.06 ± 0.04 | 0.44 ± 0.09 |
| 1801–2400 | 0.03 ± 0.03 | 0.22 ± 0.06 |
The number of licks on ethanol sippers during five minute bouts was recorded. Since animals sometimes alternated between 6% and 12% ethanol, these are combined for this analysis. Bouts were then divided into four ranges: low, medium, high, and very high. There were no differences in licking frequency during maintenance drinking. Data are presented as mean ± SEM.
The microarchitecture of bout duration is shown in Table 8. There were no viral treatment effects in 6% ethanol (F 1,15 = 0.43, p=0.52). However, there were main effects of VIRAL GROUP (F 1,15 = 6.24, p<0.05) and intervals (F 10,150 = 115.01, p<0.05), and a viral group by intervals interaction (F 10,150 = 6.18, p<0.05) for 12% ethanol, indicating that 5-HT1B overexpression increased the bout duration of 12% ethanol during maintenance drinking.
Table 8.
Duration of drinking bouts during free access maintenance drinking.
| 6% |
12% |
|||
|---|---|---|---|---|
| pHSV-GFP | pHSV-HA1B/GFP* | pHSV-GFP | pHSV-HA1 B/GFP* | |
| 1 | 109.56 ± 11.85 | 121.88 ± 17.80 | 79.56 ± 12.48 | 127.25 ± 14.48 |
| 2 | 22.56 ± 3.78 | 26.38 ± 4.57 | 11.56 ± 2.66 | 20.75 ± 3.62 |
| 3 | 2.89 ± 0.92 | 2.63 ± 1.07 | 0.67 ± 0.37 | 0.87 ± 0.30 |
| 4 | 0.33 ± 0.33 | 0.88 ± 0.35 | 0.56 ± 0.34 | 0.25 ± 0.16 |
| 5 | 0.33 ± 0.17 | 0.13 ± 0.13 | 0.11 ± 0.11 | 0.00 ± 0.00 |
| 6 | 0.00 ± 0.00 | 0.13 ± 0.13 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 7 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.22 ± 0.15 | 0.00 ± 0.00 |
| 8 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 9 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10+ | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
To assess the duration of drinking bouts, consecutive non-zero cells were tabulated, starting with one non-zero cell flanked by zero cells and ending with ten non-zero cells flanked by zero cells. There was no main effect of treatment on bout duration during initiation drinking. Data are presented as mean ± SEM
Experiment 3. Tastant Effects
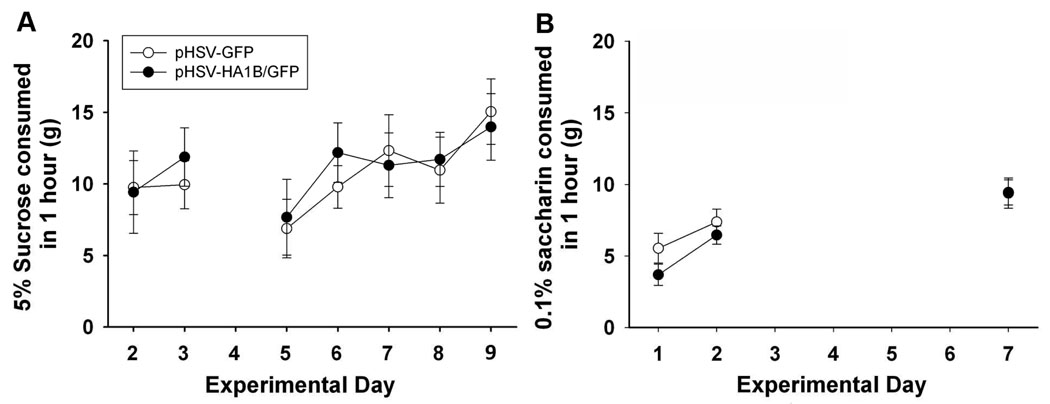
Figure 5A shows sucrose consumption during training and after augmented 5-HT1B receptor expression. There was no main effect of increased 5-HT1B receptors on consumption of a 5% sucrose solution (F 1,135 = 0.02, p=0.90). Additionally, there was no treatment effect of increased 5-HT1B receptor expression on saccharin intake (F 1,32 = 1.42, p=0.26) (Figure 5B).
