Abstract
Two Red-breasted Mergansers (Mergus serrator), one Hooded Merganser (Lophodytes cucullatus), and one Common Eider (Somateria mollissima) from a German zoological collection died of necrotizing typhlitis / typhlohepatitis within two years. Using a newly established chromogenic in-situ hybridization assay, numerous intralesional trophozoites of Tetratrichomonas gallinarum could be detected in formalin-fixed and paraffin-embedded tissues from caeca and livers of the affected birds. Partial sequencing of the 18S rRNA-gene revealed two unique nucleotide sequences very similar to T. gallinarum strains isolated from avian and human hosts. One turkey kept in the same zoological collection succumbed to histomonosis (blackhead disease) confirmed with chromogenic ISH at the time of the first duck fatalities. This turkey also harboured T. gallinarum trophozoites within necrotic cell debris in the caecal lumen, which might be epidemiologically related to the T. gallinarum infections in the ducks.
Keywords: Anatidae, Tetratrichomonas gallinarum, typhlohepatitis, protozoal parasitosis
Introduction
The flagellated protozoan parasite Tetratrichomonas gallinarum (former name Trichomonas gallinarum) has been described to cause typhlohepatitis in turkeys morphologically very similar to histomonosis (blackhead disease) either as monoinfection or as mixed infection with Histomonas meleagridis (Allen, 1941). Oral inoculation of cultured tetratrichomonads obtained from turkeys that had died of the disease induced characteristic macroscopic lesions in poults. These consisted of cheesy cores of blood-stained tissue debris in the caeca and granular, cream-colored, well defined necrotic areas in the liver that were level with or elevated above the surface. In comparison, typical lesions from H. meleagridis looked very similar but missed the granular appearance and were slightly depressed below the liver surface. T. gallinarum showed a significantly lower pathogenicity than H. meleagridis in inoculated poults and the disease took a rather chronic course (Allen, 1941).
Furthermore, T. gallinarum has been found to be associated with clinically relevant infections in various bird species, including manifestations at extraintestinal sites (Patton et al., 1996; Crespo et al., 2001). There have been repeated attempts to clarify the pathogenicity of T. gallinarum with the help of experimental infections, mostly in turkeys and chickens, with various outcomes and inconsistent pathological lesions (Allen, 1941; Kemp & Reid, 1965; Lee, 1972; Kulda, 1974; Norton, 1997).
There are only few reports of intestinal disease caused by trichomonads in ducks. For example, Tetratrichomonas anatis has been reported to cause mucopurulent sinusitis and catarrhal rhinitis, tracheitis and enteritis in juvenile ducks (Tsai, 1997). Also, next to bacteria and other protozoa, three different species of trichomonads were found to be associated with necrotic enteritis of breeder ducks by Leibovitz (1973), but T. gallinarum was not among them. In ducks and geese an experimental infection by inoculation of T. gallinarum cultures did not lead to pathological changes in the caeca in spite of large protozoal numbers (Pecka, 1991). Thus, clinically relevant intestinal infections of duck species with T. gallinarum have not been described previously.
Red-breasted Mergansers (Mergus serrator), Hooded Mergansers (Lophodytes cucullatus), and Common Eiders (Somateria mollissima) belong to the tribe Mergini, family Anatidae, order Anseriformes, and live along the continental coasts of the Northern hemisphere.
Materials and Methods
Standard pathology
Within two years two Red-breasted Mergansers (Mergus serrator), one Hooded Merganser (Lophodytes cucullatus), and one Common Eider (Somateria mollissima) kept in a zoological garden in northeastern Germany died suddenly and were submitted for necropsy. A domestic turkey from the same zoo was submitted for necropsy together with the first Red-breasted Merganser after sudden death at the same day. Sex, age and clinical history for all birds included in this study are listed in table 1.
Table 1.
