Abstract
Acute activation of adenosine monophosphate–activated protein kinase (AMPK) or jumps in cardiac work increased cardiac endothelial lipoprotein lipase (LPL), yet it is unclear whether chronic AMPK activation maintains this elevated LPL. To activate AMPK chronically, metformin at low (300 mg/kg/d) and high dose (600 mg/kg/d) was administered in drinking water for 14 days. Control, metformin-treated, and 5-amino-imidazole-4-carboxamide riboside (AICAR)–treated (0.5 mmol/L) ex vivo hearts were perfused to investigate uptake of triacylglycerol and cardiac LPL activity. For perfused rat hearts, increased uptake of labeled Intralipid and β-oxidation of Intralipid–fatty acid were noted for both AICAR (P < .05) and high-dose metformin (P < .01). Intralipid incorporation into tissue lipids was decreased by AICAR (P < .05) and increased after high-dose metformin (P < .05), the increase manifest as enhanced triacylglycerol deposition (P < .05). Low-dose metformin did not alter lipid uptake or tissue deposition. Both high-dose metformin and AICAR decreased cardiac acetyl–coenzyme A carboxylase activity (P < .01). Heparin-releasable LPL was increased after treatment with AICAR (P < .05) and high-dose metformin (P < .01). Low-dose metformin did not alter cardiac LPL. High-dose metformin doubled immunoreactive AMPK and phospho-AMPK protein (P < .001) and increased phosphorylation of p38–mitogen-activated protein kinase (P < .05). After heparin pretreatment, the rate of recruitment of LPL to the cardiac endothelium was increased by AICAR (P < .05) but not by high-dose metformin. These data suggest that AMPK activation increased cardiac endothelial LPL, yet acute and chronic activation of AMPK may yield increased LPL through differing mechanisms.
1. Introduction
Cardiac hypertrophy is characterized by poor mechanical performance and a progressive alteration in cardiac substrate utilization, a transition from a reliance upon lipids to increased glucose use to preserve the production of adenosine triphosphate (ATP) [1]. Coupled with these changes are a decrease in the phosphocreatine to ATP ratio [2,3] and an increase in the activation of adenosine monophosphate–activated protein kinase (AMPK) [4]. This has a range of effects including a decrease in the activity of acetyl–coenzyme A (CoA) carboxylase (ACC) promoting lipid oxidation through removal of the allosteric inhibitor malonyl-CoA from CPT1-mediated uptake of fatty acids (FAs) into the mitochondria [5]. Increased glucose uptake is also noted after AMPK activation [6,7]. Acute stimulation of AMPK is also associated with increased translocation of lipoprotein lipase (LPL) from the cardiomyocyte where it is synthesized to the luminal surface of the cardiac endothelium [8,9]. This increases the assimilation of triacylglycerol (TAG)-derived lipids into the cardiomyocyte for energy production. Indeed, activation of AMPK is beneficial to myocardium under metabolic stress; deletion of AMPK decreased tolerance to ischemic injury [10] and led to poor glucose uptake. By contrast, AMPK activation attenuated hypertrophy for both cultured cardiomyocytes [11] and rats with aortic constriction [12]. Yet the teleological rationale for increasing LPL translocation during cardiac hypertrophy and ischemia is unclear. The transition to glucose metabolism and the increasing reliance on glycolysis for ATP synthesis are indicative of decreased availability of oxygen; therefore, the increase in uptake of lipids through LPL appears contradictory.
It is currently unclear whether chronic activation of AMPK will facilitate increased endothelial presentation of LPL in the myocardium in a similar manner to the examples of acute activation previously noted. Generation of mice with constitutively active forms of AMPKα2 led to poor mechanical performance and significant cardiac hypertrophy, coupled with increased storage of glycogen [13]. Furthermore, investigation of the hypertrophied heart revealed a down-regulation of ATP-generating processes from all substrates. Therefore, to examine the effects of chronic AMPK activation in the absence of overt cardiac hypertrophy and without gross changes to substrate uptake and utilization, a less aggressive mechanism for activation of AMPK is required [14].
Metformin is a biguanide with insulin-sensitizing properties widely used to help normalize plasma glucose concentrations in persons with type 2 diabetes mellitus [15,16]. Metformin when added to cardiac perfusion medium has specific effects on myocardium, including protection from ischemia-reperfusion injury [17,18]; and this is coupled with increased cardiac AMPK activity [19] and also led to increased phosphorylation of endothelial nitric oxide synthase [20]. Metformin increased cardiac AMP levels in Krebs-perfused rat hearts [21]. In addition, metformin when added to perfusate increased cardiac output in hearts and increased both FA β-oxidation and glycogen synthesis [22]. By contrast, estimation of AMPK enzyme activity revealed no change for AMPKα1 or AMPKα2; and metformin did not alter AMPK/p-AMPK protein ratio, but increased p38–mitogen-activated protein kinase (MAPK) phosphorylation and protein kinase C activity [22]. Taken together, these observations suggest that metformin may have a range of effects in the myocardium, some dependent on AMPK.
The in vivo effects of metformin on myocardium are less defined; indeed, metformin was without effect on basal or insulin-stimulated glucose uptake in rat hearts [23]. In humans, metformin increased plasma clearance of chylomicron (CM) and CM remnants for overweight/nondiabetic and insulin-resistant subjects [24]; in addition, metformin increased preheparin plasma LPL mass [25], indicative of increased lipoprotein clearance.
We hypothesize that metformin will increase phosphorylation of AMPK in vivo for myocardium and therefore increase endothelial LPL and increase uptake of TAG by perfused hearts. Using the isolated perfused heart, we will investigate the impact of metformin on LPL presentation and turnover at the endothelial surface and investigate the activation of both AMPK-dependent and -independent mechanisms.
2. Materials and methods
2.1. Materials
3H-[9,10]-triolein was purchased from Amersham Biosciences (Chalfont, United Kingdom). Intralipid lipid emulsion (10% wt/vol TAG) was obtained from Fresenius Kabi (Fresenius Kabi Ltd, Runcorn, Cheshire, UK). Fatty acid–free bovine albumin, metformin, and all buffer salts were purchased from Sigma (Poole, United Kingdom). Antibodies to AMPKα2 and threonine 172 phospho-AMPKα2 were obtained from Kinasource (Dundee, Scotland). Antibodies to p38-MAPK and phospho-p38-MAPK (Thr180/Tyr182) were purchased from Cell Signaling Technologies (Danvers, MA). Ventricular balloons were constructed “in house” using Saran Wrap, Dow Chemicals, Michigan, USA polythene film.
