Abstract
Background
Intrauterine infection is a recognized cause of adverse pregnancy outcome but the source of infection is often undetermined. We report a case of stillbirth caused by Fusobacterium nucleatum that originated in the mother’s mouth.
Case
A woman with pregnancy-associated gingivitis experienced an upper respiratory tract infection at term, followed by stillbirth a few days later. Fusobacterium nucleatum was isolated from the placenta and the infant. Examination of different microbial flora from the mother identified the same clone in her subgingival plaque, but not in the supragingival plaque, vagina, or rectum.
Conclusion
F. nucleatum may have translocated from the mother’s mouth to the uterus when the immune system was weakened during the respiratory infection. This case sheds light on patient management for those with pregnancy-associated gingivitis.
INTRODUCTION
Stillbirth is a significant public health concern, accounting for 60% of perinatal deaths. Infections account for 10–25% of all stillbirths. In this report, we present a case of unusual term stillbirth caused by Fusobacterium nucleatum, a Gram-negative anaerobic bacterium prevalent in intrauterine infection but not associated with stillbirth before. We demonstrate that this organism may have originated from the mother’s oral cavity.
CASE
A 35 year old primigravid Asian woman reporting decreased fetal movement was admitted to Saint John’s Health Center at 39 weeks and 5 days of gestation. Up to the day of hospitalization, the patient had received routine prenatal care and the pregnancy was uncomplicated, with the exception of a two-vessel umbilical cord found by ultrasound. Subsequent serial ultrasounds revealed no other anatomic abnormalities. On the day of the admission, the patient reported she had last felt the baby move at approximately 5 o’clock that morning. The mother had been mildly ill with an upper respiratory tract infection for the previous three days, running a low grade fever of 100°F. There was no history of amniotic fluid leakage, bleeding, or abnormal uterine contractions. Upon admission, absence of fetal heart beat was confirmed by ultrasound. The membrane was artificially ruptured by the obstetrician, who noted slightly bloody and strong foul-smelling amniotic fluid. Over the next several hours, the patient’s labor progressed without difficulty and a significantly malodorous female stillborn infant weighing 3323 grams was delivered vaginally early the next morning. TORCH titers and parvovirus titers drawn prior to delivery were negative.