Figure 5.
Sucrose and saccharin preference after pHSV-HA1B/GFP or pHSV-GFP injection. There were no significant differences between pHSV-HA1B/GFP and pHSV-GFP groups in preference for either sucrose or saccharin solutions. Data are presented as mean ± SEM.
Experiment 4. Locomotor activity
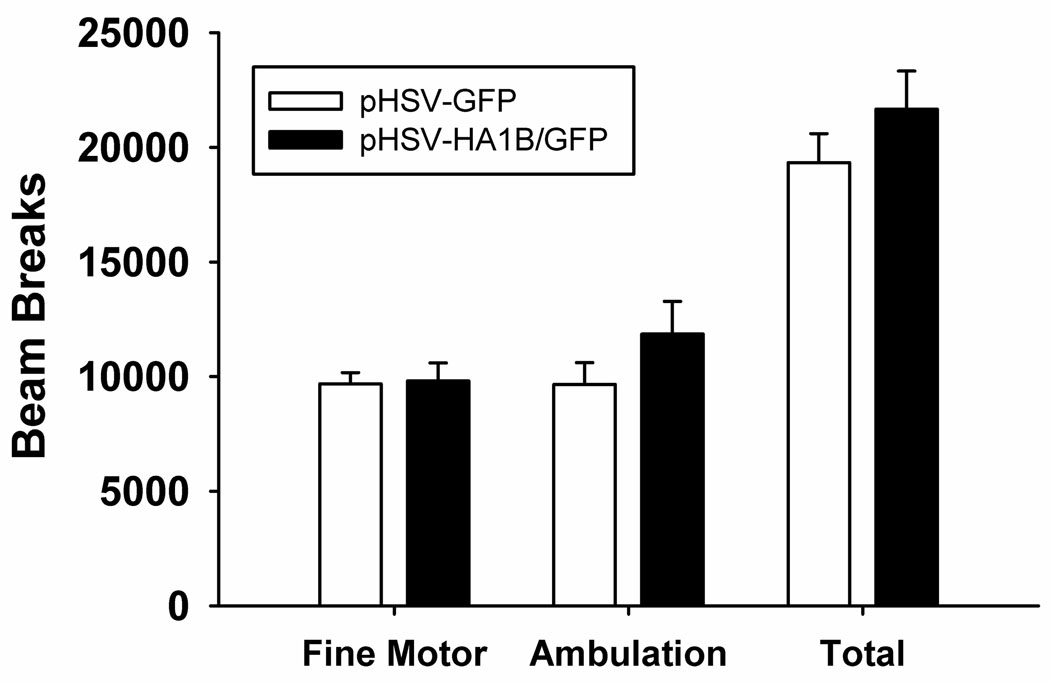
Figure 6 shows the average number of beam breaks associated with fine motor movement, ambulation, and the sum of the two types of beam breaks (total). There were no main effects of viral treatment on fine motor movement (F 1,8 = 0.02, p=0.89), ambulation (F 1,8 = 1.66, p=0.23), or overall movement (F 1,8 = 1.25, p=0.3), indicating that increased 5-HT1B expression did not alter global motor activity; also there was no indication of a shift in the circadian pattern of activity.
Figure 6.
Locomotor behavior after pHSV-HA1B/GFP or pHSV-GFP injection. There were no significant differences between pHSV-HA1B/GFP and pHSV-GFP groups in fine motor movements, ambulation, or total locomotion. Data are presented as mean ± SEM.
Table 9 shows a summary of results for initiation and maintenance phase experiments. Overall, these data indicate that overexpression of 5-HT1B receptors on NAcSh projection neurons increases the consumption of 12% ethanol while increasing high frequency licking bouts and bout number on 6% ethanol sippers. Whereas during maintenance drinking, receptor overexpression greatly increases the animals’ drinking patterns associated with higher concentrations of ethanol, but does not affect the volume of ethanol consumed.