2.2. Methods
2.2.1. Animal maintenance
Animals were maintained in accordance with the United Kingdom Home Office, Animal Scientific Procedures Act (1986) and housed at 22°C 12-hour light/12-hour dark with ad libitum access to food and water. For selected groups of animals, metformin was administered in drinking water to give 2 doses, low and high, for 2 weeks (low, 300 mg/kg; high, 600 mg/kg). Metformin solutions were made freshly daily. Throughout this period, animals had ad libitum access to both food and water. Control animals were purchased at the appropriate experimental body mass (250-300 g).
2.2.2. Radioisotope tracer preparation
Intralipid was prelabeled with 3H-[9,10]-triolein (9.0 MBq/12 mg Intralipid TAG). The Intralipid mixture was homogenized (Janke and Kunkel, Briesgau, Germany), full power 15 seconds, 4 cycles, over ice) and added to FA-free bovine albumin (final concentration, 2.0% wt/vol in perfusate) to give the final TAG working concentration (0.4 mmol/L).
2.2.3. Tissue isolation and heart perfusion
Animals were prepared surgically from fed rats as outlined previously [26]. Briefly, anesthesia was induced with halothane (3% in oxygen); and after thoracotomy, hearts were excised with lungs and thymus in situ and immersed in ice-cold Krebs-Henseleit medium. Excess tissue was dissected away, the thymus was divided to reveal the aortic arch, and the aorta was trimmed at the level of the carotid artery branches and cannulated (16-gauge cannula). Hearts were perfused in retrograde fashion as outlined previously [27]. Flow through the heart was established, and extra tissue was dissected away. An incision was made in the right ventricle, and the left atrial appendage was removed. A small flexible nonelastic balloon was inserted into the left atrium through the mitral valve and into the left ventricle. This fluid-filled balloon was attached to a fine plastic catheter and connected to a pressure transducer (MEMSCAP, Skoppum, Norway) and a graduated syringe (0-1000 μL; Hamilton, Reno, NE). Hearts were maintained at 37°C and perfused at a constant pressure (100 cm H2O) with a Krebs-Henseleit crystalloid medium supplemented with glucose (10 mmol/L) and CaCl2 (1.3 mmol/L) gassed with oxygen/CO2 (95:5). Developed pressure was measured after isovolumic contraction of the fluid-filled balloon and recorded to computer using a digital interface (AD Instruments, Chalgrove, Oxford, United Kingdom). For selected hearts, the AMPK agonist 5-amino-imidazole-4-carboxamide riboside (AICAR) (final concentration, 0.5 mmol/L) was added to the perfusate. Initial experiments utilized AICAR at concentrations up to 2 mmol/L without significantly greater affect of LPL, yet with significant decreases of cardiac function; therefore, a lower concentration was adopted for the remaining experiments.
2.2.4. Ventricular performance
The initial balloon volume was adjusted until the diastolic pressure recorded measured 0 mm Hg and the developed pressure (difference between systolic and diastolic pressures) was less than 10 mm Hg. Balloon volume was increased until diastolic pressure reached 20 mm Hg, and this was established as the “working pressure” of the myocardium. Coronary flow was estimated from timed collections of a known volume of perfusate and expressed as volume per unit mass of cardiac tissue. Coronary flow was measured as timed ejections of a known volume.
Ventricular performance was calculated off-line after the experiment using computer analysis software (Chart Version 5.0, AD Instruments). Heart rate, systolic pressure, diastolic pressure, and hence developed pressure were measured. Rate of change of pressure (+dP/dt) was calculated from the maxima of first-order derivative of pressure trace. Rate-pressure product (RPP) was calculated at each balloon volume as the product of heart rate (beats per minute) × developed pressure (millimeters of mercury).
2.2.5. Quantitation of plasma tritiated water
Metabolism of Intralipid was estimated from quantitation of tritiated water as previously described [26]. Briefly, aliquots of perfusate (1.0 mL) were extracted with chloroform/methanol (2:1) (20 mL). After addition of water (4.0 mL), tritiated water was estimated in the aqueous fraction by scintillation counting. Metabolism was calculated with reference to the specific activity at the start of the experiment.
2.2.6. Glucose metabolism
For selected hearts perfusate, glucose (10 mmol/L) was supplemented with U-14C-labeled glucose (0.185 MBq per perfusion). Effluent gases were collected from “gas-tight” perfusion apparatus into ethanolamine/ethylene glycol (2:1) solution [28], and samples of perfusate were recovered to estimate the liberation of 14C-labeled CO2 as CO2 or bicarbonate as previous detailed [26].
2.2.7. Total lipid extraction
Total cholesterol and triglycerides were also extracted from the hearts as described previously [27]. Briefly, aliquots (100 mg) of heart powder were extracted with methanol/chloroform (1:2). Extracts were evaporated to dryness and resuspended in absolute ethanol. Cardiac TAG and cholesterol were measured using commercial kits. For selected extracts, lipids were separated into phospholipids, diacylglycerol, FA, TAG, and cholesterol ester as outlined previously [26]. Briefly, ethanol extracts of tissue (100 mg) were separated on thin-layer chromatography plates (silica gel 60, 250 μm) and separated using the solvent system hexane/diethyl ether/acetic acid (70:30:1.6). Lipids were visualized using rhodamine 6G and UV light. Lipids were mechanically recovered from the thin-layer chromatography plate and quantified by liquid scintillation counting.
2.3. Estimation of 14C-glucose incorporation and total glycogen
Total cardiac glycogen and incorporation of 14C-labeled glucose into tissue glycogen were estimated as previously outlined [29]. Briefly, cardiac tissue (∼50 mg) was digested in alkali (200 μL, 30% wt/vol KOH, 70°C, 60 minutes). Glycogen was precipitated from the resulting digest after addition of 5 vol ice-cold absolute ethanol. After centrifugation, the pellet was resuspended in water; and the precipitation was repeated. Glycogen pellets were air-dried and redissolved in acetate buffer (50 mmol/L, pH = 4.5). 14C-labeled glucose incorporation was estimated after scintillation counting of an aliquot of the redissolved glycogen. The remainder of the glycogen was treated with amyloglucosidase (100 units per reaction; final volume, 0.5 mL) and digested overnight. Liberated glucose was estimated spectrophotometrically by glucose oxidase method.
