Abstract
IL-15 and IL-15 receptor alpha (IL-15Rα) play a significant role in multiple aspects of T cell biology. However, given the evidence that IL-15Rα can present IL-15 in trans, the functional capacity of IL-15Rα expressed on CD8+ T cells to modify IL-15 functions in cis is currently unclear. In the current study we explore the functional consequences of IL-15Rα expression on T cells using a novel method to transfect naive CD8+ T cells. We observed that RNA nucleofection led to highly efficient, non-toxic, and rapid manipulation of protein expression levels in unstimulated CD8+ T cells. We found that transfection of unstimulated CD8+ T cells with IL-15Rα RNA led to enhanced viability of CD8+ T cells in response to IL-15. Transfection with IL-15Rα enhanced IL-15 mediated phosphorylation of Stat5 and also promoted IL-15-mediated proliferation in vivo of adoptively transferred naïve CD8+ T cells. We demonstrated that IL-15Rα can present IL-15 via cis-presentation on CD8+ T cells. Finally, we showed that transfection with a chimeric construct linking IL-15 to IL-15Rα cell autonomously enhances the viability and proliferation of primary CD8+ T cells and cytotoxic potential of antigen-specific CD8+ T cells. The clinical implications of the current study are discussed.
Keywords: T cell proliferation, IL-15, IL-15 receptor alpha, RNA nucleofection
Introduction
IL-15 is a cytokine that regulates many aspects of innate and adaptive immune cell development and function. The roles of IL-15 in the adaptive immune response include the co-stimulation, chemotaxis, survival, and proliferation of T cells [1-8]. IL-15 is especially important for the generation and proliferation of CD8+ T cells [9, 10]. IL-15 −/− (knockout) mice have reduced numbers of CD44lo CD8+ naïve phenotype T cells and CD44hi CD8+ memory phenotype T cells [6, 10]. These mice show impaired generation of antigen specific CD8+ cells and impaired CD8+ memory T cell homeostasis following viral challenge [11].
IL-15 stimulates T cells through the shared IL-15/IL-2 receptor beta and common gamma chains (β/γc). β/γc binding activates JAK1 and JAK3 which in turn induces phosphorylation of STAT3 and STAT5 transcription factors. JAK-STAT signaling via the β/γc complex is critical for T cell survival and proliferation [12]. IL-15 also binds the IL-15 receptor alpha chain (IL-15Rα, designated here as IL-15RA). IL-15RA contributes to IL-15 mediated effects such as survival and proliferation of T cells, and the phenotypes of IL-15−/− mice and IL-15RA−/− mice are nearly indistinguishable [6, 13-16]. In contrast to the moderate binding affinity of IL-15 by the β/γc complex, IL-15RA alone binds IL-15 with high affinity.
Because IL-15RA and the β/γc complex are all expressed on the surface of T cells, it was originally proposed that IL-15 signals by binding with high affinity to a heterotrimeric IL-15Rα/β/γc complex on T cells [13-16]. However, it was subsequently demonstrated that IL-15RA bound IL-15 could be found on the surface of multiple other cell types including activated monocytes. These monocyte-bound complexes are able to signal T cell proliferation in trans [17]. Many additional mouse studies have concluded that trans-presentation of IL-15 by IL-15RA on both hematopoietic and non-hematopoietic cell types is the dominant signaling mechanism for the survival and proliferation of memory T cells in vivo [18-23]. In these reports, IL-15RA expression by non-T cells was considered critical whereas T cell expression of IL-15RA was considered dispensable for memory T cell homeostasis.
IL-15 administration via recombinant protein, viral vector, or plasmid has been shown to enhance CD8+ T cell immune responses against a variety of pathogenic diseases and IL-15 modified vaccines are currently being evaluated in clinical trials (NCT00528489, NCT00115960). In addition, modification of tumor antigen specific CD8+ T cells with IL-15 represents one of the few promising candidates for the enhancement of adoptive cellular therapies of tumors [24]. IL-15RA is expressed on the surface of activated and memory CD8+ T cells. Given the intimate functional link between IL-15 and its high affinity receptor IL-15RA, it is natural to assume that expression of IL-15RA on the surface of CD8+ T cells would influence endogenous or adoptively transferred T cell responses to natural, recombinant, or transduced IL-15. However, because of the evidence that IL-15Rα can present IL-15 in trans, the functional capacity of IL-15Rα expressed on CD8+ T cells to modify IL-15 functions in a cis fashion is currently unclear.
The contribution of IL-15RA expression on T cells to other aspects of T cell function should also not be excluded [25]. Whereas soluble IL-15 is low to undetectable in the serum of healthy individuals, the level of soluble IL-15 in disease or infection can reach very high levels in local tissues. Up to 1200 ng/mL of IL-15 has been detected in synovial fluids from rheumatoid arthritis patients [26]. In addition, synovial fluid levels of IL-15 from patients were sufficient to contribute to migration and polarization of T cells. IELs from ceoliac patients, which stained higher for levels of IL-15RA expression than IELs from healthy patients, demonstrated increased proliferation, granzyme B and perforin dependent cytotoxicity, and IFN-γ and TNF-α production upon IL-15 stimulation [27]. The interaction of IL-15 with IL-15RA on T cells may also influence CTL avidity maturation. One study reported that IL-15RA expression is higher on vaccine derived high avidity CTLs, which correlates with their longer persistence in vivo compared to their lower avidity counterparts [28].
Both memory and naive CD8+ WT T cells exhibited enhanced survival in culture at low concentrations of IL-15 in comparison to IL-15RA knockout CD8+ T cells [4]. However in these and other similar studies, the possibility remains that trans-presentation either in vivo or in culture (i.e. T cell to T cell) was responsible for the indicated effects. A recent report provided structural and biochemical evidence that IL-15RA on a mast cell line can present IL-15 in cis in vitro [29]. While this and other studies using cell lines suggest that IL-15RA can function in cis in vitro, whether IL-15RA can function in cis on primary CD8+ T cells in vitro or in vivo is still unknown.