Sex, age and clinical history
| Animal | Sex | Age (weeks) | Clinical history |
|---|---|---|---|
|
Red-breasted Merganser No. 1 (Mergus serrator) |
female | 3 | seemed healthy, sudden death |
|
Red-breasted Merganser No. 2 (Mergus serrator) |
male | 12 | reduced growth, sudden death |
|
Hooded Merganser (Lophodytes cucullatus) |
male | 10 | stopped grooming, sudden death |
|
Common Eider (Somateria mollissima) |
female | 24 | lameness, sudden death |
|
Turkey (Meleagris gallopavo f. domestica) |
male | 12 | seemed healthy, sudden death |
Tissue samples taken at necropsy from all birds including liver and caeca were fixed in 10% neutral-buffered formalin and embedded in paraffin wax. For pathohistological examination hematoxylin-eosin (HE) stain as well as periodic acid-Schiff (PAS) reaction following standard protocols were used. Native caecal tissue from the second Merganser was additionally preserved by freezing at −20° C.
Standard etiological examinations
Routine diagnostic workup included bacteriology of lung, liver and intestine using standard culture media and coproscopical examination of intestinal contents for the detection endoparasites. In addition, chlamydial infections, avian influenza viruses (AIV) and avian paramyxovirus serotype 1 (APMV-1) were excluded by PCR using standard protocols (Spackman et al., 2002; Wise et al., 2004; Ehricht et al., 2006). In the second Red-breasted Merganser, mycological investigation of lung and air sacs was carried out using standard culture media.
Chromogenic in-situ hybridization
Histological sections from liver and caecum of all birds were processed for in-situ hybridization (ISH) according to Liebhart et al. (2006) using the therein described probe specific for H. meleagridis.
Another ISH assay with a protocol similar to the assay mentioned above but specific for T. gallinarum was established. The parameters remained unchanged except for an extended colour reaction of one hour. The sequence of the digoxigenin-labelled oligonucleotide probe designed complementary to the 18S ribosomal RNA (rRNA)-gene of T. gallinarum was: 5′-TCACCGCACTGGAAAGGTGCGATCCTATTCACAATGG-3′. This sequence was checked for its specificity with Basic Local Alignment Search Tool (BLAST; www.ncbi.nlm.nih.gov/blast.cgi). A culture of T. gallinarum isolated from the caecal contents of turkeys was used as positive control (Liebhart et al., 2006). Cross-reactivity was further checked directly on various embedded cultures or tissue samples including several species of flagellates, other protozoan parasites, bacteria, fungi and viruses as listed in Mostegl et al. (2010) except for T. gallinarum.
In addition, the tissues from the turkey were investigated with double-coloured ISH using probes specific for H. meleagridis and T. gallinarum (Liebhart et al., 2006), and a previously described protocol (Richter et al., 2008).
Polymerase chain reaction and sequencing
From the first Red-breasted Merganser, DNA was extracted from paraffin-embedded hepatic tissue with a commercial kit (nexttec Genomic DNA isolation kit, Biozym Biotech Trading GmbH, Vienna, Austria). A standard polymerase chain reaction (PCR) resulting in a relatively short amplicon of 153 basepairs suitable for DNA amplification from formalin-fixed, paraffin-embedded tissue was established. The primers targeted the 18S rRNA-gene and their sequences were: F: 5′-GACCTGTCTAGCGTTGATTC-3′ and R: 5′-AGGACATCACGGACCTGTTA-3′. The annealing temperature was calculated to be 60°C and a commercial master mix was used (5Prime HotMasterMix, Eppendorf Austria GmbH, Vienna, Austria). The resulting amplicons were sequenced.
To further identify the parasites, another PCR was established, which was specific for trichomonads and included a region of higher genetic variability of the 18S rRNA-gene among different tetratrichomonad species and strains. DNA was extracted from two different localizations of frozen native caecal tissue from the second Red-breasted Merganser using the same commercial kit as described above. A standard PCR assay with an annealing temperature of 54°C was carried out with the following primers: F: 5′-AGGCACGTCATTCGACTGAG-3′ and R: 5′-ACTGCGCTGAGTCATTCYTG-3′. The resulting amplicons of 431 basepairs were cloned in competent E. coli using the TOPO TA cloning Kit (Invitrogen GmbH, Lofer, Austria) and the plasmids were extracted with Pure Link Quick Plasmid Mini Prep Kit (Invitrogen GmbH, Lofer, Austria) from four different bacterial colonies. A nucleotide sequencing reaction for each colony followed.
The resulting sequences were submitted to Basic Local Alignment Search Tool (BLAST; www.ncbi.nlm.nih.gov/blast.cgi) and aligned.
