Summary
Background
Antibodies to complexes of heparin and platelet factor 4 (PF4) are capable of causing heparin-induced thrombocytopenia (HIT). Recent evidence suggests that anti-PF4/heparin antibodies may be prothrombogenic even in the absence of thrombocytopenia and clinically-recognized HIT.
Objectives
To determine if induction of anti-PF4/heparin antibodies is an independent risk factor for early saphenous vein graft (SVG) occlusion or adverse clinical outcome after coronary artery bypass graft (CABG) surgery.
Patients/Methods
Anti-PF4/heparin antibody titers were measured in 368 patients prior to and then 4 days, 6 weeks and 6 months after CABG surgery. Serotonin release assay (SRA) and antibody isotype analysis were also performed on 6-week samples. SVG patency was determined in 297 patients 6 months after surgery by multidetector computed tomography coronary angiography.
Results
Six weeks after surgery, 52% of patients were anti-PF4/heparin seropositive and 9% were SRA positive. Six months after surgery, neither the percentage of occluded SVG (19% vs. 20%, P = NS), the percentage of patients with an occluded SVG (33% vs. 33%, P = NS) nor the incidence of adverse clinical events (21% vs. 24%, P = NS) differed between seropositive and seronegative groups. Neither IgG isotype nor SRA positivity was additionally predictive of SVG occlusion or adverse clinical outcome.
Conclusion
Induction of anti-PF4/heparin antibodies, even those capable of heparin-dependent platelet activation, is not independently associated with early SVG occlusion or adverse clinical outcomes after CABG surgery.
Keywords: anti-PF4/heparin antibodies, saphenous vein graft, thrombosis
Introduction
Unfractionated heparin (UFH) is routinely administered in high doses to patients undergoing coronary artery bypass graft (CABG) surgery. Approximately 20–55% of these patients develop antibodies against complexes of heparin and aggregates of the chemokine protein, platelet factor 4 (PF4) [1,2]. Anti-PF4/heparin antibodies are capable of causing heparin-induced thrombocytopenia (HIT), a syndrome characterized by immune complex-mediated platelet activation and procoagulant microparticle release capable of precipitating pathologic thrombosis.[3] While HIT develops in < 3% of CABG surgery patients, it is associated with a high rate of postoperative thromboembolic events, including saphenous vein graft (SVG) thrombosis [4,5].
Recent evidence suggests that anti-PF4/heparin antibodies may be capable of precipitating thrombosis even in the absence of thrombocytopenia and apparent HIT. In vitro studies reveal that anti-PF4/heparin IgG antibodies directly trigger tissue factor expression by peripheral blood monocytes, macrophages and endothelial cells [6-8]. Patients with acute coronary syndromes (ACS) and anti-PF4/heparin antibodies, but without thrombocytopenia, suffer higher rates of adverse clinical events compared with seronegative patients [9-11]. In patients undergoing CABG surgery, the preoperative presence of anti-PF4/heparin antibodies increases the risk of in-hospital complications and length of stay [12,13]. It is not known whether the induction of these antibodies as a result of CABG surgery has any untoward consequences following hospital discharge. The objective of this study is to determine whether the induction of anti-PF4/heparin antibodies, irrespective of the development of clinical HIT, adversely affects SVG patency or clinical outcomes during the first 6 months after CABG surgery.
Methods
Patient population
The Reduction in Graft Occlusion Rates (RIGOR) study was a prospective study of patients undergoing CABG surgery designed to identify novel risk factors for early SVG thrombosis. Patients were enrolled between October 2003 and October 2006 at four institutions: Johns Hopkins Hospital, Baltimore, MD; Christiana Hospital, Christiana, DE; Peninsula Regional Medical Center, Salisbury, MD; and Walter Reed Army Hospital, Washington, DC. Human subject research review board approval was obtained at all sites, which were geographically limited by the need for patients to undergo multidetector computed tomography coronary angiography (MDCTCA) at Johns Hopkins Hospital 6 months after surgery. Patients ≥ 18 years of age undergoing CABG surgery with implantation of at least one SVG were eligible for enrollment. Exclusion criteria included: (i) prior cardiac surgery; (ii) anticipated postoperative use of an oral anticoagulant or non-aspirin antiplatelet agent; (iii) allergy to aspirin or radiocontrast; (iv) renal insufficiency with a glomerular filtration rate < 30 mL min−1; (v) contraindication to beta-blockers or pulmonary disease that would preclude MDCTCA; (vi) known thrombophilia; (vii) pregnant or nursing; (viii) prior chest irradiation; and (ix) co-morbid illness likely to reduce life expectancy to < 6 months. Patients were administered aspirin (300–325 mg) within 24 h of surgery. At discharge, patients were given 250 tablets of 325 mg enteric-coated aspirin and directed to take one tablet daily for 6 months, unless modified by their physician. Pill counts were performed at all follow-up visits to assess compliance.