In the current study, we use primary unstimulated T cells to study the cis effects of IL-15RA expression in T cells. Primary unstimulated T cells provide an easily isolated, abundant, stable population of cells that are functionally responsive to IL-15. Because T cell activation, in addition to increasing IL-15RA expression levels, alters multiple other factors in T cells, the use of unstimulated T cells rather than the use of activated T cells or T cell lines allowed us to dissect out whether up-regulation of IL-15RA alone on T cells is sufficient to enhance IL-15 mediated effects. We therefore studied the effect of IL-15RA on CD8+ T cells by utilizing a novel method to transfect unstimulated murine T cells. We found that with RNA nucleofection, we consistently observed 80-95% transfection efficiency with negligible transfection toxicity of both purified unstimulated CD8+ cells from naïve mice. Using this method, we demonstrate the cis effects of IL-15RA and an IL-15/IL-15RA fusion on CD8+ T cell signaling, survival, and proliferation both in vitro and in vivo. We demonstrate a potential translational application of our findings in the functional modification of tumor antigen specific T cells with the IL-15/IL-15RA fusion construct for immunotherapy.
Results
Highly efficient, non-toxic, and rapid manipulation of protein expression levels in primary unstimulated CD8+ T cells using RNA nucleofection
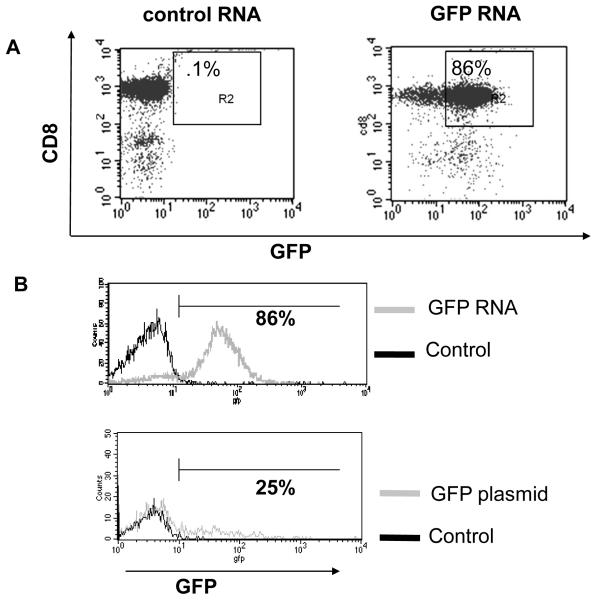
Plasmid transfection and viral transduction protocols require T cell stimulation or addition of cytokines to achieve high transfection efficiency and minimal transfection toxicity to unstimulated primary T cells. We therefore chose to use Amaxa™ nucleofection of in vitro transcribed RNA to study the role of IL-15RA in primary CD8+ T cells. In order to determine the possibility of this novel approach, we isolated unstimulated CD8+ T cells from the spleens of C57BL/6 mice and transfected them with in vitro transcribed RNA encoding various constructs. As shown in Figure 1A, Amaxa nucleofection of in vitro transcribed GFP RNA results in high transfection efficiency in CD8+ cells (86%). RNA nucleofection also results in high co-transfection efficiency (79%) (data not shown). Transfection efficiency obtained with GFP RNA was much higher than with GFP plasmid (86% vs. 25% respectively). In addition, RNA transfected cells maintained a nearly unimodal, tight expression pattern of GFP expression, which sharply contrasted the spread out GFP expression pattern in plasmid-transfected cells (Figure 1B).
Figure 1. Characterization of RNA nucleofection method used to transfect primary unstimulated CD8+ T cells.
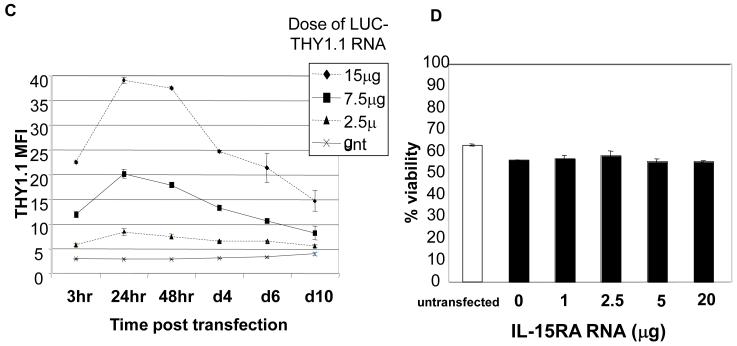
CD8+ T cells were isolated from naïve C57BL/6 mice. 1 × 106 CD8+ cells were transfected with indicated in vitro transcribed RNA or plasmid. A) Flow cytometry analysis of GFP expression at 24 h post transfection (with control RNA or GFP RNA) in CD8+ T cells. B) Histograms of GFP expression at 24 h post transfection in CD8+ T cells transfected with control RNA and GFP RNA (top panel) and CD8+ T cells transfected with control and GFP plasmid (bottom panel). C) Mean fluorescent intensity (MFI) of THY1.1 expression at different time points (mean ± SE) after transfection of CD8+ T cells with different doses of LUC-THY1.1 RNA. D) Percentage of viable CD8+ T cells at 24 h post transfection with different doses of IL-15RA RNA. Cell viability was determined with 7-AAD (mean ± SE). Data shown are of one representative experiment of two performed with transfections in triplicate.
We also noted that transfected gene expression is highly RNA dose dependent, is detectable by 3 hours, peaks at around 24 hours, and remains detectable up to 10 days post transfection (Figure 1C). Furthermore, Figure 1D shows that there was no significant difference in the percentage of viable CD8+ T cells either untransfected or transfected with increasing doses of IL-15RA RNA at 24 hours post transfection. In separate experiments, we observed similar viabilities at 24 hours post transfection with RNA encoding other genes (data not shown). This shows that the viability of the cells is not significantly affected by the RNA transfection. Taken together, our data indicate that RNA nucleofection can be employed to generate highly efficient, non-toxic, and rapid manipulation of protein expression levels in unstimulated primary T cells.
