Abstract
Recent work on cognitive control has suggested a variety of performance monitoring functions of the anterior cingulate cortex, such as errors, conflict, error likelihood, and others. Given the variety of monitoring effects, a corresponding variety of control effects on behavior might be expected. This paper explores whether conflict and error likelihood produce distinct cognitive control effects on behavior, as measured by response time. A change signal task (Brown & Braver, 2005) was modified to include conditions of likely errors due to tardy as well as premature responses, in conditions with and without conflict. The results discriminate between competing hypotheses of independent vs. interacting conflict and error likelihood control effects. Specifically, the results suggest that the likelihood of premature vs. tardy response errors can lead to multiple distinct control effects, which are independent of cognitive control effects driven by response conflict. As a whole, the results point to the existence of multiple distinct cognitive control mechanisms and challenge existing models of cognitive control that incorporate only a single control signal.
Introduction
Cognitive or executive control (Norman & Shallice, 1986) generally involves two tightly integrated functions: a performance monitoring mechanism that detects the need for control, and a mechanism that subsequently implements control. A number of studies have described a general system in the medial prefrontal cortex that first monitors the outcome of actions (Botvinick et al., 1999; Brown & Braver, 2005; Carter et al., 1998; Gehring & Knight, 2000; Ito et al., 2003; MacDonald et al., 2000) and then exerts corrective or pre-emptive control signals to optimize goal directed behavior (Botvinick et al., 2001; Brown et al., 2007; Jones et al., 2002). In the last two decades, a number of different performance monitoring signals have been described: errors (Gemba et al., 1986; Ito et al., 2003), correct responses (Ito et al., 2003; Stuphorn et al., 2000), conflict (Botvinick et al., 1999; Carter et al., 1998; MacDonald et al., 2000; Stuphorn et al., 2000), error likelihood (Brown & Braver, 2005), expected risk (Brown & Braver, 2007), task switching (Dove et al., 2000), and non-stationarity in the environmental contingencies (Behrens et al., 2007). Given the apparent variety of performance monitoring signals, the next question is whether there is a corresponding variety of control processes. Most models of cognitive control have surprisingly little to say in this regard, as the control signal in a given model generally consists of a scalar value that effectively either slows responses (Botvinick et al., 2001; Jones et al., 2002), focuses attention on task relevant rules (Norman & Shallice, 1986; Posner & DiGirolamo, 1998; Reynolds et al., 2006) or stimuli (Botvinick et al., 2001), or increases the learning rate (Behrens et al., 2007). Nonetheless, little is known about the richness and specificity of these control signals, and whether or how they may operate concurrently. In previous studies, we have explored whether multiple distinct control signals of response slowing and attentional focusing operate concurrently and interact (Brown et al., 2007).This paper further explores the question of multiple concurrent control signals.
The question to be addressed is whether concurrent control mechanisms may not only generally slow responses but also non-specifically speed up specific responses depending on the task conditions. In previous literature, the control signals driven by conflict effects are seen most clearly in that they drive a general slowing of responses in subsequent trials (Botvinick et al., 2001; Jones et al., 2002) or an attentional focusing (Botvinick et al., 2001) that slows subsequent performance with different stimuli but speeds subsequent performance with the same stimuli (Brown et al., 2007; Goschke, 2000). Studies of error likelihood monitoring have shown weak effects on response time, in which greater error likelihood increases RT in current trials (Brown & Braver, 2005), but see (Brown & Braver, 2007). This study examines a variant of the change signal task (Brown & Braver, 2007; Brown & Braver, 2005), which manipulates conditions of errors, conflict, and error likelihood, to determine whether manipulations of these factors lead to concurrent or interacting control effects on response time. Our original study of error likelihood (Brown & Braver, 2007; Brown & Braver, 2005) suggested that conflict effects may reflect error likelihood rather than conflict per se. Subsequent studies showed a surprising inverse relationship between the strength of conflict and error likelihood effects (Brown & Braver, 2007; Brown & Braver, 2005), a finding that turned out to be predicted by the error likelihood computational model (Brown & Braver, 2008) as originally published (Brown & Braver, 2005). Of particular interest for the present study is whether the likelihood of different kinds of errors can lead to multiple corresponding control effects, which are themselves distinct from control effects due to conflicts or actual error commission. To investigate this issue, the change signal task of (Brown & Braver, 2005) was modified to include separate conditions of error likelihood due to premature vs. tardy responses.
