Abstract
Previous experiments have shown that curcumin or cisplatin treatment suppresses growth of head and neck squamous cell carcinoma (HNSCC). To study the potential cooperative effect of both agents, two HNSCC cell lines were treated with curcumin or cisplatin alone or in combination. In vivo studies consisted of intravenous tail vein injection of liposomal curcumin, with intraperitoneal cisplatin, into nude mice growing xenograft HNSCC tumors. Introduction of curcumin and suboptimal concentrations of cisplatin demonstrated a significant suppressive effect compared to treatment with either agent alone. Reduced expression of cyclin D1, IkBα, phospho-IkBα and IKKβ occurred in cisplatin and curcumin treated cell lines. Confocal microscopy showed expression of IKKβ in the nucleus of the cell lines. Chromatin immunoprecipitation (ChiP) assay on DNA isolated from IKKβ immunoprecipitated samples showed PCR amplification of IL-8 promoter sequences, a binding site of NFκB, indicating an interaction between IKKβ and NFκB. Curcumin inhibited IKKβ in the cytoplasm and nucleus, leading to reduced NFκB activity, with no effect on pAKT. In vivo studies demonstrated significant growth inhibition of xenograft tumors treated with a combination of liposomal curcumin and cisplatin. Curcumin's suppressive effect was mediated through inhibition of cytoplasmic and nuclear IKKβ, resulting in inhibition of NFκB activity. Cisplatin treatment led to cellular senescence, indicating an effect mediated by p53 activation. The two agents’ mechanisms through different growth signaling pathways suggest potential for the clinical use of subtherapeutic doses of cisplatin in combination with curcumin, which will allow effective suppression of tumor growth while minimizing cisplatin's toxic side effects.
Keywords: Head and neck squamous cell carcinoma, Liposomal Curcumin, Cisplatin, Growth suppression, Nuclear factor kB
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is an aggressive cancer with a poor prognosis for advanced tumors. There were 40,490 new cases of HNSCC in the United States in 2006, accounting for about 3% of adult malignancies (1). The worldwide incidence exceeds half a million cases annually. The significant morbidity associated with current treatment modalities, which include disfiguring surgery, chemotherapy, and radiation, has led to continuing investigation of potential alternative and less toxic therapies.
Current treatment regimens for HNSCC include use of platinum-based chemotherapy such as cisplatin (2). Cisplatin is not effective when used as a single agent, but its efficacy is significantly enhanced when used with radiation therapy and/or in combination with other chemotherapeutic drugs. Thus, cisplatin/radiation or combination chemotherapy is the cornerstone of treatment of many cancers including head and neck cancer. An example of this is the combination of cisplatin and cetuximab in the treatment of metastatic/recurrent head and neck cancer (3). Initial platinum responsiveness is high but the majority of cancer patients eventually relapse with cisplatin-resistant disease. Many mechanisms of cisplatin resistance have been proposed, including changes in cellular uptake and efflux of the drug, inhibition of apoptosis and increased DNA repair. We have hypothesized that cisplatin induces cell cycle arrest through a p16/p53 dependent pathway (4). Cisplatin has a number of dose-dependent side-effects, including renal, otologic, and bone marrow suppression. Thus, a suboptimal level of cisplatin in combination with nontoxic agents would be useful for effective treatment of HNSCC.
Curcumin (diferuloylmethane) is the major component of the spice turmeric and is derived from the rhizome of the East Indian plant Curcuma longa (Figure 1A). It has been consumed as a dietary supplement for centuries and has also been shown to prevent tumor initiation, proliferation and metastasis in breast, colon, oral, and other human cancers (5). Curcumin is soluble only in organic solvents, but liposomal formulations have been studied in the treatment of pancreatic cancer (6).
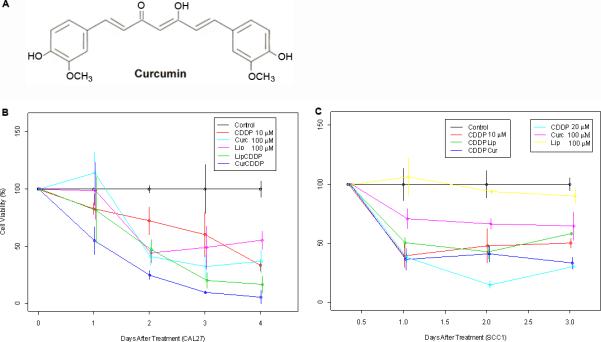
Figure 1.
Growth inhibition of HNSCC in vitro with cisplatin and curcumin. A) Molecular structure of curcumin. B) Cell viability by MTT assay was performed on CAL 27 and C) UM-SCC1 cells with cisplatin or curcumin alone or in combination. We compared the time trends (or slopes) among different groups and the estimated slopes from the lowest to the highest in CAL 27 are; untreated control (0), empty liposomes (-13.89), cisplatin alone (-15.61), liposomal curcumin (-20.80), liposomal curcumin + cisplatin (-23.35). Estimated slope values in UM-SCC1 cells are; untreated control (0), empty liposomes (-4.90), curcumin (-11.46), 10 μM of cisplatin (-15.52), curcumin + cisplatin (-22.83), and 20 μM of cisplatin (-26.79). The calculations show that empty liposomes themselves have a significant growth inhibition in CAL 27 cells over time (i.e., slope of growth inhibition) as compared to the untreated control (P=0.0015). However, the estimated slope of growth inhibition is greater with liposomal curcumin treatment in comparison to the untreated control (P < 0.0001). There is a significantly greater effect in liposomal curcumin treated cells in combination with cisplatin as compared to cells treated with cisplatin alone (P=0.0152). Statistical significance was not observed for combination treatment of liposomal curcumin and cisplatin vs liposomes and cisplatin. In the UM-SCC1 cells, liposomal effect is minimal and a significant growth inhibition over time is seen in the presence of curcumin (P=0.0064). In comparison to the untreated control, the combination treatment of curcumin with 10 μM cisplatin shows an effect (P=0.0016) similar to that seen with 20 μM of cisplatin (P=0.0011).