Measurement of anti-PF4/heparin antibodies and serotonin release assay (SRA)
Blood was collected prior to, then a median of 4 days (3–4 IQR), 5.7 weeks (4.7–6.7, IQR) and 6.2 months (6.0–6.6 IQR) after, CABG surgery, sent to the Johns Hopkins Special Coagulation Laboratory and stored at −70 °C until batch-analyzed. Serum titers of antibodies to PF4/heparin complexes were measured in duplicate using a commercially-available sandwich-type ELISA (GTI-X-HAT45; GTI Diagnostics, Waukesha, WI, USA). Bound anti-PF4/heparin antibodies were identified by a mixture of anti-human immunoglobulin secondary antibodies recognizing IgG, IgA and IgM isotypes. Coefficients of variance routinely averaged < 10%. Samples were considered positive if the mean absorbance at 405 nm was ≥ 0.4 OD units. Positive samples also underwent additional analysis with each of the isotype specific secondary antibodies separately to determine the relative amounts of IgG, IgM and IgA anti-PF4/heparin antibodies.
To determine if anti-PF4/heparin antibodies were capable of stimulating heparin-dependent platelet activation, a serotonin release assay (SRA) was performed on available heat-inactivated serum samples obtained from 322 patients 6 weeks after CABG surgery, irrespective of antibody status, as previously described [14]. Samples were considered positive only if there was both ≥ 20% release at 0.1 U mL−1 UFH and < 20% release at 100 U mL−1 UFH in at least two replicate assays using platelets from different donors. Results of the ELISA or SRA assays were not made available to clinicians in real time.
Assessment of SVG patency
We prospectively chose to assess SVG patency 6 months after surgery by MDCTCA using 16–64 row detector scanners (Aquilion; Toshiba Medical Systems Corporation, Otawara, Japan) as described in the Data Supplement. Four patients with contraindications to MDCTCA because of renal insufficiency underwent contrast MRI coronary angiography as previously described [15] and data from clinically-driven invasive coronary angiograms were used to assess SVG patency in 16 patients. Images underwent three-dimensional reconstruction and analysis using a Vitrea workstation (Vital Images, Minnetonka, MN, USA). Angiograms were analyzed by two reviewers blinded to antibody status, with each segment of ‘Y-grafts’ and ‘skip grafts’ being considered as separate SVGs according to the Society of Thoracic Surgeons (STS) criteria. SVGs were scored as patent (containing stenoses of 0–75%), significantly diseased (containing stenoses of 76–99%, that probably impaired graft flow) or occluded (containing a 100% stenosis). There was 97% concordance in assessment of SVG patency between reviewers. In cases of discordance, a third reviewer adjudicated all SVGs in that patient. Because early SVG failure is primarily due to thrombosis, rather than atherosclerosis or neointimal hyper-plasia, SVGs were considered either patent (0–99% stenosis) or occluded (100% stenosis) for the primary endpoint analysis.
Study endpoint and sample size
The primary endpoint of the RIGOR study was the percentage of SVGs occluded 6 months after CABG surgery. Secondary endpoints included: (i) the percentage of patients with at least one occluded SVG 6 months after CABG surgery; and (ii) the combined incidence of death, MI, coronary revascularization, stroke or other thromboembolic event 6 months after CABG surgery.
We estimated that a sample size of 554 SVGs in 277 patients was needed for 90% power to detect a 40% difference in SVG occlusion rate between seropositive and seronegative patients (expected rates of 32% and 19%, respectively). This assumed that: (i) an average of two SVGs would be implanted per patient; (ii) the overall SVG occlusion rate would be 25%; (iii) the antibody seropositivity rate would be 45% 6 weeks after surgery; and (iv) there would be a low degree of correlation between SVG occlusion within individual patients (intra-class correlation coefficient [rho] = 0.1). The enrollment target was set at 350 patients to accommodate an expected 6-month graft adjudication rate of 80%.
Statistical analysis
Descriptive statistics were used to characterize patients based on antibody status. We tested for differences between groups to assess for potential confounders between the relationship between antibody seropositivity and the primary outcome. Differences between patient groups were compared with t-tests, Wilcoxon rank-sum tests, chi-square tests or Fischer's exact tests, as appropriate. When the patient was the unit of analysis for the primary outcome analysis, logistic regression was used to calculate the risk ratio associated with antibody seropositivity. When the SVG was the unit of analysis, we used logistic regression accounting for clustering of grafts within patients. The confidence intervals surrounding the risk ratio in the cluster-adjusted analyses were generated using the Huber/White sandwich estimator of variance. Given that seropositive and seronegative patients were comparable in baseline and treatment characteristics, multivariate analyses was not used to adjust for differences.
Results
Study population
A total of 1034 patients were screened: 765 met all required entry criteria and 368 patients were enrolled in the study. The most common reasons for failing to enroll a screened eligible patient were the inability/unwillingness of the patient to return for the 6-month follow-up visit (45%), the operating surgeon was not participating in the study (35%) and the unavailability of study personnel to obtain consent from the patient (12%). The RIGOR study population was representative of patients undergoing isolated CABG surgery in the United States based on comparison with the STS National Database (Table S1).
