Abstract
Vagal tone (measured via respiratory sinus arrhythmia, RSA) and vagal withdrawal (measured by decreases in RSA) have been identified as physiological measures of self-regulation, but little is known how they may relate to the regulation of cognitive activity as measured through executive function (EF) tasks. We expected that baseline measures of vagal tone, thought to be an indicator of attention, would correlate with EF performance. We also predicted that vagal withdrawal would allow for the reorientation of attention that is needed to succeed on EF tasks, but too much withdrawal would be detrimental. RSA measured at baseline was indeed related to EF performance in 220 3.5-year-old children, and those who exhibited a moderate decrease in RSA during the EF tasks outperformed children whose RSA decreased by too little or too much. These findings implicate vagal tone withdrawal as a psychophysiological measure of higher cognitive processes, most likely substantiated through increases in the levels of focused attention.
Keywords: RSA, executive function, vagal withdrawal, self-regulation
The development of self-regulation has sparked a lot of recent interest, as it is implicated in successful outcomes across the lifespan (e.g., Hastings et al., 2008; Masi, Hawkley, Rickett, & Cacioppo, 2007; Movius & Allen, 2005; Phillips, Henry, Hosie, & Milne, 2006; Souza et al., 2007; Stifter & Corey, 2001). Self-regulation has been assessed independently using both physiological (e.g., Hastings et al., 2008; Souza et al., 2007) and psychological (e.g., Phillips et al., 2006) measures, and the relationship between the two approaches is emerging as a promising avenue for research. For example, the Neurovisceral Integration Model (Thayer & Lane, 2000, 2009) postulates that the cognitive, emotional, behavioral, and physiological processes involved in self-regulation all share common indices that are associated with heart rate variability. In the present study, we examine how physiological measures of self-regulation, namely the cardiac measure of vagal tone and its withdrawal, are related to cognitive regulation as measured by executive function (EF) tasks in 3.5-year-old children.
Vagal tone is responsible for the neural regulation of heart rate. The myelinated vagus nerve originates in the brainstem nucleus ambiguous and provides input to the sinoatrial node of the heart, communicating bidirectional signals between the heart and the brain that are responsible for the parasympathetic nervous system’s response to stressors in the environment (Porges, 2003). According to Porges (1991, 2003), the vagal system works to maintain a homeostatic balance between the internal and external demands of the environment. Vagal regulation in young children can be measured non-invasively by capturing respiratory sinus arrhythmia (RSA), the naturally occurring variations of heart rate during a breathing cycle.
Baseline measures of vagal tone, recorded during interactions with non-stress inducing stimuli, indicates the level of arousal along with awareness of the environment and the stimuli within that environment (Porges, 2003). Furthermore, baseline vagal tone reflects autonomic functioning and has been found to be an important predictor of attention (Linnemeyer & Porges, 1986; Suess, Porges, & Plude, 1994). Indeed, measures of baseline vagal tone have been associated with a number of phenomena across the lifespan. Infants with higher baseline levels of vagal tone demonstrate appropriate reactivity to stimuli (Huffman et al., 1998; Porges, Doussard-Roosevelt, Portales, & Suess, 1994) and higher scores in infant assessments of intelligence (Fox & Porges, 1985), whereas lower vagal tone in children is associated with externalizing behavior problems, high body mass index, depression, and sleep disruptions (El-Sheikh, Erath, & Keller, 2007). Low baseline vagal tone is also predictive of negative health outcomes (e.g., hypertension) in adults (Masi et al., 2007). In sum, higher baseline vagal tone seems to be more adaptive for physical, mental, and behavioral outcomes across all ages.
Vagal tone withdrawal, in contrast, is measured by the decrease in RSA during situations where regulation is required. Porges (1991) discusses vagal withdrawal as the ability to put on the “brake“ when reacting to events in the environment, effectively because a decrease in vagal input stimulates increases in heart rate. This process is associated with the ability to maintain an internal homeostatic balance in response to stressors in the environment and allow for increased orientation to changes in the environment (Calkins, 1997). For instance, Porges et al. (1994) found that 9-month-old infants who were unable to engage in vagal withdrawal had difficulty coping with additional attention-eliciting stimuli and exhibited a greater number of externalizing behaviors at age 3, indicating that physiological regulation of vagal tone is predictive of long-term behavioral outcomes. Vagal withdrawal predicts of a number of positive outcomes including academic performance (Blair & Diamond, 2008; Graziano, Reavis, Keane, & Calkins, 2007; Staton, El-Sheikh, & Buckhalt, 2009) and peer relationships in childhood (Wilson, Fernandes-Richards, Aarskog, Osborn, & Capetillo, 2007). In adults, greater vagal regulation has been shown to attenuate the negative heath outcomes in the midst of marital discord (Whitson & El-Sheikh, 2003).