Delivery of curcumin is limited by its poor bioavailability and insolubility in saline. Previous studies from our laboratory have shown that curcumin treatment suppresses growth of HNSCC cell lines in vitro and reduces tumor volume in vivo via topical application (7). More recently, our laboratory has demonstrated the use of liposome-encapsulated curcumin for intravenous administration of curcumin, and bioavailability studies confirmed the presence of curcumin in circulating blood and liver (8). Its growth inhibitory effect was mediated through the inhibition of transcription factor NFκB, resulting in the down-regulation of IκBα, cyclin D1, cyclooxygenase-2 (COX-2), interleukin 6 (IL-6), and interleukin 8 (IL-8) genes.
In the present study, we investigated whether curcumin would enhance the suppressive effect of cisplatin in HNSCC. Two HNSCC cell lines, CAL27 and UM-SCC1, were treated with curcumin and cisplatin individually or in combination. Nude mice with subcutaneous xenograft CAL27 tumors were administered with cisplatin alone or a combination of cisplatin and liposomal curcumin. In addition, curcumin's mechanism of action, inhibiting transactivation of NFκB, mediated through cytoplasmic and nuclear IKKβ, was studied.
MATERIALS AND METHODS
Cell lines
HNSCC cell lines CAL27 and UM-SCC1, representing oral cancers, were used. CAL27 was obtained from the American Type Culture Collection (Manassas, VA), and UM-SCC1 was obtained from Dr. Thomas E. Carey (University of Michigan, Ann Arbor, MI). CAL27 cells were characterized by ATCC using polyphasic (genotypic and phenotypic) testing methods. UM-SCC1 cells were not characterized by our laboratory after receiving from the University of Michigan. Cell lines were grown in DMEM containing high glucose (4,500 μg/mL) and 1 mmol/L glutamine, 100 IU/mL penicillin, and 10% FBS (Sigma, St. Louis, MO).
Liposomal curcumin preparation
A 9:1 ratio of lipids 1,2-dimyristoyl-sn-glycero-3-phosphocholine and 1,2-dimyristoyl-sn-glycero-3-phospho-rac-(1-glycerol) (Sigma-Aldrich, St. Louis, MO) was dissolved in tert-butanol at a concentration of 10 mg/mL. Sterile water (1/20 volume) was added and 1 part curcumin (purity 97%, Cayman Chemical, Ann Arbor, MI) was added for a final lipid:curcumin ratio of 10:1. The solution was sterile-filtered, frozen in dry ice and acetone, and lyophilized overnight. Liposomal curcumin was suspended in sterile 0.9% NaCl at 65° C to yield a 100 mmol/L stock solution.
Curcumin and cisplatin treatment of HNSCC cell lines
Cells were plated in 24-well plates, with 30,000 cells per well and allowed to grow for 24 hours. After serum starvation for 24 hours, cells were treated with cisplatin (10 μM, i.e.3 μg/ml, or 20μM, i.e.6 μg/ml) for 5 hours or with liposomal curcumin (100 μM) for 8 hours. Combination treatment consisted of addition of cisplatin 3 hours after the addition of liposomal curcumin. Untreated cells or those treated with empty liposomes alone (100 μM) were used as controls. Cells were then allowed to incubate in serum-containing media at 37°C and cell viability was measured at 24 hours. Growth assays were performed at least twice and each time in triplicates.
Cell viability assay
Growth media was aspirated out of wells and 1.0 mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (1 mg/mL in complete medium; Sigma-Aldrich, St. Louis, MO) was added to each well. Cells were then incubated at 37°C for 4 hours. The MTT solution was aspirated out of the wells, air-dried for 5 minutes, dissolved in isopropanol, and absorbance values were read in an ELISA microplate reader at 570 nm as described (4).
Western Blot analysis
Proteins were extracted from cells or xenograft tumors using RIPA lysis buffer containing a complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Western blotting was performed using 20-30ug of denatured proteins on 4-20% SDS acrylamide gels (Invitrogen, Inc., Carlsbad, CA). Proteins transferred onto nitrocellulose were hybridized following the established protocol (7) to the antibodies (cyclin D1, phospho-IkBα, IkBα, histone H1, histone H3, Santa Cruz Biotechnology, Santa Cruz, CA). All western blot analyses were performed at least twice.
Immunofluorescence
Cells were grown overnight on cover slips to semi-confluence (70-80%) and treated with liposomal curcumin for 30 minutes, 1 hour or 4 hours. Cells were then treated with TNFα for 15 minutes. Untreated cells and cells treated with empty liposomes for 4 hours were included as controls. Immunofluorescence was performed using the IKKβ, NFκB, phospho-AKT and phospho-IkBα antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA) following the established protocol (7). Confocal microscopy was performed following established protocol using a Zeiss microscope.
Chromatin immunoprecipitation (ChiP) assay
UM-SCC1 cells grown to 80% confluency were treated with TNFα for 1 hour and cross linked with 37% formaldehyde for 10 minutes at 37°C. Chromatin immunoprecipitation (ChIP) assays were performed using ChIP assay kit (Millipore, Temecula, CA) following the manufacturer's protocol. Immunoprecipitations were carried out using antibodies to IgG (Santa Cruz Biotechnology, Santa Cruz, CA), IKKβ (Cell Signaling, Danvers, MA), and NFκB (Calbiochem, Gibbstown, NJ). DNA isolated from the input as well as the immunoprecipitated samples were amplified using IL-8 and β-actin primers (12). The primers IL-8 forward 5’ GGGCCATCAGTTGCAAATC 3’, reverse 5’ TTCCTTCCGGTGGTTTCTTC 3’ and β-actin forward 5’ CAACGCCAAAACTCTCCCTC 3’ and reverse 5’ ATCGGCAAAGGCGAGGCTCTG 3’ were used in the PCR reaction. DNAs were denatured at 95°C for 1 minute, annealed at 55°C for 1 minute and extended at 72°C for 1 minute for 35 cycles. The PCR products were separated on 10% polyacrylamide gels, stained with ethydium bromide and images were captured using the Kodak Gel documentation system.
Senescence assay
CAL27 cells grown to semi confluence in 6 well plates were treated with liposomal curcumin (100 μM) for eight hours or cisplatin (20 μM) for 5 hours. Growth media was replaced and cells were grown for four days. Cells were then stained with β-galactosidase and blue senescence colonies were visualized under the microscope. Photographs were taken using a Nikon camera.