2.3.1. LPL activity
Lipoprotein lipase activity was measured as previously described [26,27]. Briefly, separate groups of hearts from control and metformin-treated rats were perfused with Krebs-Henseleit medium containing glucose (10 mmol/L) and CaCl2 (1.3 mmol/L) as outlined above. Perfusion was maintained initially in nonrecirculating mode to wash out erythrocytes. Recirculating perfusion was established and maintained for 5 minutes, after which heparin (final concentration, 10U/mL) was added and recirculated for a further 2 minutes. Samples of perfusate were isolated and frozen in liquid nitrogen. Cardiac tissue was then snap-frozen in liquid nitrogen, and cardiac mass was noted. Aliquots of postheparin perfusate and acetone-dried heart powders (10 mg) were reacted with TAG emulsion (final concentration, 5.6 mmol/L) prelabeled with 3H-[9,10]-triolein supplemented with human plasma (ratio of plasma to final reaction volume, 1:6) as a source of apolipoprotein C-II. Reactions were carried out in Tris-HCl buffer (0.1 mol/L, pH = 8.0) supplemented with FA-free bovine albumin (final concentration, 2.0% wt/vol). Incubations were carried out at 37°C, and activities were expressed per unit mass of cardiac tissue. Total cardiac LPL activity was estimated as the sum of tissue residual LPL and heparin-releasable LPL activity. For estimates of postheparin plasma LPL activity, high- (4 mol/L) and low-NaCl (0.2 mol/L)–containing buffers were used to discriminate between hepatic lipase and total plasma lipase activity, respectively, released after heparin treatment. Lipoprotein lipase was estimated as hepatic subtracted from total lipase activities.
2.3.2. Postheparin plasma
Under halothane anesthesia, blood samples were collected from the inferior vena cava for estimation of plasma glucose and TAG. Heparin was infused into the inferior vena cava (1000 U/kg body mass), and blood plasma was collected a further 2 minutes later. Plasma samples were snap-frozen in liquid nitrogen before analysis.
2.3.3. LPL recruitment to the endothelium
Hearts from control and metformin-treated rats (600 mg/kg body mass, 2 weeks) were excised from heparin-pretreated rats and perfused as outlined above in a nonrecirculating apparatus. After establishment of normal perfusion, the apparatus was transferred to recirculating and perfused for 60 minutes with Krebs-Henseleit medium supplemented with glucose (10 mmol/L) and CaCl2 (1.3 mmol/L) [30]. For selected hearts, AICAR was added to the perfusate (final concentration, 0.5 mmol/L). All hearts were unpaced. After 60 minutes, heparin (10 U/mL perfusate) was added and recirculated for 10 minutes. Aliquots of perfusate were frozen for estimation of LPL activity as outlined above.
2.3.4. ACC activity
Cardiac ACC activity was estimated by the bicarbonate fixation method of Saddik et al [31]. Briefly, tissues were homogenized in buffer containing Tris-HCl (50 mmol/L, pH = 7.5), NaF (100 mmol/L), EDTA (2 mmol/L), sucrose (0.25 mol/L), and mercaptoethanol (70 μL/100 mL). Homogenates were centrifuged (18 000 rpm, 30 minutes); and the supernatant was dialyzed against buffer containing Tris-HCl (50 mmol/L, pH = 7.5), NaF (100 mmol/L), EDTA (2 mmol/L), mercaptoethanol (10 mmol/L), and glycerol (10% vol/vol). The ACC activity was quantified in dialysate protein (100 μg) added to reaction mixture (final volume, 190μL) containing Tris-HCl (11.5 mmol/L, pH = 7.5), bovine albumin (2.9 μmol/L), mercaptoethanol (1.5 μmol/L), ATP (0.41 mmol/L), acetyl-CoA (0.21 mmol/L), magnesium acetate (0.97 mmol/L), and NaHCO3 (3.5 mmol/L) supplemented with 14C-labeled NaHCO3 (final reaction, 1.375 μCi/mL). Reactions were incubated for 5 minutes at 37°C and terminated by addition of 6% (wt/vol) perchloric acid (200 μL). Radioactivity was estimated in the aqueous fraction by liquid scintillation counting.
2.3.5. Immunoblotting for proteins
Standard Western immunoblotting techniques were used for the detection and estimation of relative amounts of p38-MAPK, phospho-p38-MAPK, muscle CPT1, AMPKα2, phospho-AMPKα2, and tubulin proteins. Briefly, cardiac tissue (50 mg) was powdered in liquid nitrogen and extracted with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors, followed by centrifugation (10 000 rpm for 10 minutes) and recovery of the supernatant. The membranes were probed with antibodies specific for muscle CPT1 (all Santa Cruz Biotechnology, Santa Cruz, CA; initial dilution, 1:1000), AMPKα2, threonine-172 phospho-AMPKα2 (both Kinasource; initial dilution, 1:1500), and mouse monoclonal anti-tubulin (Sigma; initial dilution, 1:2500). Differing sample protein loadings were used for different antibodies (phospho-AMPKα2, 20 μg; muscle CPT1 and AMPKα2, 10-15 μg; p38-MAPK and phospho-p38-MAPK [Thr180/Tyr182], 80 μg; tubulin, 10 μg). Densitometry of Western blots was estimated using ImageJ software (NIH, Bethesda, MD). Protein expression was corrected for the expression of an internal control (tubulin).
2.3.6. Statistical analysis
Statistical analysis was carried out using single factor analysis of variance with Bonferroni correction for multiple comparisons where appropriate. Data represent mean ± standard deviation.
3. Results
3.1. Postmortem
Metformin treatment was without effect on body mass gain by the rats. At postmortem, metformin was without effect on plasma TAG concentration (Table 1); however, plasma glucose concentration was decreased 20% by high-dose metformin (P < .01, Table 1).
Table 1.
Plasma metabolite concentrations after treatment with low- and high-dose metformin
| Measurement | Control | Metformin (300 mg/kg) | Metformin (600 mg/kg) | AICAR (0.5 mmol/L) |
|---|---|---|---|---|
| Body mass (g) | 277 ± 18 | 313 ± 12 | 308 ± 16 | 312 ± 18 |
| Heart mass (g) | 1.50 ± 0.23 | 1.63 ± 0.17 | 1.42 ± 0.11 | 1.43 ± 0.10 |
| Plasma glucose (mmol/L) | 10.8 ± 0.9 | 10.2 ± 1.0 | 8.0 ± 0.9⁎ | – |
| Plasma TAG (mmol/L) | 1.4 ± 0.3 | 1.1 ± 0.3 | 1.4 ± 0.2 | – |
Data represent mean ± SD (n = 6).
Statistical significance represented as significantly different from control:
P > .01.