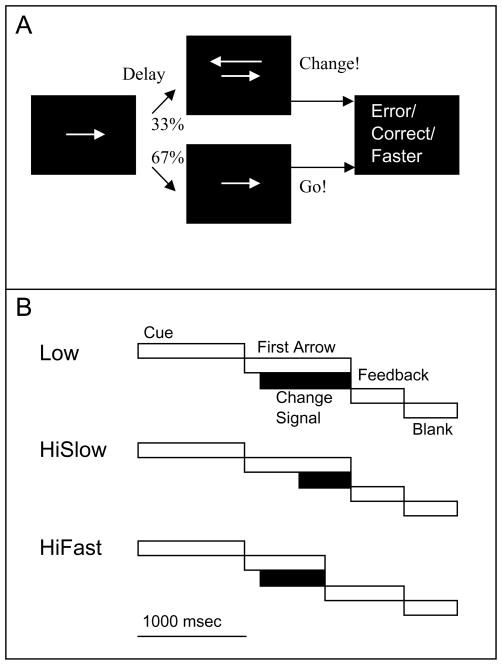
The change signal task (Brown & Braver, 2005) derives from earlier paradigms based on countermanding tasks (Husain et al., 2003; Logan & Cowan, 1984). The purpose of the change signal task (Figure 1A) is to distinguish three effects known to activate anterior cingulate cortex (ACC): response conflict (Botvinick et al., 1999; Carter et al., 1998), error commission (Gemba et al., 1986; Ito et al., 2003), and error likelihood (Brown & Braver, 2007; Brown & Braver, 2005; Magno et al., 2006). Briefly, each trial of the change signal task begins with an arrow pointing either left or right. If the arrow points left, subjects press a left button with the left index finger. If the arrow points right, then subjects press a right button with the right index finger. In one-third of trials, a second arrow appears, larger than the first arrow and above it, and pointing in the opposite direction. The second arrow instructs the subjects to, if possible, withhold the response to the first arrow and instead generate a response in the direction of the second arrow. The longer the delay between the onset of the first and second arrows becomes (i.e. the change signal delay or CSD), the more likely it is that the subject reaches the point of no return beyond which an error is inevitable. In this way, the error rate (i.e. error likelihood) can be increased by increasing the CSD. The error rate can be systematically controlled to a specific target rate by using a stairstep algorithm that adjusts the CSD after each trial (Brown & Braver, 2005). The color of the arrows is manipulated so that specific colors are paired with specific error likelihoods. In this way, conflict effects are found by comparing correct, change signal trials (i.e. second arrow appears) with correct go trials (i.e. no second arrow appears). Error effects are found by comparing incorrect change signal trials with correct change signal trials. Finally, error likelihood effects are found by comparing correct go trials with stimulus colors paired with high vs. low error likelihood trials (Brown & Braver, 2005).
Figure 1. Fast/Slow Change Signal task.
The task is a modified version of the Change Signal task of (Brown & Braver, 2005). (A) Subjects must make a button press response with either the left or right index finger corresponding to the direction of the last error shown. In the Change condition, a second arrow appears after a delay and instructs the subject to withhold a response to the first arrow and instead respond in the opposite direction based on the second arrow. Visual feedback (Correct, Error, or Faster) is given following the response. Failures to respond before the deadline lead to the feedback signal of “Faster”. (B) In the Low condition, the change signal delay (CSD) is short, meaning that the second arrow appears quickly after first, and error rates are therefore very low (around 5%). The response deadline is 1000 msec. In the HiSlow condition, the CSD is longer, and errors of commission are much more likely (around 50%). In the HiFast condition, the same CSD is used as in the low condition, but the response deadline is shortened to force errors in which the response is not generated before the deadline. The deadline is manipulated to force tardy errors in 50% of change signal trials.
For the present study, the change signal task was modified to differentially manipulate the likelihood of two different kinds of errors: premature errors as in previous work (Brown & Braver, 2005), and tardy errors (Figure 1B). In a control condition (Low), the error rate is maintained at a low level. In the High Slow condition, premature errors occur when responses to the first arrow are generated despite the appearance of a change signal (second arrow). In the High Fast condition, error likelihood is manipulated by shortening the response deadline. This leads to tardy errors as subjects fail to respond before the response deadline. Of note, error rates in the High Fast and High Slow conditions are maintained at the same level. In other words, the error likelihoods are controlled for, but the kinds of errors (tardy vs. premature) are different. The rationale for these manipulations is that the appropriate control strategies are opposite: To be successful, subjects must try to respond more quickly in the High/Fast condition but more slowly in the High/Slow condition.