Curcumin binding studies
CAL27 cells grown to semi-confluence in 100mm dishes were treated with liposomal curcumin (100 μM) for 8 hours. After media replacement, cells were grown for 24 hours and proteins were extracted from total cell lysates. Immunoprecipitation was performed with IKKβ or pS6K antibody and the precipitate was washed five times with the cell lysate buffer. Curcumin was extracted using ethyl acetate/methanol mixture, vacuum dried and MS/MS analysis was carried out on a triple quadrapole spectrophotometer as described (8). Curcumin peak identification and intensity measurement was determined using a standard curcumin run as a control. Untreated cells and cells treated with liposome alone were used as negative controls.
HNSCC xenograft tumors in mice
Five week-old female athymic nude mice (nu/nu; Harlan, Chicago, IL) were utilized for in vivo experiments. Animals were injected with 2 ×106 cells in the right flank to form xenograft tumors. Animals were housed in sterile rodent microisolator caging, with filtered cage top. Two to four animals were housed in each cage, with animals resting directly on bedding. They were given free access to sterile water and food. All cages, covers, and bedding were sterilized weekly. All animal procedures were approved by the Institutional Animal Care and Use Committee of the West Los Angeles Veterans Affairs Medical Center, in accordance with the USPHS Policy on Humane Care and Use of Laboratory Animals.
Treatment of nude mice with liposomal curcumin and cisplatin
Once subcutaneous nodules were visible at the inoculation site (Day 7-10 after inoculation), liposomal curcumin (50 mg/kg, 1mg for the 20 g mouse in a maximal volume of 100 μl) or equal amount of empty liposomes was administered via tail vein injection 3 times a week for 4 weeks. In the fourth week, cisplatin (100 μl of 7.5 μg/ml) was administered through intraperitoneal injection. There were 4 groups in the study: mice with no treatment (five), mice treated with cisplatin (five), mice treated with empty liposomes and cisplatin (five), and mice treated with liposomal curcumin and cisplatin (six). Tumor size was measured weekly using vernier calipers and tumor volume was calculated using the formula V = 4/3pW2L, where W is half of the shorter axis diameter and L is half of the longer axis diameter as described (9). At the end of the fourth week, mice were sacrificed and tumors were removed.
Statistical analysis
For the MTT optical density, cell viability was set to be 100% at baseline, and the percent of viable cells (= (viable cell count in the experiment group / mean viable cell count) × 100) per replicate was calculated at each of the post-treatment days. To examine the differences in the reduction of cell viability over time among groups, ANOVA modeling was used with the following covariates: group, time (measured in days), and a group-by-time interaction term. The model also allows heterogeneous variance across groups.
Time trends in tumor volume among experimental groups were examined using the piecewise regression model which allows for the trend after the injection of cisplatin at week 3 to be different from the trend before injection of cisplatin. Even though mice were followed over time, their IDs were not recorded due to technical difficulties. Thus the common repeated measures model cannot be used in this situation. Instead, the piecewise regression model was used, with the assumption of heterogeneous variance across groups to analyze tumor volume growth. All statistical analyses were carried out with the SAS System for Windows (Version 9.2)
RESULTS
Growth inhibition of HNSCC cell lines in vitro with liposomal curcumin and cisplatin
Two HNSCC cell lines, CAL27 and UM-SCC1, representing aggressive oral cancers were tested for growth inhibition with cisplatin or liposomal curcumin alone or in combination. All experiments were performed in triplicate in 24-well plates, and viable cell counts were measured at 24, 48, 72, and 96 hours after treatment. Empty liposomes, curcumin alone, and cisplatin alone had a significantly greater growth inhibition in CAL27 cells over time (i.e., slope of growth inhibition) as compared to the untreated control (P=0.0015, < 0.0001, and < 0.0001, respectively) (Figure 1B and 1C). There was significantly greater effect in liposomal curcumin treated cells in combination with cisplatin compared to cisplatin alone treated cells (P=0.0152). Statistical significance was not observed for combination treatment of liposomal curcumin and cisplatin vs liposomes and cisplatin. In UM-SCC1 cells, liposomes alone did not show an inhibitory effect. Significant growth inhibition over time was seen in the presence of cisplatin (P=0.0486 and p=0.0011, for 10 μM and 20 μM respectively), curcumin alone (P=0.0064), curcumin in combination with 10 μM of cisplatin (P=0.0016), and liposomes in combination with 10 μM of cisplatin (P=0.0247) as compared to control. We have also observed a significantly greater effect over time in curcumin treated cells as compared to cells treated with liposomes (P=0.0011). The combination treatment, liposomal curcumin and 10 μM of cisplatin, showed an effect similar to that seen in cells treated with 20 μM cisplatin.
Reduced expression of NFκB activated genes in curcumin and cisplatin treated cells
We have previously shown that curcumin and cisplatin downregulate the expression of genes involved in cell cycle and apoptosis and also that cisplatin induces cellular senescence through the upregulation of p16 and p53 tumor suppressor genes (4,7). Our studies have also shown that the effect of curcumin is mediated by the inhibition of IKKβ kinase resulting in reduced phosphorylation of IkBα (inhibitor of NFκB) and retention of NFκB in the cytoplasm (10). Thus, cisplatin and curcumin seem to suppress cell growth through independent mechanisms.
To verify that the two agents act via different mechanisms, we performed expression analysis of proteins collected from the drug treated cell lines. Both cisplatin and curcumin showed reduced expression of cyclin D1 and phospho-IkBα in CAL27 and UM-SCC1 cell lines (Figure 2A). Since our previous studies have shown lower level expression of these two proteins in CAL27 cells, in the present experiments, the cells were pre-treated with TNFα for 15 minutes before the addition of drugs. A higher concentration of cisplatin (20 μM) showed greater reduction in the expression of cyclin D1 and phospho-IkBα in comparison to treatment with 10 μM cisplatin. Also, combined treatment with 10 μM cisplatin and liposomal curcumin resulted in greater inhibition, equaling that of treatment with 20 μM cisplatin. Empty liposomes did not affect the expression of these two proteins. Western blot analysis showing reduced expression of IkBα and IKKβ in cisplatin (20 μM) and liposomal curcumin (100 μM) treated CAL27 cells (Figure 2B) further indicated a possible direct effect on the expression of these two proteins by cisplatin and curcumin.
