Abstract
Background
Di-(2-ethylhexyl)-phthalate (DEHP) is a widely used plasticizer that imparts flexibility to polyvinyl chloride. We have recently reported that clinically relevant concentrations of DEHP can affect electrical coupling between cardiac myocytes causing significant rhythm disturbances. The underlying causes for this effect are currently unknown.
Objectives
To use data on global mRNA expression as a tool to reveal possible pathways leading to arrhythmogenic effects of DEHP.
Methods
Rat neonatal cardiomyocyte were treated with 50 μg/mL DEHP for 72h. Extracted RNA samples were hybridized onto Affymetrix Rat Gene 1.0 ST arrays. The mRNA expression of a subset of genes was validated by qRT-PCR. In a second set of experiments, cells were treated in a concentration dependent manner to identify genes affected by low DEHP concentrations.
Results
DEHP exposure is associated with global changes in mRNA expression, with differentially expressed genes overrepresented in 47 Gene Ontology categories. Modified expression was detected for genes associated with cell electrical activity, calcium handling, adhesion and microtubular transport. For a number of key proteins, including kinesin, TGFβ2, α-tubulin, and α1 & β1 integrins, changes in mRNA levels were confirmed on the level of the protein expression. A number of genes associated with cell adhesion and electrical activity were identified as early DEHP targets as they were affected by concentrations as low as 1 μg/mL.
Conclusions
Exposure of neonatal rat cardiomyocytes to clinically relevant DEHP concentrations leads to global changes in mRNA expression. These changes help to explain arrhythmogenic effects of phthalates on these cells.
Keywords: Cardiac myocyte, microarray, gene expression, phthalate, connexin-43, calcium handling, arrhythmia
INTRODUCTION
Phthalates are used in a large variety of household and medical products as it allows stiff plastics, such as polyvinyl chloride, to become more flexible. Di-(2-ethylhexyl) phthalate (DEHP) is the most common phthalate used in PVC for medical devices and may represent up to 40% of the finished weight of the plastic. DEHP is highly hydrophobic, and as a result, it leaches from plastics following contact with blood, serum, and other lipophilic fluids (Jenke 2006). Phthalate leaching is a source of concern for human health, particularly in neonatal intensive care units (Loff, et al. 2000, Shea 2003, Sjoberg, et al. 1985). There are two main reasons for this. First, critically ill neonates undergo multiple medical interventions over a prolonged period of time and these procedures frequently employ the use of stored fluids for transfusion (nutritional, pharmacological, blood products) and flexible tubing. Multiple medical interventions can result in high DEHP exposure. It is estimated that neonatal intensive care patients can have a 26-fold higher phthalate exposure as compared to the average environmental exposure for children (Calafat, et al. 2004). The second reason is a curtailed glucuronidation pathway, which is not fully established in young children. Because glucuronidation facilitates urinary excretion of phthalates, and other xenobiotics, underdevelopment of this pathway increases the duration of exposure due to slow excretion (Leeder and Kearns 1997). Indeed, various regulatory agencies and advisory groups have expressed concern about the potential for DEHP to have adverse effects on neonates (Jahnke, et al. 2005, Kavlock, et al. 2002). There is a general awareness regarding carcinogenic, reproductive and developmental effects of phtalates (FDA: Center for Devices and Radiological Health 2002, Jahnke, et al. 2005, Shea 2003). In contrast, one rarely mentions the adverse effects of phtalates on the heart. Yet, negative inotropic or chronotropic effects have been observed following intravenous administration of DEHP or its primary metabolite, mono-(2-ethylhexyl) phthalate (MEHP), to experimental animals (Rock, et al. 1987), isolated heart preparations (Petersen, et al. 1975) and human myocardial tissue (Barry, et al. 1990). DEHP perfusion of the isolated rat heart preparation resulted in electrophysiological changes as well, notably, prolongation of the PR and QT intervals (Aronson, et al. 1978). Our recent in vitro study in rat neonatal cardiomyocytes revealed the adverse affect of DEHP on conduction velocity, network synchronicity and monolayer mechanical motion (Gillum, et al. 2009). Paradoxically, while DEHP decreased the amount of junctional connexin 43 protein, the levels of its mRNA were not affected. The data clearly pointed to multiple pathways that can be affected by DEHP exposure. The goal of this study was to determine whether DEHP induces global alterations in cardiomyocyte mRNA expression and, if so, whether these alterations can be linked to the observed changes in cell behavior. More specifically, we sought to identify molecular pathways affected by DEHP that might lead to cardiomyocyte electrical uncoupling and changes in monolayer motion and adhesion. To ensure future clinical relevance of our in vitro findings, DEHP was applied at doses and for durations that were comparable to neonatal exposure in a clinical setting (Karle, et al. 1997, Loff, et al. 2000, Sjoberg and Bondesson 1985, Sjoberg, et al. 1985).
MATERIALS & METHODS
Chemicals
Collagenase II was obtained from Worthington (Freehold, NJ). Media and porcine trypsin were obtained from Gibco BRL (Grand Island, NY). Fluo-4, nuclear stain and Trizol was purchased from Invitrogen (Eugene, OR). Cy3-conjugated AffiniPur fab fragment (donkey anti-goat or goat anti-mouse) and DyLight 488-conjugated AffiniPur fab fragment (donkey anti-rabbit) were purchased from Jackson ImmunoResearch (West Grove, PA). Glyceraldehyde-3-Phosphate Dehydrogenase antibody was purchased from Millipore (Billerica, MA). Kinesin 20a, TGFβ2, α-Integrin, and β-Integrin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Affinityscript QPCR cDNA kit and SYBR green mastermix were purchased from Stratagene (La Jolla, CA). Connexin-43, α-Tubulin, DEHP (Lot #112K3730) and all other chemicals were obtained from Sigma Chemical (St. Louis, MO) unless specified otherwise.
Cardiomyocyte culture
All experiments were performed according to the Institutional Animal Care and Use Committee of the George Washington University Medical Center, which follows Federal and State guidelines. Cardiomyocytes from 1- and 2-day old Sprague-Dawley rats were obtained by a modified enzymatic digestion procedure (Arutunyan, et al. 2001). Hearts were removed and rinsed in a cold, calcium- and magnesium-free, Hank’s Buffered Salt Solution (CMF-HBSS), and then minced into ~ 1 mm3 pieces. Tissue pieces were incubated overnight at 4°C in fresh CMF-HBSS containing 0.1 mg/mL trypsin. The next day, heart tissue was washed with fresh CMF-HBSS and treated with 0.4 mg/ml soybean trypsin inhibitor. The tissue was then collected in Leibovitz’s medium containing 0.8 mg/ml collagenase II and shaken for 30 min at 37°C. The cells were then gently triturated, passed through a cell strainer to remove any undigested pieces, and centrifuged for 5 min at 17.5 g. The pellet was resuspended in Dulbecco modified essential medium (DMEM) supplemented with 10% Fetal Bovine Serum and pre-plated for an hour to minimize the presence of fibroblasts, which attach more rapidly than myocytes. Unattached cells were then collected, counted and plated in a culture dish containing 25 mm laminin-coated glass coverslips (105 cells/cm2). Myocytes were then kept under standard culture conditions in DMEM, supplemented with 5% Fetal Bovine Serum, 10 U/ml penicillin and 1 μg/ml streptomycin.