Accession numbers
The sequence derived from the paraffin-embedded tissue from the first Red-breasted Merganser and the two sequences from the clones derived from the caecal tissue of the second Red-breasted Merganser have been deposited in Genbank under the accession numbers HM162407 – HM162409.
Results
Standard pathology and standard etiological examinations
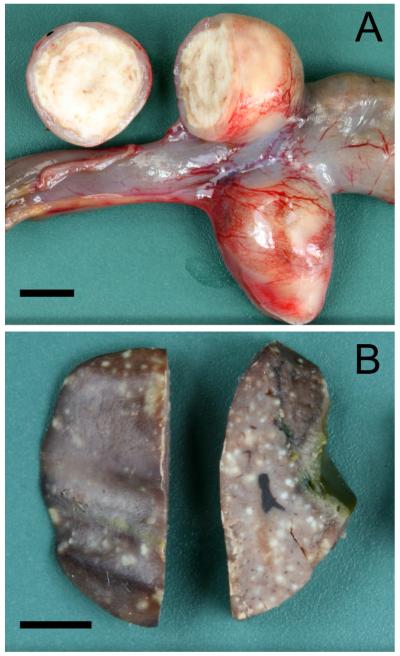
All three Mergansers and the Common Eider had a necrotizing typhlitis. The caecal lumina were filled with concentrically laminated fibrinous exsudate and necrotic debris with a brittle to firm consistency (Fig. 1a). The mucosal and submucosal layers of the caecal wall were completely necrotic down to the tunica muscularis. In addition, the first Red-breasted Merganser, the Hooded Merganser, and the Common Eider had a multifocal to coalescing necrotizing hepatitis (Fig. 1b). The necrotic foci were uniformly pale and slightly elevated above the liver surface. They had a maximum size of approximately 1 mm and were lacking a typical target shape with a darker central zone as seen in histomonosis.
Figure 1.
1a: Photograph of the caecum from the second Red-breasted Merganser with concentrically laminated fibrinous and necrotic material. Bar = 0.5 cm. 1b: Photograph of the liver from the first Red-breasted Merganser with multifocal uniformly pale tissue necrosis. Bar = 0.5 cm.
In caecal and hepatic lesions from all ducks, except for the second Red-breasted Merganser, protozoa were detected histologically. They were round to oval with a size of 6 to 11μm and distinctly PAS positive. In the caeca, they showed a strong tendency for tissue-invasion and were found in all layers of the caecal wall. In the liver, they were seen especially at the border of necrotic foci (Fig. 2).
Figure 2.
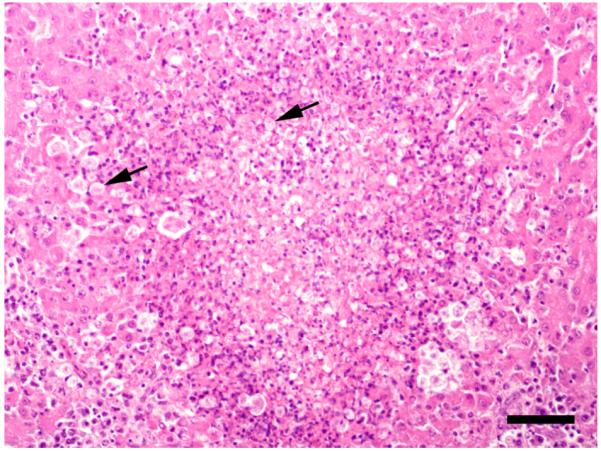
Tissue section from the liver of the first Red-breasted Merganser, focal tissue necrosis and inflammation associated with numerous tetratrichomonads (arrows). HE stain. Bar = 50 μm.
The caecal lesions in the second Red-breasted Merganser had a subacute character in comparison with the other ducks, and protozoa could not be identified in HE and PAS stained slides.
At gross as well as histopathology, the turkey showed a necrotizing typhlohepatitis consistent with histomonosis (blackhead disease). The liver contained typical target shaped concentric necrotic lesions of up to 1 cm in diameter with a dark depressed centre surrounded by a pale, slightly raised border zone. Histopathologically, numerous PAS-positive round to oval protozoa of 11 to 17 μm diameter were found in liver and caeca. The protozoa were present in high numbers in the necrotic debris filling the caecal lumen and invading the deeper layers of the caecal wall. Identical protozoa were present in massive amounts within the necrotic foci in the liver.