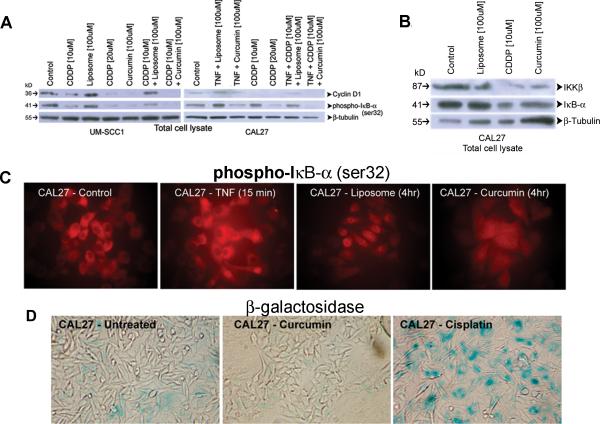
Figure 2.
Inhibition of NFκB regulated genes by cisplatin and curcumin. A) Total protein lysate extracted from CAL27 and UM-SCC1 cell lines treated with cisplatin (10 μM or 20 μM) or liposomal curcumin (100 μM) or a combination of cisplatin (10 μM) and liposomal curcumin (100 μM) were analyzed by PAGE. Untreated cells and those treated with empty liposomes (100 μM) or empty liposomes and cisplatin (10 μM) served as controls. Since CAL27 cells expressed lower level of cyclin D1, cells were also pre-treated with TNFα. Empty liposomes do not show any inhibitory effect on the expression of cyclin D1 or phospho-IkBα. However, reduced protein expression is observed with cisplatin or curcumin. The inhibitory effect of cisplatin-curcumin combination reaches that of 20 μM cisplatin, indicating usefulness of the non-toxic curcumin as a chemotherapeutic drug for adjuvant therapy. B) Hybridization of CAL27 total protein lysates to IkBα and IKKβ antibodies shows decreased expression of these two proteins in cells treated with cisplatin (20 μM) or liposomal curcumin (100 μM). C) immunofluorescence analysis of CAL 27 cells with TNFα shows increased expression of phospho-IkBα, indicating enhanced phosphorylation through IKKβ. This expression is not affected by the addition of liposomes. However, a decrease in phospho-IkBα expression level is visualized with the addition of curcumin. D) β-galactosidase assay demonstrates increased blue staining, indicating senescence-mediated cell death in cisplatin treated CAL27 cells. All pictures represent 100X magnification.
To confirm further that curcumin inhibited phospho-IkBα expression, immunofluorescence was performed on CAL27 cells treated with liposomes or liposomal curcumin. Phospho-IkBα expression was mostly seen in the cytoplasm and the expression level increased with the addition of TNFα (Figure 2C). Treatment with empty liposomes resulted in increased localization of the protein to the peri-nuclear region. However, curcumin treatment led to reduced protein expression in the cytoplasm, and in the peri-nucleus of the cells, confirming the inhibitory activity of curcumin on IKK kinase resulting in decreased phosphorylation of the inhibitor-kB-α. To demonstrate that cisplatin inhibits cell growth through a senescence pathway, CAL27 cells treated with liposomal curcumin or cisplatin were stained for the expression of β-galactosidase, a marker for cellular senescence. Untreated cells contained a low level background blue staining due to cell death from confluent cell culture in 4 days of study protocol (Figure 2D). Although there was cell killing in curcumin treated cells, there was no observable blue stain, indicating a senescence independent cell death by liposomal curcumin. As expected, cisplatin treated cells showed a high level expression of β-galactosidase, correlating to senescence mediated cell death of HNSCC cells.
Curcumin binds to IKKβ protein subunit of the IKK complex
Since we have previously shown that curcumin inhibited IKK kinase activity (10) we performed MS/MS analysis to determine whether there was a direct binding of curcumin to IKKβ protein. Cell lysates of CAL27 treated with curcumin were immunoprecipitated with IKKβ or a control pS6K antibody and the antibody bound products were extracted with organic solvents. Analysis by mass spectrometry demonstrated the presence of curcumin peak in the cell lysates. Intensity measurements using a curcumin standard showed a five-fold increased binding of curcumin to IKKβ in comparison to pS6K (Table S1) indicating an interaction between curcumin and IKKβ.