Experimental Protocol
On the third day after cell plating, cardiomyocytes formed an interconnected confluent network that exhibited rhythmic spontaneous contractions. A single dose of concentrated 50 mg/ml DEHP stock in DMSO was added directly to the cell media to achieve a final concentration of 50 μg/ml DEHP (Gillum, et al. 2009). Cardiomyocytes were treated with DEHP for 72 hours, before conducting all subsequent experiments, including: calcium transient recordings, microarray analysis, qRT-PCR, immunocytochemistry and western blot analysis. The final concentration of DMSO in DEHP-treated samples and the corresponding controls was 0.1%. Cardiomyocytes were visualized daily to monitor the appearance and beating behavior of the myocyte network. In a second set of experiments, cardiomyocytes were treated in a concentration-dependent manner (0, 1, 10 and 50 μg/ml DEHP) prior to microarray analysis, to determine whether mRNA expression is altered at lower concentrations.
Microarray sample preparation
Two sets of microarray experiments were performed, the first utilized 6 coverslips of cardiomyocytes for each treatment group (control, 50 μg/ml DEHP) and the second utilized 3 coverslips for each treatment group (0, 1, 10 and 50 μg/ml DEHP). Extensive analysis was conducted using the first set of microarray hybridizations, which enabled us to thoroughly examine the physiological effects previously reported at this concentration by our laboratory. To minimize variability, the same litter of pups was used to prepare cell monolayers for control and DEHP treated groups for each microarray experiment. RNA was isolated from cell preparations using Trizol, per the manufacturer’s instructions, and samples were treated with DNase. Samples were transferred to The Catherine Birch McCormick Center, where RNA integrity was validated and samples were processed for microarray chip hybridization.
Affymetrix gene arrays
To maximize our ability to reveal DEHP-specific changes each microarray experiment used the same litter of pups to compare control and DEHP-treated coverslips. Each coverslip was treated and processed independently. Microarray data processing, normalization (i.e., multi-array analysis [RMA]), statistical analyses and data visualization were accomplished using Genespring GX 10 software. Principle Components Analysis (PCA) demonstrated that the experimental groups of control and DEHP-treated samples were well separated by their mRNA expression profiles. Assigned groupings were also validated by examining inter-sample correlations, which demonstrated that replicates within an experimental group correlated best to other replicates within the same group. Hybridization spike-in control transcripts (i.e., BioB, BioC, BioDx, CreX) were included in the hybridization experiments, which allow one to determine whether the in vitro transcription reaction, hybridization and washing steps were performed similarly across arrays. There were no significant differences in hybridization signal intensities for the controls, indicating that there were no methodological or quality control problems in the hybridization experiments.
Ingenuity pathway analysis is a bioinformatics resource that algorithmically identifies gene networks and canonical pathways based on millions of experimentally validated interactions between proteins, genes, complexes, cells, tissues, drugs, and diseases (www.ingenuity.com; Redwood, CA). A list of differentially expressed genes consisting of unique identifiers (e.g., RefSeq, GenBank accession, Affymetrix ID) and expression values was uploaded into the IPA knowledge database. To identify gene networks, IPA rank orders each gene in the list based on its interconnectedness with other genes (triangle connectivity). Next, the top interconnected genes (focus genes) are linked together as subnetworks based on specific connectivity (the extent that gene-gene connections overlap between top focus genes), and then combined with smaller subnetworks of focus genes with lower interconnectivity through the addition of linker genes (found either in the uploaded list or IPA’s Global Molecular Network) to produce a network.
Calcium transient recording
Cells were loaded with 5 μM Fluo-4 and calcium transients were monitored at room temperature using a Zeiss LSM 510 confocal imaging system with standard 488 nm excitation/505–530 nm emission settings. Measurements were conducted in spontaneously beating cardiomyocyte cultures following 72 hour treatment with either 50 μg/ml DEHP or 0.1% DMSO control.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR (qRT-PCR) validation of microarray results were performed on an ABI Prism 7700 Sequence Detection System, as previously described (Malek, et al. 2002). Each RNA sample from cultured cardiomyocytes provided sufficient material (10–15 μg) for hybridization experiments and qRT-PCR validation assays. Total RNA was reverse transcribed using random primers as per the manufacturer’s protocol. The resulting cDNA was diluted and used as a template for qRT-PCR. Primers were selected for specificity by NCBI BLAST of the rat genome and amplicon specificity was verified by first derivative melting curve analysis using software provided by Perkin-Elmer/Applied Biosystems. Quantitation and normalization of relative gene expression was accomplished using the comparative CT method or ΔΔCT. ΔΔCT values were converted into ratios by 2−ΔΔCT and averaged across biological replicates. The expression of the “housekeeping” gene glutamate dehydrogenase was used for normalization, as this gene typically does not exhibit differential expression in our microarray assays.
Immunocytochemistry
Cardiomyocytes were fixed using a standard 4% paraformaldehyde protocol, followed by staining with connexin-43 (1:500), kinesin 20a (1:50), α-tubulin (1:800), TGFβ2 (1:50), or nuclear stain (1:300). Samples were incubated with either Cy3-conjugated AffiniPur fab fragment (donkey anti-rabbit, donkey anti-goat) or donkey anti-rabbit Dylight 488-conjugated AffiniPur fab fragment (1:1000). Images were acquired and analyzed using a Zeiss LSM 510 confocal imaging system with dye-specific filter settings.
Western blot analysis
Cells were harvested in homogenization buffer containing 250 mM sucrose, 20 mM Hepes, 1% Sodium dodecyl sulfate, 1% Triton-X100, 2 mM Dithiothreitol, 2 mM Ethylenediaminetetraacetic acid, 2 mM Ethylene-bis(oxyethylenenitrilo)tetraacetic acid, 10 mM b-Glycerophosphate, 1 mM Orthovanadate, 2 mM Phenylmethylsulfonyl fluoride, 20 μg/mL Leupeptin, 10 ug/mL Aprotinin, and 5 ug/mL Pepstatin. Cells were incubated on ice in homogenization buffer for 15 min. Samples were sonicated three times for 15 sec intervals on ice (Sonic Dismembrator, Fisher Scientific), centrifuged to clarify, and equal amounts of protein were loaded onto precise 4–20% gradient gels (Pierce). Blots were probed with α-integrin (1:500), β-integrin (1:500), α-tubulin (1:500), kinesin (1:200), TGFβ2 (1:200), and GAPDH (1:1000) for loading normalization. Blots were incubated with either goat anti-mouse, goat anti-rabbit or donkey anti-goat IgG AP conjugate (1:3000). Relative protein expression was assessed using a STORM 860 PhosphorImager.