3.2. Cardiac performance
Low- and high-dose metformin treatments were without effect on RPP for perfused hearts (Table 2). However, AICAR decreased RPP by approximately 30% for perfused hearts (P < .05, Table 2).
Table 2.
Lipoprotein lipase recruitment and cardiac performance in ex vivo perfused hearts after treatment with heparin before perfusion
| Measurement | Control | Metformin (300 mg/kg) | Metformin (600 mg/kg) | AICAR (0.5 mmol/L) |
|---|---|---|---|---|
| RPP (mm Hg/min) | 16 078 ± 2912 | 16 709 ± 2561 | 21 687 ± 4702 | 11 682 ± 1403⁎ |
| Tissue TAG (μmol/g wet mass) | 1.73 ± 0.64 | 1.72 ± 0.48 | 5.55 ± 1.83† | 1.93 ± 0.45 |
| Tissue glycogen (μmol/g wet mass) | 14.1 ± 2.9 | 15.6 ± 3.9 | 13.2 ± 2.5 | 18.0 ± 1.5 |
| ACC activity (nmol/min/mg protein) | 132 ± 16 | 109 ± 19 | 69 ± 25† | 90 ± 21† |
| LPL activity (μmol/h/g wet mass) | 18.1 ± 6.1 | ND | 24.8 ± 7.2 | 35.0 ± 9.0⁎ |
Data represent MEAN ± SD (n = 5). ND indicates not determined.
Statistical significance represented as significantly different from control:
P < .05.
P > .01.
3.3. Lipid metabolism
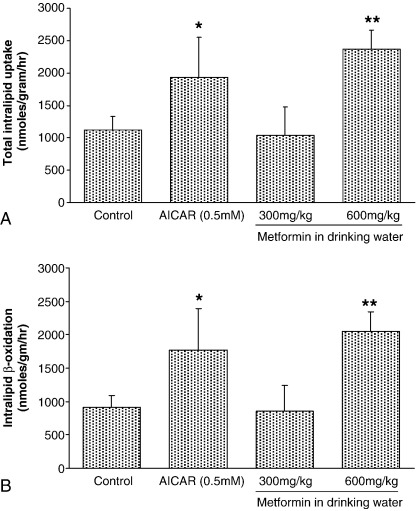
5-Amino-imidazole-4-carboxamide riboside increased uptake of Intralipid-derived FA by approximately 60% (P < .05, Fig. 1A). Low-dose metformin was without effect on Intralipid uptake by perfused hearts; however, high-dose metformin increased Intralipid uptake by perfused hearts (2.2-fold, Fig. 1A, P < .01). Fatty acid β-oxidation of Intralipid-derived FA mirrored uptake of Intralipid (Fig. 1B) and represented 90% of total uptake for all groups (Fig. 1). 5-Amino-imidazole-4-carboxamide riboside increased FA β-oxidation to 95% of total uptake (P < .01, Fig. 1). High-dose metformin increased Intralipid β-oxidation 2.2-fold (Fig. 1B, P < .01) compared with control hearts, and low-dose metformin was without affect.
Fig. 1.
Rates of (A) TAG-lipid total utilization and (B) TAG β-oxidation for perfused rat hearts after treatment with low- or high-dose metformin and AICAR. Data represent mean ± SD (n = 6 hearts in all groups). Statistical significance represented as different from control: *P < .05 and **P < .01.
3.4. Glucose metabolism
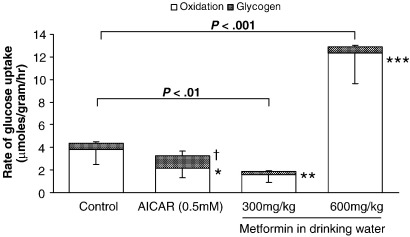
Tissue concentrations of unlabeled glycogen were unchanged after treatment with AICAR or low- or high-dose metformin (not significant [NS] for all, Table 2). Incorporation of glucose into glycogen represented 10% of the total 14C-glucose uptake for control hearts (Fig. 2). 5-Amino-imidazole-4-carboxamide riboside treatment did not affect total uptake of glucose, yet decreased oxidation of glucose (P < .05, Fig. 2) and increased deposition as glycogen (P < .05, Fig. 2). Low-dose metformin decreased total glucose uptake (P < .01, Fig. 2), decreased glucose oxidation (P < .01, Fig. 2), and preserved glycogen incorporation. High-dose metformin increased glucose uptake 3-fold (P < .001, Fig. 2) and increased glucose oxidation correspondingly (P < .001, Fig. 2). However, compared with controls, high-dose metformin preserved the incorporation of glucose into glycogen.
Fig. 2.
Rate of uptake of glucose for perfused rat hearts after treatment with low- or high-dose metformin and AICAR. Data show glucose uptake as metabolism of glucose and accumulation as glycogen. Data represent mean± SD (n = 6 hearts in all groups). Statistical significance represented as glucose oxidation different from control: *P < .05, **P < .01, and ***P < .001. Glucose deposition as glycogen different from control: †P < .05.
3.5. Tissue lipid incorporation
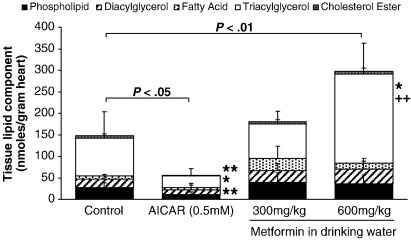
Neither AICAR nor low-dose metformin altered tissue unlabeled TAG concentration (NS for both, Table 2). However, high-dose metformin increased unlabeled TAG deposition 3-fold (P < .01, Table 2). 5-Amino-imidazole-4-carboxamide riboside significantly decreased the deposition of Intralipid-FA as tissue lipids in the perfused heart (Fig. 3, P < .05). When separated into individual lipid classes, AICAR significantly decreased Intralipid deposition as phospholipids (P < .01), TAG (P < .05), and cholesterol ester (Fig. 3, P < .01). Low-dose metformin was without effect on tissue 3H-labeled lipid accumulation or the individual lipid classes (Fig. 3, NS). High-dose metformin increased Intralipid incorporation into total lipids (P < .01, Fig. 3). High-dose metformin also increased lipid deposition as TAG (P < .05, Fig. 3). When compared with low-dose metformin, high-dose metformin significantly increased lipid accumulation as TAG (P < .01, Fig. 3).
Fig. 3.
Incorporation of Intralipid into tissue lipids for perfused rat hearts. Data show Intralipid incorporation into individual lipid classes. Data represent mean ± SD (n = 6 hearts in all groups). Statistical significance represented as different from control: *P < .05 and **P < .01. Effect of increasing metformin dose: +P < .05.
