Abstract
Systemic lupus erythematosus (SLE) is prototypic autoimmune disease characterized by the production of autoantibodies to DNA among other nuclear molecules. These antibodies can form immune complexes that promote pathogenesis by stimulating cytokine production and depositing in the kidney to instigate nephritis. The antigens that form these complexes arise from the blood nucleome, a pool of circulating macromolecules comprised of DNA, RNA and nuclear proteins released from cells. Cell death is a major source of these molecules, releasing DNA in a process that can be modeled in mice by the administration of cells killed ex vivo. In the mouse model, the appearance of blood DNA requires macrophages and differs between males and females. This finding raises the possibility that augmented levels of extracellular DNA and other nuclear antigens can contribute to the increased frequency of SLE in females. Extracellular DNA can occur in both a soluble and particulate form, with microparticles generated in vitro displaying antigenically active DNA. Together, these findings suggest that cell death is an important event in lupus pathogenesis and can provide a supply of blood DNA essential for immune complex formation.
Keywords: systemic lupus erythematosus, immune complexes, DNA, nucleome, apoptosis, macrophages
Take Home Messages
Anti-DNA antibodies can form immune complexes that stimulate cytokine production and deposit in the kidney.
The blood nucleome consists of DNA, RNA and nuclear proteins that circulate in the blood in normal and pathological conditions.
Cell death is a major source of DNA in the blood nucleome.
Macrophages influence the generation of circulating DNA from dead and dying cells.
DNA in the blood has both soluble and particulate components.
Systemic lupus erythematosus (SLE) is prototypic autoimmune disease that causes a wide range of inflammatory manifestations and results from both inherited and environmental factors. While SLE is clinically heterogeneous, the expression of antibodies to components of the cell nucleus (antinuclear antibodies or ANA) is almost invariable among patients. The targeted antigens include DNA, RNA and complexes of nucleic acids with proteins to form the nucleosomal structure. Critical to the role of ANAs in pathogenesis is their interaction with nuclear material to form immune complexes [1]. This material, which exits cells to enter the circulation, can be termed the blood nucleome. In this conceptualization, the blood nucleome comprises DNA, RNA and the proteins with which nucleic acids are associated.
As shown in studies on human and murine lupus, antibodies to DNA (anti-DNA) among other ANA can form immune complexes that impact disease by stimulation of cytokine production by plasmacytoid dendritic cells and renal deposition to incite inflammation and glomerular injury. Cytokine stimulation, in particular type 1 interferon, reflects the intrinsic immune activity of DNA which can by modified by its binding to antibody that can affect its uptake into cells and access to internal nucleic acid sensors [2-4]. These sensors include toll-like receptor 9 (TLR9) as well as non-TLR receptors. In addition to promoting cytokine production, immune complexes can impact on pathogenesis by deposition in the kidney to instigate glomerulonephritis. Once deposited in the kidney, the complexes can activate complement to intensify inflammation [5-6].
Components of Immune Complexes
By definition, an immune complex disease has two essential elements: antigen and antibody. For immune complex diseases that arise after infection, the source of the antigen is obvious. It is the infecting organism. Without the organism, whether viral or bacterial, neither an antibody response nor complex formation can occur. In such diseases, the generation of the antibody response raises few mechanistic issues since the antibody response follows the classical rules of the normal immune system.
In contrast, immune complex disease in autoimmunity may reflect two distinct pathogenetic disturbances: autoantibody production and the generation of extracellular autoantigens. In general, research in this field has focused on the generation of autoantibodies since the phenomenon of autoimmunity is closely related to the most fundamental question in immunology: the self-non-self discrimination. Such studies, however, do not directly address the source of antigen to drive responses or form immune complexes. For complexes to form in lupus, nuclear material must leave the cell, a complicated and unusual situation given the polymeric structure of DNA and its localization in the nucleus.
The Blood Nucleome
Along with proteins, lipids and carbohydrates, extracellular DNA is a prominent component of the blood, with increased levels of DNA occurring widely with disease (Table1). As this list indicates, a rise of DNA occurs with conditions characterized by inflammation, cell death or both [7-9]. Importantly, DNA in the blood shows laddering, suggesting that apoptotic cells are a major source of extracellular DNA and that DNA exits cells as part of the massive rearrangement of the cell as death proceeds.
Table 1.
Conditions with Increased Blood DNA
| SLE |
| Pregnancy |
| Eclampsia |
| Malignancy |
| Sepsis |
| Trauma |
| Pulmonary infarction |
The role of cell death in creating a pool of extracellular DNA is important in the context of SLE in view of evidence that patients with lupus have either an increase in the amount of cell death or impairment in the clearance of dead and dying cells [10]. As such, patients with lupus may have an augmented supply of self DNA antigen to stimulate the autoantibody production or form pathogenic complexes.