RESULTS
Physiological changes in DEHP-treated cardiomyocytes
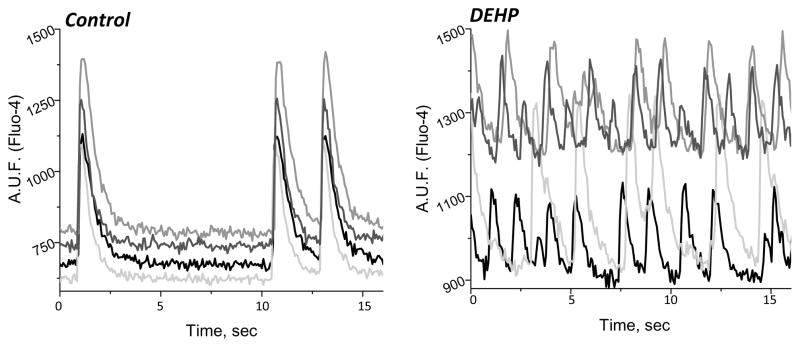
We previously reported that delays in conduction velocity become more severe with increasing DEHP concentration and/or exposure time, which ultimately leads to a loss of cardiac network synchronicity (Gillum, et al. 2009). Figure 1 illustrates the physiological effects of phthalate exposure upon treating confluent cardiomyocyte layers with 50 μg/mL DEHP for 72 hours. In control samples, cardiomyocytes were beating synchronously and exhibited homogenous activation wavefronts with fast conduction velocity. In contrast, DEHP-treated myocytes showed marked uncoupling between different regions of the cell monolayer, leading to slow propagation of fractured wavefronts (Gillum, et al. 2009). This effect was attributed to a smaller amount of punctuated connexin-43 staining in DEHP-treated samples. In addition, DEHP-treated samples exhibited an unusual “waterbed”-like pattern of motion (Gillum, et al. 2009). The latter could not be explained by diminished electrical coupling, as pharmacological inhibition of gap junctions did not reproduce this effect. To reveal the multiple pathways that are affected by DEHP exposure we sought to comprehensively assess mRNA expression, as detailed below.
Figure 1. Physiological changes in cardiomyocyte behavior caused by DEHP (50 μg/mL) treatment.
(A) Calcium transient measurements were recorded from 4 regions of interest on a cardiomyocyte monolayer using Fluo-4, a calcium indicator dye. DEHP treatment (50 μg/mL) results in marked uncoupling between the different regions of the cell network (right) compared with untreated control samples (left).
Genome-wide gene expression in DEHP treated samples
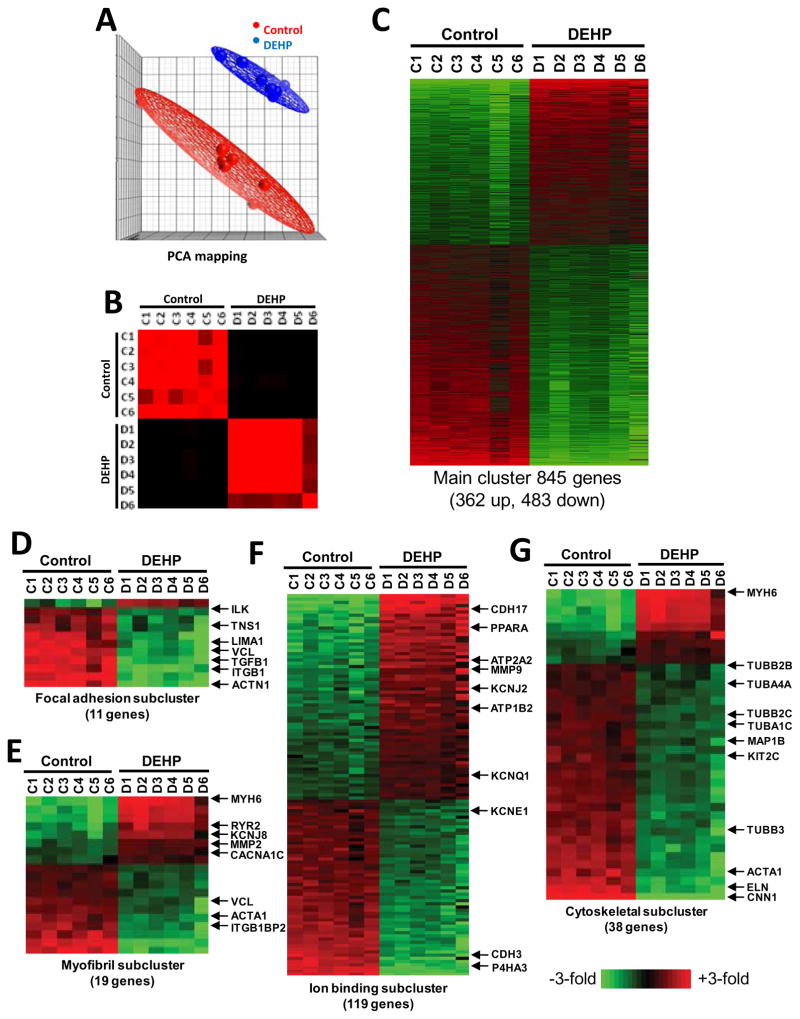
For the first set of experiments, six coverslips of cardiomyocytes were used for each treatment group (control, 50 μg/mL DEHP) to isolate total RNA and process for microarray chip hybridization. Array data was imported into GeneSpring GX 10 and principle components analysis demonstrated that the two experimental groups of control (red spheres) and DEHP samples (blue spheres) were well separated by their mRNA expression profiles (Fig 2A). Assigned groupings were also validated by examining inter-sample correlations, which demonstrated that replicates within an experimental group correlated best to other replicates within the same group (Fig 2B). A t-test with 10% false discover rate to correct for multiple testing (Benjamini and Hochberg 1995) was then employed to identify statistically significant gene expression differences between control and 50 μg/mL DEHP-treated cardiomyocytes. At a 1.5-fold expression difference cut-off, a total of 845 mRNAs were differentially expressed. Of these 845 differentially expressed mRNAs, 362 were up-regulated and 483 were down-regulated by DEHP treatment (Fig 2C). The expression differences (up or down) ranged from 1.5 to 11-fold. Gene Ontology (GO) analysis revealed that differentially expressed genes were significantly over-represented in 47 GO categories of which four are shown (P<0.05 after adjustment for multiple testing. Categories of relevance to physiological changes observed after DEHP treatment of cardiac myocytes included: focal adhesion (GO:0005925, GO:0008357), myofibril (GO:0030016), ion binding (GO:0043167) and cytoskeletal (GO:0005856) (Fig 2D–G).
Figure 2. Microarray profiling of control and DEHP-treated (50 μg/mL) cardiomyocytes using gene and theme-based approaches.
(A) Principle components analysis shows that the two treatment groups (control, DEHP) have different mRNA expression profiles. Control samples are shown as red dots and samples corresponding to cardiomyocytes treated with 50 μg/ml DEHP are shown in blue. (B) Assigned groupings were also validated by examining inter-sample correlations, which demonstrated that replicates within an experimental group correlated best to other replicates within the same group. (C) A t-test with 10% false discover rate (FDR) to correct for multiple testing was employed to identify statistically significant gene expression differences between control and DEHP-treated cardiomyocytes. At a 1.5-fold expression difference cut-off, a total of 845 mRNAs were differentially expressed. Of these 845 differentially expressed mRNAs, 362 were up-regulated and 483 were down-regulated by DEHP treatment. Gene Ontology (GO) analysis revealed that differentially expressed genes were significantly over-represented in 47 GO categories of which 4 are shown, including: focal adhesion (D), myofibril (E), ion binding (F), and cytoskeletal (G). Examples of key genes of interest are noted to the right of each GO category (p<0.05 after adjustment for multiple testing).