A number of researchers (e.g., DeGangi, DiPietro, Greenspan, & Porges, 1991; Richards & Casey, 1991) have used vagal tone suppression within a task as a measure of sustained attention. Although the majority of these studies involve infants, Suess et al. (1994) assessed the roles of both baseline RSA and vagal withdrawal in middle childhood throughout a continuance performance task. They reported that baseline RSA was predictive of performance in the first 3 minute block of the task and that vagal withdrawal occurred in subsequent blocks, providing compelling evidence that these cardiac measures are associated with attentional capacity and the ability to sustain attention.
Although vagal withdrawal has been typically associated with positive outcomes (e.g., Morgan, Alkins, Steffian, Coric, & Southwick, 2007), extremely low and high levels of vagal withdrawal have been associated with negative outcomes. For instance, Calkins, Graziano, and Keane (2007) found that compared to control children, children at risk for externalizing behaviors exhibited lower levels of vagal withdrawal. In addition, excessively high vagal withdrawal has occasionally been linked to deficits in emotional functioning (Beauchaine, 2001), suggesting that it interferes with coping or self-regulation. For example, children with behavior problems caused by anxiety, displayed greater vagal withdrawal than children with no behavior problems (Calkins et al., 2007). Perhaps excessive physiological regulation interferes with the ability to process environmental stimuli in a flexible manner, and if so, decrements in adaptive self-control behaviors should become apparent in these contexts.
Most vagal tone studies examine its relation to emotion or behavioral regulation (e.g., Lane et al., 2009). We contend that, in addition, it is highly likely that moderate vagal withdrawal would facilitate EF because of the need to stimulate (but not over-stimulate) increases in heart rate to cope with the stressful demands inherent in the execution of higher order thought processes. The goal of the current study was to examine how vagal tone processes relate to performance on EF measures in 3.5-year-old children. EF is a cognitive structure comprised of multiple subcomponents including attention, working memory, and inhibitory control that may very well form the basis of self-regulation skills. As 3- to 4-year-old children are entering a period of rapid and intense development of EF skills (Carlson, 2005; Espy, 1997; Kirkham, Cruess, & Diamond, 2003; Munakata & Yerys, 2001; Zelazo, Muller, Frye, & Marcovitch, 2003), it is important to determine whether the emergence of these skills is related to physiological measures of regulation. If a relation does exist, then we can begin to take steps toward early intervention to avoid potentially maladaptive development of cognitive functioning. We chose two measures of EF – the number recall task and the Children’s Stroop Test – as an index of cognitive self-regulation in children.
In the current study, vagal tone was assessed via RSA measurements taken during a baseline phase where children were watching a video, and again while children performed the EF tasks. We hypothesized that as a measure of attention regulation, RSA measured at baseline would positively predict performance on EF tasks. In addition, children who displayed a moderate decrease in RSA during EF tasks were predicted to outperform peers who displayed relatively little or too much vagal withdrawal.
Method
Participants
Participants were taking part in the first wave of an ongoing longitudinal study. The final sample consisted of 220 3.5-year-old children (M = 41.89 months; SD = 2.38): 52% female; 53.2% Caucasian, 35.9% African American, 6.4% other ethnicities. Poverty level was assessed via parental self report using a scale ranging from 1 (less than $200 / month) to 15 (more than $10,000 / month) yielding a wide range of poverty scores (M = 8.9, SD = 3.3).
Measures
Vagal tone
Heart period was measured from two disposable pediatric electrodes placed on the child’s chest that were connected to a preamplifier, the output of which was processed through a vagal tone monitor (Series 2000 Mini-Logger, Mini Mitter Co., Inc. Bend, OR) for R-wave detection. Data files were edited and analyzed using Cardio batch/Edit software (Brain-Body Center, University of Illinois at Chicago, Chicago, IL). Porges’ (1985) method of calculating RSA was used, and was calculated every 30 seconds for the baseline period and every 15 seconds during the cognitive tasks; the score used was an average across all epochs in a given task. Following previous research (Calkins, 1997; Moore & Calkins, 2004) difference scores for calculating vagal withdrawal were computed by subtracting episode RSA from baseline RSA, with positive values indicating greater vagal withdrawal.