Nuclear expression of IKKβ protein in HNSCC cell lines
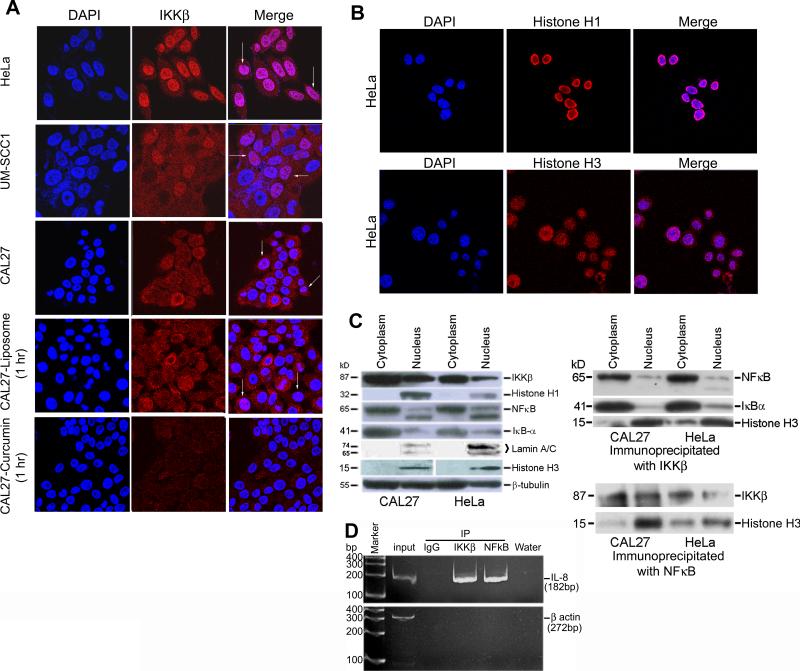
To verify further whether curcumin binding resulted in the inhibition of IKKβ expression as was seen in western blotting, confocal microscopy was performed. We observed expression of IKKβ both in the cytoplasm and in the nucleus of CAL27 and UM-SCC1 cell lines (Figure 3A). Although the nuclear presence of the IKKα protein has been demonstrated before (11), there is only one recent report indicating the expression of IKKβ protein in the nucleus of normal and neoplastic B-lymphocytes (12). Thus, for the first time, we have seen IKKβ expression in the nucleus of HNSCC cell lines. To confirm this finding, we used two different IKKβ antibodies and HeLa cells, a cervical cancer cell line shown before to contain cytoplasmic IKKβ expression. Expression of IKKβ in the HeLa cells was seen in the cytoplasm as well as in the nucleus of the cells (Figure 3A). Co-staining with DAPI (left column), a nuclear specific stain showed magenta staining of the nucleus (last column in figure 3A) confirming nuclear expression of IKKβ in the HeLa and HNSCC cell lines. While treatment with liposomes did not have an effect on the expression of IKKβ, liposomal curcumin treatment resulted in an inhibitory effect both in the cytoplasmic and nuclear expression of this protein (last row in figure 3A). As a confirmation to cytoplasmic and nuclear localization of IKKβ, we performed confocal microscopy for nuclear specific proteins histones H1 and H3. The results showed complete localization of histone H1 to the nucleus (Figure 3B). Expression of histone H3 was seen as speckles inside the nucleus as wells in the peri-nuclear/cytoplasmic region (Figure 3B). Western blot hybridization studies of the cytoplasmic and nuclear extracts of CAL27 and HeLa cells further confirmed the presence of IKKβ in the nucleus of these two cell lines (Figure 3C). Immunoprecipitation with nuclear IKKβ from CAL27 cells and hybridization to histone H3 antibody showed binding of histone H3, indicating a role for nuclear IKKβ in chromatin remodeling (Figure 3C). There was also hybridization to NFκB in these immunoblots indicating an interaction between NFκB and IKKβ proteins in the cytoplasm as well as in the nucleus. These interactions were confirmed by the hybridization of IKKβ antibody to the immunoprecipitates of cytoplasmic and nuclear NFκB (Figure 3C). Finally, ChiP assay showed amplification of IL-8 promoter sequences (NFκB binding site) in the DNA isolated from the IKKβ antibody immunoprecipiated samples indicating an interaction between IKKβ and NFκB (Figure 3D). IL-8 promoter amplification was not seen with the IgG antibody precipitated samples used as a control. There was no amplification of β-actin promoter sequences in any of the immunoprecipiated samples again used as a control in the Chip assay.
Figure 3.
Nuclear expression of IKKβ in HNSCC cell lines. A) Confocal microscopy with a Zeiss microscopic system performed on HeLa, CAL27, and UM-SCC1 cells show cytoplasmic and nuclear localization of IKKβ in all three cell lines. However, nuclear expression is more prominent in HeLa and UM-SCC1 cell lines. Co-staining with DAPI shows magenta staining of the nuclei confirming nuclear localization of IKKβ in all three cell lines. Cytoplasmic staining is also visualized in these photographs. While treatment with liposomes does not affect the expression of IKKβ in CAL27 cells, treatment with liposomal curcumin results in reduced expression of the protein in the nucleus and cytoplasm (last row). Arrows point to nuclear magenta staining indicating costaining of DAPI and IKKβ. All pictures represent 63X magnification. B) Confocal microscopy on HeLa cells shows nuclear expression of histones H1 and H3. Costaining with DAPI indicates presence of a small fraction of histone H3 in the peri-nuclear/cytoplasmic region. Speckle like staining of histone H3 could indicate localization to nucleosomes involved in chromatin remodeling. C) Western blot analysis of cytoplasmic and nuclear fractions of CAL27 and HeLa cells with IKKβ demonstrates the presence of IKKβ in both the cell compartments. While histones H1 and H3 and lamin A-C used as a controls represents proteins of the nucleus, β-tubulin is present both in the cytoplasm and nucleus. Immunoprecipitation with IKKβ antibody followed by western blot analysis shows predominant binding of nuclear histone H3 to IKKβ, indicating a role for IKKβ in chromatin remodeling. Minor hybridization seen in the cytoplasmic fraction could represent hybridization to the cytoplasmic H3 protein. Binding of nuclear IKKβ to NFκB is also seen in the immunoblots . The cytoplasmic IKKβ binds to NFκB and IkBα, confirming its role in the phosphorylation and ubiquitination of IkBα. The interaction between NFκB and IKKβ in the nucleus and cytoplasm is confirmed by the immunoprecipitation of proteins with NFκB antibody followed by hybridization to IKKβ. D) ChiP assay performed on DNA isolated from IKKβ and NFκB immunoprecipiated samples show a PCR product of 182 bp for IL-8 promoter sequences. PCR product is not seen in the IgG immunoprecipiated sample. While the input DNA is positive for the 272 bp β-actin promoter sequence, the immunoprecipiated samples do not show the PCR product confirming the specificity of the ChiP assay. and H3. Costaining with DAPI indicates presence of a small fraction of histone H3 in the peri-nuclear/cytoplasmic region. Speckle like staining of histone H3 could indicate localization to nucleosomes involved in chromatin remodeling. C) Western blot analysis of cytoplasmic and nuclear fractions of CAL27 and HeLa cells with IKKβ demonstrates the presence of IKKβ in both the cell compartments. While histones H1 and H3 and lamin A-C used as a controls represents proteins of the nucleus, β-tubulin is present both in the cytoplasm and nucleus. Immunoprecipitation with IKKβ antibody followed by western blot analysis shows predominant binding of nuclear histone H3 to IKKβ, indicating a role for IKKβ in chromatin remodeling. Minor hybridization seen in the cytoplasmic fraction could represent hybridization to the cytoplasmic H3 protein. Binding of nuclear IKKβ to NFκB is also seen in the immunoblots . The cytoplasmic IKKβ binds to NFκB and IkBα, confirming its role in the phosphorylation and ubiquitination of IkBα. The interaction between NFκB and IKKβ in the nucleus and cytoplasm is confirmed by the immunoprecipitation of proteins with NFκB antibody followed by hybridization to IKKβ. D) ChiP assay performed on DNA isolated from IKKβ and NFκB immunoprecipiated samples show a PCR product of 182 bp for IL-8 promoter sequences. PCR product is not seen in the IgG immunoprecipiated sample. While the input DNA is positive for the 272 bp β-actin promoter sequence, the immunoprecipiated samples do not show the PCR product confirming the specificity of the ChiP assay.