Pathology
Placenta: The placenta was relatively small (5th percentile for 39 weeks gestation) and had a single umbilical artery. Acute chorioamnionitis with umbilical phlebitis, chorionic vasculitis, and foci of recent nonocclusive chorionic vessel thrombosis was noted. Unusual features of the chorioamnionitis in this case include an unusually severe acute deciduitis with foci of decidual necrosis in the placental membranes and a single focus of acute deciduitis in the decidua basalis (Figure 1). Gram negative bacilli were observed in the amnion and subchorion by Gram stain and placental culture was positive for Fusobacterium nucleatum.
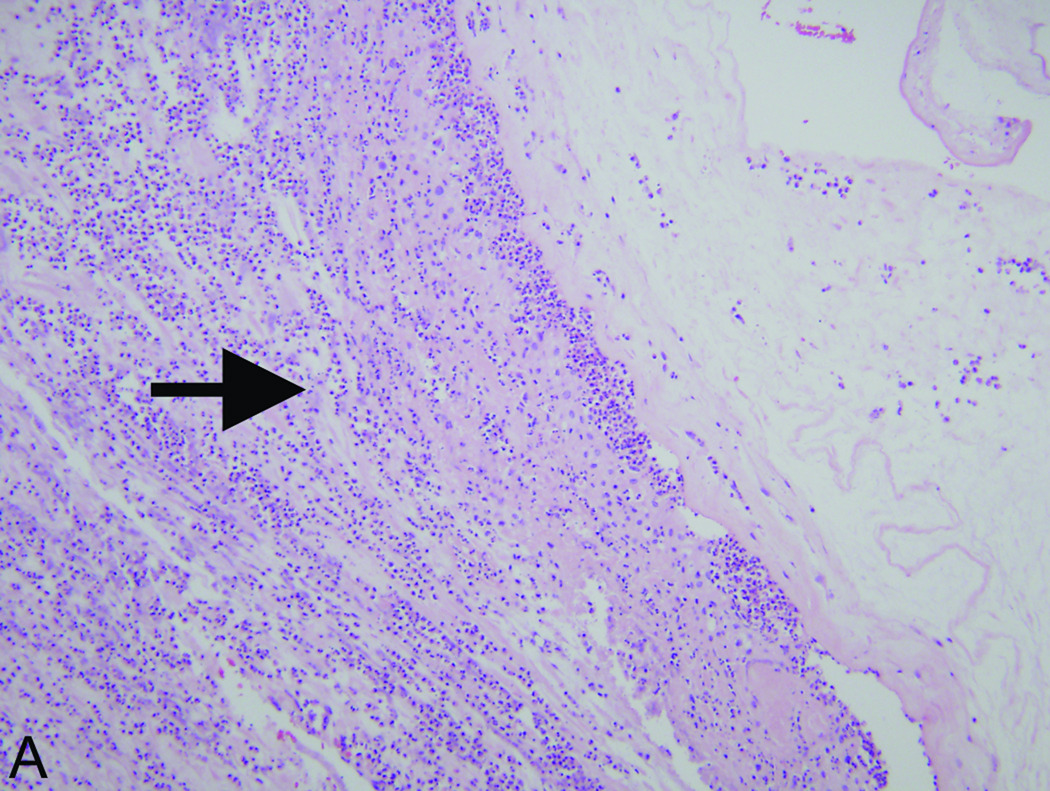
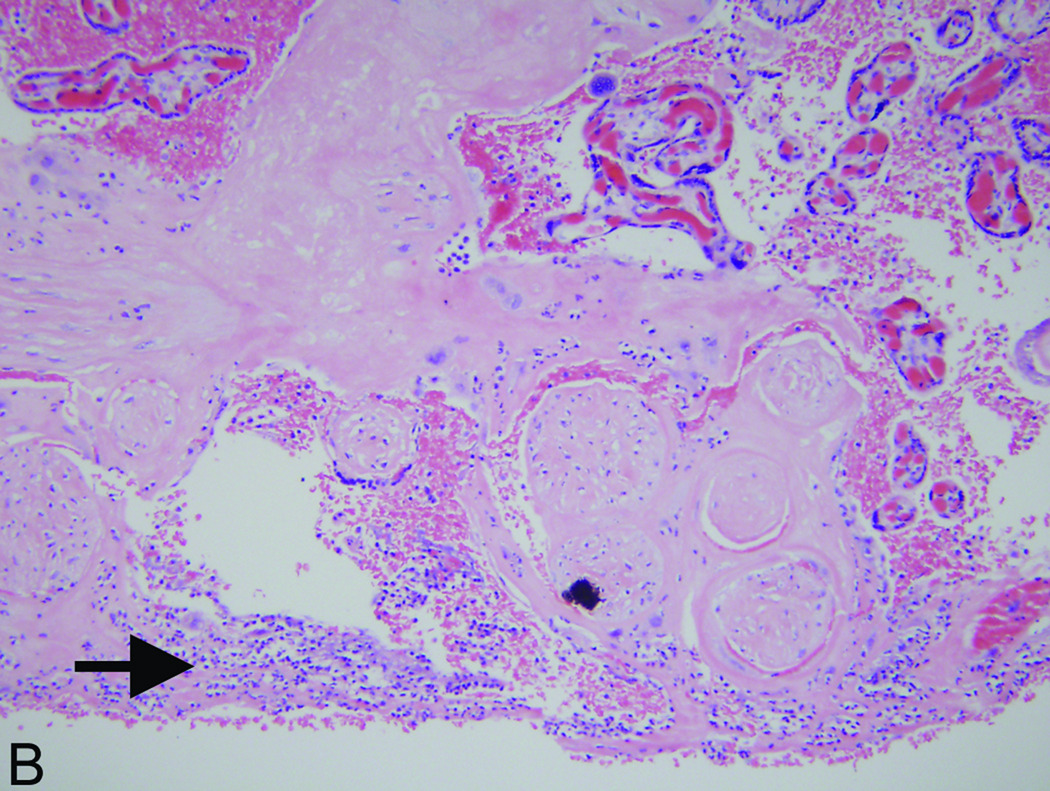
Figure 1.
Accentuated decidual inflammatory response in acute chorioamnionitis caused by Fusobacterium nucleatum. (A) Decidua capsularis (hemotoxylin and eosin [H&E] stain, 200× magnification): There is an unusually extensive and intense neutrophilic infiltrate in the decidua capsularis (arrow). (B) Decidua basalis (H&E, 200× magnification): Focus of acute deciduitis in the decidua basalis underlying the intervillous space (arrow). These foci are rarely seen in typical cases of acute chorioamnionitis.
Autopsy: The mother consented to a complete autopsy of the infant. Minimal maceration and autolysis of organs were observed, consistent with fetal demise less than 12 hours prior to delivery. No congenital anomalies were noted and the fetus was appropriate for gestational age by autopsy measurements. There was diffuse venous congestion. Organ weights were unremarkable, with the exception of the lungs which were twice the normal weight and showed histologic evidence of massive intraalveolar hemorrhage. Giemsa stain revealed multiple foci of filamentous bacteria in the bronchi with no accompanying inflammatory response. Filamentous bacteria were also seen in large numbers in the stomach without transit to the colon, suggesting relatively recent acquisition. F. nucleatum was isolated from both the lung and stomach as pure culture using the routine culturing methods in the clinical microbiology laboratory at St. John’s Health Center.
