Abstract
Research into transplantation strategies to treat spinal cord injury (SCI) is frequently performed in rodents, but translation of results to clinical patients can be poor and a large mammalian model of severe SCI is needed. The pig has been considered an optimal model species in which to perform preclinical testing, and the Yucatan minipig can be cloned successfully utilizing somatic cell nuclear transfer (SCNT). However, induction of paralysis in pigs poses significant welfare and nursing challenges. The present study was conducted to determine whether Yucatan SCNT clones could be used to develop an SCI animal model for cellular transplantation research. First, we demonstrated that transection of the sacrocaudal spinal cord in Yucatan SCNT clones produces profound, quantifiable neurological deficits restricted to the tail. We then established that neurospheres could be isolated from brain tissue of green fluorescence protein (GFP) transfected SCNT clones. Finally, we confirmed survival of transplanted GFP-expressing neural stem cells in the SCI lesion and their differentiation into glial and neuronal lineages for up to 4 weeks without immunosuppression. We conclude that this model of sacrocaudal SCI in Yucatan SCNT clones represents a powerful research tool to investigate the effect of cellular transplantation on axonal regeneration and functional recovery.
Introduction
Spinal cord injury (SCI) is a common, high morbidity problem that impacts quality of life and causes significant economic loss worldwide (Rossignol et al., 2007). Although the clinical management of SCI patients has improved and numerous different strategies to limit secondary injury processes have been developed (Coutts and Keirstead, 2007), functional improvements are restricted, and many patients are left with significant neurological disability. Advances in the understanding of stem cell biology have led to a surge in interest in cellular transplantation therapy of SCI, and numerous experimental studies offer hope for achieving functional recovery (Coutts et al., 2007; Markay-Sim et al., 2008; Nandoe et al., 2008; Rossignol et al., 2007). Fetal neural stem cells, mesenchymal stem (stromal) cells from a variety of sources, Schwann cells, and olfactory nerve-ensheathing cells have been transplanted into different models of SCI with variable and often contradictory results (reviewed in Louro and Pearce, 2008).
One of the most problematic issues faced when translating a cellular intervention from the benchtop to the bedside is the predictive validity of findings made in animal models (Regenberg et al., 2009). In addition to inevitable differences in experimental and clinical injuries, there are significant biological differences between rodents and humans such as life span and body size (Regenberg et al., 2009). Simple morphologic differences can limit the validity of rodent models at predicting factors such as successful axonal regeneration, and prevent assessment of issues such as successful delivery of a large volume of cells.
The pig has been considered an optimal model species for preclinical therapeutic trials for human disease due to the similarity of its morphology and physiology (Dath et al., 2007; Rydevik et al., 1990). However, commercial pig breeds can be challenging to use for biomedical purposes because of their large size; adults frequently weigh more than 200 kg. There is therefore interest in breeds such as the Yucatan minipig that have the advantage of small size and a gentle disposition. Recently, Estrada et al. (2008) reported successful cloning of the Yucatan minipig utilizing somatic cell nuclear transfer (SCNT) with both oocytes and recipients from commercial occidental breeds. In addition, work by several groups has demonstrated the usefulness of SCNT for generation of genetically manipulated swine (reviewed by Aigner et al., 2010), making pigs the only large omnivore in which complex transgenic manipulations are possible. These important advances make it possible to develop Yucatan minipig SCNT clones that can be modified to express cellular markers and can be manipulated genetically as needed. Given the advantages of a porcine model, and the possibility of transgenic manipulation of both donors and recipients, we hypothesized that Yucatan minipig SCNT clones could be used to develop an SCI animal model for cellular transplantation research. In the present study, we cultured neurospheres isolated from fetal brain tissue of green fluorescence protein (GFP) transfected Yucatan SCNT lines, and transplanted these cells into a novel and humane model of SCI in Yucatan minipig SCNT clones. We confirmed survival of transplanted GFP-expressing porcine neural stem cells (GFP–pNSCs) and their differentiation into glial and neuronal lineages.
Materials and Methods
The experimental protocols used in this study were approved by North Carolina State University Institutional Animal Care and Use Committee.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO) unless otherwise indicated.
Production of GFP transfected Yucatan fetal fibroblasts and SCNT clones
Porcine fetal fibroblasts were obtained from previously established frozen Yucatan fibroblast cell lines (Estrada et al., 2008) and grown to confluence. The cells were trypsinized and resuspended in 800 μL Ham F-10 nutrient media (Mediatech Manassas, VA). Linearized pCAG-GFP DNA from Addgene plasmid 11150 (Matsuda and Cepko, 2004) and linearized pgk-Puro (Chen and Bradley, 2000) were added to the cell suspension at a concentration of 2 μg total DNA per 1 million cells and coelectroporated with two pulses at 450 volts for 1 msec. The cell suspension was then resuspended in the appropriate amount of alpha-MEM (HyClone Laboratories Inc., Logan, UT), including 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, plated into 10-mm dishes and incubated at 37°C in 5% CO2 in air with humidity. After 24 h of culture, the media was replaced with selection media containing 2.5 μg/mL puromycin and the cells were allowed to grow until colonies had formed. GFP-positive cells were assessed by fluorescent microscopy using a filter with an excitation of 488 nm and an emission of 509 nm. These fibroblasts were used for SCNT cloning to generate GFP-transfected Yucatan SCNT clones using the method previously reported by our research group (Estrada et al., 2008).