Additional pathological findings and the results of the standard etiological examinations for all birds included in this study are listed in table 2.
Table 2.
Results of standard etiological examinations and additional pathological findings
| Animal | Bacteriology / Mycology |
Coproscopy | Concurrent Pathology |
PCR* |
|---|---|---|---|---|
|
Red-breasted Merganser No. 1 |
Campylobacter jejuni |
neg. | none | neg. |
|
Red-breasted Merganser No. 2 |
Campylobacter
coli, Aspergillus fumigatus |
neg. | Aspergillosis, Amyloidosis |
neg. |
|
Hooded Merganser |
no pathogenic bacteria |
neg. | Amyloidosis | neg. |
| Common Eider | no pathogenic bacteria |
neg. | none | neg. |
| Turkey | no pathogenic bacteria |
neg. | none | neg. |
Chlamydia spp., Chlamydophila spp., avian influenzaviruses, avian paramyxovirus serotype
Chromogenic in-situ hybridization
All protozoa present in the liver and / or caeca from the Mergansers and the Common Eider showed a positive signal with the T. gallinarum probe (Fig. 3) and no signal with the H. meleagridis probe. Positive signals were visible mainly inside or at the border of necrotic lesions in both organs. In the second Merganser with the subacute typhlitis, low numbers of tetratrichomonads were located inside the necrotic cell debris but they did not invade the tissue as in the other ducks.
Figure 3.
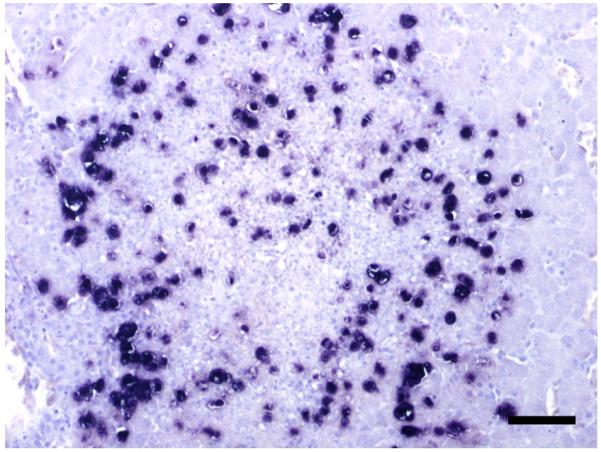
Tissue section from the liver of the first Red-breasted Merganser, T. gallinarum are clearly visible as dark purple spots. ISH. Bar = 50 μm.
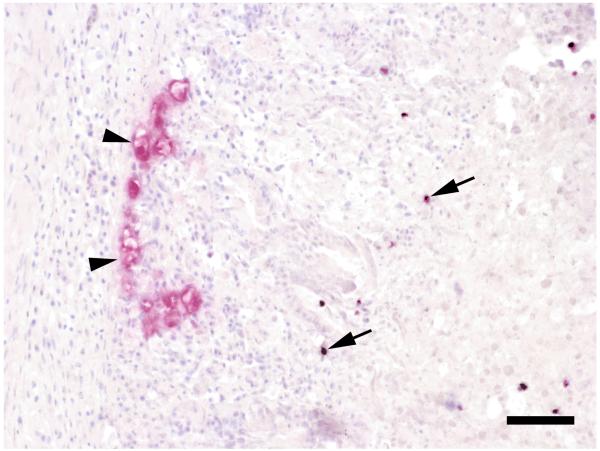
ISH from the tissues of the turkey were positive for H. meleagridis in liver and caeca. In addition, tetratrichomonads were present in the caeca but not in the liver. The double-coloured ISH showed two distinctly coloured types of protozoa in the caeca (Fig. 4). The histomonads were primarily located at the border between the necrotic debris and the inflamed caecal tissue and some were found to be invading the caecal wall. They had a size of 12 to 16 μm. The tetratrichomonads on the other hand were located closer to the lumen mainly within the necrotic debris. With a size of 5 to 8 μm they were generally smaller than the histomonads.
Figure 4.