Source of F. nucleatum infection
To investigate the possible source of F. nucleatum infection, vaginal, introitus, and rectal swabs, and supragingival and subgingival plaque samples were collected from the mother. The patient reported excessive gum bleeding during her pregnancy. Following the identification of F. nucleatum from the stillborn infant, a full-mouth periodontal examination was performed at three weeks postpartum, revealing minimal gingival inflammation and no signs of periodontitis. Thus, the patient may have had pregnancy-associated gingivitis.
The bacteria isolated from the stillbirth infant were designated F. nucleatum strain 708. The central part of the region corresponding to the 16S–23S rRNA genes was amplified by PCR using universal primers as previously described 1. Based on the amplified sequence, Fusobacterium-specific primers were designed and used to analyze the following samples collected from the patient: pooled full-mouth supragingival plaque, pooled full-mouth subgingival plaque, and introitus, vaginal and rectal swabs.
Using universal primers, bacterial DNA was amplified from all samples, indicating adequate sample collection at different sites. When Fusobacterium-specific primers were used, bacterial DNA was amplified only from the supragingival and subgingival plaque samples, but not from the other samples (Table 1). Clone libraries were generated using the Fusobacterium-specific PCR amplicons. A group of 10–24 random clones from each library was sequenced. The 16S rRNA portion of each sequence was used to BLAST against the Human Oral Microbiome Database (HOMD) for species identification (Table 1). F. nucleatum 708 belonged to subspecies (ss) animalis Oral Taxon 420. Two of the 24 clones from the subgingival library also belonged to this taxon (Table 1). Furthermore, one of these two clones matched F. nucleatum 708. No F. nucleatum ss animalis Oral Taxon 420 was identified among the 19 clones analyzed from the supragingival plaque library. Taken together, the results of PCR and clone analysis indicate that F. nucleatum was present in the mother’s subgingival flora but absent from her supragingival, vaginal, or rectal floras.
Table 1.
Identification of Fusobacteria species in the mother’s plaque samples.
| Samples | identificationa | # clones analyzeda |
|---|---|---|
| F. nucleatum 708 | F. nucleatum ss animalis Oral Taxon 420 | 10 |
| Subgingival plaque | F. nucleatum ss animalis Oral Taxon 420 | 2b |
| F. nucleatum ss polymorphum Oral Taxon 202 | 5 | |
| F. nucleatum ss vincentii Oral Taxon 200 | 17 | |
| Supragingival plaque | F. naviforme Oral Taxon 200 | 2 |
| F. nucleatum ss polymorphum Oral Taxon 202 | 14 | |
| F. nucleatum ss vincentii Oral Taxon 200 | 3 | |
| Introitus swab | ---c | |
| Vaginal swab | ---c | |
| Rectal swab | ---c | |
16S rRNA clone libraries were generated for F. nucleuatm 708, subgingival plaque, and supragingival plaque, respectively, by cloning the Fusobacterium-specific amplicons. Randomly selected clones were sequenced and BLASTed against the Human Oral Microbiome Database. The taxon was identified if the sequence identify was >98.9%.
One of these two clones had 100% sequence match in the 16S rRNA region with F. nucleatum 708.
No fusobacterial DNA was amplified from these samples.
COMMENT
A PubMed search in November 2009 using keywords of “stillbirth”, “Fusobacterium”, and “oral bacteria” revealed no prior cases of oral bacteria causing stillbirth. {AQ: The first sentence of the Comment section was moved from the Introduction.} Studies from our group and others have demonstrated that F. nucleatum is one of the most prevalent species in intrauterine infection, predominantly identified in cases of preterm birth 2, 3. Although intrauterine infection of F. nucleatum has long been suspected to originate from the oral cavity, this hypothesis has not been proven in humans until now. In this unusual case of term stillbirth, F. nucleatum was found in the mother’s oral cavity, but not in her vaginal or rectal flora. A total of four Fusobacteria taxa were identified from the subgingivcal and supragingival plaque samples, among which three were F. nucleatum taxa. These results indicate that F. nucleatum is part of the normal flora in the mother’s mouth. No F. nucleatum was detected in the mother’s vaginal or rectum samples, thus an ascending route of infection was unlikely.