Anti-PF4/heparin antibody induction
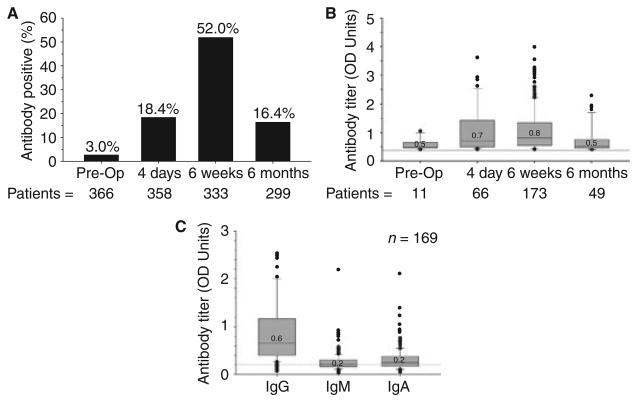
Figure 1(A, B) shows the percentage of anti-PF4/heparin seropositive patients and their respective median OD units prior to, and up to 6 months after, CABG surgery. Six weeks after surgery, 52% (173 of 333) of patients were positive for anti-PF4/heparin antibodies. Isotype analysis was performed on available serum samples from 169 of these patients (Fig. 1C) and revealed the predominant isotype contributing to the OD of the mixed antibody titer to be IgG in 81%, IgM in 9% and IgA in 10%. Considering all patients, 39% (127 of 329) had IgG antibody titers ≥ 0.4 OD units 6 weeks after CABG surgery. Forty-nine (16%) patients tested positive for anti-PF4/heparin antibodies 6 months after CABG surgery. Forty (82%) of these patients had no further exposure to UFH after discharge, indicating persistence of antibodies induced prior to or during the index hospitalization.
Fig. 1.
Induction of anti-PF4/heparin antibodies after CABG surgery. (A) Percentage of patients seropositive for ant-PF4/heparin antibodies (titer ‡ 0.4 OD units) at each time point. (B) Antibody titer of seropositive patients at each time point. Median values with interquartile range (boxes), 5% and 95% confidence intervals (bars) and individual outliers are shown. Gray line demarks titer of 0.4 OD units. (C) Titer of IgG, IgM and IgA isotypes in 169 antibody positive patients 6 weeks after CABG surgery. Median values with IQR (boxes), 5% and 95% confidence intervals (bars) and individual outliers are shown. Gray line demarks titer of 0.4 OD units.
The baseline characteristics of patients stratified by antibody status 6 weeks after CABG surgery are shown in Table 1. Other than a higher incidence of former tobacco use in seronegative patients, there were no significant differences between patients in the two antibody groups or patients in whom antibody status was not determined. The operative and postoperative characteristics of patients stratified by antibody status at 6 weeks are shown in Table 2. Anti-PF4/heparin seropositive patients received slightly more UFH during surgery than seronegative patients (45 000 units vs. 40 500 units, respectively, P = 0.02), though the cumulative doses received during the index hospitalization were similar. Platelet counts did not differ between antibody groups preoperatively, on postoperative day 4, and at hospital discharge or postoperative day 7 (whichever occurred first). HIT was diagnosed by the treating physicians in five patients, four during the index hospitalization and one within 2 weeks of discharge. All of these patients tested positive for anti-PF4/ heparin antibodies 6 weeks after surgery.
Table 1.
Preoperative patient characteristics stratified by anti-PF4/heparin antibody status 6 weeks after CABG surgery
| Antibody positive (n = 173) |
Antibody negative (n = 160) |
Missing (n = 35) |
|
|---|---|---|---|
| Age, years | 63 ± 10 | 65 ± 10 | 66 ± 10 |
| Body mass index, kg m−2 (median, IQR) | 29 [25–33] | 29 [26–33] | 31 [25–34] |
| Men | 141 (82%) | 120 (75%) | 23 (66%) |
| Race (white) | 151 (87%) | 138 (86%) | 24 (69%) |
| Hypertension | 141 (82%) | 131 (82%) | 30 (86%) |
| Hyperlipidemia | 137 (79%) | 133 (83%) | 25 (71%) |
| Diabetes | 70 (40%) | 50 (31%) | 15 (43%) |
| Current tobacco | 42 (24%) | 43 (27%) | 13 (37%) |
| Former tobacco | 113 (65%) | 121 (76%)* | 23 (66%) |
| Prior myocardial infarction | 72 (42%) | 66 (41%) | 18 (51%) |
| Myocardial infarction in past 90 days | 53 (31%) | 48 (30%) | 12 (34%) |
| Prior percutaneous coronary intervention | 35 (20%) | 33 (21%) | 11 (31%) |
| History of congestive heart failure | 27 (16%) | 22 (14%) | 6 (17%) |
| Cerebral/peripheral vascular disease | 30 (17%) | 37 (23%) | 12 (34%) |
| Chronic obstructive pulmonary disease | 14 (8%) | 16 (10%) | 4 (11%) |
| History of atrial fibrillation | 8 (5%) | 9 (5%) | 2 (6%) |
| History of venous thromboembolism, % | 6 (3%) | 5 (3%) | 4 (11%) |
| History of HIT | 0 | 0 | 0 |
| UFH exposure in past 12 months | 6 (3%) | 9 (6%) | 2 (6%) |
| Urgent/emergent surgery | 122 (70%) | 93 (58%) | 28 (80%) |
| euroSCORE [median, IQR] | 3 [2–5] | 3 [2–6] | 5 [3–7] |
| Preoperative ejection fraction < 50% | 74 (43%) | 69 (43%) | 18 (51%) |
| Three-vessel or left main coronary disease | 148 (86%) | 147 (92%) | 32 (91%) |
IQR, interquartile range.