The hypotheses to be explored here are: (1) that an increased error likelihood resulting from tardy responses will yield distinct control effects in subsequent trials, namely that subsequent responses will be faster regardless of subsequent trial conditions; (2) that an increased error likelihood due to premature responses will lead to slower responses in subsequent trials; and (3) that these effects of error likelihood will be distinct from the control effects induced by response conflict. Evidence consistent with these hypotheses will advance understanding of the richness and specificity of cognitive control signals.
Materials and Methods
With informed consent, subjects (n=21, 11 female, mean age 24, range 18 to 41) performed a Fast/Slow Change Signal task (Brown & Braver, 2007; Brown & Braver, 2005) as follows. All procedures were approved by the Indiana University Human Subjects Committee. Subjects were seated in front of a CRT computer screen and performed six blocks of 150 trials of the task. Stimuli were presented in the center of a black screen. Each trial began as a cue of colored dashes “—“ was presented for 1000 msec. There were three error likelihood conditions (described below): Low error likelihood, High Fast error likelihood, and High Slow error likelihood. The color of the dashes was paired with the error likelihood condition. Cue colors were blue (RGB 128,128,255), yellow (255, 255, 0), and white (255,255,255), and the pairings of cue color with error likelihood and likely error type were counterbalanced across subjects. Subsequent to the cue, arrows were formed by adding a greater-than or less-than symbol to the end of the dashes, as in “-->” in Courier font. The Cue and Go signals were presented in 18 point font, and the Change signal (if it appeared) was presented directly above the Go signal in 36 point font. The change signal consists of two dashes and a greater- or less-than symbol forming an arrow pointing in the opposite direction from the first arrow (Go signal). The appearance of the second arrow (the Change signal) countermanding the first satisfies the definition of stimuli that elicit response conflict. For the Low error likelihood condition, the response deadline was 1000 msec after the onset of the first arrow. The CSD for the low condition was initialized at 250 msec and adjusted with an asymmetric staircase algorithm to maintain a target rate of 5% errors. For the High Slow condition, the CSD was initialized at 400 msec and adjusted to maintain a target rate of 50% errors. For the High Fast condition, the CSD was the same as the Low condition, but the response deadline was initialized at 750 msec and adjusted to maintain a target rate of 50% errors, i.e. tardy errors consisting of a failure to respond before the response deadline. Immediately after the response deadline, feedback was presented onscreen to the subject until 1500 msec had elapsed since the onset of the first arrow. Feedback consists of the words “Correct”, “Error” (in the case of premature responses), or “Faster” (in the case of tardy or missing responses) presented in the center of the screen, in the same font color as the error likelihood color cue for that trial. Each trial ends with a black screen for 500 msec. Thus, each trial lasts 3000 msec. Two thirds of the trials were Go trials, in which only a single arrow appeared. The remaining one third of trials were Change trials, in which the second arrow appeared. The High Fast, High Slow, and Low error likelihood conditions were presented with equal probability, randomly, and with replacement from trial to trial. To summarize the task conditions, the factors of error likelihood (Low, High Fast, and High Slow) were fully crossed with the factor of Go vs. Change. The response outcomes for each trial could be correct, premature response errors (errors of commission), or tardy errors (no response trials).
At the beginning of each session, subjects were instructed how to do the task and given a small number of practice trials to ensure that they understood the task and were able to perform it. Practice trials ended as soon as it was clear that the subject understood the task instructions and was able to perform the task. Subjects were not informed about the meaning of the cue colors in terms of their association with different error likelihoods. Thus, all error likelihood effects in Go trials are the result of learning from experience.