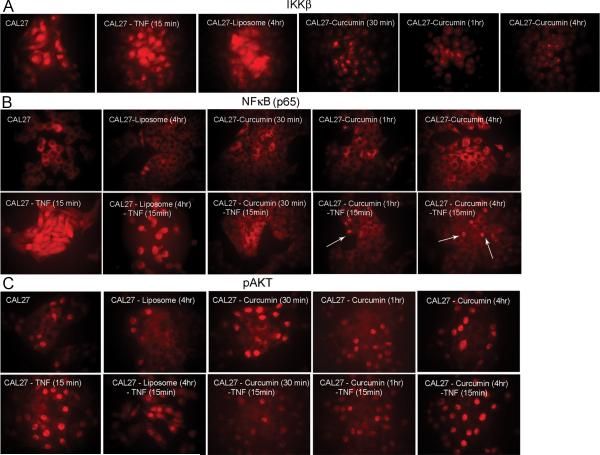
To verify the effect of curcumin on nuclear IKKβ and on NFκB, liposome and liposomal curcumin treated CAL27 cells were studied by immunofluorescence. Expression was also measured with the addition of TNFα, an NFκB upregulating molecule. IKKβ expression was seen in the nucleus and there was minimal effect with the addition of TNFα (Figure 4A). Treatment with liposome did not alter the expression. However, reduced expression was observed in as little as 30 minutes post curcumin treatment. The presence of IKKβ in nucleosome-like particles again demonstrated a possible role of this protein in chromatin remodeling. Expression of NFκB was seen in the cytoplasm and increased nuclear transport occurred with the addition of TNFα (Figure 4B). NFκB expression in liposome treated cells resembled that of control cells. Reduction in the expression of NFκB could be seen in curcumin treated cells. However, the inhibitory effect was pronounced with the blockage of NFκB transport in the presence of TNFα, confirming curcumin's inhibitory effect on the transcriptional activation of NFκB. Here the results clearly demonstrate curcumin's effect on cytoplasmic IKKβ leading to the inhibition of IKKβ kinase activity. Finally, we did not see an inhibitory effect on pAKT expression in the presence or absence of TNFα (Figure 4C), confirming our previous finding that curcumin inhibited NFκB by an AKT independent mechanism in HNSCC cells.
Figure 4.
Confirmation of IKKβ protein expression in the nucleus. A) Immunofluorescence studies show inhibition of nuclear IKKβ with a 30 minute curcumin treatment and the inhibition is greater for longer treatments. The speckle-like particles possibly represent nucleosomes involved in chromatin remodeling. B) NFκB is mostly localized to the cytoplasm and is transported to the nucleus with the addition of TNFα. While there is no difference in NFκB expression in liposome treated cells, curcumin treated cells show inhibition of nuclear transport and this inhibition is clearly visible after the addition of TNFα. C) Absence of an effect on pAKT points to an AKT independent downregulation of NFκB by curcumin. Arrows indicate cells with nuclear expression. All pictures represent 100X magnification.
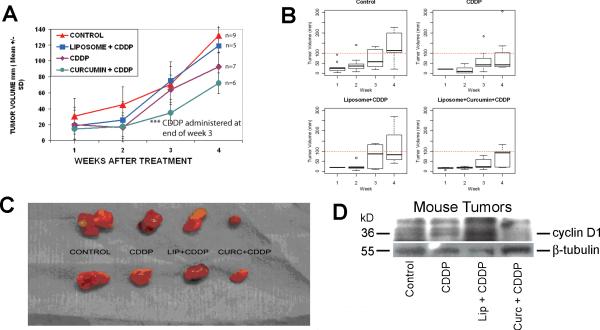
Enhanced growth inhibition of in vivo xenograft tumors with combination (liposomal curcumin and cisplatin) therapy
CAL27 xenograft tumors were grown in nude mice and tumor dimensions measured weekly with calipers. Once it was evident that xenograft tumors were forming (7 days), liposomes, and liposomal curcumin in saline were injected via the tail vein three times a week for four weeks. In the fourth week, mice also received cisplatin by intraperitoneal injection. A slower tumor growth rate of liposomal curcumin treated mice for the first three weeks confirmed our earlier findings (8). Results from the piecewise regression model indicated that in comparison to the control, a greater inhibitory effect over time (i.e., estimated slope of growth) was seen with the curcumin – cisplatin combination treatment before and after receiving the cisplatin. However, the estimated difference in slopes of growth did not reach statistical significance (P = 0.1098). A boxplot is a convenient way of graphically depicting groups of numerical data through their five-number summaries (minimum, lower quartile, median, upper quartile, and maximum). The tumor volume over the first three weeks for cisplatin alone (fig. 5B, top right) and curcumin – cisplatin (bottom right) did not go beyond 100mm (dash-line) except one observation at week 3 in the cisplatin alone group. In addition, a smaller variation in tumor volume is seen one week after the injection of cisplatin (week 4) for these two groups. This analysis again showed growth inhibition of xenograft tumors in the combination treatment in comparison to cisplatin alone or the controls (fig. 5B). The tumor size shown in figure 5C demonstrated growth inhibition with cisplatin alone and an enhanced growth reduction with the inclusion of curcumin in the combination treatment. Western blot analysis demonstrated a marginal inhibitory effect on the expression of cyclin D1 in cisplatin treated tumors (Figure 5D). However, liposomal curcumin treatment in combination with cisplatin resulted in a marked decrease in cyclin D1 expression correlating to the inhibitory effect on tumor growth.