P = 0.04, all others P ≥ 0.05.
Table 2.
Operative and postoperative characteristics of patients stratified by anti-PF4/heparin antibody status 6 weeks after CABG surgery
| Antibody positive (n = 173) |
Antibody negative (n = 160) |
|
|---|---|---|
| Cardiopulmonary bypass | 171 (98%) | 154 (96%) |
| Pump time, minutes (median, IQR) | 76 [63–95] | 75 [59–99] |
| Total grafts per person (median, IQR) | 3 [3–4] | 3 [3–4] |
| SVG per person (median, IQR) | 2 [1–3] | 2 [1–3] |
| Patients with number of SVGs | ||
| 1 | 50 (29%) | 47 (29%) |
| 2 | 63 (36%) | 71 (44%) |
| ≥ 3 | 45 (26%) | 42 (26%) |
| Protamine use | 170 (98%) | 157 (98%) |
| Aprotinin use | 28 (16%) | 20 (13%) |
| ASA use at hospital discharge | 172 (99%) | 160 (100%) |
| ASA use at 6 months | 152 (99%; n = 153) | 138 (99%; n = 139) |
| Thienopyridine use at hospital discharge | 14 (8%) | 22 (14%) |
| Thienopyridine use at 6 months | 13 (8%; n = 153) | 20 (14%; n = 139) |
| Oral anticoagulant use at hospital discharge | 22 (13%) | 12 (8%) |
| Oral anticoagulant use at 6 months | 11 (7%; n = 153) | 5 (4%; n = 139) |
| UFH use | ||
| Preoperative UFH during hospitalization | 89 (51%) | 59 (37%) |
| Preoperative dose, units (median, IQR) | 42 600 [16 920–78 659] (n = 89) | 39 855 [19 450–65 857] (n = 59) |
| Intraoperative dose, units (median, IQR) | 45 000 [38 000–57 000] | 40 500 [34 000–55 000]* |
| Post-operative UFH during hospitalization | 115 (66%) | 122 (76%) |
| Postoperative dose, units (median, IQR) | 65 000 [45 000–105 000] (n = 115) | 60 000 [45 000–110 000] (n = 122) |
| Total hospital dose, units (median, IQR) | 106 000 [66 575–18 5000] | 97 775 [69 500–161 616] |
| UFH after discharge | 19 (11%) | 20 (13%) |
| Platelet transfusion during or after CABG | 70 (40%) | 56 (35%) |
| Baseline preoperative platelet count (103 mm−3) [median, IQR] | 225 [196–265] | 211 [177–263] |
| Platelet count on postoperative day 4 (103 mm−3) [median, IQR] | 190 [158–225] | 183 [143–225] |
| Platelet count at discharge or postoperative day 7 (103 mm−3) [median, IQR] | 247 [199–307] | 245 [198–309] |
| Patients clinically diagnosed with HIT | 5 (3%) | 0† |
IQR, interquartile range.
P = 0.02
P = 0.04, all others P-values ≥ 0.05.
Heparin-dependent platelet activation
The SRA was positive in 14% (23 of 169) of anti-PF4/heparin seropositive patients, including only two of the five patients clinically diagnosed with HIT. Twenty-two (96%) of these patients had IgG titers ≥0.4 OD units. The SRA was also positive in 5% (7 of 153) of patients seronegative for anti-PF4/heparin antibodies, though the average per cent serotonin release was < 50% in six of these patients. One of these seven patients tested borderline positive for anti-PF4/heparin antibodies using an ELISA kit from a different manufacturer (Asserachrom HPIA; Diagnostica Stago Inc., Parsippany, NJ, USA) and four had high titers of anti-HLA antibodies (data not shown), which have been reported to cause a false-positive SRA [16].
Saphenous vein graft patency
Patency status was determined for 642 SVGs in 297 patients (81% of enrollees) a median of 189 days (182–202 IQR) after CABG surgery. One hundred and thirty-three (21%) SVGs were totally occluded at follow-up, while only 17 (3%) contained a high-grade (75–99%) stenosis. Over 80% of the SVGs scored as patent had no or only minimal luminal irregularities. Ninety-nine (33.3%) patients had at least one occluded SVG and 27 (9%) had occlusion of all implanted SVGs.