Microarray validation
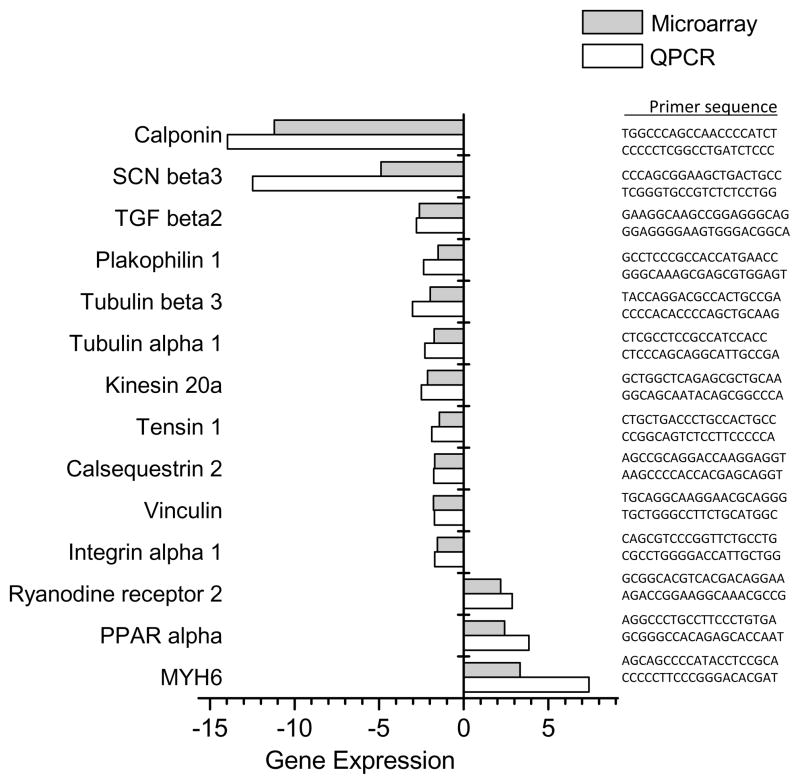
Differential expression of a subset of relevant genes was validated using qRT-PCR (Fig 3). PCR primers were selected for specificity by NCBI BLAST of the rat genome and amplicon specificity was verified by first derivative melting curve analysis using software provided by Perkin-Elmer/Applied Biosystems. Data obtained from qRT-PCR analysis strongly correlated with that of microarray data (Fig 3). Validated genes included those related to calcium handling (ryanodine receptor, calsequestrin, calponin, cardiac myosin), ion channels (sodium voltage-gated channels, potassium rectifier channels), cell adhesion (tensin, vinculin, plakophilin, integrin) and genes linked to connexin-43 expression and/or transport (tubulin, kinesin, TGFβ2).
Figure 3. Quantitative real-time PCR validation of microarray data.
RNA was isolated from cell preparations using Trizol and samples were DNase treated. Each RNA sample provided sufficient material for hybridization experiments and quantitative real-time PCR. Primers were selected for specificity using NCBI Blast, and glutamate dehydrogenase was used for normalization (forward: CTCTGCTGTCCCGCAACCCG, reverse: GTCGTCTTCGCGGTCGGTGG). Fold change data were calculated from quantitative real-time PCR experiments using the delta-delta-CT method and normalized to control value (white bars). Changes in gene expression were correlated with data obtained from microarray hybridizations (gray bars). Corresponding primer sequences are listed to the right of each sample.
Western blot and immunocytochemistry validation
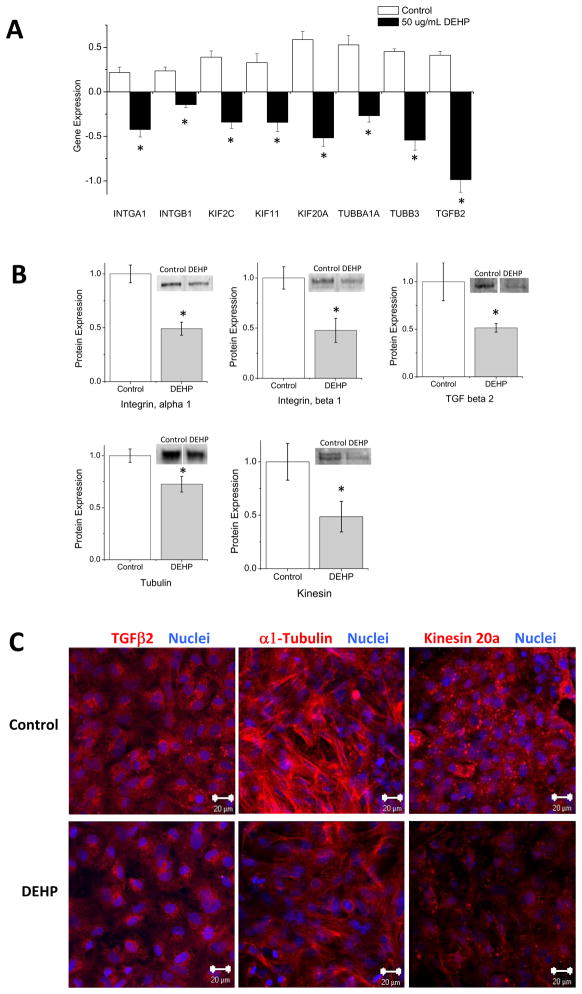
For most relevant targets we used alternative means of validation to determine whether changes in mRNA expression correlated with modifications in protein expression and/or localization. Differential mRNA expression of tubulin and kinesin genes correlated with a reduction in protein expression (Fig 4A,B). This downregulation may translate to a decrease in microtubule formation and/or motor protein trafficking. Modified expression was confirmed in a number of other proteins of interest, including TGFβ2, α1-Integrin and β1-Integrin, all of which correlated with the altered mRNA expression first revealed by microarray analysis and validated by qRT-PCR. Immunocytochemistry was used to visually verify these changes in protein expression and/or localization (Fig 4C).
Figure 4. Changes in gene expression correlate with modification in total protein expression.
(A) Analysis of microarray data revealed changes in mRNA expression following DEHP treatment (50 μg/mL). Integrin α1 (INTGA1), integrin β1 (INTGB1), kinesin 2C (KIF2C), kinesin 11 (KIF11), kinesin 20a (KIF20A), tubulin α1α (TUBBA1A), tubulin β3 (TUBB3), transforming growth factor beta 2 (TGFB2) mRNA expression was decreased following DEHP treatment. (B) Western blot analysis was performed on control and DEHP-treated (50 μg/mL) cardiomyocytes to verify that differential gene expression correlated with protein expression. Samples were quantitated and normalized to GAPDH. Control samples significantly overexpress α1-Integrin, β1-Integrin, α-Tubulin, TGFβ2 and total kinesin relative to DEHP samples. (C) Immunocytochemistry of control and DEHP-treated (50 μg/mL) cardiomyocytes showed less staining for TGFβ2 (left), α-Tubulin (middle), and kinesin 20a (right) in DEHP samples. Images were acquired using a Zeiss LSM 510 confocal system. (Nuclei = blue, size bar = 20 μm) (*p < 0.05).