3.6. LPL activity
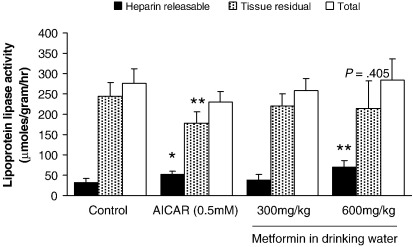
For control perfused hearts, heparin-releasable LPL represented 10% of total tissue LPL (Fig. 4). This ratio was preserved for low-dose metformin–treated hearts. 5-Amino-imidazole-4-carboxamide riboside treatment was without effect on total LPL for perfused hearts (NS, Fig. 4), yet heparin-releasable LPL increased 100% compared with control hearts (P < .05, Fig. 4). There was a corresponding decrease in tissue residual LPL activity (P < .01 compared with control, Fig. 4), implying translocation between tissue residual and heparin-releasable pools. High-dose metformin increased heparin-releasable LPL 3-fold (P < .01, Fig. 4) and was without effect on tissue residual or total LPL activities (NS, Fig. 4). No change was noted for LPL messenger RNA expression in hearts isolated from any group (data not shown).
Fig. 4.
Heparin-releasable and tissue LPL activity from rat hearts after treatment with low- or high-dose metformin and AICAR. Total LPL estimated as sum of heparin-releasable and tissue residual LPL. Data represent mean ± SD (n = 6 hearts in all groups). Statistical significance represented as different from control: *P < .05 and **P < .01.
3.7. Western blot analysis
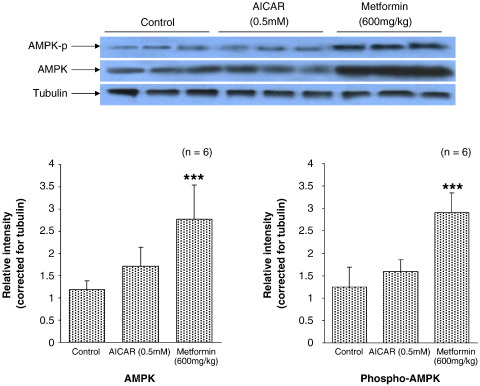
High-dose metformin increased immunodetectable AMPK 2-fold compared with control hearts (P < .001, Fig. 5). In addition, high-dose metformin increased phosphorylation of AMPK 2.5-fold compared with control hearts (P < .001, Fig. 5). 5-Amino-imidazole-4-carboxamide riboside was without effect on tissue AMPK or phospho-AMPK levels as detected by Western blot (NS, Fig. 5). Low-dose metformin was also without effect on either immunodetectable AMPK or phospho-AMPK (data not shown).
Fig. 5.
Representative Western blot analysis showing relative abundance of phospho-AMPK, AMPK, and tubulin proteins after treatment with low- or high-dose metformin and AICAR. Individual protein levels corrected for tubulin internal control for each sample. Data represent mean ± SD (n = 6 hearts in all groups). Statistical significance represented as different from control: ***P < .001.
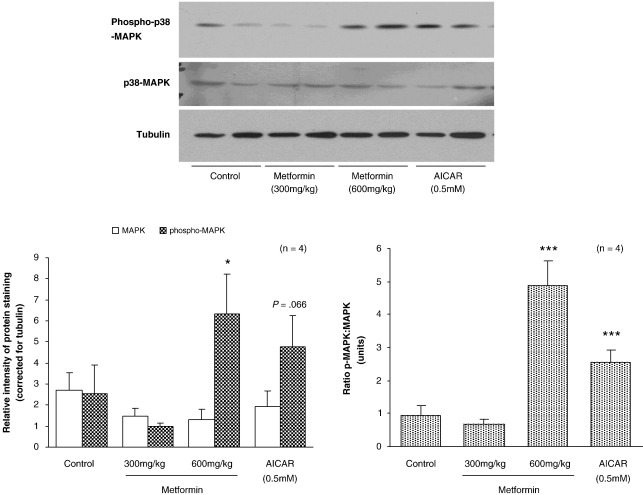
Estimation of p38-MAPK content of hearts was unaffected by treatment with low- or high-dose metformin or perfusion with AICAR (Fig. 6). High-dose metformin increased phospho-p38-MAPK in hearts 2.5-fold (P < .05, Fig. 6). 5-Amino-imidazole-4-carboxamide riboside treatment of perfused hearts increased phospho-p38-MAPK protein content 2-fold (NS, Fig. 6). Estimation of the phospho-p38-MAPK/p38-MAPK ratio to quantify the shift in phosphorylation revealed that, for both AICAR and high-dose metformin, the ratio was increased (P < .001, Fig. 6). Low-dose metformin was without effect on the phospho-p38-MAPK/p38-MAPK ratio.
Fig. 6.
Representative Western blot analysis showing relative abundance of phospho-p38-MAPK, MAPK, and tubulin proteins after treatment with high-dose metformin and AICAR. Individual protein levels corrected for tubulin internal control for each sample. Data represent mean ± SD (n = 4 hearts in all groups). Statistical significance represented as different from control: *P < .05.
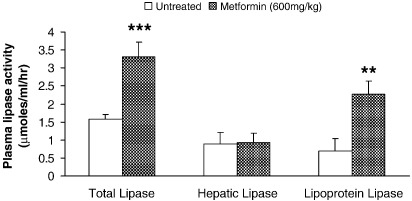
3.8. Postheparin lipase activity
High-salt active plasma lipase (hepatic lipase) represented approximately 60% of total plasma lipase activity isolated from heparin-pretreated rat plasma (Fig. 7). High-dose metformin treatment of rats doubled heparin-releasable lipase activity in plasma (P < .001, Fig. 7), but was without effect of lipase activity in high-salt buffer (Fig. 7). By calculation, LPL activity was increased 3-fold after high-dose metformin treatment (P < .01, Fig. 7).
Fig. 7.
Estimation of postheparin plasma lipase activity for rats after treatment with high-dose metformin. Total and hepatic lipase activities determined after use of low– and high–salt concentration reaction buffer. Lipoprotein lipase activity determined by subtraction of hepatic from total lipase activities. Data represent mean ± SD (n = 6 hearts in all groups). Statistical significance represented as different from control: **P < .01 and ***P < .001.
3.9. Cardiac recruitment of LPL
Lipoprotein lipase recruitment to the cardiac endothelium estimated after 60-minute perfusion with glucose only revealed that metformin pretreatment of rats was without affect of translocation of LPL to the heparin-releasable compartment (table 2). 5-Amino-imidazole-4-carboxamide riboside treatment of perfused hearts doubled LPL recruitment to the heparin-releasable compartment (18.1 ± 6.1 μmol/[h g wet mass], control vs 35.0 ± 9.0 μmol/[h g wet mass], AICAR treated; P < .05; Table 2).