Transfection of primary unstimulated CD8+ T cells with IL-15RA RNA leads to enhanced viability of CD8+ T cells in response to IL-15
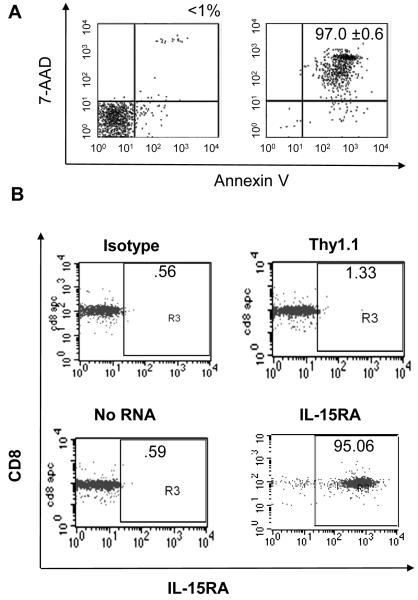
Purified unstimulated CD8+ T cells die by neglect within a few days of in vitro culture unless supplemented with growth factors such as IL-15 [4, 30]. We noted that within 4 days of in vitro culture >90% of unstimulated purified splenic CD8+ T cells undergo apoptotic or necrotic cell death as determined by annexin V and 7-AAD and staining. In our experiments, Annexin V staining was nearly completely overlapped by 7-AAD staining (Figure 2A), and we used 7-AAD staining to analyze total T cell death in future assays. In order to determine if over-expression of IL-15RA alone in unstimulated CD8+ T cells was sufficient to increase their survival to death by neglect, we isolated CD8+ T cells from the spleens of naïve C57BL/6 mice and transfected them with IL-15RA RNA, or THY1.1 RNA or no RNA as controls. As shown in Figure 2B, without additional in vitro stimulation, IL-15RA was undetectable by flow cytometry analysis in untransfected purified splenic CD8+ T cells. However, IL-15RA could be over expressed by nucleofection of IL-15RA RNA (Figure 2B). Furthermore, CD8+ T cells transfected with increasing doses of IL-15RA RNA showed correspondingly increased surface expression of IL-15RA (Supporting Information Figure 1A). We then plated IL-15RA RNA or control transfected CD8+ T cells in rIL-15 and assessed viability after 4 days of culture. We observed that there was a statistically significant increase in the percentage viability of CD8+ T cells transfected with IL-15RA RNA compared to no RNA or THY1.1 RNA transfected cells (Figures 2C and D). The extent of the survival response of CD8+ T cells to IL-15 appeared to be dependent on the level IL-15RA expressed on the surface of the T cell (Supporting Information Figure 1B). The effect was not dependent on endogenous IL-15RA since CD8+ T cells from IL-15RA KO mice yielded similar results (data not shown).
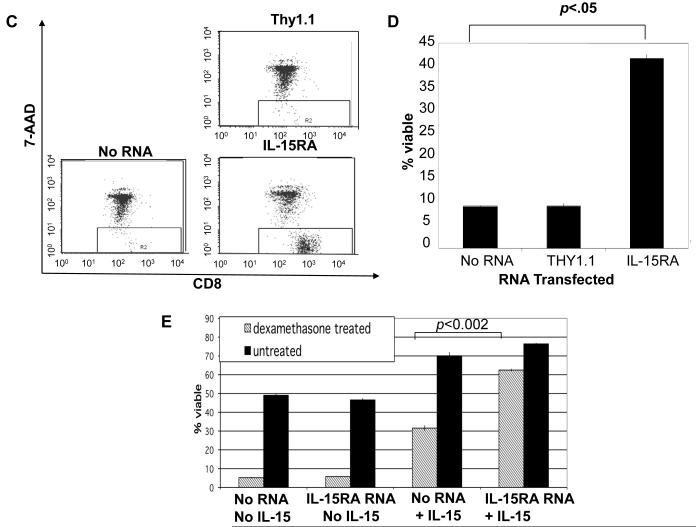
Figure 2. Effects of IL-15RA expression on the viability of primary unstimulated CD8+ T cells.
CD8+ T cells were isolated from naïve C57BL/6 mice. 1 × 106 cells were transfected with no RNA, THY1.1 or IL-15RA RNA. A) Representative flow cytometry data demonstrating 7-AAD and annexin V staining of CD8+ T cells prior to culture (left panel) and after 4 days of culture (right panel) in media alone (mean ± SE of triplicate cultures). B) Representative flow cytometry data of IL-15RA expression at 24 hours after RNA transfection. C) Representative flow cytometry data of CD8+ T cell viability following IL-15RA RNA-transfection or control RNA-transfection. The transfected cells were cultured for 4 days post transfection in the presence of 5 ng/mL IL-15 and then analyzed by 7-AAD staining. D) Percentage viability of CD8+ T cells cultured for 4 days post transfection in the presence of 5 ng/mL IL-15 (mean ± SE). The data shown here are representative of three experiments performed. P values were determined using the student’s t-test and were adjusted for 3 comparisons. E) Percentage viability of transfected CD8+ T cells treated with or without 10 uM dexamethasone in the presence or absence of 5 ng/mL IL-15 (mean ± SE). 16 hours after treatment with dexamethasone, cells were stained with CD8 and 7-AAD to determine viability. Data shown are representative of two experiments performed.
The robust correlation of RNA dose with level of surface expression allowed us to match the IL-15RA expression level in unstimulated T cells with the level found in memory phenotype or activated cells. transfection of 2.5 μg of RNA into unstimulated CD8+ T cells resulted in surface IL-15RA levels that were similar to or less than IL-15RA levels on T cells stimulated for 2 days with CD3, CD28, and IL-2 or levels on memory phenotype (CD44hi) T cells stimulated with IFN-γ and LPS for 3 days (Supporting Information Figure 1C). This dose of IL-15RA RNA that reflected physiologic levels of IL-15RA in activated and memory phenotype T cells significantly enhanced unstimulated T cell viability in response to IL-15 (Supporting Information Figure 1B and C).
IL-15 has been shown to preferentially influence CD44hi memory phenotype cells. Even after transfection of IL-15RA RNA and addition of IL-15 to culture, at the 5 ng/mL dose of IL-15, only <40% of IL-15RA transfected cells survive a 4 day culture. We therefore wondered if there was a preferential survival and resultant enrichment of CD44hi cells in the remaining population of transfected cells after 4 days culture with IL-15. Surprisingly, at the dose of IL-15 indicated, we did not observe a significant increase in the ratio of CD44hi cells versus CD44lo cells after transfection with IL-15RA and 4 days culture in the presence of IL-15 (Supporting Information Figure 2A). In contrast, we did observe an increase in the ratio of C44hi cells to CD44lo cells after culture in the presence of LPS and IFN-γ or high dose IL-15 (50 ng/mL) regardless of transfection with IL-15RA (data not shown). Thus IL-15RA expression enhanced both memory phenotype and naïve phenotype CD8+ T cell survival.