Experimental Modeling of the Blood Nucleome
In view of compelling evidence that cell death can produce blood DNA, my laboratory began experiments to model this process and explore potential differences in DNA translocation during apoptosis and necrosis. In one of the model systems we investigated, we administered to mice varying doses of cells induced to undergo either apoptosis or necrosis ex vivo. For most of these studies, we have used the Jurkat T cell leukemia line [11]. This cell line was derived from a male and has a Y chromosome which, in our experiments, allows tracking of the DNA. When Jurkat cells are administered to a female mouse, amplification of a Y chromosome sequence provides unequivocal proof of the origin of any circulating DNA in the blood.
Using this experimental design, we demonstrated that administration of Jurkat cells to female mice leads to the dose-dependent appearance in the blood of DNA as assessed with the dye PicoGreen that binds double stranded DNA. The DNA appeared in the blood after several hours and, by 24 hours, levels returned to baseline. Interestingly, the levels of DNA, the presence of laddering as shown by gel electrophoresis and the kinetics of DNA appearance in the blood were similar whether the administered cells were apoptotic or necrotic [11].
While these results could suggest that in vivo dead cells rapidly break down to release their contents, we were interested in the role of macrophages in this process since these phagocytes can scavenge dead cells to promote their elimination. To determine the effects of macrophages on the release process, we administered the Jurkat cells to mice in which macrophages were eliminated by treatment with clodronate, a bis-phosphonate that induces macrophage death following uptake. The treatment with clodronate itself led to a large peak of blood DNA, likely because of macrophage killing and the absence of a phagocytic system for clearance. Following the return of DNA to baseline, we administered the dead cells to the macrophage-deficient mouse. The results of this experiment were striking since we found that, in mice without macrophages, a peak in blood DNA did not occur following the administration of Jurkat cells [11].
To explain these findings, we suggested that, with a large number of dead and dying cells, macrophages attempting to phagocytose this material undergo apoptosis, die and release both their DNA as well as DNA from the engulfed cell. With a lower number of administered dead cells, the macrophage can digest the material and prevent the generation of blood DNA. In the absence of macrophages, the dead and dying cells may undergo a gradual disintegration which fails to increase levels of DNA in the blood. The role of inflammatory cells in this process was confirmed in studies on the effects of dexamethasone treatment as well as the responses of mice in which peritonitis was induced prior to the administration of dead cells [12-13].
Since females and males differ immunologically, we wondered whether the system would work similarly with male mice as recipients. We therefore repeated these experiments using male recipients. The results were notable since the levels of DNA in the blood in the male mice were significantly lower than that of the females when receiving comparable number of Jurkat cells [14]. Castration of female mice led to responses similar to that of male mice, suggesting a role of sex hormones in the clearance of dead and dying cells and generation of extracellular DNA. In the context of SLE, these results could suggest that sex hormones can influence the generation of the blood nucleome and therefore the supply of autoantigens that can drive autoantibody production or form immune complexes for renal deposition. Such effects could contribute the dramatically higher levels of lupus in women.
Microparticles as a Source of DNA in the Nucleome
The blood nucleome has both soluble and particulate components that likely reflect the different ways in which nucleic acids exit cells as well as the stability of released nucleic acids in the circulation. Whereas DNA is distributed in both soluble and particulate compartments, RNA is primarily particulate. The major particulates in the blood are termed microparticles [15-17]. These particles are membrane-bound vesicles that are released from cells that undergo activation or apoptosis. Importantly, microparticles, which may correspond to blebs that form during apoptosis, contain both DNA and RNA. As we have and others have shown, microparticles generated in vitro (and the closely related blebs and apoptotic bodies) can bind monoclonal antinuclear antibodies as well as sera of patients with SLE [18-20], suggesting that nuclear antigens are on the particle surface or otherwise accessible to antibody interaction (Figure 1). As a result, microparticles may be a source DNA-containing immune complexes, with their intrinsic immunologically activity and their cargo of nuclear molecules promoting activity which may involve both TLR and non-TLR signaling systems. The relative contribution of soluble vs particulate DNA in forming immune complexes from the blood nucleome and promoting pathogenesis is an important area of ongoing investigation.
Figure 1.