GFP–pNSC preparation
Forty-five-day-old fetuses of the GFP transfected Yucatan minipig SCNT clones were used to generate neurospheres. A modified method was used based on previously reported neurosphere culture techniques (Deleyrolle and Reynolds, 2009; Ghashghaei et al., 2007; Jacquet et al., 2009). Briefly, after euthanasia, brains were removed and placed into a balanced salt solution (Gibco-BRL, Gaithersburg, MD), and then grossly dissected, minced, and digested in enzyme solution in a 37°C for 40 min. The enzymatic digestion was stopped with addition of inhibitory solution and incubated at 37°C for 2 min. The enzyme solution was composed of dissociation medium (DM) with cysteine (3.2 mg/mL) and papain (10 μl/mL) added. The inhibitory solution was composed of DM with bovine serum albumin (10 mg/mL) and trypsin inhibitor (10 mg/mL) added. DM was composed of Na2SO4 (98 mM), K2SO4 (30 mM), MgCl2 (5.8 mM), CaCl2 (0.25 mM), HEPES (1 mM), glucose (20 mM), phenol red (0.001%), and NaOH (0.125 mN). The tissue was triturated to a single cell suspension, centrifuged at 1200 rpm for 5 min, and resuspended in serum-free NEP basal medium [Neurobasal™ medium (Gibco-BRL) supplemented with 2% B27 (Gibco-BRL), 1% N2 (Gibco-BRL), and 0.1 % penicillin–streptomycin (Gibco-BRL)]. The total number of viable cells was determined by manual count on a hemocytometer; cell viability was assessed using 0.4% trypan blue. Suspended single cells were plated on a noncoated bacterial plate at a concentration of 1 × 106/6 cm plate in NEP basal medium [Neurobasal™ medium (Gibco-BRL) supplemented with 2% B27 (Gibco-BRL), 1% N2 (Gibco-BRL), and 0.1% penicillin–streptomycin (Gibco-BRL)] with 10 ng/mL basic fibroblast growth factor (bFGF) (bFGF, Invitrogen, Carlabad, CA) and 100 ng/mL recombinant human epidermal growth factor (EGF) (rhEGF, Invitogen). Cells were maintained at 37°C in humidified 5% CO2 tissue culture incubator. Once neurospheres had formed, half of the media was replaced every 3 to 5 days.
In order to confirm that the neurospheres were indeed neural stem cells, neurospheres derived from brain tissue of non-GFP transfected clones were differentiated into neural lineages. Neurospheres were dissociated and plated onto chamber slides (LAB-TEK®, NalgeNunc Inc., New York) with NEP basal medium with 3% FBS. This differentiation medium was replaced every other day for 7 days. After 7 days, immunocytochemistry was performed to identify the cell lineages present. Cells were fixed, permeabilized with 0.3% Triton X-100 and blocked with 10% normal goat serum for 1 h and then incubated overnight at 4°C with mouse-anti-A2B5 (1:200, MAB312, Millipore, Bedford, MA), rabbit-anti-GFAP (1:1000, DAKO, Carpinteria, CA) and mouse-anti-Tuj1 (1:500, Covance, Richmond, CA) primary antibodies. The next morning, the cells were incubated with the following secondary antibodies for 1 h: Alexa 488 conjugated goat–antirabbit, and Cy3 conjugated goat–antimouse (1:500; all from Invitrogen). Cell nuclei were stained with DAPI (Vector Laboratory Inc., Burlingame, CA), and staining was visualized with a fluoromicroscope (AZ 100 Macro/microscope Nikon, Japan).
Induction of SCI and transplantation of GFP–pNSCs
Experimental animals
Five Yucatan SCNT clones (aged 10.4 ± 2.41 months and weighing 29.5 ± 9.48 kg) generated in the initial cloning study (Estrada et al., 2008) were allowed to grow to adulthood for this transplantation study (control group; n = 2, GFP–pNSCs transplantation group; n = 3). With the exception of the GFP marker these pigs were genetically identical to the GFP-transfected SCNT clones.
Tail behavioral assessment
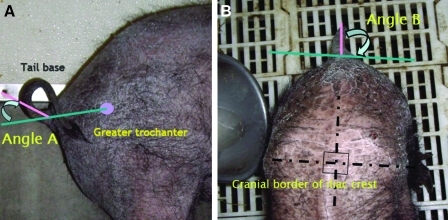
The five Yucatan SCNT clones were trained for 2 months prior to SCI induction to describe their normal tail behavior and to familiarize them to handling and testing of tail responses. Training included observation of the response to food treats, hand clapping, touching their back, scratching their flank, and digitally pinching the tail end. The pigs were trained until their responses were consistent. Once trained, each pig's responses to different stimuli were videotaped 1 week before lesion induction. A marker was placed on the skin overlying the greater trochanter of the femur and lateral photographs of the pigs' hind quarters taken when the pig was relaxed with its tail in neutral position to allow calculation of the dorsal angle (Fig. 1A). Photographs were also taken of the dorsal lumbar spine to document the neutral lateral deviation of the tail (Fig. 1B). Normal lateral tail movement was videotaped from above the pig to calculate the speed of movement using a computer-based video analysis program (Microsoft® Window Movie Maker).