Gene network analysis
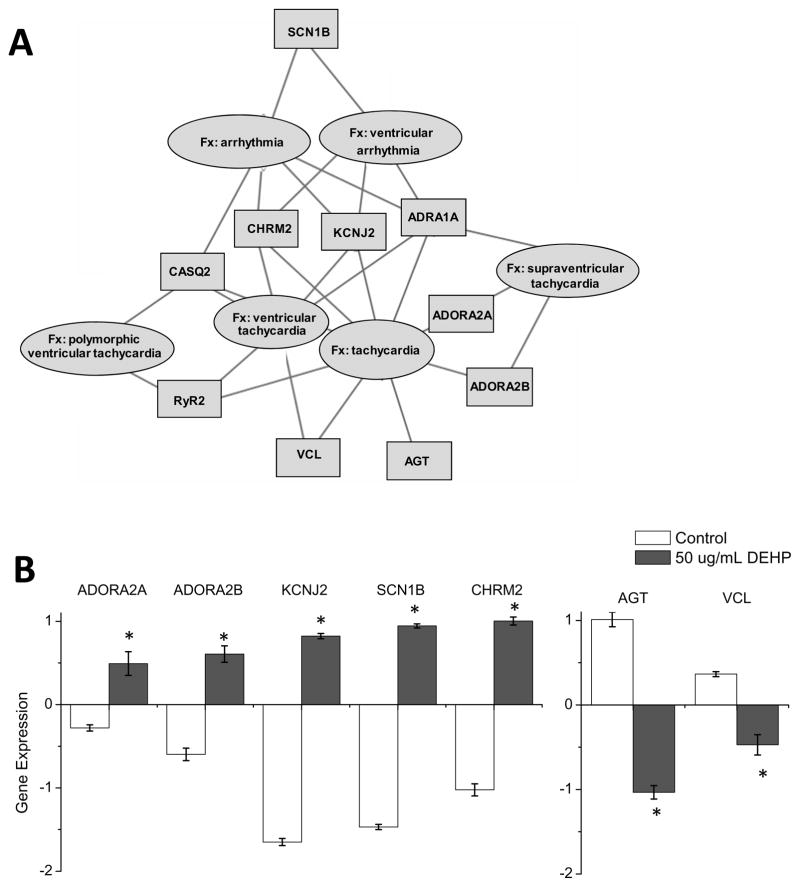
To identify gene networks associated with DEHP treatment, differentially expressed genes identified in Fig 2 were imported into Ingenuity Pathway Analysis (IPA) for network analysis. IPA represents a higher order analysis strategy that can be employed to identify biological themes associated with gene expression information (Hu, et al. 2009). A number of gene networks relevant to myocardial function were identified. The latter included differentially expressed mRNAs associated with cardiac electrical disturbances, including arrhythmia and tachycardia (Fig. 5A). The affected genes included voltage-gated Na+ channels and potassium inward-rectifying channels (Fig 5B). Additional studies will be required to confirm if changes in mRNA expression correlate with modifications in protein expression, localization and/or channel activity.
Figure 5. Alteration of cardiac gene expression is associated with gene networks for electrical disturbances.
Genes with altered expression following treatment with DEHP (50 μg/mL) were analyzed by Ingenuity pathway analysis. (A) The cartoon displays differentially expressed mRNAs that are associated with disturbances in electrical activity (i.e., tachycardia and arrhythmia). (B) Raw data for a few of the differentially expressed genes, normalized to control samples. Adenosine a2a receptor (ADORA2A), Adenosine a2b (ADORA2B), potassium-inward rectifying channel (KCNJ2), sodium voltage-gated channel (SCN1B) and the cholinergic muscarinic channel isoform 2 (CHRM2) were all overexpressed in DEHP-treated (50 μg/mL) samples. Angiotensinogen (AGT) and vinculin (VCL) displayed decreased expression following DEHP treatment. (*p ≤ 0.05)
Modification of calcium handling
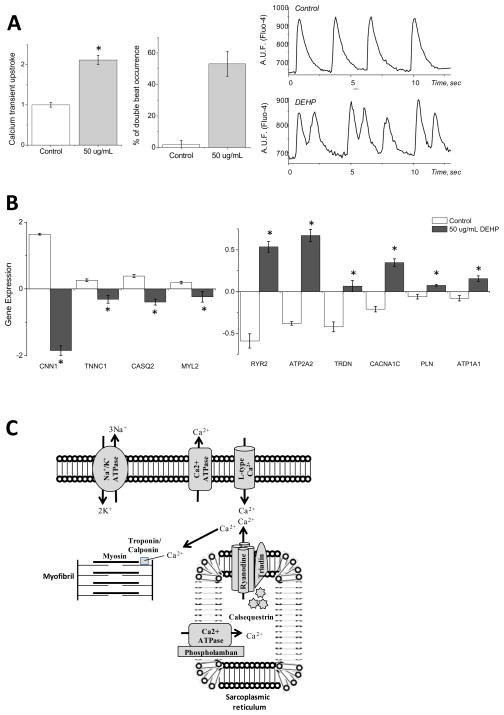
A comparison between the morphology of calcium transients in control and 50 μg/mL DEHP-treated samples revealed a significant prolongation in transient duration and an increase in the incidence of spontaneous double beats (Fig 6A). The latter can lead to triggered activity, pointing to the potential arrhythmogenicity of phthalates via their adverse effects on calcium handling. Indeed, microarray analysis revealed significant changes in a number of key calcium handling proteins (Fig 6B, C). DEHP-treated samples displayed diminished gene expression for calponin, troponin C and calsequestrin 2 (Fig 6B, C). Conversely, DEHP-treatment enhanced the expression of other key calcium handling genes, including: ryanodine receptor 2, cardiac calcium transporting ATPase, triadin, voltage-dependent l-type calcium channel, phospholamban, sodium/potassium transporting ATPase and cardiac myosin heavy chain 6 (Fig. 6B&C).
Figure 6. Modifications in gene expression explain calcium handling dynamics in DEHP-treated cardiomyocytes.
(A) Control and DEHP-treated (50 μg/mL) cardiomyocytes were loaded with Fluo-4 to analyze calcium transient morphology. DEHP-treated samples showed a significant increase in calcium transient upstroke duration compared to control (left). In addition, a large number of traces show evidence of double transients, indicating increased arrhythmogenicity (center). Individual calcium transients recorded from control and DEHP-treated cardiomyocytes are shown on the right. (B) Numerous calcium handling genes were shown to be differentially expressed in DEHP-treated samples (50 μg/mL) versus control. Calponin (CNN1), troponin C (TNNC1), calsequestrin 2 (CASQ2) expression were decreased following DEHP treatment. Ryanodine receptor 2 (RYR2), cardiac calcium transporting ATPase (ATP2A2), triadin (TRDN), voltage-dependent l-type calcium channel (CACNA1C), phospholamban (PLN), sodium/potassium transporting ATPase β2 (ATP1B2) and cardiac myosin heavy chain (MYH6) expression were increased following DEHP treatment. (C) Cartoon illustrating key players involved in calcium handling within cardiomyocytes, which are modified following DEHP exposure.