We next performed a short-term assay for CD8+ T cell survival using the glucocorticoid analogue dexamethasone to accelerate cell death. Despite shorter culture conditions of 16 hours, we observed that CD8+ T cells transfected with IL-15RA and treated with IL-15 demonstrate higher % viability in the presence of dexamethasone compared to similarly treated no RNA transfected cells (Figure 2E). We noted in this assay that in contrast to the longer term survival assay, in which cells required IL-15RA transfection for survival, at the same dose of IL-15 tested, IL-15 enhanced cell survival regardless of IL-15RA transfection. The difference in results between these two short and longer term survival assays may be explained by the constitutive presence of the intermediate affinity β/γc receptor and the known ability of IL-15RA to recycle and re-present IL-15 to the cell surface. Thus, because of the ability of the β/γc receptor to signal survival in the absence of IL-15RA, IL-15RA binding and recycling of IL-15 may prolong the effects of IL-15 in vitro, but is not essential for IL-15 mediated short-term survival.
In order to confirm that the enhancement of viability by over-expression of IL-15RA is specific to IL-15, we transfected CD8+ T cells with IL-15RA or IL-2RA and then treated the cells with IL-15 or IL-2. We observed that IL-15RA overexpression led to significant enhancement of IL-15-mediated survival, but not IL-2 mediated T cell survival. On the other hand, IL-2RA overexpression led to significant enhancement of IL-2-mediated survival, but not IL-15 mediated T cell survival (Supporting Information Figure 3). We confirmed by CFSE dilution analysis that the pro-survival effect observed was independent of proliferation. At the doses of IL-15 used in our survival assays (less than or equal to 5 ng/mL IL-15), there was no significant proliferation by day 4 in any of the transfected groups (data not shown). Taken together, our data indicate that overexpression of IL-15RA alone in CD8+ T cells is sufficient to enhance survival responses to IL-15 in vitro.
IL-15RA enhances phosphorylation of Stat5
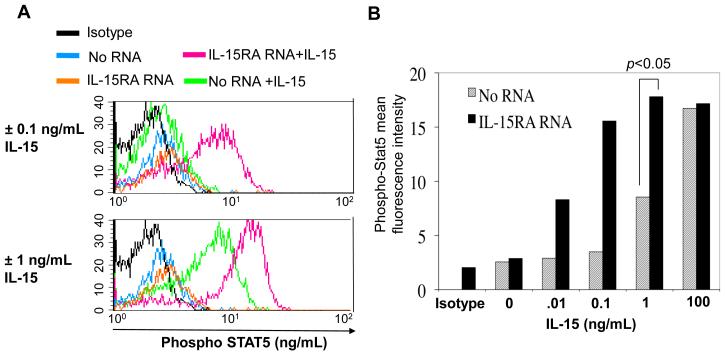
In order to determine the short-term downstream effects of IL-15 signaling, we studied the effect of IL-15RA expression on Stat5 phosphorylation. We transfected CD8+ T cells with IL-15RA RNA and stimulated them for 40 minutes with IL-15. We then stained the cells for phosphorylated Stat5 (phospho Stat5) and analyzed them by flow cytometry analysis. Figure 3A and B show that IL-15 stimulation increases the staining intensity of phosphorylated STAT5 in CD8+ T cells in a dose-dependant manner. Furthermore, IL-15RA transfected cells stained even more intensely for phosphorylated STAT5 compared to no RNA transfected cells in response to IL-15. Thus, our data suggest that over-expression of IL-15RA alone on unstimulated CD8+ T cells augments short term effects of IL-15 signaling, i.e. IL-15 mediated phosphorylation of Stat5.
Figure 3. Characterization of the effect of IL15RA expression on STAT5 phosphorylation.
CD8+ T cells were isolated from naïve C57BL/6 mice. 1 × 106 cells were transfected with no RNA, or IL-15RA RNA. 24 hours after transfection, CD8+ T cells were cultured with increasing doses of IL-15 for 40 minutes. Transfected cells that were not cultured with IL-15 were used as a control. Cells were stained for intracellular phospho-STAT5 and analyzed by flow cytometry. A) STAT5 phosphorylation in cells transfected with no RNA versus IL-15RA RNA treated with 0.1 (upper panel) or 1 ng/mL IL-15 (lower panel). B) Mean fluorescence intensity of phospho-STAT5 in IL15RA RNA or no RNA transfected cells treated with increasing doses of IL-15. The data represent three experiments performed at the 1 ng/mL dose with transfections and treatments in duplicate. P value for comparison at 1 ng/mL dose was determined using the student’s t-test.
IL-15RA presents IL-15 via cis-presentation on CD8+ T cells
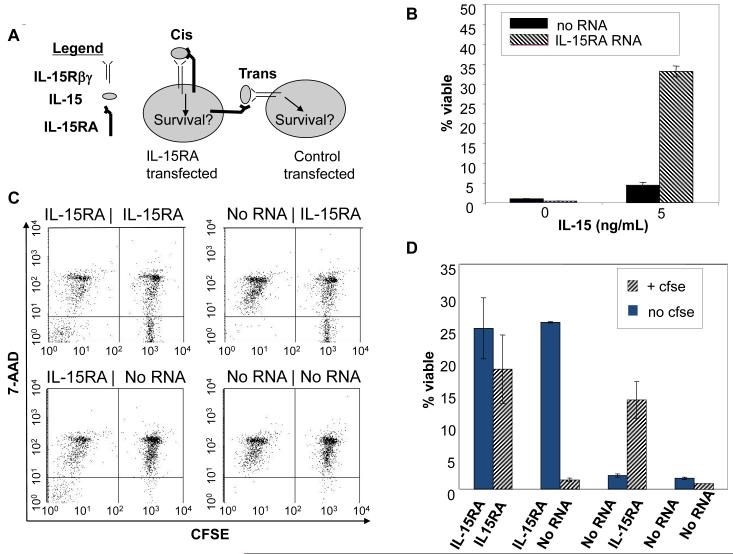
Two plausible modes of IL-15-mediated T cell signaling in T cell cultures are: cis-presentation by IL-15 via IL-15RA on transfected T cells, or trans-presentation of IL-15 to bystander T cells by IL-15RA on transfected T cells (Figure 4A). To confirm our hypothesis that IL-15RA can present IL-15 via cis-presentation on CD8+ T cells, it was necessary to rule out the possibility of trans-presentation occurring in our in vitro cultures. We thus transfected CD8+ T cells with IL-15RA RNA or no RNA and plated them in 6 well plates to minimize contact between the cells (Supporting Information Figure 4A). The T cells were then stimulated with IL-15 for 4 days. As shown in Figure 4B, in spite of no observable cell-cell contact, there was a significant increase in viability of CD8+ T cells transfected with IL-15RA RNA compared to the no RNA transfected cells. As a second confirmation to rule out trans-presentation, we performed a cell mixing experiment. Purified CD8+ T cells were differentially labeled with or without CFSE to distinguish IL-15RA RNA or no RNA transfected cells and mixed at a ratio of 1:1. These cells were then plated in 96 well plates with IL-15 and centrifuged briefly to maximize potential cell contacts (Supporting Information Figure 4B). As shown in Figures 4C and D, the control RNA transfected cells did not demonstrate a significant increase in viability in the presence of IL-15, when mixed with IL-15RA RNA transfected cells. The same results were observed when the labeling was swapped. We also observed similar results when the differentially labeled and transfected cells were mixed in the shorter dexamethasone survival assay (data not shown). Thus, our data indicate that IL-15RA can present IL-15 via cis-presentation on CD8+ T cells.