Table 9.
Summary of the effects of 5-HT1B overexpression on drinking behavior.
| Initiation |
Maintenance |
|||
|---|---|---|---|---|
| 6% | 12% | 6% | 12% | |
| Volume of ethanol consumed | ↔ | ↑ | ↔ | ↔ |
| Average number of bouts/day | ↑ | ↔ | ↔ | ↑ |
| Average number of bouts | ↑ | ↔ | ↔ | ↑ |
| Average number of licks/day | ↔ | ↔ | ↔ | ↑ |
| Bout duration | ↔ | ↔ | ↑ | ↑ |
| High frequency licking bouts | ↑ (combined %) | ↔ (combined %) | ||
Discussion
Numerous studies have suggested a role for the 5-HT1B receptor in alcoholism; however, specific brain regions and mechanisms are still being elucidated. Previously we found that overexpression of 5-HT1B receptors in NAcSh projection neurons increased voluntary consumption of ethanol (Hoplight et al., 2006). The present study demonstrates that both the preference for low vs. high ethanol concentration and the pattern of drinking behavior are altered by these receptors during both initiation and maintenance of ethanol drinking.
During initiation, augmented 5-HT1B receptor expression increased the preference for 12% ethanol without changing the pattern of consumption over time. While the overall consumption of 6% ethanol did not change, there was a shift toward more frequent drinking bouts on this concentration. It is important to note that the overall consumption of ethanol did not change, the ratio of 12% to 6% increased in the animals with increased 5-HT1B receptor expression. During maintenance of established drinking, increased 5-HT1B receptor expression did not affect the volume of ethanol consumed due to between-subject variability, but did have dramatic effects on the pattern of intake, especially with the 12% ethanol, increasing the number of bouts, average number of licks, and bout duration. These results indicate a subtle shift in the animals’ ethanol drinking, such that more time was spent interacting with the 12% sipper. Taken as a whole, these results suggest a role for 5-HT1B receptors during both the initiation and maintenance of ethanol drinking behavior.
Increased ethanol consumption, as well as drinking pattern changes after 5-HT1B overexpression are presumably due to altering the balance of rewarding and aversive effects of ethanol, since these receptors also increase conditioned place preference to cocaine (Barot et al., 2007; Neumaier et al., 2002). There is, however, evidence that the rewarding effects of ethanol were enhanced as these 5-HT1B receptors modulate the mesolimbic reward pathway and indirectly enhance dopaminergic tone in the NAcSh by negatively regulating NAcSh GABAergic projection neurons that synapse onto dopamine projection neurons in the VTA (Yan et al., 2004). In turn, these neurons release dopamine in the NAcSh; since self-administration of ethanol has been shown to elicit patterned firing VTA neurons (Janak et al., 1999) and ethanol free-choice paradigms increase dopamine efflux specifically in the NAcSh (Rebec et al., 1997). Therefore, 5-HT1B receptors in NAcSh projection neurons enhance disinhibition of VTA neurons thereby facilitating reward mechanisms and driving elevations in drinking. The present results suggest that this occurs by changing the microarchitecture of drinking behavior as well as the preference for higher concentration ethanol, while not affecting the overall ethanol consumption.
Approximately 95% of striatal neurons are medium spiny neurons. In these studies, viral vectors were used to alter gene expression in the ventral striatum, and specifically to target NAcSh projection neuron circuitry. Viral effects on drinking are attributed to this population of neurons , however, it is important to note that striatal interneurons have been shown to play a role in ethanol drinking behavior (Herring et al., 2004). Based on the preponderance of data on 5-HT1B receptors expressed in NAcSh neurons, they are thought to regulate GABA release from terminals in VTA and ventral pallidum to alter reward mechanisms. Since we have previously shown that the virally expressed 5-HT1B receptors are transported to VTA (Barot et al., 2007), we think that this is the predominant physiological effect of the transgenic receptors but we cannot rule out some contributions of interneurons. Promoter-specific vectors are currently being developed in order to address this issue further.