Modified cell layer motion
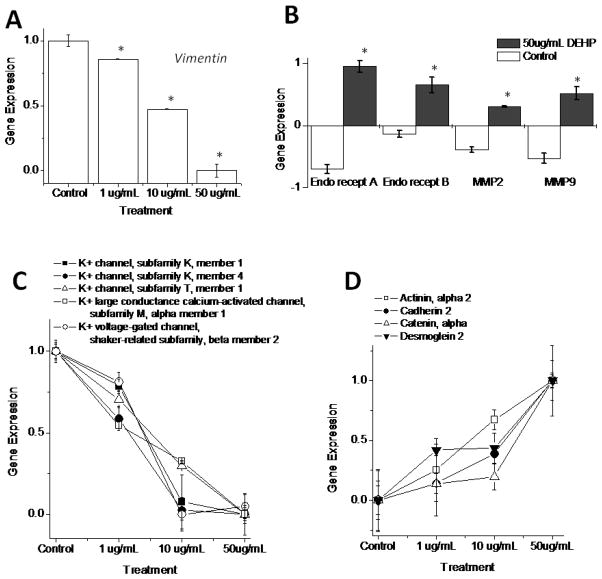
In addition to electrical disturbances, DEHP treatment led to an unusual pattern of monolayer contractile motion [nicknamed “waterbed” effect due to its visual appearance (Gillum, et al. 2009). This observation suggested a decrease in cell attachment to the underlying surface, although the molecular mechanisms behind this effect are currently unknown. We previously showed that DEHP diminishes the expression of triton-insoluble vimentin protein, which constitutes fibroblast intermediate filaments. Indeed, microarray analysis revealed changes in vimentin gene expression (Fig 7A). A second explanation for the observed changes in contractile monolayer motion is enhanced endothelin receptor (EDNRA, EDNRB) expression in DEHP-treated samples (Fig 7B). Indeed, both matrix metalloproteinase 2 and 9 (MMP2, MMP9) were upregulated in DEHP-treated cardiomyocytes (Fig 7B).
Figure 7. Genes targeted at low concentrations of DEHP are linked to multiple pathways, including: cell motion, cell adhesion and ionic channels.
(A) Vimentin gene expression decreased in a concentration-dependent pattern following DEHP exposure (1–50 μg/mL). (B) Endothelin receptors have been shown to influence the expression of connexin-43 and matrix metalloproteinases. Upregulation of endothelin receptors (ETA, ETB) and gelatinase matrix metalloproteinase genes (MMP2, MMP9) were observed in DEHP-treated (50 μg/mL) samples compared with control. (C) The gene expression of numerous potassium channels was decreased following DEHP exposure in a concentration-dependent manner (1–50 μg/mL). These include the potassium channels KCNK1, KCNK4 and KCNT1, as well as the potassium large conductance calcium-activated channel (KCNMA1) and the potassium voltage-gated channel (KCNAB2). (D) Multiple cellular adhesion genes were also modified in a concentration-dependent manner, following DEHP treatment. These include α2-actinin (ACTN2), cadherin-2 (CDH2), α-catenin (CTNNA1) and desmoglein-2 (DSG2). (*p ≤ 0.05)
Concentration-dependent responses
A second set of microarray experiments were conducted to determine whether DEHP induces changes in mRNA expression at concentrations lower than 50 ug/mL. In these experiments, three coverslips of cardiomyocytes were used per treatment group (0, 1, 10 and 50 μg/mL DEHP). Differential mRNA expression was observed in all gene families of interest, including those related to calcium handling, protein trafficking, cell motility and arrhythmogenesis. Many of these genes were altered at 10 ug/mL DEHP dose, however a few were altered at concentrations as low as 1 ug/mL. These included vimentin (Fig 7A), potassium ion channels (Fig 7C) and genes involved in mechanical adhesion junctions (Fig 7D). One can suggest using these genes as early indicators of DEHP effects on cardiac tissue.
DISCUSSION
DEHP is found in a variety of medical products, including: bags and tubing for the administration of blood, plasma, intravenous fluids, and total parenteral nutrition; nasogastric and enteral feeding tubes, umbilical catheters, and tubing used in hemodialysis, cardiopulmonary bypass, and extracorporeal membrane oxygenation (ECMO) blood circuits. Since DEHP is lipophilic, it will readily partition from the PVC medical device into whole blood, plasma, platelet concentrate, lipid-containing fluids (such as IV lipid emulsion), total parenteral nutrition solution, and solutions containing Polysorbate 80 and other formulation aids used to solubilize many intravenous medications (Jenke 2006). Therefore, DEHP exposure increases dramatically with multiple medical interventions and whole blood products can reach as high as 620 μg/mL DEHP concentration (FDA: Center for Devices and Radiological Health 2002). Notably, in our studies DEHP was applied at doses and for durations that were comparable to neonatal exposure in a clinical setting (Karle, et al. 1997, Loff, et al. 2000, Sjoberg and Bondesson 1985, Sjoberg, et al. 1985).
The main focus of this paper was to demonstrate that DEHP exposure causes differential expression of genes that can be associated with cardiac arrhythmias. Yet, many other pathways are also differentially expressed in response to DEHP. Affected GO categories included cholesterol & sterol biosynthetic and metabolic processes, which is in perfect agreement with numerous reports about endocrine effects of DEHP in a variety of cells and tissues (Kavlock, et al. 2002). The cell cycle and regulation of cell growth were also on the list of significantly affected GO categories. Cardiac myocytes are terminally differentiated cells and as of today there are no published data linking DEHP and myocardial hypertrophy. Yet, the above mentioned data suggests that such a link could be further explored. Other major class of affected GO categories pointed to genes governing focal and cell-substrate adhesion, actin filaments and stress fibers (stress fibers connect to the sites where cell anchoring occurs, i.e. focal adhesions). This finding is in good agreement with our previous observations that monolayers of DEHP-treated myocytes exhibit dramatically different patterns of contractile motion (Gillum, et al. 2009). Our future studies will systematically compare DEHP affected GO categories including PCR & western blot based validation of altered protein expression together with parallel measurements of associated phenotypical changes.