Figure 4. Characterization of the presentation of IL-15 by IL-15RA on CD8+ T cells.
A)Schematic diagram to demonstrate two possible modes (cis vs. trans) of IL-15 presentation by IL-15RA on CD8+ T cells in the presence of IL-15. B) Percentage of viable CD8+ T cells transfected with IL-15RA or no RNA (mean ± SE). CD8+ T cells were isolated from naïve C57BL/6 mice. 1 × 106 cells were transfected with IL-15RA or no RNA. 1 ×105 transfected cells were diluted in 6 well plates to minimize cell-cell contact and cultured ± IL-15 (5 ng/ml). Four days after transfection, cells were stained with 7-AAD and CD8α and analyzed by flow cytometry. C & D. Flow cytometry analysis for the viability of CFSE-labeled and -unlabeled CD8+ T cells. Splenocytes isolated from naïve C57BL/6 mice were labeled with or without CFSE. 1 × 106 labeled or unlabeled CD8+ T cells were transfected with either IL-15RA RNA or no RNA. Within 2 hours post transfection, CFSE labeled cells were mixed with unlabeled cells at a 1:1 ratio. The mixed cells were plated in 96-well U-shaped plates and centrifuged briefly to enhance cell-cell contact. The mixed cells were cultured ± IL-15 (0.5 ng/ml). Four days after transfection, cells were stained with 7-AAD and CD8α and analyzed by flow cytometry. C) Representative flow cytometry data of the viability of CFSE labeled and unlabeled CD8+ T cells as determined by 7-AAD staining. D) Percentage of viable CFSE labeled and unlabeled CD8+ T cells at 4 days post transfection (mean ± SE). Data shown are from one representative experiment of two performed.
Transfection with IL-15RA promotes IL-15-mediated proliferation of adoptively transferred CD8+ T cells
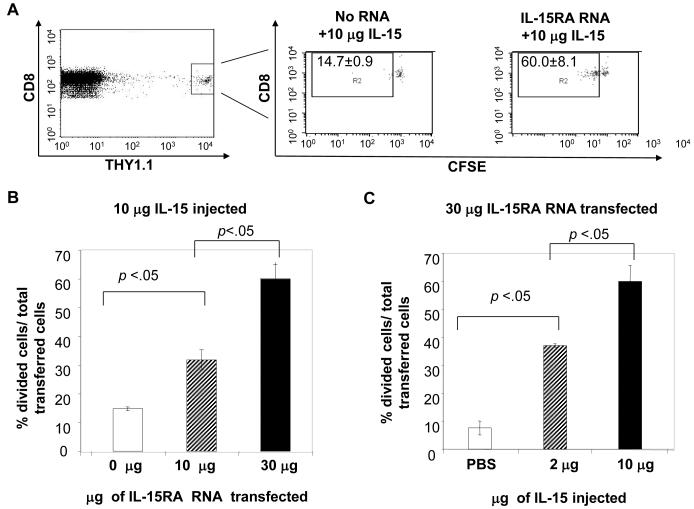
In order to determine if transfection with IL-15RA can promote IL-15-mediated proliferation of adoptively transferred unstimulated CD8+ T cells, we isolated CD8+ T cells from THY1.1 congenic mice and labeled them with CSFE. We transfected these cells with no RNA or varying doses of IL-15RA RNA and adoptively transferred them into THY1.2 mice. 24 hours later mice were injected i.p. with IL-15. 7 days later, splenocytes from the recipient mice were isolated and analyzed by flow cytometry staining. Transferred cells were distinguished from endogenous cells by first gating on CD8+ and 7-AAD negative cells to exclude dead cells and subsequently gating on THY1.1 and CD8+ cells. Figure 5A, far left panel is representative flow cytometry data demonstrating gating on Thy1.1+ and CD8+ cells (transfected cell population). To determine in vivo kinetics of IL-15RA expression on IL-15RA RNA transfected CD8+ T cells we stained cells for surface IL-15RA and analyzed IL-15RA surface expression on the transfected cell population. We observed that in the absence of injected IL-15, IL-15RA RNA transfected CD8+ T cells demonstrated significantly higher IL-15RA surface expression than no RNA transfected cells for at least 7 days after adoptive transfer (unpublished observations). We then analyzed the transfected cell population for proliferation by CFSE dilution. Figure 5A, right panels are representative flow cytometry data demonstrating CFSE dilution of the transfected cell population. The box in the upper left hand corner marks the population of cells that have undergone at least one cell division. As shown in Figure 5A, right panels and Figure 5B, IL-15RA transfected cells demonstrated significantly more proliferation than no RNA transfected cells in response to injected recombinant IL-15 and the amount of cells proliferating correlated with the amount of IL-15RA RNA transfected into the cell. As shown in Figure 5C, IL-15RA transfected cells also demonstrated a dose-dependent proliferation in the presence of increasing amounts of IL-15. We did not observe enhancement of proliferation in cells transfected with irrelevant RNA used as controls (data not shown). Thus, our data indicate that expression of IL-15RA on CD8+ T cells promotes the IL-15-mediated proliferation of adoptively transferred primary CD8+ T cells.
Figure 5. Characterization of the proliferation of adoptively transferred IL-15RA transfected CD8+ T cells in response to injected recombinant IL-15.