Number recall task
The number recall subtest of the Kaufman Assessment Battery for Children (Kaufman & Kaufman, 1983) has been used previously as a measure of cognitive control, an important component of EF, in 3.5-year-old children (see Leerkes, Paradise, O’Brien, Calkins, & Lange, 2008). On each trial, the experimenter recited a series of numbers, and children were asked immediately to repeat the numbers back in the same sequence. The task was terminated after three consecutive errors. The raw score was computed as the difference between the ceiling item and the total number of errors.
The Children’s Stroop Test
In the Children’s Stroop Test (Gerstadt, Hong, & Diamond, 1994), children were shown a series of 16 cards. Half of the cards were black with yellow moons and stars, to which children were to respond “day.“ The remaining cards were white with a yellow sun on each card, to which children were to respond “night.“ Children were given two practice trials, and then presented with the cards in a fixed order. Each child’s score was the number of correct responses to the 16 test trials.
Procedure
Children were brought into the laboratory to participate in a battery of tasks (most of which are not presented in the current paper) that assessed cognitive and emotional development in one session that lasted approximately two hours. Approximately 20 minutes into the session, RSA was measured during a baseline period while children watched a non-stress inducing video (4.5 minutes long). Then, approximately 25 minutes later, RSA was further assessed during administration of the number recall task (typically lasting between 2 and 2.5 minutes) and, about 10 minutes after that, on the Children’s Stroop Test (typically lasting between 1 and 1.5 minutes).
Results
The mean poverty score (M = 8.9) was used for the two children whose parents did not report income. The poverty score correlated significantly with both the number recall test, r(218) = .134, p = .048, and the Children’s Stroop test, r(218) = .133, p = .049. For all further analyses, the poverty score was used as a covariate.
Decreases in RSA across the two EF tasks were highly correlated, r(218) = .59, p < .001, justifying an aggregate measure of vagal withdrawal calculated by summing the standardized withdrawal scores. Based on these aggregate withdrawal (AW) scores, children were divided into one of 3 categories by identifying the lowest and highest 50 scores as extreme. Thus, the groups were Low Withdrawal (n = 50, AW < −1.38), Moderate Withdrawal (n = 120), and High Withdrawal (n = 50, AW > 1.31).
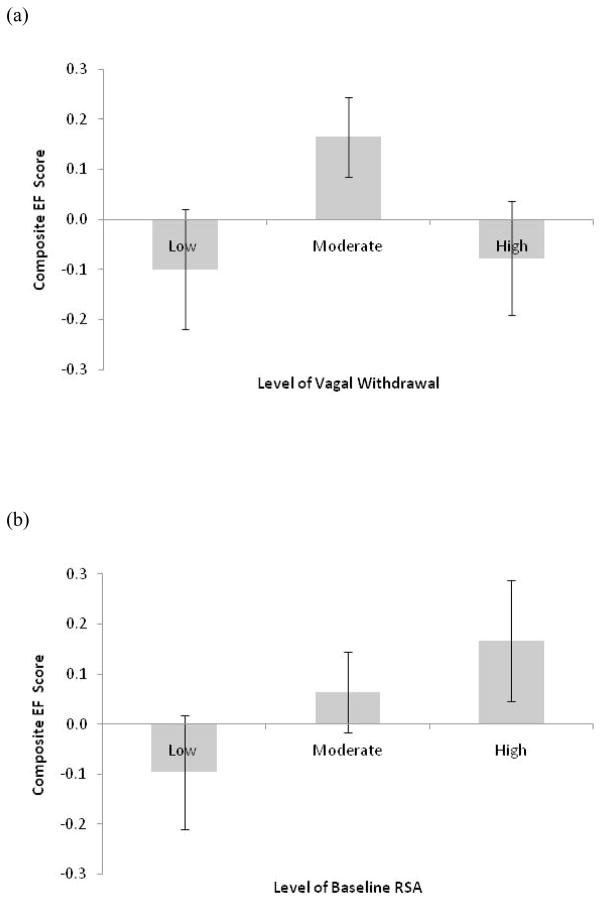
We conducted a multivariate analysis of covariance (MANCOVA) assessing the effects of sex and level of vagal withdrawal on both EF tasks. The analyses revealed a marginal effect of the level of vagal withdrawal, Wilks’ λ = 0.96, F(4, 424) = 2.1, p = .08, ηp2 = 0.02, but no effect of sex, Wilks’ λ = 0.98, F(2, 212) = 1.6, p = .20, ηp2 = 0.01, nor an interaction between the two variables, Wilks’ λ = 0.99, F < 1, ηp2 = 0.01. Our primary analysis was based on our prediction that children who engaged in moderate withdrawal would perform better than children who engaged in low or high withdrawal. To that end, we carried out an a priori quadratic contrast (1 –2 1) for the MANCOVA and found that children who exhibited moderate withdrawal outperformed their peers who exhibit either high or low withdrawal, Wilks’ λ = 0.97, F(2, 212) = 3.6, p = .03, η2 = 0.03 (see Figure 1a).