Results
Error likelihood manipulation
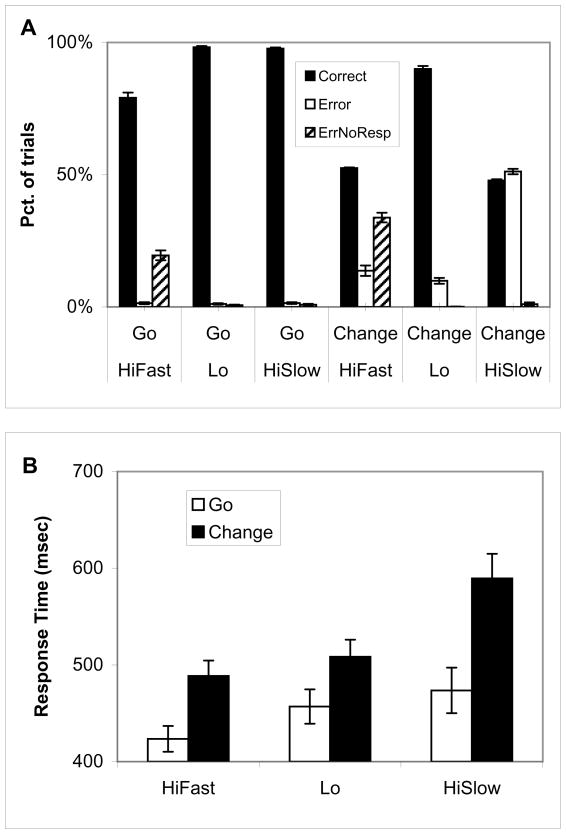
The first analysis confirmed the validity of the error likelihood manipulation (Figure 2A). In the Change conditions, the average error rates for High Fast and High Slow were 47.5% and 52.2%, respectively, which is close to the target of 50% errors. The error rate for Low trials was 10%, which is close to the 5% target and well below the 50% error rate target for high error likelihood conditions. Of these errors, tardy errors (i.e. no response or response too late) constituted 33.8% of High Fast Change trials, 1.03% of High Slow Change trials, and less than 0.1% of Low Change trials. Thus, the majority of errors in the High Fast condition were tardy errors, while errors in the other conditions consisted almost exclusively of premature responses. Of note, in the High Fast Go (i.e. no change signal) condition, the response deadline forced an average tardy error rate of 19.5%, further contributing to the overall majority of tardy rather than premature errors in the High Fast condition.
Figure 2. Current trial effects.
(A) Trials are mostly correct in the Go and Change Lo conditions. Premature errors (errors of commission, labeled as Error) occur in about 50% of HiSlow Change trials, and tardy errors (errors of omission, labeled as ErrNoResp) are most common in the HiFast condition. (B) In the current trial a Change signal (i.e. a second arrow appears) leads to longer response times relative to Go trials (with only one arrow presented to the subject). Also, response times are fastest for the HiFast condition and slowest for the HiSlow condition.
Current trial RT effects
Given inconsistent prior results of error likelihood effects on response time (Brown & Braver, 2007; Brown & Braver, 2005), a foundational question is whether error likelihood manipulations yield response time effects. Analysis of current correct trial effects on response time (Figure 2B) revealed a main effect of error likelihood condition (High/Fast, High/Slow, and Low/Slow), F(2,40, MSe = 2337) = 26.32, p <0.0001. There was a main effect of Go vs. Change, F(1,20, MSe=1308)=144.55, p < 0.0001. The error likelihood effects were not limited to change trials. When the analysis was restricted to correct go trials with the three error likelihood conditions, the effects of error likelihood remained, F(2,40, MSe=1234)=11.08, p < 0.0002. Further tests with correct, go trials revealed a trend toward slower response times for High Slow vs. Low conditions, F(1,20, MSe=737)=3.95, p = 0.06. Response times were faster in the High Fast condition vs. the Low condition, F(1,20, MSe=610)=19.29, p < 0.0003. The fact that response times are faster the High Fast condition may be due to anticipation of tardy errors as driven by the associated color cue, but it may also be due to a truncation of the response time distribution by the earlier response deadline. The latter possibility is suggested by the higher percentage of tardy errors in the High Fast conditions, for both Go and Change trials (Figure 2A). The analyses of previous trial RT effects below will demonstrate that the High Fast trial effects are consistent with a control signal driven by anticipated tardy errors. Overall, both error likelihood and conflict (Change vs. Go condition) manipulations led to response time effects in the current trial.