Tissue section from the caecum of the turkey, on the left hand side H. meleagridis stained in red (arrowheads) invade the mucosal tissue, on the right hand side scattered T. gallinarum stained dark purple (arrows) can be seen closer to the caecal lumen. Double-coloured ISH. Bar = 50 μm.
Polymerase chain reaction and sequencing
The 153 bp sequence derived from the paraffin-embedded tissue from the first Red-breasted Merganser showed a 96% similarity with 13 T. gallinarum sequences from various birds, mainly Gallifomes and Anseriformes, four Tetratrichomonas spp. sequences from humans as well as two from unidentified hosts, and one termite gut symbiont, when submitted for BLAST search. Sequences from other strains of T. gallinarum and other Tetratrichomonas species followed with less similarity. All four clones derived from the caecal tissue of the second Red-breasted Merganser had a length of 431 basepairs. Three of them had exactly the same sequence, being 98% similar to eight T. gallinarum sequences from Galliformes, Anseriformes and one unidentified bird, and two human Tetratrichomonas sp. sequences. All of these were found amongst those with the highest similarity from the other PCR assay described above, too. The fourth clone showed four differing nucleotides when compared to the other clones resulting in a slightly decreased similarity of 97% to sequences from tetratrichomonads mainly consistent with those mentioned with the first Merganser above.
An alignment of the resulting sequences from the bacterial clones compared to one from T. gallinarum from the GenBank database is shown in figure 5.
Figure 5.
Alignment of partial 18S rRNA sequences of T. gallinarum from the GenBank and the second Red-breasted Merganser.
Discussion
This is the first report of fatal necrotizing typhlitis / typhlohepatitis in duck species associated with T. gallinarum similar to tetratrichomonosis in turkeys. The partially sequenced T. gallinarum strains show unique nucleotide sequences in parts of the 18S rRNA-gene compared to sequences listed in the GenBank database. Tetratrichomonads as well as histomonads were specifically detected intralesionally with previously reported and newly established chromogenic ISH assays conducted on formalin-fixed and paraffin-embedded tissue samples.
The pathogenicity of different T. gallinarum strains may vary among avian host species, such as turkeys, chickens and ducks. Also, based on the results from experimental infections and molecular genetic studies, protozoa phenotypically identified as T. gallinarum seem to form a heterogenous group regarding their virulence and genotype, and there may be cryptic species hidden within this group (Cepicka et al., 2005). This variability has to be kept in mind, since in natural infections as well as in conventional cultures used for experimental infections multiple morphologically undistinguishable strains may be present.
Bacteria or other parasites, such as Eimeria spp., may play an important role in the pathogenicity of T. gallinarum (Norton, 1997). Similarly, in infections with the more virulent H. meleagridis in turkeys, the presence of caecal bacteria plays an important role in the development of clinical disease (McDougald, 2005). The birds described in this study had no concurrent parasitic infection at the time of their death. Except for Campylobacter spp. from the Red-breasted Mergansers, the enteric bacterial flora of these birds included mainly coliform bacteria and Escherichia coli, which might have had an influence on the pathogenicity of the tetratrichomonads. All affected birds were juvenile or subadult individuals, which might have had an additional influence on the course of the disease. Also, as seaducks they all belonged to the tribe Mergini, while ducks belonging to other tribes kept in the same collection have been unaffected.
In turkeys, coinfections of H. meleagridis and T. gallinarum occur frequently (Allen, 1941; Kulda, 1974). In the present study, a coinfection with both parasites was demonstrated in the caecum of the turkey, which died from blackhead disease. Using double-coloured ISH, the tissue distribution of both parasites could be assessed and compared directly to each other. Since the tetratrichomonads were present only within the caecal necrotic debris and not at the interface between necrosis and inflamed tissue or in the liver lesions as were the histomonads, it is presumed, that the present strains of tetratrichomonads revealed no high pathogenicity for the turkey. Thus, turkeys might be subclinical carriers of some strains of T. gallinarum, which may have the potential to induce disease in various duck species living in the same zoological collection. Further studies are required to elucidate the complete epidemiology that led to the recurrent fatal diseases in the ducks presented in this article.
Acknowledgements
The excellent supportive technical work of Karin Fragner and Klaus Bittermann is gratefully acknowledged. Thanks go to Dr. Dieter Liebhart and Prof. Michael Hess from the Clinic for Avian, Reptile and Fish Medicine, University of Veterinary Medicine Vienna for providing well defined protozoal control material.