3.10. ACC activity
5-Amino-imidazole-4-carboxamide riboside treatment of perfused hearts decreased ACC activity by 30% (P < .01, Table 2); however, low-dose metformin was without affect on ACC activity (NS, Table 2). High-dose metformin decreased ACC activity by 50% compared with control (P < .01, Table 2).
4. Discussion
We demonstrate that at a high dose, metformin is able to increase the presentation of LPL at the cardiac endothelium and that this may result from a direct activation of AMPK in the myocardium [32]. Interestingly, this effect may not arise from an increase in the rate of recruitment to the endothelium because, after heparin pretreatment to deplete endothelial LPL, the recruitment of LPL to the endothelium after 60-minute perfusion is unchanged by metformin when compared with control, but increased by acute AICAR treatment.
High-dose metformin and AICAR increased the uptake of Intralipid-derived FA and did not increase phosphorylation of AMPK protein yet led to LPL translocation to endothelium [8]. Low-dose metformin treatment was without effect on the endothelial LPL, the phosphorylation of AMPK, or, more critically, the uptake of Intralipid into the myocardium. None of the treatments used altered LPL messenger RNA expression (data not shown) or total LPL activity, suggesting that both AICAR and high-dose metformin alter distribution of an existing pool of enzyme rather than promoting synthesis of fresh enzyme. The majority (∼90%) of lipid uptake was diverted directly to β-oxidation, in agreement with our previous results [27]. The concentrations of AICAR used in the initial experiments were sufficient to increase the translocation of LPL to the endothelium over the course of the 60-minute perfusion and increased lipid uptake into the myocardium without apparent effect on AMPK protein phosphorylation. This may reflect allosteric activating effects of AMP/ZMP without direct phosphorylation as previously noted [33]. 5-Amino-imidazole-4-carboxamide riboside also significantly decreased accumulation of Intralipid as tissue lipids that may represent alterations to the activity of glycerol-3-phosphate acyl transferase, the first committed step in synthesis of TAG and phospholipid, and negatively regulated acutely by AMPK [34]. We also illustrate the downstream activation of p38-MAPK after metformin treatment and AICAR. More importantly, we document decreased ACC activity after both AICAR and high-dose metformin treatment despite no overt phosphorylation of AMPK protein. Adenosine monophosphate–activated protein kinase can phosphorylate downstream targets and affect cellular processes including FA β-oxidation [33]. Similar studies after activation of AMPK (in vivo) for rats document no change to utilization of glucose [35], yet AICAR was also noted to increase translocation of glucose transporter–4 to the cardiac endothelium and facilitate increased glucose uptake for perfused hearts [7]. We note the preservation of glucose uptake by AICAR coupled with maintained unlabeled tissue glycogen, yet a change in the fate of glucose characterized by increased deposition as glycogen. This is at odds with previous experiments detailing preserved glycogen levels and increased rates of glycogen synthesis [22]. Interestingly, high-dose metformin increased glucose uptake with little impact on glycogen deposition, implying increased demands for ATP. Given the preservation of glucose-FA “Randle” cycle in hearts, the LPL-mediated increase in FA uptake might be expected to decrease glucose utilization [36]; and the increased FA β-oxidation levels may simply represent increased FA supply through LPL [37], as no changes to CPT1 protein were noted (data not shown). The increased FA β-oxidation after high-dose metformin is similar to that previously observed for FA-perfused hearts [22]. Experiments adopting the same high-dose regimen for metformin document increased citrate synthase and β-hydroxyacyl-CoA dehydrogenase for soleus muscle via peroxisome proliferator-activated receptor-γ coactivator-1α, implying increased FA β-oxidation [38]. Decreased ACC activity through phosphorylation by AMPK [39] may diminish cellular concentrations of malonyl-CoA [40], enhancing FA translocation into the mitochondria and measured as increased rates of β-oxidation in these perfusions. Chronic exposure to high-dose metformin led to accumulation of cardiac TAG, indicative of increased lipid uptake reflecting prolonged increase in uptake of plasma lipids through LPL. Previous experiments document a reduction in cardiac TAG for FA-perfused hearts after exposure to metformin [22], a discrepancy with our experiment that may reflect the increase (in vivo) in cardiac LPL in the intact animal leading to lipid accumulation in excess of requirements for β-oxidation.
Previous experiments suggest that direct activation of AMPK after acute increases in cardiac work [41] and with the pharmacologic agonist AICAR increased the recruitment of LPL to the endothelial surface from the subendothelial layers [42]. Our experiments confirm that, after perfusion of heparin-pretreated hearts, now devoid of active endothelial LPL, AICAR increased LPL translocation to the endothelial surface; and this increase is met by transfer of tissue residual LPL activity to the endothelial surface. Such increased recruitment is not noted for metformin-treated hearts. This may result from the continuous activation of AMPK, initially increasing recruitment of LPL from subendothelial compartments through increased translocation from cardiomyocytes; but after prolonged activation, the pool becomes depleted. Our data support acute changes to LPL relying on a pool of available enzyme in the cardiomyocyte. We speculate that, after prolonged activation (chronic metformin exposure), this becomes depleted and increases in LPL transfer must be met through direct use of freshly transcribed protein; yet this is not reflected in changes to tissue residual LPL for metformin-treated hearts.
It is striking that high dose metformin increased postheparin plasma LPL in the rat, mirroring changes noted for the myocardium. Previous experiments document that myocardium contains only 5% of the whole-body endothelial LPL activity [43], and recent observation demonstrates increased LPL activity for metformin-treated L6 skeletal muscle cells in culture [44]. We anticipated decreased plasma TAG [45] in the presence of increased postheparin plasma LPL; however, none was recorded. We cannot rule out changes to food intake and hence CMs as a consequence of metformin exposure; however, previous observations suggest that for mice and rats the anorectic effects of metformin are modest and transient [46,47]. We also cannot discount that changes to lipogenic rates in the rat increased hepatically derived TAG. However, hepatocyte culture with metformin suggests metformin was without effect on lipogenesis rates [48]; and 7-day exposure to low-dose metformin led to decreased very low-density lipoprotein secretion by perfused livers [45].