Splenocytes isolated from naïve C57BL/6 THY1.1 congenic mice were labeled with CFSE. CD8+ T cells were transfected with no RNA, 10 μg, or 30 μg IL-15RA RNA. 1 × 106 /mouse of the transfected cells were transferred i.v. into C57BL/6 THY1.2 mice (2 mice per group). 24 hours later, mice were injected with indicated doses of IL-15 or PBS. 7 days later, splenocytes were analyzed by flow cytometry for 7-AAD, CD8α, and THY1.1. expression. 7-AAD− gated cells and further gated on THY1.1+ and CD8+ (transfected cell population) and analyzed for CFSE dilution. A) Representative flow cytometry analyses of CFSE dilution of adoptively transferred IL-15RA transfected CD8+ T cells. The left panel demonstrates gating of THY1.1+, CD8+ cells. Right 2 panels demonstrate flow cytometry analyses of CFSE dilution of transferred cells transfected with different amounts of IL-15RA RNA. Box represents % of transferred cells that had undergone one or more divisions (means ± SD). B) Bar graph depicting the percentage of transfected cells that had undergone one or more divisions at day 7 after 10 μg IL-15 treatment at varying doses of IL-15RA RNA (mean ± SE). C) Bar graph depicting the percentage of cells transfected with 30 μg IL-15RA RNA, which had undergone one or more divisions at day 7 after treatment with varying doses of IL-15 (mean ± SE). Data shown are from one representative experiment of two performed. p-values adjusted for 2 comparisons determined by pairwise student’s t-test.
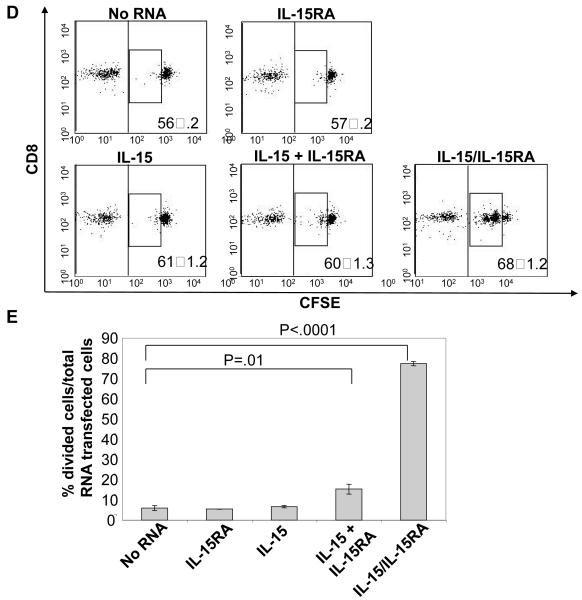
Transfection with IL-15 + IL-15RA or a chimeric construct linking IL-15 to IL-15RA autonomously enhances the viability and proliferation of CD8+ T cells
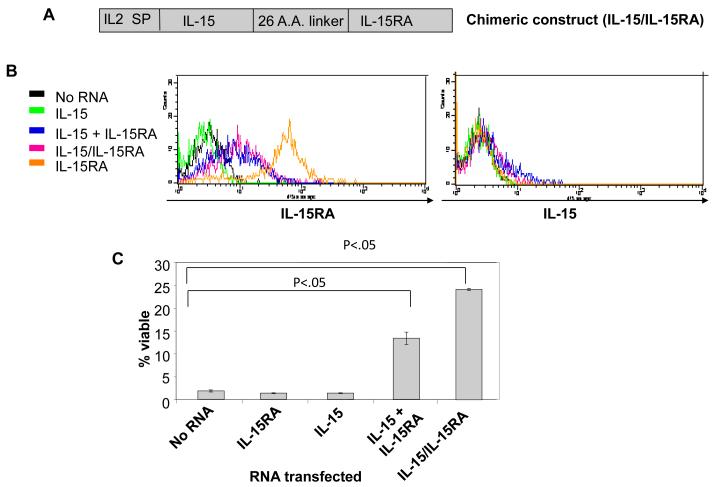
We wondered if IL-15RA expressed on the surface of CD8+ T cells would influence the functional response to IL-15 produced by the T cells themselves. Furthermore, to determine if direct interaction of IL-15 with T cells, in the absence of soluble IL-15 interaction with surrounding cells, is sufficient to drive in vivo proliferation, we generated an IL-15/IL-15RA fusion construct. This construct would prohibit the release of free IL-15 by keeping IL-15 permanently bound to IL-15RA on the surface of the CD8+ T cell and allow expression of IL-15 and IL-15RA from a single construct. We linked IL-15 to IL-15RA using a strategy similar to one previously described [31]. We also replaced the IL-15 signal peptide with the stronger IL-2 signal peptide [32], and called the construct IL-15/IL-15RA (Figure 6A). For comparison, the signal peptide of unlinked IL-15 was also replaced with the signal peptide for IL-2. As seen in Figure 6B, expression of IL-15RA was detected at similar levels on the surface of CD8+ T cells transfected with either IL-15 RNA+ IL-15RA RNA or IL-15/IL-15RA RNA. Surface expression of IL-15 was also detectable at comparable levels but the staining intensity was low compared to IL-15RA staining. The low level of staining is likely a result of interference of antibody binding to IL-15 by the IL-15RA interaction with IL-15 as has previously been suggested [33].
Figure 6. Effect of IL-15 + IL-15RA or a chimeric construct linking IL-15 to IL-15RA on viability and proliferation of transfected CD8+ T cells.