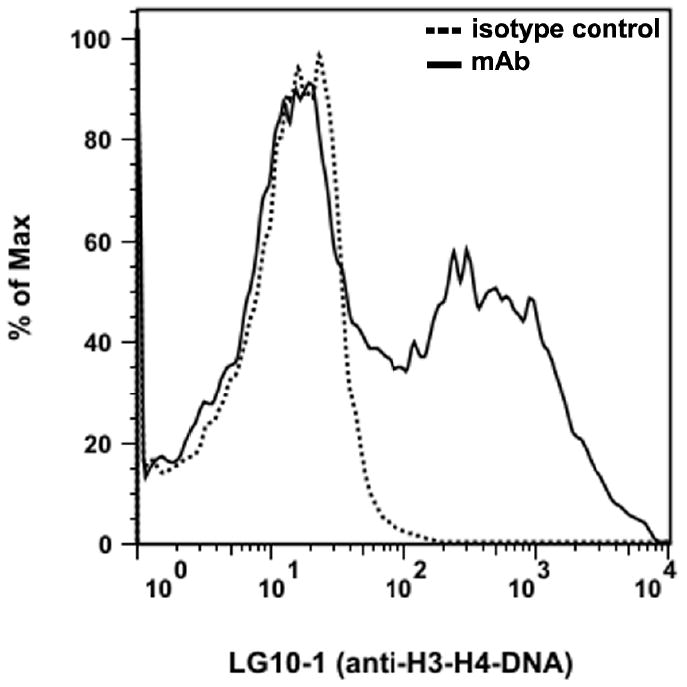
The binding of a monoclonal anti-nucleosomal antibody to microparticles generated in vitro. Microparticles were prepared from the medium of Jurkat cells induced to undergo apoptosis by treatment with staurosporine. The binding of a murine monoclonal antibody (LGl0-1, a generous gift of Dr. Marc Monestier) with specificity for the H3-H4-DNA complex was assessed by flow cytometry. As these data indicate, the antibody can bind significantly to the microparticles indicating nucleosomal antigen accessibility.
Acknowledgments
These studies were supported by a VA Merit Review grant and NIH Grant A1082402
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ardoin SP, Pisetsky DS. Developments in the scientific understanding of lupus. Arthritis Res Ther. 2008;10:218. doi: 10.1186/ar2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and –independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian J, Avalos AM, Mao S-Y, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 2007;8:487–497. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda K, Richez C, Uccellini MB, Richards RJ, Bonegio RG, Akira S, Monestier M, Corley RB, Vigilianti GA, Marshak-Rothstein A, Rifkin IR. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–3117. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. doi: 10.1681/ASN.2008010112. [DOI] [PubMed] [Google Scholar]
- 6.Kalaaji M, Fenton KA, Mortensen ES, Olsen R, Sturfelt G, Alm P, Rekvig OP. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71:664–672. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 7.Lui YYN, Lo YMD. Circulating DNA in plasma and serum: biology, preanalytical issues and diagnostic applications. Clin Chem Lab Med. 2002;40:962–968. doi: 10.1515/CCLM.2002.169. [DOI] [PubMed] [Google Scholar]
- 8.Tsang JCH, Lo YMD. Circulating nucleic acids in plasma/serum. Pathology. 2007;39:197–207. doi: 10.1080/00313020701230831. [DOI] [PubMed] [Google Scholar]
- 9.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch R-D, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 10.Munoz LE, Lauber K, Schiller M, Manfredi AA, Hermann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 11.Jiang N, Reich CF, 3rd, Pisetsky DS. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood. 2003;15:2243–2250. doi: 10.1182/blood-2002-10-3312. [DOI] [PubMed] [Google Scholar]
- 12.Jiang N, Pisetsky DS. The effect of dexamethasone on the generation of plasma DNA from dead and dying cells. Am J Pathol. 2004;164:1751–1759. doi: 10.1016/S0002-9440(10)63733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang N, Pisetsky DS. The effect of inflammation on the generation of plasma DNA from dead and dying cells in the peritoneum. J Leukoc Biol. 2005;77:296–302. doi: 10.1189/jlb.0704411. [DOI] [PubMed] [Google Scholar]
- 14.Pisetsky DS, Jiang N. The generation of extracellular DNA in SLE: the role of death and sex. Scand J Immunol. 2006;64:200–204. doi: 10.1111/j.1365-3083.2006.01822.x. [DOI] [PubMed] [Google Scholar]
- 15.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 16.Sellam J, Proulle V, Jüngel A, Ittah M, Miceli RC, Gottenberg JE, Tori F, Benessiano J, Gay S, Freyssinet JM, Mariette X. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 18.Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz H-M. Autoantigens are translocated into small apoptotic bodies during early states of apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 19.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 20.Reich CF, 3rd, Pisetsky DS. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp Cell Res. 2009;315:760–768. doi: 10.1016/j.yexcr.2008.12.014. [DOI] [PubMed] [Google Scholar]