FIG. 1.
Reference lines for measurement of lateral and ventral tail deviation. (A) Ventral deviation (angle A) was calculated from a line connecting the greater trochanter of the femur to the tail base. (B) Lateral deviation (angle B) was calculated from the middle of the tail to a line parallel with a line drawn between the cranial border of the iliac crests. (See color version of this figure at www.liebertonline.com).
Induction of sacrocaudal SCI and GFP–pNSCs transplantation
An SCI was induced in the sacrocaudal spinal cord of Yucatan SCNT clone to induce paralysis of the tail while sparing pelvic limb, rectal, and bladder function (Lim et al., 2008). The pigs were sedated by intramuscular administration of medetomidine (Domitor®, Pfizer, New York) at a dose of 20 μg/kg and ketamine (Ketaset®, Fort Dodge, KS) at a dose of 5-mg/kg. Once sedated, isoflurane (Isoflo®, Abbott, Abbott park, IL) was administered by mask until tracheal intubation was possible. Anesthesia was maintained by inhalation of isoflurane–oxygen mixture via a closed-circuit gas anesthetic unit (Matrx®VMS, New York). Physiologic measures monitored during anesthesia included body temperature, heart and respiratory rate, oxygenation (using a pulse oximeter), and CO2 levels (using a capnogram). Pigs were placed in sternal recumbency and surgical preparation performed on the skin of the caudal lumbar spine. A midline skin incision was made extending from the fifth lumbar to the second sacral vertebra. After dissection through the subcutaneous fat, epaxial spinal musculature was reflected to expose the last two lumbar and the sacral vertebrae. A dorsal laminectomy of the seventh lumbar and first two sacral vertebrae was performed using rongeurs and a high-speed pneumatic burr. Epidural fat was carefully removed by suction to expose the cauda equina. The meninges of the conus medullaris were incised to expose the sacral and caudal spinal cord segments using tenotomy scissors. A peripheral nerve stimulator (0.5∼0.8 mA, Stimuplex® Dig RC Peripheral Nerve Stimulator, B/Braun, USA) with stimulating needle (22 G × 2 inches, Stimuplex® insulated needle, B/Braun) was used to stimulate the caudal and sacral nerve roots. Stimulation of a caudal nerve root induced flexion of the tail, while stimulation of a sacral nerve root induced a twitch in the pelvic limbs and anal contraction, which was often accompanied by tail flexion. Sequential stimulation of nerve roots allowed identification of the last sacral spinal cord segment. The spinal cord was transected at the junction of the last sacral and first caudal spinal cord segment (Fig. 2A) using tenotomy scissors. A small piece of gelfoam (Gelfoam®, Pfzer Inc.) was inserted into the resulting defect, and then the meninges were sutured using 6.0 PDS (Ethicon, Somerville, NJ). In the cell transplantation group, 3 × 106 GFP-pNSC cells suspended in 30 μL of artificial CSF (Ghashghaei et al., 2007; Jacquet et al., 2009) were loaded into a 10-μL Hamilton syringe (Hamilton, Reno, NY) with a 26-gauge needle attached. The needle was loaded three times for three injections to be made. The syringe was placed into a stereotactic frame, and the needle tip was positioned into the spinal cord defect. Ten microliters of the cell suspensions (1 × 106 cells) were injected at each spinal cord/gelfoam junction and the final 10 μL was injected into the center of the gelfoam. The soft tissues and skin were closed routinely. Meloxicam (Metacam®, Boehringer Ingelheim, Vetmedica, Inc., St. Joseph, MO) was administered by intramuscular injection at a dose of 0.4 mg/kg for analgesia during closure of the incision. Postoperatively, the pigs' rectal temperature, heart, and respiratory rate, and responsiveness were monitored continuously until they were extubated and then every 30 min until they were resting comfortably. Intravenous hydromorphone (Dilaudid-HP®) was administered at a dose of 0.01 mg/kg on extubation and then repeated as needed over the next 24 h. A transdermal fentanyl patch (75 μg/h)(Fentanyl®, Mylan, Morgantown, WV) was placed to administer fentanyl continuously for 3 days. In addition, oral meloxicam (Metacam®, Boehringer Ingelheim) was given at a dose of 0.4 mg/kg for 1 week after surgery. Neurological assessment was performed daily postoperatively. Tail movements and sensation, pelvic limb strength, anal tone, voluntary urination, and defecation were noted. The tail behavioral responses were assessed daily postoperatively for 14 days and then once a week for 1 month.