Splenocytes isolated from naïve C57BL/6 THY1.1 congenic mice were differentially labeled with or without CFSE. CD8+ cells were transfected with no RNA, IL-15RA, IL-15, IL-15 + IL-15RA, or IL-15/IL-15RA RNA. A) Schematic diagram of the chimeric IL-15/IL-15RA construct composed of IL-2 signal peptide fused to IL-15 and linked to IL-15RA via a 26 a.a. poly-proline linker. B) Representative flow cytometry data demonstrating IL-15RA (left) and IL-15 (right) surface expression level on CD8+ T cells at 24 hours after transfection. C) Viability in vitro of transfected CD8+ T cells cultured for 7 days post transfection in the absence of IL-15 (mean ± SE). Cells were stained with 7-AAD to determine viability. p-value adjusted for 2 comparisons determined by student’s t-test. D & E. 5 × 105 (per mouse) of transfected CFSE labeled CD8+ T were mixed with the reference population and transferred into THY1.2 mice as described in the Materials and Methods. 7 days later, lymph node cells were analyzed by flow cytometry for 7-AAD, CD8α, and THY1.1 expression. D) Representative flow cytometry data depicting the CFSE dilution of the 7-AAD− gated THY1.1+CD8+ T cells. Numbers represent %-transfected cells of the total transferred cells (mean ± SD). The middle box represents the population of cells that had undergone proliferation. E) Bar graph depicting the percentage of RNA transfected cells that had undergone at least one cell division, 7 days after transfer (mean ± SE). F) Representative flow cytometry data of CFSE dilution of gated THY1.1+CD8+ T cells (top panels) and percentage of CFSE labeled or unlabeled cells of the total gated THY1.1+CD8+ T transferred cells at 7 days after transfer (bottom graphs) (mean ± SE). 5 × 105 (per mouse) of untransfected CFSE labeled cells were mixed with CFSE unlabeled IL-15/IL-15RA transfected cells at a 1:1 ratio or CFSE labeling was swapped between the untransfected cells and the IL-15/IL-15RA transfected cells. Mixed cells were transferred i.v. into C57BL/6 THY1.2 mice (2 mice per group). 7 days later, splenocytes were analyzed by flow cytometry. Numbers represent % of total gated THY1.1+CD8+ T cells (transferred cells). The data shown here are representative of two experiments performed. p-values were determined by student’s t-test.
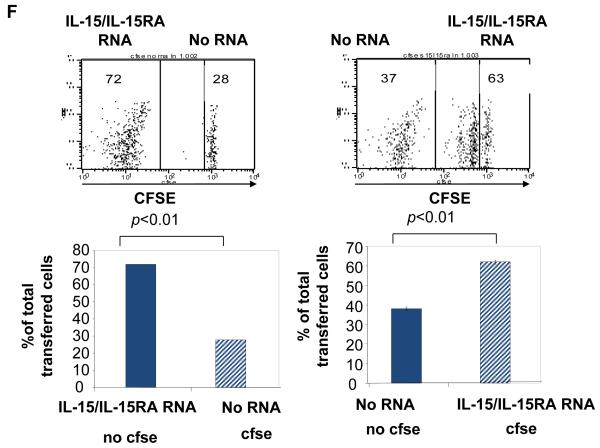
We found that transfection of primary unstimulated CD8+ T cells with either IL-15 RNA + IL-15RA RNA or IL-15/IL-15RA RNA induced significant levels of sustained STAT5 phosphorylation compared to IL-15RA RNA transfected cells (Supporting Information Figure 5). This combined with data from Figure 3 indicates that intracellular signaling generated by IL-15/IL-15RA fusion is similar to the signaling generated by normal IL-15. Furthermore, as shown in Figure 6C, transfection of CD8+ T cells with either IL-15 RNA+ IL-15RA RNA or IL-15/IL-15RA RNA enhanced T cell survival to death by neglect in 7 days of culture, whereas transfection of IL-15 RNA or IL-15RA RNA alone did not. At the same time, we also tested whether IL-15 RNA + IL-15RA RNA or IL-15/IL-15RA RNA transfection could induce proliferation in vivo of primary unstimulated CD8+ T cells. We mixed the CFSE labeled transfected CD8+ T cells with a CFSE unlabeled reference population at a ratio of 1.5:1 and injected each group into THY1.2 mice. 7 days later, in vivo injected cells were analyzed by flow cytometry for proliferation as previously described for Figure 5. Accumulation was determined by comparing the transfected cell population with the reference population. As shown in Figures 6D and 6E, we observed that transfection with IL-15 RNA + IL-15RA RNA or IL-15/IL-15RA RNA led to significant proliferation (Figure 6D middle boxes and 6E) of transferred CD8+ T cells compared to transfection with IL-15 RNA alone or IL-15RA RNA alone. Interestingly, the IL-15/IL-15RA fusion construct resulted in much more pronounced enhancement of proliferation (Figure 6D middle boxes and 6E) and accumulation (Figure 6D bottom right numbers) than IL-15 + IL-15RA. This dramatic increase in bioactivity of the IL-15/IL-15RA fusion construct compared to mixed and transfected IL-15 + IL-15RA is consistent with a previous report describing an IL-15/IL-15RA sushi domain recombinant fusion protein that exhibited a 10 fold increase in bioactivity in vitro compared to recombinant IL-15 mixed with recombinant IL15RA [31]. We further analyzed the level of CD44 expression on IL-15/IL-15RA transfected CD8+ cells 7 days after adoptive transfer. In contrast to our results comparing the in vitro survival of CD44hi cells versus CD44lo cells after transfection, we observed that CD44hi cells are seemingly enriched in the divided proportion of cells induced by transfection with IL-15/IL-15RA RNA (Supporting Information Figure 2B). This may be a result of preferential division of CD44hi cells or a result of induced differentiation of IL-15/IL-15RA transfected cells into CD44hi cells. Because cells manipulated via our RNA transfection procedure are not amenable to cell sorting, we have yet to establish which of these is the case.
We performed a dye swapping experiment similar to that performed in Figure 4 to rule out the possibility that T cell to T cell trans-presentation or trans-presentation by contaminating other cell types was playing a role in the proliferation we observed in vivo. As shown in Figure 6F, even when control transfected cells were mixed and co-injected in mice at a ratio of 1:1 with IL-15/IL-15RA transfected cells, we only observed significant proliferation and accumulation in the cells transfected with IL-15/IL-15RA. Thus, our data demonstrates that IL-15RA can present IL-15 in cis to drive cell autonomous CD8+ T cell proliferation in vivo.
Proliferation and IFN-γ response of E7 tumor antigen specific CD8+ T cells transfected with IL-15/IL-15RA
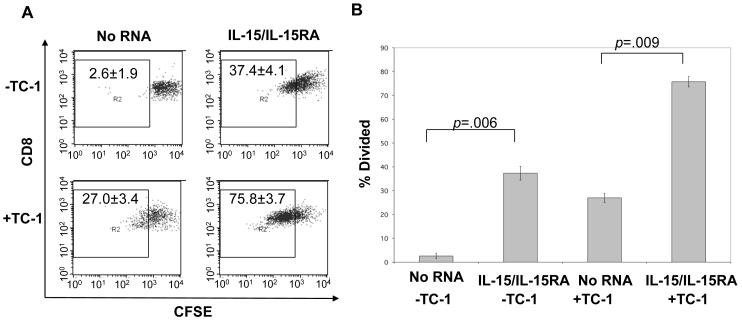
It has been previously shown that transduction of tumor antigen specific T cells with IL-15 is one of the few strategies that effectively enhances anti-tumor effects of adoptively transferred T cells [24]. To demonstrate a potential translational application of our findings, we transfected an E7 tumor antigen specific CD8+ T cell line with IL-15/IL-15RA RNA and analyzed their proliferation in the presence or absence of antigen stimulation in vitro. As shown in Figure 7A and 7B, E7 tumor antigen specific T cells transfected with IL-15/IL-15RA RNA demonstrated enhanced proliferation 3 days after transfection and culture in the presence or absence of antigen stimulation compared to no RNA transfected controls.