Connexin-43 trafficking & localization
Microarray data confirmed our previous PCR findings that DEHP treatment does not diminish the level of connexin-43 mRNA, despite the dramatic decrease in gap-junctional connexin-43 protein (Gillum, et al. 2009). Connexin-43 is a very dynamic protein that requires assembly, trafficking to the cell membrane, stabilization and eventually turnover; the average lifespan of connexin-43 protein is a mere 1.5 hours (Lauf, et al. 2002). DEHP exposure produced changes in a number of tubulin (TUBB2B, TUBB2C, TUBB3, TUBA1A, TUBA1B, TUBA1C, TUBA4A) and kinesin genes (KIF2C, KIF4, KIF5C, KIF11, KIF18A, KIF20A). A decrease in the mRNA expression of these genes may explain the diminished gap junctional connexin-43 protein expression. Gap junction hemichannels migrate to the cell membrane via microtubules, which have insertion sites along the cell surface (Lauf, et al. 2002). Heterodimerized alpha- and beta-tubulins form the major component of microtubules. Kinesins are motor proteins that move along microtubules carrying protein cargo toward the plasma membrane(Hirokawa and Noda 2008, Shaw, et al. 2007). Of the differentially expressed mRNAs, all tubulin and kinesins were downregulated in DEHP-treated samples. Indeed, the disruption of microtubules has been shown to reduce connexin-43 incorporation into gap junctions (George, et al. 1999), and phthalate exposure has been shown to alter the organization of microtubules (Nakagomi, et al. 2001). This observation offers the most plausible explanation for the reduced amount of functional connexin-43 protein we observed in DEHP-treated cardiomyocytes (Gillum, et al. 2009). The second mechanism which may explain the observed decrease in gap-junctional connexin-43 protein is a reduction in growth factor secretion. Cardiomyocytes have been shown to release angiotensin II (Tamura, et al. 1998), endothelin-1 (Yamazaki, et al. 1996), vascular endothelial growth factor (Li, et al. 1997) and transforming growth factor-beta (Villarreal and Dillmann 1992). Subsequent upregulation of connexin-43 expression and an increase in conduction velocity has been attributed to secretion of these growth factors (Zhuang, et al. 2000). Microarray data analysis of DEHP-treated samples revealed modifications in the mRNA expression of angiotensinogen (AGT), transforming growth factor-beta (TGFB1, TGFB2, TGFB3), vascular endothelial growth factor (VEGFC, VEGFA) and endothelin-1 (END1) (Figs 2-4&7). Therefore, DEHP-induced alterations in growth factor secretion can be an additional factor that influences connexin-43 translation and/or stability in phthalate-treated samples.
Cardiomyocyte layer motion
Analysis of microarray data suggested possible mechanisms that may explain the effect on monolayer contractile motion caused by DEHP treatment. These include modifications in focal adhesion complexes, endothelin receptors, matrix metalloproteinase (MMP) and vimentin gene expression. Upregulation of endothelin receptors results in remodeling of MMPs and downregulation of connexin-43 through a common pathway (Peng, et al. 2010). These MMPs are termed “gelatinases” and are known to degrade basement membrane proteins (Ahmed, et al. 2006, Malla, et al. 2008). An upregulation in MMP mRNA expression was observed following treatment with DEHP. Such degradation can lead to the “waterbed”-like irregular monolayer motion described previously (Gillum, et al. 2009). Interestingly, an enhanced expression of MMP in Sertoli cells was reported after cell exposure to mono-ethylhexyl phthalate (MEHP), the primary metabolite of DEHP (Yao, et al. 2010).
Our previous studies indicated diminished expression of triton-insoluble vimentin protein expression following DEHP-treatment of cardiomyocyte layers. Vimentin constitutes an essential part of fibroblast intermediate filaments (Wang and Stamenovic 2002). We hypothesized that DEHP effects vimentin expression, which impacts the stiffness of the cardiac fibroblast layer found beneath cardiomyocytes in primary cultures. Indeed, microarray analysis revealed changes in vimentin gene expression in a concentration-dependent manner beginning at 1 μg/mL DEHP.
Calcium handling & arrhythmogenesis
Microarray analysis revealed that DEHP exposure alters the expression of key genes associated with ion channel and calcium handling proteins. This provides an explanation for DEHP-induced changes in calcium transient morphology and the occurrence of multiple double transients observed in DEHP samples. The latter can lead to triggered activity (Gyorke and Terentyev 2008, Katra, et al. 2007), pointing to the potential arrhythmogenicity of phthalates via their adverse effects on calcium handling. DEHP-treated samples displayed diminished gene expression for calponin, troponin C and calsequestrin 2. Downregulation of the calcium binding proteins, calsequestrin and calponin, have been shown to trigger arrhythmia via calcium leak (Chopra, et al. 2007, Gyorke, et al. 2004). Interestingly, Park et al. observed a decrease in calponin gene expression in the insect Chironomus riparius, beginning as early as 1 hour after DEHP exposure (Park and Kwak 2009).
DEHP-treatment enhanced the expression of other key calcium handling genes, including: ryanodine receptor 2, cardiac calcium transporting ATPase, triadin, voltage-dependent l-type calcium channel, phospholamban, sodium/potassium transporting ATPase and myosin heavy chain 6. An influx of calcium through the L-type calcium channel serves as a trigger for calcium-induced calcium release via the ryanodine receptor (Fabiato and Fabiato 1979, Nabauer, et al. 1989)Calcium reuptake kinetics has been shown to influence triggered activity and spontaneous calcium release via the ryanodine receptor (Katra, et al. 2007). Overexpression of triadin has been shown to predispose cardiac myocytes to arrhythmia, via its interaction with the ryanodine receptor (Terentyev, et al. 2005).
Lastly, our studies identified several genes, including members of the potassium channel and mechanical adhesion families, which are significantly affected by DEHP concentrations as low as 1 μg/mL (Fig. 7). PCR assessment of these genes may help to gauge early effects of DEHP on the cardiac function of most susceptible patients.
Conclusion
By assessing global changes in gene expression, we were able to identify several pathways that can be responsible for adverse effect of phthalates on cardiac muscle cells. Our analysis of altered gene expression was based on in vitro exposure of isolated rat cardiac muscle cells to DEHP. Additional steps will be necessary to establish the risk of DEHP-containing tubing in clinical settings. These steps include in vivo DEHP treatment of animals using clinically relevant routes of DEHP exposure, followed by electro- and echocardiographic measurements of cardiac function and validation of these conclusions in human subjects.
Acknowledgments
Acknowledgements & grant information: The authors thank Luther Swift for isolation of cardiomyocytes and Yi Lian and Dr. Sydney Fu for their assistance with microarray processing. This work was supported by the National Institutes of Health (HL076722 to NS, 1F32ES019057 to NGP and CA120316 to NHL) and the GWU Catherine Birch McCormick Center.