Figure 5.
Inhibition of CAL 27 mouse xenograft tumors with cisplatin or cisplatin-curcumin combination. Mice were treated with empty liposomes or liposomal curcumin for 3 weeks after the appearance of tumor nodules. Intraperitoneal injection of cisplatin was administered on the fourth week and a week later tumors were excised. A) Tumor volume was calculated using the method described in material and methods. As compared to control, the results show tumor growth inhibition with cisplatin treatment. A greater inhibitory effect was seen with the curcumin – cisplatin combination treatment before and after receiving the cisplatin. However, the estimated difference in slopes of growth between the curcumin – cisplatin combination and control did not reach statistical significance (P = 0.1098). B) A boxplot is a convenient way of graphically depicting groups of numerical data through their five-number summaries (minimum, lower quartile, median, upper quartile, and maximum). The analysis demonstrates reduced growth of the xenograft tumors in the combination treatment in comparison to other groups. C) Representative tumors show reduced growth with cisplatin treatment and greater tumor growth inhibition with the cisplatin-curcumin combination treatment. D) Western blot analysis of proteins isolated from the xenograft tumors show a marginal reduction in cyclin D1 expression in cisplatin treated tumors in comparison to the untreated controls. However, treatment with the combination of cisplatin and curcumin shows significant reduction in the expression of cyclin D1 correlating to tumor size reduction in the combination treatment.
DISCUSSION
Cisplatin's mechanism of action includes cell cycle arrest and initiation of apoptosis (13). We and others have shown that cisplatin induces cellular senescence through activation of p53 and p16 proteins, and there is strong evidence that p53 plays a role in cisplatin sensitivity. It also appears that cisplatin-induced growth arrest in human cancer cells has characteristics of senescence in addition to apoptosis (14). In the present investigation we used HNSCC cell lines containing mutated p53 and lacking p16 expression. The senescence-associated β-galactosidase activity was seen in the CAL27 cells following cisplatin exposure, suggesting that growth of cancer cells containing mutated p53 function can also be inhibited by cisplatin via nuclear transportation of p53 protein (4). Previous results further suggest that functional expression of p16 augments the effect of cisplatin in cell killing. Because p16 and p53 are functionally active in the nucleus, it is likely that cisplatin plays an essential role in the nuclear transport and stabilization of these proteins for cell cycle arrest and apoptosis. We have shown here that in the presence of cisplatin, there is reduced expression of NFκB regulated proteins cyclin D1 and phospho-IkBα, thereby indicating control of NFκB transactivation by cisplatin through the nuclear p53 protein.
Tumor cells evade apoptosis by downregulation of apoptotic proteins and upregulation of antiapoptotic proteins. Expression of growth-signaling pathways gives them survival advantage and allows them to resist therapy-induced apoptosis (15). We and others have shown curcumin-mediated head and neck cancer cell growth inhibition occurs through the inhibition of NFκB activity (8,16,17,18). Recently, we have reported that the inhibitory effect of curcumin on NFκB is by the inhibition of IKK kinase (10). In the present investigation we have demonstrated that this inhibition could be due to direct interaction between curcumin and IKKβ. Further, for the first time we have demonstrated the presence of IKKβ in the nucleus whose function seems to be related to transcriptional activation of NFκB by chromatin remodeling. Additional studies including chromatin immunoprecipitation (CHIP assay) will be required to determine the proteins recruited by IKKβ for this NFκB transactivation.
Transcription factor NFκB and the serine/threonine kinase Akt play critical roles in cancer cell survival and have been shown to be activated in various malignancies (19, 20). Some have shown that curcumin acts via an AKT dependent pathway in cell growth regulation (21, 22). Previous studies in our laboratory revealed that liposomal curcumin treatment suppressed the activation of NFκB without affecting the expression of pAKT or its downstream target phospho-S6 kinase (8). Here we have confirmed using immunofluorescence that curcuimn affects NFκB expression via an AKT independent mechanism.
Recent investigative trials include gene targeted therapies in HNSCC, including cetuximab, bevacizumab, and erlotinib targeted against EGFR, the epidermal growth factor receptor (23-27). Although anti-EGFR therapies are active in some patients, eventually disease in nearly all patients will become refractory to therapy (28). Mutations in the EGFR receptor seem to play a significant role in the resistance to EGFR therapy. Thus, efforts are underway to identify alternate therapies, including the use of curcumin in combination with radiation and/or chemotherapeutic drugs in hepatic, ovarian, and HNSCC (29-31).
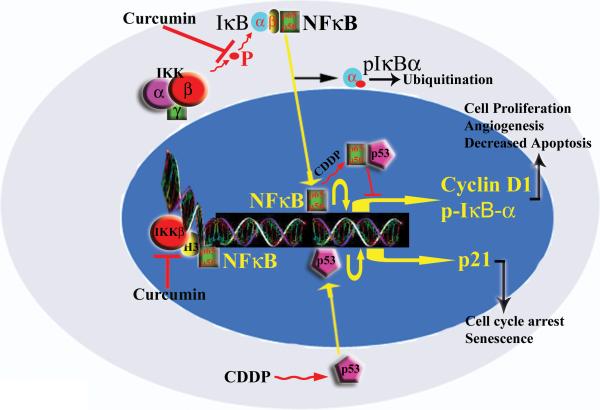
In conclusion, we have used the combination of curcumin and cisplatin to demonstrate enhanced growth suppression in HNSCC. We believe this occurs through two different molecular pathways. Curcumin affects NFκB transactivation by inhibiting IKK kinase activity both in the cytoplasm and nucleus, possibly through an interaction with IKKβ (Figure 6). We hypothesize that nuclear IKKβ in combination with histone H3 is involved in chromatin remodeling of the NFkB transcription binding sites. Inhibition of IKKβ by curcumin therefore results in reduction in NFkB occupied sites in chromatin leading to a decrease in NFkB mediated transcription. Cisplatin, in contrast, downregulates NFκB through a p53 mediated pathway. We therefore believe that the therapeutic potential of cisplatin will be enhanced with the addition of curcumin, with lower, less toxic doses of cisplatin required for its cytotoxic effect.
Figure 6.