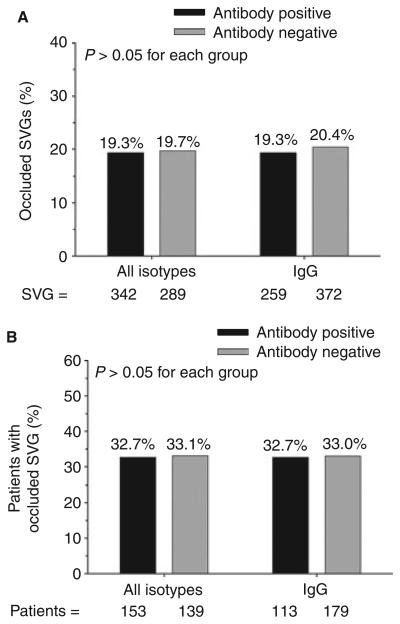
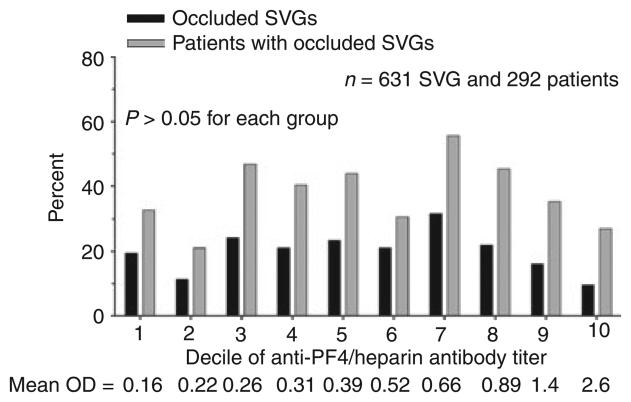
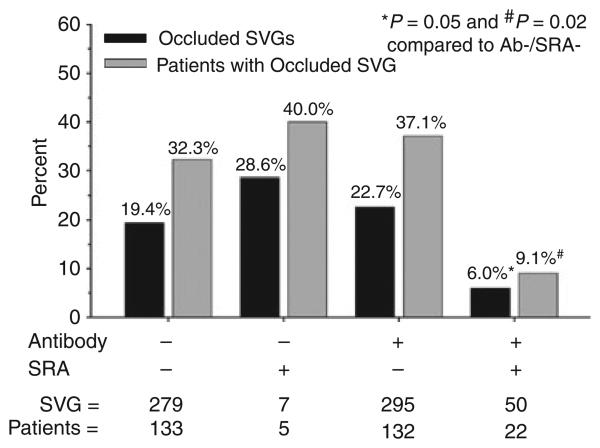
We found no differences with respect to the percentage of occluded SVGs or the percentage of patients with at least one occluded SVG when patients were stratified by either mixed anti-PF4/heparin antibody or IgG isotype seropositivity (Fig. 2). Because there is evidence that a titer threshold of 0.4 OD units may be too low to accurately identify pathologic anti-PF4/heparin antibodies by ELISA [17], SVG outcomes were also stratified by deciles of OD units. We found no association between antibody titer and SVG occlusion, analyzed either on a per-graft or on a per-patient basis (Fig. 3). Surprisingly, seropositive patients with a positive SRA had a lower rate of SVG occlusion compared with patients who were both antibody and SRA negative (Fig. 4).
Fig. 2.
Association of anti-PF4/heparin antibody status with SVG outcome. (A) Percentage of SVG occlusion 6 months after CABG surgery in patients stratified by total or IgG isotype antibody status 6 weeks after CABG surgery. (B) Percentage of patients with at least one occluded SVG 6 months after CABG surgery stratified by total or IgG isotype antibody status 6 weeks after CABG surgery
Fig. 3.
Association of anti-PF4/heparin antibody titer with SVG outcome. Percentage of occluded SVG and percentage of patients with at least one occluded SVG 6 months after CABG surgery stratified by deciles of total anti-PF4/heparin antibody titer 6 weeks after CABG surgery.
Fig. 4.
Association of anti-PF4/heparin antibody and SRA status with SVG outcome. Percentage of occluded SVG and percentage of patients with at least one occluded SVG 6 months after CABG surgery stratified by both antibody and SRA status 6 weeks after CABG surgery.
Clinical outcomes
Table 3 shows 6-month clinical outcomes for 321 patients stratified by antibody status. By univariate analysis there were no differences between antibody groups with respect to the incidence of any of the major adverse cardiac endpoints (MACE), stroke, thromboembolic event or their composite. Stratifying patients by IgG isotype seropositivity or antibody plus SRA positivity similarly revealed no significant differences in 6-month clinical outcomes (data not shown). Not unexpectedly, the composite adverse outcome occurred more frequently in patients diagnosed clinically with HIT compared with those without HIT (80% vs. 21%, respectively, P < 0.01).
Table 3.
Univariate analysis of 6-month clinical outcomes in patients stratified by anti-PF4/heparin antibody status 6 weeks after CABG surgery
| Antibody positive (n = 168), % |
Antibody negative (n = 153), % |
Risk ratio (95% confidence interval)* |
|
|---|---|---|---|
| Death | 6 (3) | 5 (3) | 1.09 (0.34–3.51) |
| Myocardial infarction | 16 (10) | 17 (11) | 0.85 (0.45–1.63) |
| Percutaneous coronary revascularization | 11 (6) | 13 (8) | 0.77 (0.36–1.66) |
| Repeat CABG surgery | 0 | 0 | – |
| Venous thromboembolism | 11 (6) | 7 (4) | 1.43 (0.57–3.59) |
| Stroke | 7 (4) | 3 (2) | 2.12 (0.56–8.07) |
| Composite endpoint | 35 (21) | 36 (24) | 0.89 (0.59–1.33) |
| Composite endpoint or SVG occlusion (n = 292) | 69 (45) | 61 (44) | 1.03 (0.79–1.32) |
All P-values ≥ 0.05.
Discussion
The major findings of this study are as follows. (i) Induction of high titers of anti-PF4/heparin antibodies occurs frequently after CABG surgery and can persist for up to 6 months. (ii) A significant proportion of these antibodies are capable of mediating heparin-dependent platelet activation in the early post-operative period as determined by the SRA. (iii) Induction of anti-PF4/heparin antibodies, even those associated with a positive SRA, is not independently associated with early SVG occlusion or adverse clinical outcomes after CABG surgery.