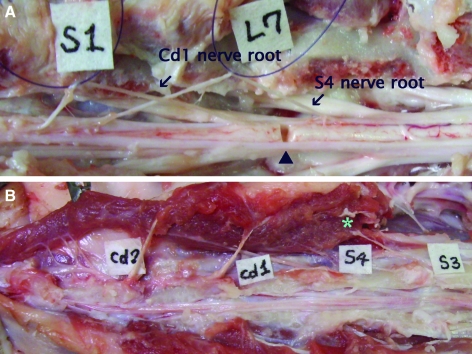
FIG. 2.
The cauda equina (spinal cord and lumbar, sacral, and caudal nerve roots) in Yucatan SCNT clones. (A) Following durotomy, the S4 and Cd1 nerve roots can be seen originating from the spinal cord at the level of the L7 vertebra (arrows). The square labels indicate the identity of the vertebrae. The optimal location for the transverse section is indicated (arrowhead). (B) Branches of the third sacral nerves innervate the proximal coccygeal muscles (*). (See color version of this figure at www.liebertonline.com).
Histology and immunocytochemistry
Histological evaluation was performed at 1 week (GFP–pNSCs transplantation group; n = 1) and 4 weeks (control group; n = 2, GFP–pNSCs transplantation group; n = 2) after surgery. The animals were deeply anesthetized and perfused transcardially with 4% paraformaldehyde at room temperature. A 10-cm block of the spinal cord tissue containing the lesioned area was collected and sectioned in half longitudinally. Half of the tissue was embedded in paraffin wax for routine histological analysis. Six-micrometer longitudinal sections were cut and stained with hematoxylin and eosin.
The remaining half of the spinal cord was cryoprotected with 30% sucrose for 72 h and embedded in OCT compound. Twenty-micrometer longitudinal sections were cut on a cryostat and mounted on Superfrost Plus slides (Fisher, Houston, TX). Sections were permeabilized with 1% Triton X-100, and nonspecific binding sites were subsequently blocked with 10% normal goat serum. After 1 h of blocking, tissue sections were incubated overnight at 4°C with chicken-anti-GFP (1:200, Invitrogen), mouse-anti-Nestin (1:500, Covance), mouse or rabbit-anti-GFAP (1:1000, DAKO), and mouse-anti-Tuj1 (1:500, Covance) primary antibodies. The sections were then incubated with the following secondary antibodies for 1 h: Alexa 488 conjugated goat–antichicken or goat–antirabbit, and Cy3 conjugated goat–antimouse (1:500; all from Invitrogen) and counterstained with Nissl (Invitrogen) to visualize cell nuclei. Immunofluorescence was visualized using confocal microscope (Confocal Microscope DIGITAL ECLIPSE C1 Plus® Nikon).
Results
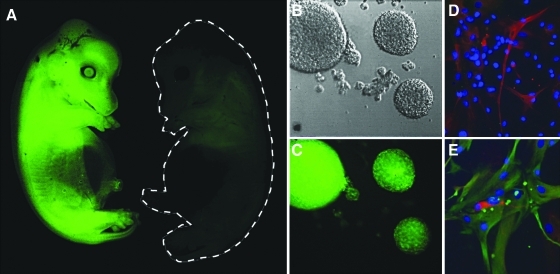
GFP-transfected Yucatan SCNT clones were successfully generated (Fig. 3A) and neurosphere cultures expressing GFP were produced from fetal brain tissues (Fig. 3B and C). Single dissociated neural cells were proliferated to form clusters termed neurospheres for 7 days in the presence of bFGF and EGF. Spheres that were more than 150 μm in diameter were harvested, redissociated into single cells mechanically, and replated in medium with mitogenic growth factors leading to the generation of “secondary spheres.” We repeated this procedure up to six passages. Both secondary and tertiary passages of GFP transfected neurospheres were used for cell transplantation. Differentiation of the neurospheres was performed on second and third passages of non- GFP transfected brain tissue derived neurospheres. In vitro differentiation by mitogen withdrawal and presence of serum for 7 to 10 days yielded expression of three neural lineage markers: GFAP, Tuj1, and A2B5 (Fig. 3D and E). Prior to surgery, the Yucatan SCNT clones held their tails with the end curled up while standing, walking, or running (Fig. 1), and they responded to stimuli by lateral wagging, ventroflexion, or dorsiflexion. Food treats elicited a tail wag, hand clapping, and touching the flank produced ventroflexion, scratching the flank produced wagging and ventroflexion, and pinching the tail produced both dorsi- and ventroflexion. Preoperatively, the mean velocity of lateral tail movement was 10.2 ± 1.78 fields per second (fps) in normal Yucatans and tail deviation was 18.79 ± 14.61 degrees for angle A and zero degrees for angle B (Fig. 1). Postoperatively, all pigs were able to stand and walk within 1 h of surgery with only mild pelvic limb weakness. Pelvic limb strength recovered to normal 1 day after surgery. There was no alteration in anal tone, bladder, or bowel function. In all five pigs, the tail was paralyzed immediately postoperatively and was held in ventroflexion with no voluntary movement. However, it tended to deviate laterally. Tail deviation was negative 67.06 ± 6.43 degrees for angle A and 53.12 ± 16.57 degrees for angle B (Fig. 4). There was no sensation in all but the proximal 1.35 ± 0.2 cm of the tail. In control pigs and transplanted pigs the lateral deviation gradually improved up to 37.2 ± 14.09 degrees, but there was no return of voluntary function and no change in sensation over 4 weeks in either controls or transplanted pigs.