Evidence is accumulating that oral bacteria play a significant yet previously unrecognized role in intrauterine infection leading to preterm birth 1, 2. As one of the major microbiomes in the human body, the oral flora serves as a microbial reservoir. Part of the reason that the role of oral bacteria was underestimated may be because the majority of oral species are uncultivated, hence cannot be detected by the routine culturing methods employed by the hospital microbiology laboratories. For instance, an uncultivated oral species, Bergeyella, which had never been identified in intrauterine infection, has been repeatedly detected in amniotic fluids associated with preterm birth using 16S rRNA gene-based culture-independent technology 1, 2. Likewise, we speculate that a certain portion of the unexplained stillbirths may be caused by previously unrecognized oral bacteria, translocated to the uterus independent of the ascending vaginal route. Further systematic analysis is needed to determine the prevalence of oral bacteria in stillbirth.
How do oral bacteria invade into the uterus? Previous studies in animals have provided clues. Hematogenous injection of orally-related F. nucleatum into pregnant mice resulted in specific bacterial colonization in the placenta causing localized inflammation 4, 5. The bacteria first colonized in the mouse decidua by crossing the endothelium, followed by proliferation and spreading to fetal membranes, amniotic fluid, and fetus, and eventually causing fetal demise 4. The pattern of infection is similar to the case reported here. It is plausible that F. nucleatum translocated from the mother’s mouth hematogenously. Supporting this notion is the mother’s report of excessive gum bleeding during pregnancy. The mother might have had pregnancy-associated gingivitis, i.e. plaque-induced inflammation of the gingiva, which has been reported to be present among at least three quarters of the pregnant population 6. Such a condition would increase transient bacteremia. The upper respiratory tract infection might have weakened the mother’s immune system enough to provide a window of escape for the bacteria to colonize in the uterus. In the murine model described above, F. nucleatum was completely cleared from the maternal circulation after 24 hours of injection. However, once colonized in the immune privileged placenta, the bacteria proliferated quickly and caused fetal death within 3 days 4. This time frame coincides with the time the mother became ill prior to the fetal demise.
Pathologic findings in this case support the above scenario. Acute chorioamnionitis was stage 2, a pattern estimated to occur after approximately 12–24 hours of infection. The placenta was small for gestational age and had foci of laminar necrosis in the decidua, which may have allowed small numbers of decidual bacteria to grow more readily. There was an unusual amount of membrane acute deciduitis and a focus of acute decidutis in the decidua basalis. Both findings are unusual in ascending infections and the latter was a prominent feature of the hematogenous murine model described above. Autopsy findings suggest relatively recent and sudden death, possibly due to endotoxemia as evidenced by marked venous congestion, massive intraalveolar hemorrhage, and the lack of an inflammatory response to infection.
Periodontal disease has been recognized as a risk factor for adverse pregnancy outcome. However, the efficacy of periodontal treatment on birth outcome has been inconsistent 7, 8. Interestingly, the Lopez trial, which focused on treating pregnancy-associated gingivitis, reported that the incidence of preterm/low birth weight in the group receiving periodontal and maintenance therapies was reduced to 2.14% from 6.71% in the control group, with an odds ratio (OR) of 3.26. Following adjustment of several known risk factors, an OR of 2.76 (95% CI 1.29–5.88; P=0.008) was obtained. The authors concluded that gingivitis was an independent risk factor for preterm/low birth weight, a finding consistent with the case reported here 7. This case sheds light on patient management for those with pregnancy-associated gingivitis who also contract additional infections. Prophylactic antibiotic therapy may be considered in the presence of multiple infections to prevent prolonged bacteremia and potential hematogenous translocation of the oral bacteria into the uterus.
Acknowledgments
The authors thank Dr. Floyd Dewhirst for consultation on the use of the Human Oral Microbiome Database. Supported in part by grants from the National Institute of Dental and Craniofacial Research RO1 DE 14924, KO2 DE 16102, and R21 DE17165 to Y.W.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented at the Gordon Research Conference on Periodontal Diseases held on Aug 2–7, 2009, at the Colby-Sawyer College in New London, New Hampshire.
REFERENCES
- 1.Han YW, Ikegami A, Bissada NF, Herbst M, Redline RW, Ashmead GG. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J Clin Microbiol. 2006;44:1475–1483. doi: 10.1128/JCM.44.4.1475-1483.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 6.Arafat AH. Periodontal status during pregnancy. J Periodontol. 1974;45:641–643. doi: 10.1902/jop.1974.45.8.2.641. [DOI] [PubMed] [Google Scholar]
- 7.Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol. 2005;76:2144–2153. doi: 10.1902/jop.2005.76.11-S.2144. [DOI] [PubMed] [Google Scholar]
- 8.Michalowicz BS, Hodges JS, Diangelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]