SVGs are the most widely used conduits for CABG surgery. Unlike arterial conduits, SVGs are highly susceptible to thrombotic occlusion during the first postoperative year, with reported occlusion rates of 25–45% in two recent large clinical trials [18,19]. SVGs are particularly well-suited to imaging by MDCTA because of their large lumenal diameters, limited motion during the cardiac cycle and paucity of calcification. This is borne-out in comparative angiographic studies where pooled results utilizing 8, 16 and 64 detector row scanners report a sensitivity and specificity of 99% and 100%, respectively, for detecting SVG occlusion [20]. The pattern of SVG failure in our patients was essentially an ‘all or none’ phenomenon, consistent with thrombosis, rather than neointimal hyperplasia or atherosclerosis, being the primary mechanism of early SVG failure. Given the exposure to large doses of UFH and the high rate of postoperative thrombotic events, including SVG thrombosis, patients undergoing CABG surgery are therefore an ideal study population in which to test the hypothesis that anti-PF4/heparin antibodies are prothrombotic even in the absence of clinically apparent HIT.
The results of our study fail to support this hypothesis and are at odds with several previously published prospective clinical studies of patients with cardiovascular disease. Mattioli et al. [9] were the first to report that, among 124 ACS patients without thrombocytopenia, those who became antibody seropositive had a higher MACE rate at 1 year than those who remained seronegative (66% vs. 44%, P < 0.01). However, none of the MACE endpoints individually were statistically significant and there was no description of or control for the treatment received during the index hospitalization, including coronary artery revascularization procedures. In a similar population of 252 ACS patients, Matsuo et al. [11] reported that thrombotic events, including myocardial infarction and stent thrombosis, occurred in 7 (32%) of the 22 patients who became anti-PF4/heparin antibody seropositive 4 weeks after receiving UFH but in only 6 (2.6%) of 230 patients who remained seronegative (odds ratio for thrombosis of 17.4, P < 0.0001). Notably, however, two of the seven patients who developed thrombotic events were diagnosed with HIT and the remaining five patients were anti-PF4/heparin antibody seronegative at the time of the event, suggesting that factors other than anti-PF4/heparin antibodies may have been responsible.
Our data are consistent with several small studies involving patients undergoing major vascular surgery that failed to show a significant independent association between anti-PF4/heparin antibody induction and adverse clinical events [21-23]. The most relevant of these was a recent study by Alexy et al. [23], who measured anti-PF4/heparin antibody levels and heparin-induced platelet activation serially for 4 weeks in 79 patients undergoing infrainguinal bypass surgery. Twenty-two (32%) patients seroconverted, among which five (22%) had evidence of heparin-dependent platelet activation. Early graft thrombosis was only detected in three (4%) patients, all of whom remained seronegative. The strength and totality of evidence to date does not therefore support the concept that anti-PF4/heparin antibody induction during the index hospitalization in the absence of HIT increases the subsequent risk of adverse events in patients with cardiovascular disease. What is not answered by these studies is whether subsequent exposure to UFH in patients with pre-existing anti-PF4/heparin antibodies is prothrombogenic in the absence of HIT. Several prior studies suggest this possibility, including two demonstrating that patients undergoing CABG surgery with preoperative anti-PF4/heparin antibodies have longer hospital stays and worse outcome in the immediate postoperative period compared with seronegative patients [10,12,13]. We found no significant association between preoperative antibody seropositivity and either SVG occlusion or adverse clinical outcome (data not shown), though its low incidence in our patient population precludes adequate investigation of this relationship.
A surprising finding of our study was that SVG occlusion was less frequent in patients who were both anti-PF4/heparin antibody and SRA positive. This did not appear to be explained by treatment bias as: (i) the results of neither the antibody ELISA nor SRA were provided to clinicians in real time; (ii) only two of the 22 antibody/SRA positive patients were clinically suspected of HIT (neither suffered early SVG occlusion); and (iii) the incidence of oral anticoagulant and/or thienopyridine use at the time of hospital discharge was modest and did not differ between antibody/SRA positive and negative groups (13.6% vs. 21.1%, respectively, P = 0.6). SRA is considered to be the closest to a ‘gold standard’ assay for the diagnosis of HIT. The criteria for SRA positivity used in the current study, ≥ 20% serotonin release with low-dose and < 20% release with high-dose heparin, has been used by us and others in prior studies [14,24]. Recently, some laboratories have considered clinically significant anti-PF4/heparin antibodies to be those that cause ≥ 50% serotonin release [3]. Applying this criterion for positivity in our patient population would have increased the specificity of the SRA by eliminating six of the seven false-positive results that occurred in antibody-negative patients but would not have significantly altered the percentage of occluded SVGs. Mechanistically, it is difficult to envision how anti-PF4/heparin antibodies that are capable of inducing heparin-induced platelet activation could protect against SVG thrombosis and we suspect that the lower SVG occlusion rate in this group was a random association. We therefore take a conservative view of the data and conclude only that anti-PF4/heparin antibody seropositivity, even with associated SRA positivity, does not predict adverse outcome in an unselected population of CABG patients with a low index of suspicion for HIT.