Figure 1.
(a) Performance on EF tasks by level of vagal withdrawal. (b) Performance on EF tasks by level of RSA baseline
Note: For the purpose of exposition, an EF composite was created by averaging the standardized scores of both the Stroop and number recall tests.
Similar to previous studies measuring vagal withdrawal (e.g., Calkins, 1997), we found that baseline RSA was correlated with vagal withdrawal (using the aggregate score), r(218) = 0.51, p < .001, which speaks to the interdependence of the two measures. To determine whether a linear or non-linear relationship existed between baseline RSA and EF performance, we also identified the lowest 51 (due to a tie) and highest 50 scores as extreme, yielding Low (n=51), Moderate (n=119), and High (n=50) baseline RSA groups. The corresponding MANCOVA on both measures of EF performance revealed a marginal effect of the level of baseline RSA, Wilks’ λ = 0.96, F(4,424) = 2.3, p = .06, ηp2 = 0.02, but no effect of sex, Wilks’ λ = 0.99, F < 1, ηp2 = 0.01, nor an interaction between the two variables, Wilks’ λ = 0.99, F < 1, ηp2 = 0.01. Importantly, though, an a priori linear contrast (−1 0 1) revealed a significant linear relation, Wilks’ λ = 0.96, F(2,212) = 4.4, p = .01, η2 = 0.04 (see Figure 1b).
Discussion
The current study assessed the relationship between EF performance and two measures of cardiac activity: baseline vagal tone, indicative of individuals’ attentional abilities and awareness of their environment, and vagal withdrawal, indicative of the ability to adjust to changes in the environment. Consistent with our hypotheses, baseline vagal tone was positively associated with performance on EF tasks at 3.5 years of age. Previous research in adults has demonstrated that higher baseline vagal tone is associated with better attentional abilities (see Porges, 1991), which suggests that individual differences in regular cardiac functioning is a determinant in higher functioning processes even as young as 3.5 years of age (Porges et al., 1994). This is in line with a number of studies that have demonstrated that high levels of baseline RSA in infancy is predictive of measures of attention and intelligence later in life (e.g., Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997; Fox & Porges, 1985). The present study expands on this literature by demonstrating that baseline RSA measured in young children was predictive of EF functioning within the same experimental session.
Further, we found that children who displayed moderate levels of vagal withdrawal outperformed children who exhibited either high or low levels of withdrawal. Success on EF tasks requires the ability to orient attention toward the relevant aspects of the stimuli, coupled with the ability to focus attention intently (or sustain attention) in the face of distracting information. For example, in the Children’s Stroop Test, successful children must first orient attention to the stimulus card and process the relevant details of the scene (e.g., moon and stars) and then focus intently on the rule (e.g., say ’day’) in the face of the environmental distracter (e.g., the inclination to respond ’night’). Similarly, in the number recall test, successful children first orient attention to the orally presented stimuli, and then focus intently on repeating back the elements in the appropriate order in the face of the distraction of having to hold the remaining elements in mind. Consistent with polyvagal theory (Calkins, 1997; Porges et al., 1994), failure to suppress vagal tone reflects shortcomings in the ability to orient attention to environmental stressors, and this explains the relative disadvantage for children with low levels of withdrawal as compared to those with moderate levels of withdrawal. In contrast, children with extremely high amounts of vagal withdrawal may be physiologically over-aroused and this hinders their ability to focus their attention intently in the face of distraction. This is consistent with previous findings of the association between childhood anxiety and high amounts of vagal withdrawal (Calkins et al., 2007).