Mechanism of action of curcumin and cisplatin. The available data suggests NFκB activation to be inhibited through a p53 directed mechanism by cisplatin. Inhibition of NFκB seems to occur through the downregulation of IKKβ by curcumin. H3 refers to histone H3, bound to IKKβ forming a nucleosomal complex.
Supplementary Material
Acknowledgements
We thank Dr. Yasutada Akiba for confocal microscopy and Dr. Ke Wei Zhao for help in acquiring photographic images.
Financial support: The study was supported by funds from VAGLAHS, West Los Angeles Surgical Education Research Center, UCLA Academic Senate grant (M. Wang), NIH (R21 CA116826-01 to M. Wang) and Merit grant from the Veterans Administration, Washington, DC (E.S. Srivatsan).
Abbreviations list
- HNSCC
head and neck squamous cell carcinoma
- ChiP
chromatin immunoprecipitation
- NFκB
nuclear factor kappa B
- IKKβ
inhibitor of NFκB kinase subunit β
- IkBα
inhibitor of NFκB
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. “Cancer statistics, 2006”. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Stordal B, Davey R. A systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: role of BRCA1. Curr Cancer Drug Targets. 2009;9:354–65. doi: 10.2174/156800909788166592. [DOI] [PubMed] [Google Scholar]
- 3.Burtness B, Goldwasser M, Flood W, et al. “Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study”. J Clin Oncol. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 4.Yip HT, Chopra R, Chakrabarti R, et al. Cisplatin-induced growth arrest of head and neck cancer cells correlates with increased expression of p16 and p53. Archives of otolaryngology--head & neck surgery. 2006;132:317–26. doi: 10.1001/archotol.132.3.317. [DOI] [PubMed] [Google Scholar]
- 5.Reuter S, Eifes S, Dicato M, et al. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76:1340–51. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Braiteh FS, Kurzrock R, et al. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–31. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 7.LoTempio M, Veena MS, Steele H, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–02. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Veena MS, Stevenson K, et al. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kB by an AKT-Independent pathway. Clin Cancer Res. 2008;14:6228–36. doi: 10.1158/1078-0432.CCR-07-5177. [DOI] [PubMed] [Google Scholar]
- 9.Kunnumakkara Ab, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to {gamma}-radiation by targeting nuclear factor -{kappa} B-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 10.Cohen A, Veena MS, Srivatsan ES, et al. Suppression of IL-6 and IL-8 production in head and neck cancer cells with curcumin via inhibition of Ikappa beta kinase (IKK). Arch Otolaryngol Head Neck Surg. 2009;135:190–7. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 11.Anest V, Hanson JL, Cogswell PC, et al. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–63. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 12.Fu L, Lin-Lee YC, Pham LV, Tamayo AT, et al. BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood. 2009;113:4627–36. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 14.Rebbaa A, Zheng X, Chu F, Mirkin BL. The role of histone acetylation versus DNA damage in drug-induced senescence and apoptosis. Cell Death Differ. 2006;13:1960–7. doi: 10.1038/sj.cdd.4401895. [DOI] [PubMed] [Google Scholar]
- 15.Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 16.Jobin C, Bradham CA, Russo MP, et al. Curcumin blocks cytokine-mediated NF-kB activation and proinflammatory gene expression by inhibiting inhibitory factor I-kB kinase activity. J Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- 17.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-KB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6:1276–82. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 19.Van Waes C. Nuclear Factor-kB in development, Prevention, and Therapy of Cancer. Clin Cancer Res. 2007;13:1076–82. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 20.Pugazhenti S, Akhov L, Selvaraj G, et al. Regulation of heme oxygenase-1 expression by demethoxy curcumoids through Nrf2 by a PI3-kinase/Akt mediated pathway in mouse B-cells. Am J Physiol Endocrinol Metab. 2007;293:E645–55. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- 21.Tomita M, Matsuda T, Kawakami H, et al. Curcumin targets Akt cell survival signaling pathway in HTLV-1-infected T-cell lines. Cancer Science. 2006;97:322–7. doi: 10.1111/j.1349-7006.2006.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Aoki H, Takada Y, Kondo S, et al. Evidence that curcumin suppresses the growth of Malignant Gliomas in vitro and in vivo through induction of autophagy: Role of Akt and Extracellular-Signal Regulated Kinase Signaling Pathways. Molecular Pharmacology. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 23.Lin C, Calvo E, Papadopolus K, et al. Phase I study of cetuximab, erlotinib, and bevacizumab in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology. 2009;63:1065–71. doi: 10.1007/s00280-008-0811-x. [DOI] [PubMed] [Google Scholar]
- 24.Laimer K, Spizzo G, Gastl G, et al. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: A TMA-based immunohistochemical analysis. Oral Oncol. 2007;43:193–8. doi: 10.1016/j.oraloncology.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Agra IM, Carvalho AL, Pinto, et al. Biological Markers and Prognosis in Recurrent Oral Cancer after Salvage Surgery. Arch Otolaryngol Head and Neck Surg. 2008;134:743–9. doi: 10.1001/archotol.134.7.743. [DOI] [PubMed] [Google Scholar]
- 26.William WN, Kim ES, Herbst RS. Cetuximab therapy for patients with advanced squamous cell carcinomas of the head and neck. Nature Reviews Clinical Oncology. 2009;6:132–3. doi: 10.1038/ncponc1321. [DOI] [PubMed] [Google Scholar]
- 27.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized Phase II Trial of Erlotinib or Standard Chemotherapy in Patients with Advanced Non-Small-Cell Lung Cancer and a Performance Status of 2. Journal of Clinical Oncology. 2008;26:863–9. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 28.Harandi A, Zaidi A, Stocker A, et al. Clinical Efficacy and Toxicity of Anti-EGFR Therapy in Common Cancers. Journal of Oncology. 2009;2009:1–14. doi: 10.1155/2009/567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nortarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone, or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Letters. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 30.Chirnomas D, Taniguchi T, De La Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 31.Khafif A, Lev-Ari S, Vexler A, et al. Curcumin: a potential radio-enhancer in head and neck cancer. Laryngoscope. 2009;119:2019–26. doi: 10.1002/lary.20582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.