Previous trial RT effects
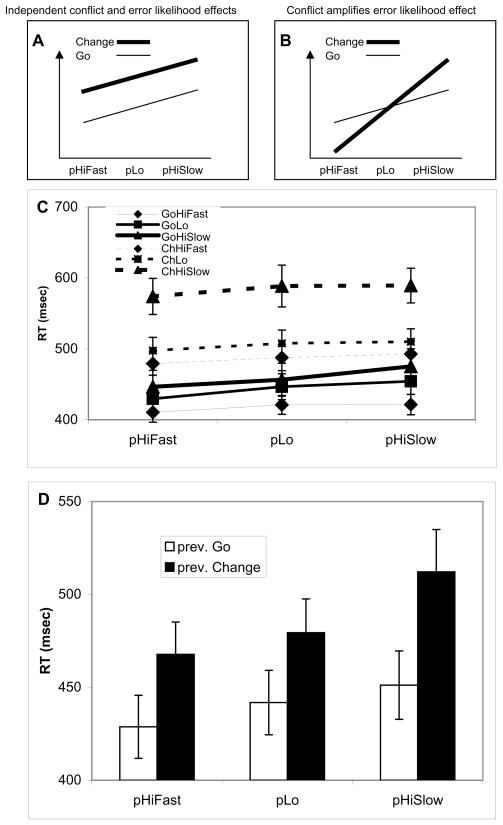
Cognitive control effects are seen most clearly in how a preceding trial condition influences current trial RT (Botvinick et al., 2001; Jones et al., 2002). If error likelihood effects lead to changes in cognitive control consistent with avoiding the potential error, then a higher likelihood of tardy errors should lead to faster responses in subsequent trials (Figures 3A, B). Likewise, a higher likelihood of premature response errors should lead to slower responses in subsequent trials. Furthermore, if conflict effects are in fact potent indicators of impending error likelihood, then adding a change signal (i.e. conflict) to a given error likelihood condition (relative to the go condition) might be expected to increase the perceived error likelihood and therefore amplify the subsequent-trial control effect exerted in response to that error likelihood condition (Figure 3B). Alternatively, if control effects due to conflict are distinct and separate from those due to error likelihood, then no interaction between conflict and error likelihood effects would be predicted in subsequent correct, go trials (Figure 3A).
Figure 3. Previous and current trial effects.
(A, B) These two panels depict competing hypotheses about the nature of conflict and error likelihood effects. If the two effects are independent, then the results in panel D should look like panel A. If conflict reflects underlying error likelihood computations, then panel D should look like panel B. (C) RT effects as a function of both current and previous trial conditions, restricted to trials where both previous and current responses were correct trials, and the previous trial was a Go trial. The legend depicts current trial conditions, and the “p” prefix on the horizontal axis denotes previous trial conditions. (D) Effects of previous trial effects on current trial RT, where previous and current trials are correct Go trials. Panel D is consistent with the hypothesis represented in panel A but not panel B.
To explore the question of these competing hypotheses, the response times were submitted to a full factorial analysis using factors of current and previous trial error likelihood conditions, looking only at conditions in which the current and previous trial responses were correct, go trials (Figure 3C). For current correct, go trials in which the previous trial was also a go trial, the percentage of trials remaining after selecting for previous correct trials was consistent with Figure 2A: 80.3% of previous High Fast trials, 97.5% of previous High Slow trials, and 98.2% of previous Low trials had correct previous trial responses. Analysis of these trials showed that in addition to the main effect of current error likelihood condition (F(2,40, MSe=3809)=7.36, p < 0.002), there was also a main effect of previous trial error likelihood condition (F(2,40, MSe=289)= 25.16, p < 0.0001), as well as a slight interaction between current and previous error likelihood conditions (F(4,80, MSe=220.7)=2.95, p <0.03). Further analysis of the previous error likelihood condition effects (Figure 3C,D) showed that a previous High Fast condition speeded up response time relative to a previous Low condition (428.8 ms vs. 441.8 ms, t(20)=4.25, p < 0.0004). Likewise, a previous High Slow condition slowed response time relative to a previous Low condition (451.2 ms vs. 441.8 ms, t(20)=3.93, p < 0.001). It must be emphasized that the effects of speeding and slowing were carried over into subsequent trials, a finding that is consistent with the hypothesis of two distinct cognitive control effects, one that speeds up responses given a likelihood of errors due to shorter response deadlines, and one that slows down responses given a likelihood of errors due to premature responses. Also, it is not clear how the present results could be accommodated by a priming based account (Mayr et al., 2003); the results show distinct effects of both speeding up and slowing down responses in subsequent trials, even when restricting the analysis to trials without conflict (change signals).