FIG. 3.
A fetus of a GFP-transfected Yucatan SCNT clone is shown (A). Nontransfected clones do not express GFP (outlined in gray). Neurospheres isolated from these fetal brain tissues expressing GFP are shown (B and C). Differentiated cells are shown stained with A2B5 (red, D) GFAP (green, E), and TUJ1 (red, E). (See color version of this figure at www.liebertonline.com).
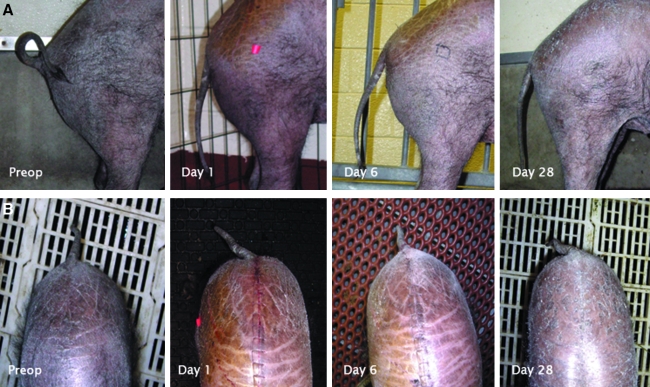
FIG. 4.
Ventrodorsal (A) and lateral (B) tail position before and after sacrocaudal spinal cord transection in Yucatan SCNT clones. (See color version of this figure at www.liebertonline.com).
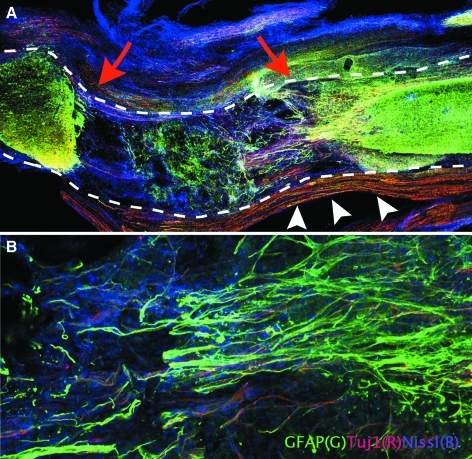
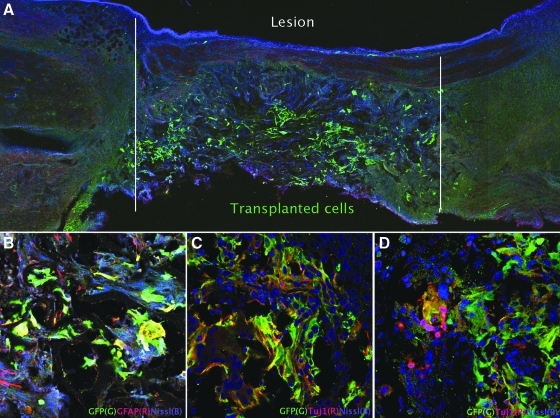
Histopathology confirmed complete transection of the spinal cord (Fig. 5A), with astrocytic scarring at the ends of the lesion (Fig. 5A). Intact sacral (peripheral) nerves were seen around the spinal cord as expected (Fig. 5A). Immunofluorochemistry revealed numerous GFAP positive cells with filamentous processes extended into the transection site at 4 weeks in the controls (Fig. 5B). In the GFP–pNSCs transplantation group, GFP-positive cells were distributed throughout the transection site at 1 week after lesioning (Fig. 6A). Transplanted pNSCs clearly expressed GFP and also coexpressed GFAP or Tuj1 at 1 week and 4 weeks. GFP–pNSCs expressing Tuj1 and GFAP were present (Fig. 6B and C), In the transplanted animals, Tuj1 + /GFP − cells derived from the host clustered around GFP-positive cells (Fig. 6D) at 4 weeks after transplantation. GFP–pNSCs did not express nestin at either 1 or 4 weeks.
FIG. 5.
Histopathologic findings at 4 weeks after transection. (A) The outline of the spinal cord is highlighted by the dashed lines; the spinal cord is completely transected (red arrows) ( × 4). The sacral peripheral nerves running alongside the spinal cord are indicated by white arrowheads. (B) Immunocytochemistry revealed GFAP positive cells (green) extending into the transection site ( × 20). (See color version of this figure at www.liebertonline.com).
FIG. 6.
Transplantation of GFP–pNSCs into the sacrocaudal spinal cord transection site. Transplanted cells survived, and differentiated to glial and neuronal lineages. (A) The GFP–pNSCs were detected using anti-GFP antibody 1 week after transplantation (green). (B and C) Clusters of GFP-positive cells double labeled with GFAP (B) or Tuj1 (C) within a lesion are shown. (D) Tuj1 positive (red), host-derived cells can be seen around GFP expressing cells at 4 weeks. (See color version of this figure at www.liebertonline.com).