We considered the possibility that alterations in drinking behavior were a result of modulation of taste preference by testing whether increasing 5-HT1B receptors in these neurons altered the preference for other tastants. Increased 5-HT1B receptor expression did not impact consumption of either the sweet or the sweet-bitter liquid as compared to GFP controls. While it is still possible that the taste of 6% and 12% ethanol were affected by 5-HT1B expression, it seems most likely that the rewarding rather than taste properties were involved, especially given previous results with cocaine that did not involve oral ingestion (Barot et al., 2007; Neumaier et al., 2002).
Our work agrees with a previous study that specifically addressed the role of 5-HT1B receptors in the reward circuit, which showed that intraperitoneal injection of 1–2 g/kg of ethanol, an amount that is similar to what our animals consume per day, resulted in increased dopamine levels in both the NAcSh and VTA (Yan et al., 2005). These increases were attenuated by administration of a 5-HT1B antagonist into the VTA, suggesting that the 5-HT1B receptors on NAcSh terminals in the VTA are involved in ethanol-induced DA increase. It is important to note that while the dose of ethanol in this study is similar to the volume consumed by our animals, dopamine efflux was only measured for 120 minutes after the ethanol dose, while our animals consumed the bulk of their ethanol during the lights-off portion of the light cycle. Although the timecourse between the two studies is different, both indicate a role for this receptor in ethanol effects. The present study directly implicates 5-HT1B receptors expressed on NAcSh projection neurons, and demonstrates their role in altering the pattern of ethanol consumption.
An important issue to address is the apparent disconnect between the increased volume of 12% ethanol consumed and increased licking activity on 6% drinking spouts during initiation. Volume consumed and licking patterns are not always tightly correlated. Previous studies have demonstrated that the pattern of alcohol drinking over time is not always strongly associated with the volume consumed, and can be associated with other factors such as genotype or metabolism. For example, Bell and colleagues showed that changes in patterned drinking and variations in licking behavior in alcohol preferring rats were correlated with blood ethanol levels in some cases, not volume consumed (Bell et al., 2006). In another study, Gill, et al showed that the frequency of ethanol drinking bouts was correlated with aldehyde dehydrogenase and catalase activity (Gill et al., 1996), suggesting that drinking patterns do reflect meaningful neurocorrelates. Furthermore, ethanol concentrations in the NAc of mice have been positively correlated with licking behavior (Griffin et al., 2007), supporting the idea that altering the function of NAc neurons changes licking behavior. While the NAc integrates a broad range of neural inputs that affect patterning, including circadian inputs from the hypothalamus, we did not observe that 5-HT1B receptor levels caused substantial changes in circadian drinking behavior. Therefore, these 5-HT receptors appear to impact the pattern of drinking over shorter rather than longer time intervals.
The expression timecourse of our HSV construct is such that peak expression occurs at around four days post injection; then the virus slowly dissipates until it is generally gone at 10 days post injection (Barot et al., 2007). This allows us to manipulate behavioral outcomes with temporal precision, but also hinders us from measuring long term behavioral effects (which could be possible with longer-lasting promoter regulation or a different virus such as AAV). In the present experiments, virus was onboard during the behavioral testing, until the last two days, when we expected to see a dramatic decrease in the drinking behavior of animals with augmented 5-HT1B receptor expression. In the initiation experiment, a subtle drop in the number of bouts was observed in the last three days of intake measurements, while during maintenance, there was a dramatic drop in the number of bouts on day 10, as would be expected with the timecourse of expression of this virus. As-is, our viral construct precludes us from measuring behavior over more extended periods of time; we are currently working on developing vectors with longer expression times that we will use in future studies.