List of abbreviations
- DEHP
Di-(2-ethylhexyl) phthalate
- TGF
tumor growth factor
- MEHP
Mono-(2-ethylhexyl) phthalate
- DMSO
Dimethyl sulfoxide
- MMP
Matrix metalloproteinase
- DMEM
Dulbecco modified essential medium
- FBS
Fetal Bovine Serum
- PCA
Principle Components Analysis
- qRT-PCR
Quantitative real-time RT-PCR
- GO
Gene Ontology
Footnotes
Financial interests:
NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- Aronson CE, Serlick ER, Preti G. Effects of di-2-ethylhexyl phthalate on the isolated perfused rat heart. Toxicol Appl Pharmacol. 1978;44:155–169. doi: 10.1016/0041-008x(78)90295-8. [DOI] [PubMed] [Google Scholar]
- Arutunyan A, Webster DR, Swift LM, Sarvazyan N. Localized injury in cardiomyocyte network: a new experimental model of ischemia-reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2001;280:H1905–15. doi: 10.1152/ajpheart.2001.280.4.H1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry YA, Labow RS, Keon WJ, Tocchi M. Atropine inhibition of the cardiodepressive effect of mono(2-ethylhexyl)phthalate on human myocardium. Toxicol Appl Pharmacol. 1990;106:48–52. doi: 10.1016/0041-008x(90)90104-3. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–34. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- FDA: Center for Devices and Radiological Health. Safety Assessment of Di(2-ethylhexyl)phthalate (DEHP) Released from PVC Medical Devices. 2002. [Google Scholar]
- George CH, Kendall JM, Evans WH. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J Biol Chem. 1999;274:8678–8685. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- Gillum N, Karabekian Z, Swift LM, Brown RP, Kay MW, Sarvazyan N. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol Appl Pharmacol. 2009;236:25–38. doi: 10.1016/j.taap.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke S, Gyorke I, Terentyev D, Viatchenko-Karpinski S, Williams SC. Modulation of sarcoplasmic reticulum calcium release by calsequestrin in cardiac myocytes. Biol Res. 2004;37:603–7. doi: 10.4067/s0716-97602004000400014. [DOI] [PubMed] [Google Scholar]
- Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Hu VW, Nguyen A, Kim KS, Steinberg ME, Sarachana T, Scully MA, Soldin SJ, Luu T, Lee NH. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: altered pathways in neuronal development and steroid biosynthesis. PLoS One. 2009;4:e5775. doi: 10.1371/journal.pone.0005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke GD, Iannucci AR, Scialli AR, Shelby MD. Center for the evaluation of risks to human reproduction--the first five years. Birth Defects Res B Dev Reprod Toxicol. 2005;74:1–8. doi: 10.1002/bdrb.20028. [DOI] [PubMed] [Google Scholar]
- Jenke D. Extractable substances from plastic materials used in solution contact applications: an updated review. PDA J Pharm Sci Technol. 2006;60:191–207. [PubMed] [Google Scholar]
- Karle VA, Short BL, Martin GR, Bulas DI, Getson PR, Luban NL, O’Brien AM, Rubin RJ. Extracorporeal membrane oxygenation exposes infants to the plasticizer, di(2-ethylhexyl)phthalate. Crit Care Med. 1997;25:696–703. doi: 10.1097/00003246-199704000-00023. [DOI] [PubMed] [Google Scholar]
- Katra RP, Oya T, Hoeker GS, Laurita KR. Ryanodine receptor dysfunction and triggered activity in the heart. Am J Physiol Heart Circ Physiol. 2007;292:H2144–51. doi: 10.1152/ajpheart.00924.2006. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeder JS, Kearns GL. Pharmacogenetics in pediatrics. Implications for practice. Pediatr Clin North Am. 1997;44:55–77. doi: 10.1016/s0031-3955(05)70463-6. [DOI] [PubMed] [Google Scholar]
- Li J, Hampton T, Morgan JP, Simons M. Stretch-induced VEGF expression in the heart. J Clin Invest. 1997;100:18–24. doi: 10.1172/JCI119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loff S, Kabs F, Witt K, Sartoris J, Mandl B, Niessen KH, Waag KL. Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers. J Pediatr Surg. 2000;35:1775–1781. doi: 10.1053/jpsu.2000.19249. [DOI] [PubMed] [Google Scholar]
- Malek RL, Irby RB, Guo QM, Lee K, Wong S, He M, Tsai J, Frank B, Liu ET, Quackenbush J, Jove R, Yeatman TJ, Lee NH. Identification of Src transformation fingerprint in human colon cancer. Oncogene. 2002;21:7256–7265. doi: 10.1038/sj.onc.1205900. [DOI] [PubMed] [Google Scholar]
- Malla N, Berg E, Uhlin-Hansen L, Winberg JO. Interaction of pro-matrix metalloproteinase-9/proteoglycan heteromer with gelatin and collagen. J Biol Chem. 2008;283:13652–13665. doi: 10.1074/jbc.M709140200. [DOI] [PubMed] [Google Scholar]
- Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Nakagomi M, Suzuki E, Usumi K, Saitoh Y, Yoshimura S, Nagao T, Ono H. Effects of endocrine disrupting chemicals on the microtubule network in Chinese hamster V79 cells in culture and in Sertoli cells in rats. Teratog Carcinog Mutagen. 2001;21:453–462. doi: 10.1002/tcm.1032. [DOI] [PubMed] [Google Scholar]
- Park K, Kwak IS. Calponin gene expression in Chironomus riparius exposed to di(2-ethylhexyl) phthalate. Environ Toxicol. 2009;24:555–562. doi: 10.1002/tox.20463. [DOI] [PubMed] [Google Scholar]
- Peng HJ, Dai DZ, Ji H, Dai Y. The separate roles of endothelin receptors participate in remodeling of matrix metalloproteinase and connexin 43 of cardiac fibroblasts in maladaptive response to isoproterenol. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Petersen SV, Lyman DJ, Roll DB, Swinyard EA. Final Report. Contract NIH-NHLI-73-2098-B. PB-250. Washington, DC: National Technical Information Service; 1975. Toxicology of plastic devices having contact with blood; p. 102. [Google Scholar]
- Rock G, Labow RS, Franklin C, Burnett R, Tocchi M. Hypotension and cardiac arrest in rats after infusion of mono(2-ethylhexyl) phthalate (MEHP), a contaminant of stored blood. N Engl J Med. 1987;316:1218–9. doi: 10.1056/NEJM198705073161915. [DOI] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea KM. Pediatric Exposure and Potential Toxicity of Phthalate Plasticizers. Pediatrics. 2003;111:1467–1474. doi: 10.1542/peds.111.6.1467. [DOI] [PubMed] [Google Scholar]
- Sjoberg P, Bondesson U. Determination of di(2-ethylhexyl) phthalate and four of its metabolites in blood plasma by gas chromatography-mass spectrometry. J Chromatogr. 1985;344:167–175. doi: 10.1016/s0378-4347(00)82017-4. [DOI] [PubMed] [Google Scholar]
- Sjoberg P, Bondesson U, Sedin G, Gustafsson J. Dispositions of di- and mono-(2-ethylhexyl) phthalate in newborn infants subjected to exchange transfusions. Eur J Clin Invest. 1985;15:430–436. doi: 10.1111/j.1365-2362.1985.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Umemura S, Nyui N, Hibi K, Ishigami T, Kihara M, Toya Y, Ishii M. Activation of angiotensinogen gene in cardiac myocytes by angiotensin II and mechanical stretch. Am J Physiol. 1998;275:R1–9. doi: 10.1152/ajpregu.1998.275.1.R1. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–8. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992;262:H1861–6. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- Wang N, Stamenovic D. Mechanics of vimentin intermediate filaments. J Muscle Res Cell Motil. 2002;23:535–540. doi: 10.1023/a:1023470709071. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, Takano H, Yazaki Y. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87:316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]