Discussion
The results of the present study demonstrate that transection of the sacrocaudal spinal cord in Yucatan minipigs induces quantifiable tail paralysis without causing impairment in pelvic limb, urinary, or bowel functions. GFP–pNSCs can be transplanted into the injured spinal cord, and survive and differentiate into glial and neuronal cell types without the need for immunosuppression. To establish whether transplanted pNSCs could lead to functional recovery, a controlled study involving a larger cohort of patients with adequate duration of follow up is needed.
Models of SCI have been developed to investigate the pathophysiology of CNS damage, the functional neuroanatomy of motor, sensory, and autonomic pathways, and the effect of different therapies on outcome of SCI (Bernards and Akers, 2006; Lytle et al., 2009; Meletis et al., 2008). However, the predictive validity of these animal models when evaluating therapies can be problematic, resulting in a failure of translation of research findings to the clinical setting (Regenberg et al., 2009). Traditionally, rodent models have been used to investigate SCI; reproducible injury techniques have been developed along with accurate methods of outcome evaluation (Basso et al., 1996; Sharp et al., 2009). However, although extremely useful to investigate certain aspects of SCI, these rodent models do not capture all aspects of human disease. For example, the distance of an axonal injury site from the neuronal cell body and the distance over which axonal regeneration needs to occur in humans cannot be modeled in rodents.
To address these issues, there is a move to model SCI in larger mammals, and both canine and primate SCI models have been developed (Lim et al., 2007). These models have drawbacks including limited public acceptance of the experimental use of these species, and inability to perform complex genetic manipulations. Use of the pig addresses these problems; these large mammals are amenable to complex genetic manipulation (Aigner et al., 2010), and are genetically comparable to humans, with a similar genome size and extensive conserved homology with the human genome (Frönicke et al., 1996). However, producing a severe thoracolumbar SCI in pigs to cause paralysis poses significant concerns in terms of humane management of the subject. In order to avoid pelvic limb paralysis, and yet develop a model of complete spinal cord transection suitable for the investigation of axonal regeneration, we hypothesized that transection of the sacral spinal cord would produce paralysis of the tail only. This approach limits the welfare consequences and practical nursing challenges associated with inducing pelvic limb and bladder paralysis in a large mammal. Although there is a porcine model of cauda equina compression designed to model human low-back pain syndrome and sciatica (Olmarker et al. 1991), there are no other reports of an acute SCI model affecting the sacrocaudal spinal cord in pigs.
Spinal cord injury models that target the tail have been investigated in the rat and cat (Bennett et al., 1999; Ritz et al., 1992a, 1992b; Walker et al., 1998). A detailed neuroanatomical study in the rat described the descending supraspinal and propriospinal projections to the sacrocaudal spinal cord, showing their similarity to projections to the pelvic limbs (Masson et al., 1991). Lesions of the sacrocaudal spinal cord in these species induces paralysis of the tail only, and the effects of eliminating supraspinal control to the tail are quantifiable and produce spasticity comparable to that observed after SCI in humans (Ritz et al., 1992a, 1992b; Walker et al., 1998). In our study, transection of the sacrocaudal spinal cord in Yucatan minipig SCNT clones also induced paralysis of the tail. Localization of the site of transection was based on a previous anatomical survey, which indicated that the fourth sacral and first caudal nerve roots originated from the spinal cord at the level of the seventh lumbar vertebra, approximately 5 to 10 mm apart in Yucatan SCNT clones (Lim et al., 2008). Accidental injury to the more proximal sacral spinal cord could result in paralysis of the bladder and external anal sphincter and so a nerve root stimulator was used to identify the nerves innervating the tail muscles during surgery. This allowed precise transection of the spinal cord producing paralysis restricted to the tail.
Prior to lesion induction, the pigs were trained so that their responses to stimuli were repeatable. The pigs were found to be sociable and easy to train. The response to stimuli was consistent among pigs, but the resting behavior (e.g., tail wagging) did vary between individuals. Speed of lateral tail movement, the angle through which the tail moved and the neutral position of the tail were all repeatable measures with little variation across the group. Sacrocaudal spinal cord transection changed the angles at which the tail was held, and caused complete paralysis, eliminating all responses to stimuli. There was no evidence of recovery of tail function for 4 weeks after transection in all pigs. The presence of nociception in the proximal part of the tail observed the day after surgery was consistent with anatomical findings in which cutaneous nerve branches from the third sacral spinal cord segment innervated the proximal tail in Yucatan SCNT clones (Lim et al., 2008) (Fig. 2B). There was no change in the nociceptive field over the 4 weeks evaluated postoperatively, indicating that there was no recovery of sensory function below the level of the spinal cord transection. The histopathological findings confirmed complete section of the spinal cord with sprouting of GFAP-positive processes forming the prominent glial scar typically seen after SCI (Schultz, 2005).