A potential limitation of the RIGOR study design relates to the assessment of anti-PF4/heparin antibody seroconversion 6 weeks after CABG surgery. Based on a prior study, it was assumed that antibody titers would peak around this time point and persist for several months [25]. Recent evidence suggests that antibody titers peak earlier, 10–12 days after heparin exposure, and decline thereafter in some patients [26]. It is therefore possible that some patients with a low-level antibody response might have been incorrectly classified as seronegative by measuring antibody titers 6 weeks after surgery. We do not believe that this alters the conclusions of the study based on: (i) the number of patients misclassified is likely to be small given that the 52% antibody seroconversion rate in our patient population was as high or higher than that reported in prior studies where titers were measured earlier after CABG surgery [1,2]; and (ii) there was no observable dose-response relationship between the magnitude of the antibody titer at 6 weeks and the rate of SVG occlusion (Fig. 3).
We did not prospectively define criteria for diagnosing HIT in our study population but instead relied on a clinical diagnosis made by the treating physicians. It is certainly possible that not all five patients diagnosed with HIT truly had the disorder (as three of the five were SRA negative) and conversely that some patients with HIT may have gone undiagnosed. As the goal of the RIGOR study was to investigate the prothrombotic potential of anti-PF4/heparin antibodies irrespective of HIT, an accurate diagnosis of the condition does not affect the endpoints or conclusions of the study. Most patients develop thrombocytopenia after CABG surgery as a result of cardiopulmonary bypass [1]. This, along with the high rate of antibody seroconversion, makes accurate diagnosis of HIT and identification of patients at increased risk for thromboembolic events difficult in this patient population. Ongoing analysis of the RIGOR data is aimed at evaluating the diagnostic criteria for HIT and determining which most accurately predict adverse outcome.
In summary, induction of anti-PF4/heparin antibodies consequent to UFH exposure is not an independent risk factor for either early SVG thrombosis or adverse clinical outcome within the first 6 months after CABG surgery. Nonetheless, early SVG thrombosis in the modern era remains substantial, highlighting the need for new research into the mechanisms of SVG failure and therapies to prevent it.
Supplementary Material
Acknowledgements
This work received logistic and material support from the Johns Hopkins General Clinical Research Center (funded by the National Institutes of Health [M01RR000052]) and funding support from The Medicines Company (Parsipany, NJ). The principal and co-investigators were solely responsible for the design of the RIGOR study, the collection, management and analysis of data, as well as writing of the manuscript. We thank K. Laws for research coordination, K. McNicholas, T.C. Villines and J.C. Todd for patient recruitment, K.M. Citro for assistance with regulatory documentation, C. McKaughan and J.T. Jani for technical help with the antibody assays, R.G. Weiss and M. Stuber for assistance with the MRI angiography, D.G. Holmack for assistance data management, and A. Jani and R. Cosgriff for general assistance.
Footnotes
Addendum
Study design: T.J. Gluckman, J.B. Segal, S.P. Schulman, E.P. Shapiro, T.S. Kickler, J.V. Conte and J.J. Rade. Biochemical analysis:M.M.Prechel,J.M. Walenga and T.S. Kickler. Image analysis: T.J. Gluckman, E.P. Shapiro, I. Shafique and J.J. Rade. Data interpretation and analysis: T.J. Gluckman, J.B. Segal and J.J. Rade. Manuscript drafting/editing: T.J. Gluckman, J.B. Segal, S.P. Schulman and J.J. Rade.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data Supplement. Assessment of SVG patency.
Table S1. Baseline characteristics of patients enrolled in the RIGOR study and from isolated CABG surgery patients extracted from the 2007 STS National Database.
Table S2. Modality used to assess vein graft patency in 297 enrolled patients.
Table S3. Reasons for not obtaining graft patency data in 71 of the 368 enrolled patients.