The results of previous trial effects (Figure 3D) discriminate between the two competing hypotheses of Figure 3A,B. Response time is slower in correct, go trials for previous correct change vs. go trials (pChange (468 ms) vs. pGo (429 ms), t(20)=5.55, p < 0.0001). Critically, there is no interaction between previous error likelihood condition and previous change vs. go conditions when looking at previous correct, current correct go trials (F(2,40, MSe=907)=2.39, p=0.10). Even when both the current and previous trials are correct High Fast trials (current go trials only), a previous change vs. go trial slows RT in subsequent trials (452 vs. 423 msec., t(41)=5.81, p<10−6). These results are consistent with the hypothesis of independent control mechanisms driven separately by conflict and error likelihood (Figure 3A), but they are inconsistent with the hypothesis that conflict effects amplify error likelihood effects (Figure 3B).
Error effects on response time
If the likelihood of a tardy error in the Hi/Fast condition leads to greater speeding up in subsequent trials even when no tardy error occurs, then it might be expected that actual tardy errors in previous High Fast trials would also lead to speeding up in current correct, go trials. To examine this, the data were submitted to an ANOVA of previous High Fast tardy error vs. correct trials crossed with previous go vs. change conditions, restricting the analysis to current correct, go trials. The results show that previous tardy errors vs. correct responses generally speeded subsequent correct, go RTs (427 vs. 448 msec respectively, F(1,20, MSe=757)=12.78, p < 0.002). At the same time, previous change vs. go trials generally slowed RTs (452 vs. 423 msec, respectively, F(1,20, MSe=363)=45.98, p < 10−5). There was also a small interaction such that previous tardy errors vs. correct led to less conflict-induced slowing (F(1,20, MSe=527)=4.64, p < 0.05). These findings are notable for several reasons. First, they suggest a multiplicity of error-driven control signals, namely that tardy errors specifically can lead to speeding up as well as the slowing down effects reported (Laming, 1968) and simulated (Botvinick et al., 2001; Jones et al., 2002) previously. Second, they show that conflict vs. errors can under certain circumstances lead to distinct or even competing control effects. This suggests that theories of error signals as a reflection of underlying conflict detection (Carter et al., 1998) may be incomplete. Regarding premature (as distinct from tardy) response errors, there was a surprising absence of effects in subsequent trials: for conditions of current correct go trials, a previous premature response error vs. correct responses did not slow responses in subsequent trials (F(1,20, MSe=4050)=0.74, p = 0.40).
Practice effects
Previous studies of the Change Signal task showed evidence consistent with learning of the error likelihood effects across an experimental session, even though the effects did not reach significance (Brown & Braver, 2005). The current study yielded similar results. To test for practice effects, each session was divided into sets of the first three blocks vs. the last three blocks, and the response times were analyzed for current correct trials. An ANOVA was performed on error likelihood condition (High Fast, High Slow, Low) by conflict (Go, Change) by time (first three vs. last three blocks), with subject as the random factor. The results showed a main effect of time (F(1,20, MSe=10413)=14.07, p < 0.002), such that response time was faster in later blocks (466 ms) vs. earlier blocks (514 ms). There was also an interaction of time by conflict (F(1,20, MSe=565)=7.85, p < 0.02), such that the conflict effect was smaller in the later blocks (69 ms) vs. the earlier blocks (86 ms). There was no significant interaction of time by error likelihood condition (F(2,40, MSe=693)=0.53, p = 0.59) for current trial response time, indicating that the error likelihood effects were already established relatively early in the session, possibly during the practice trials.
Discussion
The results of effects of previous trial conditions are consistent with multiple distinct cognitive control effects. The hypothesis that increased error likelihood of tardy responses will lead to speeding up in subsequent trials was confirmed, as was the hypothesis that increased error likelihood of premature responses will lead to slowing in subsequent trials. Critically, the results are also consistent with the third hypothesis, that cognitive control effects due to conflict vs. error likelihood are distinct. The results thus suggest that conflict monitoring and error likelihood monitoring by ACC may constitute at least partly distinct processes.
The results are consistent with the existence of three separate cognitive control mechanisms, each of which is adaptive in the task context. First, an increased likelihood of premature response errors, even in the absence of response conflict, leads to a general slowing of responses in subsequent trials. This is adaptive as slower responses in general may reduce the likelihood of premature errors on Change trials. Second, an increased likelihood of tardy response errors leads to a general speeding of responses in subsequent trials. This is also adaptive, as faster responses may help avoid failures to respond in time. It should be noted that although the results are consistent with distinct monitoring/control mechanisms, it is also possible that the monitoring mechanisms responsible for speeding up vs. slowing down may drive a single final common control pathway, albeit in opposite directions.