As 3.5-year-old children are entering a developmental stage that is characterized by rapid growth in cognitive flexibility, it would be beneficial to isolate psychophysiological causes of and correlates with the development of this skill. Future research should concentrate on discovering associations between vagal withdrawal and EF performance at different ages, and importantly, whether vagal withdrawal at an early age is predictive of cognitive functioning later in development.
In summary, the findings of the current study demonstrated a positive relationship between baseline vagal tone and performance on cognitive tasks measuring inhibition and working memory in children. Furthermore, this study supports the notion that those who engage in moderate amounts of physiological regulation have better performance on measures of EF, and that over-regulation can be maladaptive for optimal cognitive processing.
Acknowledgments
This research was supported by NICHD grant HD050806 to UNCG.
Contributor Information
Stuart Marcovitch, Email: s_marcov@uncg.edu.
Janet Leigh, Email: jlhaisli@uncg.edu.
Susan D. Calkins, Email: sdcalkin@uncg.edu.
Esther M. Leerkes, Email: emleerke@uncg.edu.
Marion O’Brien, Email: m_obrien@uncg.edu.
A. Nayena Blankson, Email: ablanks1@spelman.edu.
References
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano P, Keane S. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. Children’s sleep and adjustment: The moderation role of vagal regulation. Journal of Sleep Research. 2007;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Espy KA. The Shape School: Assessing executive function in preschool children. Developmental Neuropsychology. 1997;13:495–499. doi: 10.1207/s15326942dn2601_3. [DOI] [PubMed] [Google Scholar]
- Fox NA, Porges SW. The relation between neonatal heart period pattern and developmental outcome. Child Development. 1985;56:28–37. [PubMed] [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: Performance of children 3½-7 years old on a Stroop-like day-night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Graziano PA, Reavis RD, Keane SP, Calkins SD. The role of emotion regulation in children’s early academic success. Journal of School Psychology. 2007;45:3–19. doi: 10.1016/j.jsp.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, Vyncke JD. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development. 2008;79:45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children (K-ABC) Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–476. [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Paradise M, O’Brien M, Calkins SD, Lange G. Emotion and cognition processes in preschoolers. Merrill Palmer Quarterly. 2008;54:102–124. [Google Scholar]
- Linnemeyer SA, Porges SW. Recognition memory and cardiac vagal tone in 6-month-old infants. Infant Behavior and Development. 1986;9:43–56. [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movius HL, Allen JJB. Cardiac vagal tone, defensiveness, and motivational style. Biological Psychology. 2005;68:147–162. doi: 10.1016/j.biopsycho.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Yerys BE. All together now: When dissociations between knowledge and action disappear. Psychological Science. 2001;12:335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Alkins DE, Steffian G, Coric V, Southwick S. Relationship between cardiac vagal tone and performance in male military personnel exposed to high stress: Three prospective studies. Psychophysiology. 2007;44:120–127. doi: 10.1111/j.1469-8986.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Henry JD, Hosie JA, Milne AB. Age, anger regulation and well-being. Aging & Mental Health. 2006;10:250–256. doi: 10.1080/13607860500310385. [DOI] [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. 4,510,944. US Patent. 1985 April 16;
- Porges SW. Vagal tone: An autonomic mediator of affect. In: Garber J, Dodge A, editors. The development of emotional regulation and dysregulation. Cambridge: Cambridge University Press; 1991. pp. 111–128. [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales LA, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year-olds. Developmental Psychobiology. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Souza GGL, Mendonca-de-Souza ACF, Barros EM, Coutinho EFS, Oliveira L, Mendlowicz MV, Figueira I, Volchan E. Resilience and vagal tone predict recovery from acute social stress. The International Journal on the Biology of Stress. 2007;10:368–374. doi: 10.1080/10253890701419886. [DOI] [PubMed] [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. Respiratory sinus arrhythmia and cognitive functioning in children. Developmental Psychobiology. 2009;51:249–258. doi: 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Corey JM. Vagal regulation and observed behavior in infancy. Social Development. 2001;10:189–201. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-aged children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Whitson S, El-Sheikh M. Marital conflict and health: Processes and protective factors. Aggression and Violent Behavior. 2003;8:283–312. [Google Scholar]
- Wilson BJ, Fernandes-Richards S, Aarskog C, Osborn T, Capetillo D. The role of emotion regulation in the social problems of boys with developmental delays. Early Education and Development. 2007;18:201–222. [Google Scholar]
- Zelazo PD, Muller U, Frye D, Marcovitch S. The development of executive function. Monographs of the Society for Research in Child Development. 2003;68:11–119. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]