There is a lot of interest in the use of stem cell transplantation to treat SCI (Nandoe et al., 2009). There are a variety of mechanisms by which cellular transplantation could improve outcome, including replacement of damaged cells with integration into the host circuitry (Hooshmand et al., 2009; Sharp et al., 2009), neuroprotection, and production of growth factors (Hwang et al., 2009, Someya et al., 2008), and support of regeneration of endogenous cells (Ghashghaei et al., 2007; Meletis et al., 2008). However, although many different stem cell transplantation strategies have been evaluated for their ability to produce a functional improvement in animal models of SCI, pathological findings frequently do not match with clinical improvement and the fate of transplanted cells is often unclear. Transplanted cells can be tracked using gene-targeted transgenic animals (Price et al., 2006) or donor-specific markers (in the case of xenografts) (Ostrander et al., 2001), and many transplantation studies use allografts or xenografts that can be tracked in this manner. However, this approach requires the use of immunosuppression to prevent rejection of the grafts, complicating experiments because of the potential for immunosuppressive drugs to cause secondary infections and toxicity in the recipient and their unknown effect on cell differentiation and survival (Wijdicks, 2001). The enhanced GFP gene has proven a useful marker and in the present study, Yucatan SCNT clone fetuses were successfully generated using GFP expressing fibroblasts from frozen Yucatan fibroblast cell lines established previously (Estrada et al., 2008). Neurospheres were isolated from GFP + Yucatan minipig SCNT clones fetuses and transplanted into adult clones of the same line (Estrada et al., 2008). Use of genetically identical Yucatan SCNT clones alleviated the need for immunosuppression, and the GFP marker allowed identification of transplanted cells. GFP-labeled cells were found throughout the lesion at 1 and 4 weeks after transplantation and coexpressed either GFAP or Tuj1. This provided clear proof of survival of transplanted cells and confirmed the ability of transplanted cells to differentiate into both neuronal and glial lineages. There was no improvement in tail function in the transplanted pigs, but this preliminary study was designed to demonstrate that tail paralysis could be induced and transplanted cells could survive without immunosuppression in a small number of pigs. Ongoing work would involve optimization of transplantation protocols and long-term outcome evaluation in a much larger cohort of control and transplanted pigs.
To summarize, this proof-of-concept study demonstrated that transection of the sacrocaudal spinal cord in Yucatan minipigs SCNT clones produces profound, quantifiable neurological deficits restricted to the tail. GFP-labeled neural stem cells derived from the same pig clones could be successfully transplanted directly into the SCI lesion at the time of injury and survived for up to 4 weeks without immunosuppression. There was no functional recovery in the transplanted group over the 4 weeks after injury, but unlike the control group, histological findings revealed that GFAP- or Tuj1-positive cells derived from the transplanted GFP–pNSCs were diffusely located within the spinal cord lesion. We conclude that this model of sacrocaudal SCI in Yucatan SCNT clones represents a powerful research tool to investigate the effect of cellular transplantation on axonal regeneration and functional recovery.
Acknowledgments
This work was sponsored by a grant from the Center for Comparative Medicine and Translational Research, College of Veterinary Medicine, North Carolina State University, and a Fellowship award in Korea Research Foundation (J.-H. Lim) (E00046). T.G. is supported by NIH grant R01NS062182 and a grant from American Federation for Aging Research and J.A.P. is supported by NIH grant HL51587.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Aigner B. Renner S. Kessler B., et al. Transgenic pigs as models for translational biomedical research. J. Mol. Med. 2010;88:653–664. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bennett D.J. Gorassini M. Fouad K., et al. Spasticity in rats with sacral spinal cord injury. J. Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bernards C. Akers T. Effect of postinjury intravenous or intrathecal methylprednisolone on spinal cord excitatory amino-acid release, nitric oxide generation, PGE2 synthesis, and myeloperoxidase content in a pig model of acute spinal cord injury. Spinal Cord. 2006;44:594–604. doi: 10.1038/sj.sc.3101891. [DOI] [PubMed] [Google Scholar]
- Chen Y.T. Bradley A. A new positive/negative selectable marker, puDeltatk, for use in embryonic stem cells. Genesis. 2000;28:31–35. doi: 10.1002/1526-968x(200009)28:1<31::aid-gene40>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Coutts M. Keirstead H.S. Stem cells for the treatment of spinal cord injury. Exp. Neurol. 2008;209:368–377. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Dath R. Ebinesan A.D. Porter K.M., et al. Anatomical measurements of porcine lumbar vertebrae. Clin. Biomech. 2007;22:607–613. doi: 10.1016/j.clinbiomech.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Deleyrolle L.P. Reynolds B.A. Isolation, expansion, and differentiation of adult Mammalian neural stem and progenitor cells using the neurosphere assay. Methods Mol. Biol. 2009;549:91–101. doi: 10.1007/978-1-60327-931-4_7. [DOI] [PubMed] [Google Scholar]
- Estrada J.L. Collins B. York A., et al. Successful cloning of the Yucatan minipig using commercial/occidental breeds as oocyte donors and embryo recipients. Cloning Stem Cells. 2008;10:287–296. doi: 10.1089/clo.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frönicke L. Chowdhary B. Scherthan H., et al. A comparative map of the porcine and human genomes demonstrates ZOO-FISH and gene mapping-based chromosomal homologies. Mamm. Genome. 1996;7:285–290. doi: 10.1007/s003359900084. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T. Weimer J.M. Schmid R.S., et al. Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev. 2007;21:3258–3271. doi: 10.1101/gad.1580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshmand M.J. Sontag C.J. Uchida N., et al. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS One. 2009;11:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D.H. Kim B.G. Kim E.J., et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;22:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet B.V. Salinas-Mondragon R. Liang H., et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.H. Jung C.S. Byeon Y.E., et al. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J. Vet. Sci. 2007;8:89–94. doi: 10.4142/jvs.2007.8.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.H. Piedrahita J.A. Ghashghaei H.T., et al. Development of a tail paralysis model in the Yucatan minipig. Annu. Symp. ESVN. 2008. pp. 81–82.