This work was partially funded by the Austrian Science Fund (FWF), Project No. P20926-B17.
Literature
- Allen EA. Macroscopic differentiation of lesions of Histomoniasis and Trichomoniasis in turkeys. American Journal of Veterinary Research. 1941;2:214–217. [Google Scholar]
- Cepicka I, Kutisova K, Tachezy J, Kulda J, Flegr J. Cryptic species within the Tetratrichomonas gallinarum species complex revealed by molecular polymorphism. Veterinary Parasitology. 2005;128:11–21. doi: 10.1016/j.vetpar.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Crespo R, Walker RL, Nordhausen R, Sawyer SJ, Manalac RB. Salpingitis in Pekin ducks associated with concurrent infection with Tetratrichomonas sp. and Escherichia coli. Journal of veterinary diagnostic investigation. 2001;13:240–245. doi: 10.1177/104063870101300309. [DOI] [PubMed] [Google Scholar]
- Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Molecular and Cellular Probes. 2006;20:60–63. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kemp RL, Reid WM. Pathogenicity studies on Trichomonas gallinarum in domestic poultry. Poultry Science. 1965;44:215–221. doi: 10.3382/ps.0440215. [DOI] [PubMed] [Google Scholar]
- Kulda J, Suchankova E, Svoboda S. Studies on pathogenicity of Tetratrichomonas gallinarum in mice and turkey poults. Acta veterinaria Brno. 1974;43:53–64. [Google Scholar]
- Lee DL. Changes in the ultrastructure of the caecum of chickens caused by Trichomonas gallinarum. Parasitology. 1972;65:71–76. doi: 10.1017/s0031182000044243. [DOI] [PubMed] [Google Scholar]
- Leibovitz L. Necrotic enteritis of breeder ducks. American Journal of Veterinary Research. 1973;34:1053–1061. [PubMed] [Google Scholar]
- Liebhart D, Weissenböck H, Hess M. In-situ hybridization for the detection and identification of Histomonas meleagridis in tissues. Journal of Comparative Pathology. 2006;135:237–242. doi: 10.1016/j.jcpa.2006.08.002. [DOI] [PubMed] [Google Scholar]
- McDougald LR. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Diseases. 2005;49:462–476. doi: 10.1637/7420-081005R.1. [DOI] [PubMed] [Google Scholar]
- Mostegl MM, Richter B, Nedorost N, Maderner A, Dinhopl N, Kulda J, Liebhart D, Hess M, Weissenböck H. Design and validation of an oligonucleotide probe for the detection of protozoa from the order Trichomonadida using chromogenic in situ hybridization. Veterinary Parasitology. 2010;171:1–6. doi: 10.1016/j.vetpar.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton RA. Pathogenicity of a strain of Trichomonas gallinarum in turkeys and its possible interaction with cecal coccidia. Avian Diseases. 1997;41:670–675. [PubMed] [Google Scholar]
- Patton CS, Patton S. Tetratrichomonas gallinarum encephalitis in a mockingbird (Mimus polyglottos) Journal of Veterinary Diagnostic Investigation. 1996;8:133–137. doi: 10.1177/104063879600800126. [DOI] [PubMed] [Google Scholar]
- Pecka Z. Pathogenicity of Tetratrichomonas gallinarum. Veterinarni Medicina. 1991;36:183–188. [PubMed] [Google Scholar]
- Richter B, Fragner K, Weissenböck H. Simultaneous detection of protozoa in the tissues of snakes by double in situ hybridization. Microscopy Research and Technique. 2008;71:257–259. doi: 10.1002/jemt.20546. [DOI] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SS, Chang TC, Kuo M, Itakura C. Respiratory and intestinal trichomoniasis in mule ducks. Avian Pathology. 1997;26:651–656. doi: 10.1080/03079459708419241. [DOI] [PubMed] [Google Scholar]
- Wise MG, Suarez DL, Seal BS, Pedersen JC, Senne DA, King DJ, Kapczynski DR, Spackman E. Development of a real-time reverse-transcription PCR for detection of newcastle disease virus RNA in clinical samples. Journal of Clinical Microbiology. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]