The divergent effects of low- vs high-dose metformin in vivo are complex. Low-dose metformin preserved normal metabolic function, whereas high-dose metformin increased substrate metabolism without altering cardiac work, suggestive of metabolic inefficiency. Indeed, previous observations suggest that metformin at high dose may directly inhibit complex I of the electron transport chain, thus decreasing metabolic efficiency of the substrates used [49,50]. Decreased metabolic efficiency after high-dose metformin may suggest a mechanism resulting in both the increased β-oxidation of FA and increased glucose oxidation without altered cardiac work (estimated as RPP). By contrast, others report improved myocardial energy status, measured as increased phosphocreatine concentrations, after acute exposure to lower doses of metformin for perfused hearts [22] and improved mitochondrial performance after metformin treatment of chronic coronary artery-ligated mice [14]. This discrepancy reflects an improvement of coupling of oxidative phosphorylation at low concentration of metformin [14,22], followed by direct inhibition of complex I and mitochondrial inefficiency at higher doses [49,50]. 5-Amino-imidazole-4-carboxamide riboside is known to be a negative inotrope and chronotrope [33]; and given the intimate connection between cardiac work and metabolism maintenance of cardiac performance, our estimates of metabolite oxidation may be underestimates to meet decreased work.
5. Concluding remarks
We confirm that acute activation of AMPK by AICAR led to increased LPL presentation at the endothelium. Moreover, long-term metformin treatment increased LPL presentation at the cardiac endothelium possibly through an AMPK-dependent mechanism; and yet this did not simply represent increased rates recruitment from the subendothelial compartment.
Acknowledgment
The authors are grateful to Dr Y Cheng (School of Clinical and Experimental Medicine, University of Birmingham) for assistance with the “Western” immunoblotting and to the British Heart Foundation (Project Grant 06/007) for their financial support of this work.
References
- 1.Allard M.F., Schőnekess B.O., Henning S.L., English D.R., Lopaschuk G.D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol Heart. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 2.Massie B.M., Schaefer S., Garcia J., McKirnan M.D., Schwartz G.G., Wisneski J.A. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91:1814–1823. doi: 10.1161/01.cir.91.6.1814. [DOI] [PubMed] [Google Scholar]
- 3.Conway M.A., Allis J., Ouwerkerk R., Niioka T., Rajagopalan B., Radda G.K. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- 4.Allard M.F., Parsons H.L., Saeedi R., Wambolt R.B., Brownsey R. AMPK and metabolic adaptation by the heart to pressure overload. Am J Physiol. 2007;292:H140–H148. doi: 10.1152/ajpheart.00424.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kudo N., Gillespie J.G., Kung L., Witters L.A., Schulz R., Clanachan A.S. Characterisation of 5'AMP-activated protein kinase in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Holman G.D. Long-term metformin treatment stimulates cardiomyocyte glucose transport through and AMP-activated protein kinase–dependent reduction in GLUT4 endocytosis. Endocrinology. 2006;147:2728–2736. doi: 10.1210/en.2005-1433. [DOI] [PubMed] [Google Scholar]
- 7.Russell R.R., Bergeron R., Shulman G.I., Young L.H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 8.An D., Pulinilkummil T., Qi D., Ghosh S., Abrahani A., Rodrigues B. The metabolic “switch” AMPK regulates cardiac heparin-releasable lipoprotein lipase. Am J Physiol. 2005;288:E246–E253. doi: 10.1152/ajpendo.00211.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kim M.S., Kewalramani G., Puthanveetil P., Lee V., Kumar U., An D. Acute diabetes modulates trafficking of cardiac lipoprotein lipase through p38 mitogen-activated protein kinase–dependent actin cytoskeleton organisation. Diabetes. 2008;57:64–76. doi: 10.2337/db07-0832. [DOI] [PubMed] [Google Scholar]
- 10.Xing Y., Musi N., Fujii N., Zou L., Luptak I., Hirshman M.F. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 11.Stuck B.J., Lenski M., Böhm M., Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem. 2008;283:32562–32569. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Yin R., Chen D., Liu D., Wang D., Yang Q. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. Cell Biochem. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad F., Arad M., Musi N., He H., Wolf C., Branco D. Increased α2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation. 2005;112:3140–3148. doi: 10.1161/CIRCULATIONAHA.105.550806. [DOI] [PubMed] [Google Scholar]
- 14.Gundewar S., Calvert J.W., Jha S., Toedt-Pingel I., Ji S.Y., Nunez D. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey C.J. Metformin—an update. Gen Pharmacol. 1993;24:1299–1309. doi: 10.1016/0306-3623(93)90411-p. [DOI] [PubMed] [Google Scholar]
- 16.Klip A., Leiter L.A. Cellular mechanism of action of metformin. Diabetes Care. 1990;13:696–704. doi: 10.2337/diacare.13.6.696. [DOI] [PubMed] [Google Scholar]
- 17.Legtenberg R.J., Houston R.J., Oeseburg B., Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metabol Res. 2002;34:182–185. doi: 10.1055/s-2002-26705. [DOI] [PubMed] [Google Scholar]
- 18.Sambandam N., Abrahani M.A., St. Pierre E., Al-Ata O., Cam M.C., Rodrigues B. Localisation of lipoprotein lipase in the diabetic heart: regulation by acute changes in insulin. Arterioscler ThrombVasc Biol. 1999;19:1526–1534. doi: 10.1161/01.atv.19.6.1526. [DOI] [PubMed] [Google Scholar]
- 19.Solskov L., Løfgren B., Kristiansen S.B., Jessen N., Pold R., Nielsen T.T. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24hr after administration. Basic Clin Pharmacol Toxicol. 2008;103:82–87. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 20.Calvert J.W., Gundewar S., Jha S., Greer J.J.M., Bestermann W.H., Tian R. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS–mediated signalling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., He H., Balschi J.A. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol. 2007;293:H457–H466. doi: 10.1152/ajpheart.00002.2007. [DOI] [PubMed] [Google Scholar]
- 22.Saeedi R., Parsons H.L., Wambolt R.B., Paulson K., Sharma V., Dyck J.R.B. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol. 2008;294:H2497–H2506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 23.Fischer Y., Thomas J., Rosen P., Kammermeir H. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology. 1995;136:412–420. doi: 10.1210/endo.136.2.7835271. [DOI] [PubMed] [Google Scholar]