- Louro J. Pearse D.D. Stem and progenitor cell therapies: recent progress for spinal cord injury repair. Neurol. Res. 2008;30:5–16. doi: 10.1179/174313208X284070. [DOI] [PubMed] [Google Scholar]
- Lytle J.M. Chittajallu R. Wrathall J.R., et al. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57:270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A. Feron F. Cochrane J., et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson R.L., Jr. Sparkes M.L. Ritz L.A. Descending projections to the rat sacrocaudal spinal cord. J. Comp. Neurol. 1991;1:120–130. doi: 10.1002/cne.903070111. [DOI] [PubMed] [Google Scholar]
- Matsuda T. Cepko C.L. Electroporation and RNA interface in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K. Barnabé-Heider F. Carlén M., et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandoe Tewarie R.S. Hurtado A. Bartels R.H., et al. Stem cell-based therapies for spinal cord injury. J. Spinal Cord Med. 2009;32:105–114. doi: 10.1080/10790268.2009.11760761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmarker K. Holm S. Rosenqvist A.L., et al. Experimental nerve root compression. A model of acute, graded compression of the porcine cauda equina and an analysis of neural and vascular anatomy. Spine. 1991;16:61–69. [PubMed] [Google Scholar]
- Ostrander R.V. Goomer R.S. Tontz W.L., et al. Donor cell fate in tissue engineering for articular cartilage repair. Clin. Orthop. Relat. Res. 2001;389:228–237. doi: 10.1097/00003086-200108000-00032. [DOI] [PubMed] [Google Scholar]
- Price E.M. Prather R.S. Foley C.M. Multipotent adult progenitor cell lines originating from the peripheral blood of green fluorescent protein transgenic swine. Stem Cells Dev. 2006;15:507–522. doi: 10.1089/scd.2006.15.507. [DOI] [PubMed] [Google Scholar]
- Regenberg A. Mathews D.J.H. Blass D.M., et al. The role of animal models in evaluating reasonable safety and efficacy for human trials of cell-based interventions for neurologic conditions. J. Cereb. Blood Flow Metab. 2008;29:1–9. doi: 10.1038/jcbfm.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz L.A. Bailey S.M. Murray C.R., et al. Organizational and morphological features of cat sacrocaudal motoneurons. J. Comp. Neurol. 1992a;318:209–221. doi: 10.1002/cne.903180206. [DOI] [PubMed] [Google Scholar]
- Ritz L.A. Friedman R.M. Rhoton E.L., et al. Lesions of cat sacrocaudal spinal cord: a minimally disruptive model of injury. J. Neurotrauma. 1992b;9:219–230. doi: 10.1089/neu.1992.9.219. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Schwab M. Schwartz M., et al. Spinal cord injury: time to move? J. Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydevik B.L. Pedowitz R.A. Hargens A.R., et al. Effects of acute, graded compression on spinal nerve root function and structure. An experimental study of the pig cauda equina. Spine. 1991;16:487–493. doi: 10.1097/00007632-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Schultz S.S. Adult stem cell application in spinal cord injury. Curr. Drug Targets. 2005;6:63–73. doi: 10.2174/1389450053345046. [DOI] [PubMed] [Google Scholar]
- Sharp J. Frame J. Siegenthaler M., et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya Y. Koda M. Dezawa M., et al. Reduction of cystic cavity, promotion of axonal regeneration and sparing, and functional recovery with transplanted bone marrow stromal cell-derived Schwann cells after contusion injury to the adult rat spinal cord. J. Neurosurg. Spine. 2008;9:600–610. doi: 10.3171/SPI.2008.9.08135. [DOI] [PubMed] [Google Scholar]
- Walker C. Vierck C.J. Ritz L.A. Balance in the cat: role of the tail and effects of sacrocaudal transection. Behav. Brain Res. 1998;91:41–47. doi: 10.1016/s0166-4328(97)00101-0. [DOI] [PubMed] [Google Scholar]
- Wijdicks E.F. Neurotoxicity of immunosuppressive drugs. Liver Transpl. 2001;7:937–942. doi: 10.1053/jlts.2001.27475. [DOI] [PubMed] [Google Scholar]