Third, conflict leads to a general slowing of subsequent trial responses. This is an appropriate response, because whenever there is a change signal, slower responses may allow more time to respond effectively to a delayed change signal. In that regard, the results are consistent with conflict as reflecting a likelihood of premature response errors, leading to a general slowing effect (Botvinick et al., 2001; Jones et al., 2002). Of note, the results do not discriminate whether the conflict effect reflects an actual conflict computation per se or instead an underlying prediction of error likelihood; the results simply show that change signals lead to subsequent trial slowing, which is a response consistent with reducing the likelihood of errors in the event that more change signals appear in future trials.
The present results differ from earlier studies of the Change Signal task, in which response time effects were not consistently found (Brown & Braver, 2007; Brown & Braver, 2005). Several factors may account for the differences. First, the present study used shorter inter-trial intervals (ITI) than the previous studies. Longer ITIs were necessary in previous studies due to the importance of varying inter-trial intervals in event-related fMRI (Dale, 1999). Correspondingly, the mean RTs are shorter in this study relative to the previous studies. It may be that with longer ITIs, RTs show a ceiling effect near the response deadline as subjects focus on accuracy over speed in order to maximize the rate of correct trials per unit time (Bogacz et al., 2006).
The issue of multiple cognitive control signals is not universally appreciated in previous work. Existing studies cast ACC activity as generally slowing responses (Botvinick et al., 2001; Brown & Braver, 2005; Jones et al., 2002), increasing attentional focus (Botvinick et al., 2001; Posner & DiGirolamo, 1998), or increasing learning rates (Behrens et al., 2007). A model of multiple interacting control signals in task switching has recently been proposed (Brown et al., 2007).
As a whole, the results point to the existence of multiple, distinct cognitive control mechanisms that contribute to RT effects across sequences of trials. The lack of interaction between conflict and error likelihood effects on subsequent trial RT points to the existence of at least partly distinct cognitive control mechanisms that detect conflict vs. error likelihood. These results contribute to a growing body of literature suggesting multiple distinct cognitive control effects driven by ACC and challenge existing models of cognitive control.
Acknowledgments
The author thanks E. Dinh for help with data collection. Supported by AFOSR FA9550–07–1–0454, NIDA 1 R03 DA023462–01, a NARSAD Young Investigator Award to JWB, and the Sidney R. Baer, Jr. Foundation.
References
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10(9):1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev. 2006;113(4):700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JC. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom L, Fissel K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brown J, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cog Aff Behav Neurosci. 2007;7(4):266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Brown J, Reynolds J, Braver TS. A Computational model of fractionated conflict-control mechanisms in task switching. Cognitive Psychology. 2007;55:37–85. doi: 10.1016/j.cogpsych.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned Predictions of Error Likelihood in the Anterior Cingulate Cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Res. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll DC, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: An event-related fMRI study. Cognitive brain research. 2000;9(1):103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosci Lett. 1986;70(2):223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Goschke T. Intentional reconfiguration and involuntary persistence in task set switching. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge: The MIT Press; 2000. pp. 331–3555. [Google Scholar]
- Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nat Neurosci. 2003;6(2):117–118. doi: 10.1038/nn1005. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown J, Schall JD. Performance Monitoring by Anterior Cingulate Cortex During Saccade Countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jones AD, Cho R, Nystrom LE, Cohen JD, Braver TS. A computational model of anterior cingulate function in speeded response tasks: Effects of frequency, sequence, and conflict. Cog Aff Behav Neurosci. 2002;2(4):300–317. doi: 10.3758/cabn.2.4.300. [DOI] [PubMed] [Google Scholar]
- Laming DRJ. Information theory of choice reaction times. London: Academic Press; 1968. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91(3):295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal cortex and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26(18):4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6(5):450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-regulation. Vol. 4. Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Posner MI, DiGirolamo G. Conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. Cambridge: MIT Press; 1998. [Google Scholar]
- Reynolds JR, Braver TS, Brown J, Stigchel S. Computational and Neural Mechanisms of Task Switching. Neurocomputing. 2006;69(10):1332–1336. [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]