- 24.Grosskopf I., Ringel Y., Charach G., Maharshak N., More R., Iaina A. Metformin enhances clearance of chylomicrons and chylomicron remnants in nondiabetic mildly overweight glucose-intolerant subjects. Diabetes Care. 1997;20:1598–1602. doi: 10.2337/diacare.20.10.1598. [DOI] [PubMed] [Google Scholar]
- 25.Ohira M., Miyashita Y., Ebisuno M., Saiki A., Endo K., Koide N. Effect of metformin on serum lipoprotein lipase mass levels and LDL particle size in type 2 diabetes mellitus patients. Diabetes Res Clin Pract. 2007;78:34–41. doi: 10.1016/j.diabres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Hauton D., Bennett M.J., Evans R.D. Utilisation of triacylglycerol and non-esterified fatty acid by the working rat heart: myocardial lipid substrate preference. Biochim Biophys Acta. 2001;1533:99–109. doi: 10.1016/s1388-1981(01)00146-9. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y., Hauton D. Cold acclimation induces physiological cardiac hypertrophy and increases assimilation of triacylglycerol metabolism through lipoprotein lipase. Biochim Biophys Acta. 2008;1781:618–626. doi: 10.1016/j.bbalip.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffay H., Alvarez J. Liquid scintillation counting of carbon-14. Use of ethanolamine-ethylene glycol monomethyl ether-toluene. Analytical Chem. 1961;33:612–615. [Google Scholar]
- 29.Lavery G.G., Hauton D., Hewitt K.N., Brice S.M., Sherlock M., Walker E.A. Hypoglycaemia with enhanced hepatic glycogen synthesis in recombinant mice lacking hexose-6-phosphate dehydrogenase. Endocrinology. 2007;148:6100–6106. doi: 10.1210/en.2007-0963. [DOI] [PubMed] [Google Scholar]
- 30.Pulinilkunnil T., Abrahani A., Varghese J., Chan N., Tang I., Ghosh S. Evidence for rapid “metabolic switching” through lipoprotein lipase occupation of endothelial-binding sites. J Mol Cell Cardiol. 2003;35:1093–1103. doi: 10.1016/s0022-2828(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 31.Saddik M., Gamble J., Witters L.A., Lopaschuk G.D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 32.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longnus S.L., Wambolt R.B., Parsons H.L., Brownsey R.W., Allard M.F. 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol. 2003;284:R936–R944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- 34.Muoio D.M., Seefeld K., Witters L.A., Coleman R.A. AMP-activated protein kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in the liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 35.Shearer J., Fueger P.T., Rottman J.N., Bracy D.P., Martin P.H., Wasserman D.H. AMPK stimulation increases LCFA but not glucose clearance in cardiac muscle in vivo. Am J Physiol. 2004;287:E871–E877. doi: 10.1152/ajpendo.00125.2004. [DOI] [PubMed] [Google Scholar]
- 36.Randle P.J. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Longnus S.L., Wambolt R.B., Barr R.L., Lopaschuk G.D., Allard M.F. Regulation of myocardial fatty acid oxidation by substrate supply. Am J Physiol. 2001;281:H1561–H1567. doi: 10.1152/ajpheart.2001.281.4.H1561. [DOI] [PubMed] [Google Scholar]
- 38.Suwa M., Egashira T., Nakano H., Sasaki H., Kumgai S. Metformin increases PGC-1α protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- 39.Ho R.C., Fujii N., Witters L.A., Hirshman M.F., Goodyear L.J. Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signalling in skeletal muscle. Biochem Biophys Res Comm. 2007;362:354–359. doi: 10.1016/j.bbrc.2007.07.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson D.M., Brown J.D., Fillmore N., Condon B.M., Kim H.J., Barrow J.R. LKB1 and the regulation of malonyl-CoA and fatty acid oxidation in muscle. Am J Physiol. 2007;293:E1572–E1579. doi: 10.1152/ajpendo.00371.2007. [DOI] [PubMed] [Google Scholar]
- 41.An D., Kewalramani G., Qi D., Pulinilkunnil T., Ghosh S., Abrahani A. β-Agonist stimulation produces changes in cardiac AMPK and coronary lumen LPL only during increased workload. Am J Physiol. 2005;288:E1120–E1127. doi: 10.1152/ajpendo.00588.2004. [DOI] [PubMed] [Google Scholar]
- 42.Qi D., Kuo K.H., Abrahani A., An D., Qi Y., Heung J. Acute Intralipid infusion reduces cardiac luminal lipoprotein lipase but recruits additional enzyme from cardiomyocytes. Cardiovasc Res. 2006;72:124–133. doi: 10.1016/j.cardiores.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Ruge T., Bergo M., Hultin M., Olivecrona G., Olivecrona T. Nutritional regulation of binding sites for lipoprotein lipase in rat heart. Am J Physiol. 2000;278:E211–E218. doi: 10.1152/ajpendo.2000.278.2.E211. [DOI] [PubMed] [Google Scholar]
- 44.Ohira M., Miyahita Y., Murano T., Watanabe F., Shirai K. Metformin promotes induction of lipoprotein lipase in skeletal muscle through activation of adenosine monophosphate-activated protein kinase. Metabolism. 2009;58:1408–1414. doi: 10.1016/j.metabol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Tessari P., Tiengo A. Metformin treatment of rats with diet-induced overweight and hypertriglyceridemia decreases plasma triglyceride concentrations, while decreasing triglyceride and increasing plasma ketone body output by the isolated perfused liver. Acta Diabetol. 2008;45:143–145. doi: 10.1007/s00592-008-0032-0. [DOI] [PubMed] [Google Scholar]
- 46.Bailey C.J., Flatt P.R., Ewan C. Anorectic effect of metformin in lean and genetically obese hyperglycaemic (ob/ob) mice. Arch Int Pharmacodyn Ther. 1986;282:233–239. [PubMed] [Google Scholar]
- 47.Rouru J., Huupponen R., Pesonen U., Koulu M. Subchronic treatment with metformin produces anorectic effect and reduces hyperinsulinemia in genetically obese Zucker rats. Life Sci. 1992;50:1813–1820. doi: 10.1016/0024-3205(92)90066-x. [DOI] [PubMed] [Google Scholar]
- 48.Melin B., Cherqui G., Blivet M.J., Caron M., Lascois O., Capeau J. Dual effect of metformin in cultured rat hepatocytes: potentiation of insulin action and prevention of insulin-induced resistance. Metabolism. 1990;39:1089–1095. doi: 10.1016/0026-0495(90)90171-8. [DOI] [PubMed] [Google Scholar]
- 49.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho C., Corriera S., Santos M.S., Seiça R., Oliveira C.R., Moreira P.I. Metformin promotes isolated liver mitochondria impairment. Mol Cell Biochem. 2008;308:75–83. doi: 10.1007/s11010-007-9614-3. [DOI] [PubMed] [Google Scholar]