The present data agree with past studies that show that 5-HT1B receptors are important in ethanol intake behavior. However, the methods in our study were slightly different than those from our previous work (Hoplight et al). Although both studies found that augmenting the expression of 5-HT1B receptors on NAc projection neurons enhances the animals’ interaction with 12% ethanol, the former study found so using three-bottle choice access to ethanol and water, while the latter study found so using a forced access period before giving choice. The intention of slightly altering the ethanol presentation method was to eliminate learning cues that may render interpretation of the data more difficult. Additionally, the purpose of the present studies was to directly test the role of 5-HT1B receptors on NAcSh projection neurons in two different aspects of behavior (acquisition and maintenance of drinking). While directly comparing the Hoplight 2006 study with the present study would not be entirely accurate, both studies seem to indicate a role for these receptors in ethanol consumption, especially that of higher ethanol concentration. In conclusion, we have shown shifts in the pattern of ethanol intake after manipulation of 5-HT1B receptor expression on NAcSh projection neurons. Overexpression of the 5-HT1B receptors in NAcSh is sufficient to alter drinking behavior during both initiation and maintenance of ethanol consumption. These results may provide further insights into the development of serotonergic agents to modify problem drinking of alcohol in humans.
Acknowledgements
The authors state no known conflicts of interest. The authors thank Dr. Michele Kelly for editorial comments and Daniel Eskenazi, Ph.C. for microscopy assistance. This work was generously supported by AA015981 (JFN) and DA00007278 (ARF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alen F, Moreno-Sanz G, Isabel de Tena A, Brooks RD, Lopez-Jimenez A, Navarro M, Lopez-Moreno JA. Pharmacological activation of CB1 and D2 receptors in rats: predominant role of CB1 in the increase of alcohol relapse. Eur. J. Neurosci. 2008;27:3292–3298. doi: 10.1111/j.1460-9568.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Badawy AA. Tryptophan metabolism in alcoholism. Adv. Exp. Med. Biol. 1999;467:265–274. doi: 10.1007/978-1-4615-4709-9_33. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb. Exp. Pharmacol. 2009:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur. J. Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol. Biochem. Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Berglund K, Fahlke C, Berggren U, Eriksson M, Balldin J. Personality profile in type I alcoholism: long duration of alcohol intake and low serotonergic activity are predictive factors of anxiety proneness. J. Neural Transm. 2006;113:1287–1298. doi: 10.1007/s00702-005-0412-3. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Hen R, Olivier B. Ethanol intake is not elevated in male 5-HT(1B) receptor knockout mice. Eur. J. Pharmacol. 2000;403:95–98. doi: 10.1016/s0014-2999(00)00527-6. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol. Biochem. Behav. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Casu MA, Pisu C, Lobina C, Pani L. Immunocytochemical study of the forebrain serotonergic innervation in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:341–351. doi: 10.1007/s00213-003-1663-z. [DOI] [PubMed] [Google Scholar]
- Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007:86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat. Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- David V, Segu L, Buhot MC, Ichaye M, Cazala P. Rewarding effects elicited by cocaine microinjections into the ventral tegmental area of C57BL/6 mice: involvement of dopamine D1 and serotonin1B receptors. Psychopharmacology (Berl) 2004;174:367–375. doi: 10.1007/s00213-003-1767-5. [DOI] [PubMed] [Google Scholar]
- De Montis MG, Grappi S, Gambarana C, Leggio B, Nanni G, Scheggi S, Tagliamonte A. Sardinian alcohol-preferring rats show low 5-HT extraneuronal levels in the mPFC and no habituation in monoaminergic response to repeated ethanol consumption in the NAcS. Brain Res. 2004;1006:18–27. doi: 10.1016/j.brainres.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann. N. Y. Acad. Sci. 2006;1094:193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- Fehr C, Grintschuk N, Szegedi A, Anghelescu I, Klawe C, Singer P, Hiemke C, Dahmen N. The HTR1B 861G>C receptor polymorphism among patients suffering from alcoholism, major depression, anxiety disorders and narcolepsy. Psychiatry Res. 2000;97:1–10. doi: 10.1016/s0165-1781(00)00215-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol. Psychiatry. 2008;63:207–213. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, New Jersey: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Gill K, Amit Z, Smith BR. The regulation of alcohol consumption in rats: the role of alcohol-metabolizing enzymes-catalase and aldehyde dehydrogenase. Alcohol. 1996;13:347–353. doi: 10.1016/0741-8329(96)00006-7. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Aissi F, Batel P, Ades J, Cohen-Salmon C, Hamon M, Boni C, Lanfumey L. Reappraisal of the serotonin 5-HT(1B) receptor gene in alcoholism: of mice and men. Brain Res. Bull. 2002;57:103–107. doi: 10.1016/s0361-9230(01)00641-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res. 2007;1138:208–213. doi: 10.1016/j.brainres.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. USA. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur. J. Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Rockville, MD: National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 1–13. [Google Scholar]
- Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J. Neural Transm. 2002;109:513–521. doi: 10.1007/s007020200042. [DOI] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol. Clin. Exp. Res. 2004;28:588–597. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding YS, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol. Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, C.o.L.S., National Research Council. Guide for the Care and Use of Laboratory Animals. NATIONAL ACADEMY PRESS; 1996. [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J. Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch. Gen. Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc. Assoc. Am. Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Liang KaZ, SL Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Maurel S, Schreiber R, De Vry J. Role of 5-HT1B, 5-HT2A and 5-HT2C receptors in the generalization of 5-HT receptor agonists to the ethanol cue in the rat. Behav. Pharmacol. 1998;9:337–343. [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin and ethanol preference. Recent Dev. Alcohol. 1989;7:187–209. doi: 10.1007/978-1-4899-1678-5_10. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes Brain Behav. 2009;8:346–355. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab M, Haghparast A, Hassanpour-Ezatti M. Effects of reversible inactivation of the ventral tegmental area on the acquisition and expression of morphine-induced conditioned place preference in the rat. Behav. Brain Res. 2009;198:466–471. doi: 10.1016/j.bbr.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Mokrovic G, Matosic A, Hranilovic D, Stefulj J, Novokmet M, Oreskovic D, Balija M, Marusic S, Cicin-Sain L. Alcohol dependence and polymorphisms of serotonin-related genes: association studies. Coll. Antropol. 2008;32 Suppl 1:127–131. [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol. Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol. Sci. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J. Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J. Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J. Pharmacol. Exp. Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- Paxinos GaW, Charles . The Rat Brain: in Stereotaxic Coordinates. Third edn. Academic Press; 1986. 2nd edition (November 1986) [Google Scholar]
- Pierucci-Lagha A, Feinn R, Modesto-Lowe V, Swift R, Nellissery M, Covault J, Kranzler HR. Effects of rapid tryptophan depletion on mood and urge to drink in patients with co-morbid major depression and alcohol dependence. Psychopharmacology (Berl) 2004;171:340–348. doi: 10.1007/s00213-003-1588-6. [DOI] [PubMed] [Google Scholar]
- Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, Ansseau M. The short allele of the serotonin transporter promoter polymorphism influences relapse in alcohol dependence. Alcohol Alcohol. 2008;43:398–400. doi: 10.1093/alcalc/agn015. [DOI] [PubMed] [Google Scholar]
- Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol. Clin. Exp. Res. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am. J. Med. Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Risinger FO, Bormann NM, Oakes RA. Reduced sensitivity to ethanol reward, but not ethanol aversion, in mice lacking 5-HT1B receptors. Alcohol. Clin. Exp. Res. 1996;20:1401–1405. doi: 10.1111/j.1530-0277.1996.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Doan AM, Vickrey AC. Oral operant ethanol self-administration in 5-HT1b knockout mice. Behav. Brain Res. 1999;102:211–215. doi: 10.1016/s0166-4328(99)00012-1. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W, Leffel JK, Chester JA, Froehlich JC. Effects of forced alcohol drinking on alcohol-water choice in three pairs of rat lines selectively bred for differences in alcohol preference. Alcohol. 2009;43:105–118. doi: 10.1016/j.alcohol.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, O'Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol. Biochem. Behav. 2000;66:129–136. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Wilson AW, Costall B, Neill JC. Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J. Psychopharmacol. 2000;14:340–346. doi: 10.1177/026988110001400402. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Feng MJ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Res. 2005;1060:126–137. doi: 10.1016/j.brainres.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res. 2004;1021:82–91. doi: 10.1016/j.brainres.2004.06.053. [DOI] [PubMed] [Google Scholar]