References
- 1.Pouplard C, May MA, Regina S, Marchand M, Fusciardi J, Gruel Y. Changes in platelet count after cardiac surgery can effectively predict the development of pathogenic heparin-dependent antibodies. Br J Haematol. 2005;128:837–41. doi: 10.1111/j.1365-2141.2005.05381.x. [DOI] [PubMed] [Google Scholar]
- 2.Everett BM, Yeh R, Foo SY, Criss D, Van Cott EM, Laposata M, Avery EG, Hoffman WD, Walker J, Torchiana D, Jang IK. Prevalence of heparin/platelet factor 4 antibodies before and after cardiac surgery. Ann Thorac Surg. 2007;83:592–7. doi: 10.1016/j.athoracsur.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–65. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuitunen A, Suojaranta-Ylinen R, Raivio P, Kukkonen S, Lassila R. Heparin-induced thrombocytopenia following cardiac surgery is associated with poor outcome. J Cardiothorac Vasc Anesth. 2007;21:18–22. doi: 10.1053/j.jvca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Liu JC, Lewis BE, Steen LH, Grassman ED, Bakhos M, Blakeman B, Wrona L, Leya F. Patency of coronary artery bypass grafts in patients with heparin-induced thrombocytopenia. Am J Cardiol. 2002;89:979–81. doi: 10.1016/s0002-9149(02)02252-x. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Engl J Med. 1987;316:581–9. doi: 10.1056/NEJM198703053161004. [DOI] [PubMed] [Google Scholar]
- 7.Pouplard C, Iochmann S, Renard B, Herault O, Colombat P, Amiral J, Gruel Y. Induction of monocyte tissue factor expression by antibodies to heparin-platelet factor 4 complexes developed in heparin-induced thrombocytopenia. Blood. 2001;97:3300–2. doi: 10.1182/blood.v97.10.3300. [DOI] [PubMed] [Google Scholar]
- 8.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin-8. Blood. 2001;98:1252–4. doi: 10.1182/blood.v98.4.1252. [DOI] [PubMed] [Google Scholar]
- 9.Mattioli AV, Bonetti L, Sternieri S, Mattioli G. Heparin-induced thrombocytopenia in patients treated with unfractionated hearin: prevalence of thrombosis in a 1 year follow-up. Ital Heart J. 2000;1:39–42. [PubMed] [Google Scholar]
- 10.Williams RT, Damaraju LV, Mascelli MA, Barnathan ES, Califf RM, Simoons ML, Deliargyris EN, Sane DC. Anti-platelet factor 4/heparin antibodies: an independent predictor of 30-day myocardial infarction after acute coronary ischemic syndromes. Circulation. 2003;107:2307–12. doi: 10.1161/01.CIR.0000066696.57519.AF. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Tomaru T, Kario K, Hirokawa T. Incidence of heparin-PF4 complex antibody formation and heparin-induced thrombocytopenia in acute coronary syndrome. Thromb Res. 2005;115:475–81. doi: 10.1016/j.thromres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Slaughter TF, White WD, Welsby IJ, Greenberg CS, El Moalem H, Ortel TL. Preoperative anti-PF4/heparin antibody level predicts adverse outcome after cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1567–72. doi: 10.1016/j.jtcvs.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 13.Kress DC, Aronson S, McDonald ML, Malik MI, Divgi AB, Tector AJ, Downey FX, III, Anderson AJ, Stone M, Clancy C. Positive heparin-platelet factor 4 antibody complex and cardiac surgical outcomes. Ann Thorac Surg. 2007;83:1737–43. doi: 10.1016/j.athoracsur.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman TJ, Segal JB, Fredde NL, Saland KE, Jani JT, Walenga JM, Prechel MM, Citro KM, Zidar DA, Fox E, Schulman SP, Kickler TS, Rade JJ. Incidence of antiplatelet factor 4/heparin antibody induction in patients undergoing percutaneous coronary revascularization. Am J Cardiol. 2005;95:744–7. doi: 10.1016/j.amjcard.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Desai MY, Lai S, Barmet C, Weiss RG, Stuber M. Reproducibility of 3D free-breathing magnetic resonance coronary vessel wall imaging. Eur Heart J. 2005;26:2320–4. doi: 10.1093/eurheartj/ehi357. [DOI] [PubMed] [Google Scholar]
- 16.Prechel MM, Woznica MA, McDonald MK, Jeske WP, Walenga JM. The impact of Non-H:PF4 antibodies on results of the serotonin release assay for heparin-induced thrombocytopenia. ASH Annu Meet Abstr. 2005;106:3952. [Google Scholar]
- 17.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6:1304–12. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 19.Widimsky P, Straka Z, Stros P, Jirasek K, Dvorak J, Votava J, Lisa L, Budesinsky T, Kolesar M, Vanek T, Brucek P. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation. 2004;110:3418–23. doi: 10.1161/01.CIR.0000148139.79580.36. [DOI] [PubMed] [Google Scholar]
- 20.Jones CM, Athanasiou T, Dunne N, Kirby J, Aziz O, Haq A, Rao C, Constantinides V, Purkayastha S, Darzi A. Multi-detector computed tomography in coronary artery bypass graft assessment: a meta-analysis. Ann Thorac Surg. 2007;83:341–8. doi: 10.1016/j.athoracsur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Calaitges JG, Liem TK, Spadone D, Nichols WK, Silver D. The role of heparin-associated antiplatelet antibodies in the outcome of arterial reconstruction. J Vasc Surg. 1999;29:779–85. doi: 10.1016/s0741-5214(99)70203-x. [DOI] [PubMed] [Google Scholar]
- 22.Lindhoff-Last E, Eichler P, Stein M, Plagemann J, Gerdsen F, Wagner R, Ehrly AM, Bauersachs R. A prospective study on the incidence and clinical relevance of heparin-induced antibodies in patients after vascular surgery. Thromb Res. 2000;97:387–93. doi: 10.1016/s0049-3848(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 23.Alexy T, Tucker S, Boyle S, Rowe VL, Weaver FA, Liebman HA. Heparin-platelet factor 4 antibodies are frequent after vascular surgery but are not a frequent cause of graft thrombosis or thrombocytopenia. J Vasc Surg. 2008;48:377–81. doi: 10.1016/j.jvs.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Warkentin TE, Hayward CP, Smith CA, Kelly PM, Kelton JG. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J Lab Clin Med. 1992;120:371–9. [PubMed] [Google Scholar]
- 25.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–92. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 26.Greinacher A, Kohlmann T, Strobel U, Sheppard JA, Warkentin TE. The temporal profile of the anti-PF4/heparin immune response. Blood. 2008;113:4970–6. doi: 10.1182/blood-2008-08-173062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.