Figure 7. Characterization of the proliferation and IFN-γ production of IL-15/IL-15RA transfected E7 tumor antigen specific CD8+ T cells in the presence or absence of TC-1 tumors.
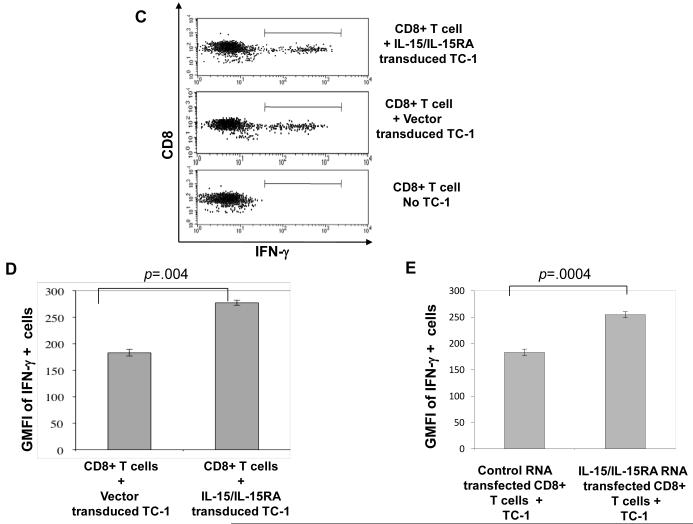
A&B) CFSE labeled T cells from an E7 specific T cell line were transfected with indicated RNA followed by stimulation with irradiated TC-1 tumor cells (5:1 T cell: tumor cell). 3 days later, cells were analyzed by flow cytometry for proliferation by CFSE dilution. A. Flow cytometry data representing CFSE dilution of no RNA or IL-15/IL-15RA RNA transfected E7 specific T cells in the presence or absence of TC-1 stimulation. Numbers represent % of CD8+ T cells that had undergone at least on CFSE dilution (mean ± SD). B. Percentage of E7-specific T cells that had undergone at least one CFSE dilution 3 days after transfection with no RNA or IL-15/IL-15RA RNA and culture ± TC-1 (mean ± SE). C-E) CD8+ T cells were isolated from mice vaccinated with E7 antigen as described in the Materials and Methods. 1 × 106 cells were transfected with control RNA or IL-15/IL-15RA RNA, cultured for 4 hours at a ratio of 5:1 with TC-1 tumor cells transduced with IL-15/IL-15RA or empty vector and analyzed by flow cytometry. C. Representative flow cytometry data demonstrating gating of intracellular IFN-γ+ cells in control RNA transfected CD8+ T cells either unstimulated or stimulated with empty vector or IL-15/IL-15RA transduced TC-1 tumor cells. D. MFI (mean ±SE) of IFN-γ staining in IFN-γ+ gated control RNA transfected CD8+ cells stimulated with empty vector or IL-15/IL-15RA transduced TC-1 tumor cells. E. MFI (mean ±SE) of IFN-γ staining in empty vector transduced TC-1 stimulated and IFN-γ+ gated CD8+ T cells transfected with control RNA or IL-15/IL-15RA RNA. The data shown here are representative of two experiments performed with transfections and stimulations performed in triplicate. p-values determined by student’s t-test.
In other experiments, we have transduced TC-1 tumor cells with retrovirus encoding IL-15/IL-15RA or empty vector and demonstrated that IL-15/IL-15RA transduced T cells express high levels of surface IL-15/IL-15RA expression. We have seen that IL-15/IL-15RA transduced TC-1 tumor cells enhance CD8+ T cell signaling, activation, and proliferation in trans [34]. Stimulation of CD8+ T cells with IL-15 has been shown to prime CD8+ T cells to produce higher levels or IFN-γ upon stimulation with antigen. We were interested in knowing whether IL-15/IL-15RA expression in tumor cells or T cells could enhance IFN-γ production in tumor antigen specific CD8+ T cells either in trans or in cis respectively. Because we have observed that our continually cultured E7 T cell line produces already very high levels of IFN-γ upon TC-1 stimulation, we reasoned that the use of ex-vivo T cells from E7 antigen vaccinated mice would provide a more physiologically relevant mixed population of IFN-γ producing CD8+ T cells. We therefore transfected ex-vivo CD8+ T cells from E7 antigen vaccinated mice with IL-15/IL-15RA RNA or control RNA. Following transfection, T cells were stimulated with vector transduced or IL-15/IL-15RA transduced TC-1 cells. As shown in Figure 7C and 7D, primary CD8+ T cells from E7 tumor antigen vaccinated mice, produced higher levels of IFN-γ on a per cell basis, after ex-vivo stimulation with IL-15/IL-15RA transduced TC-1 tumor cells compared to vector transduced TC-1 cells. As shown in Figure 7E, CD8+ T cells transfected with IL-15/IL-15RA RNA also demonstrated enhanced IFN-γ production following TC-1 tumor stimulation compared to T cells transfected with control RNA. Thus expression of IL-15/IL-15RA in either TC-1 tumor cells or antigen primed T cells enhances, in trans or in cis, antigen stimulated IFN-γ production in CD8+ T cells. Therefore RNA transfection of tumor antigen specific T cells with IL-15/IL-15RA RNA represents a potentially potent strategy for the modification of cells for adoptive T cell transfer therapy.
Discussion
In the current study, we addressed the functional effects of IL-15RA expression on primary unstimulated and tumor antigen specific CD8+ T cells. Our results suggest that IL-15RA expression on CD8+ T cells is sufficient to confer enhanced signaling, survival, and in vivo proliferative responses to IL-15 presented in cis. Furthermore, IL□15/IL□15RA expression alone promotes cell autonomous primary unstimulated CD8+ T cell survival and proliferation in vitro and in vivo and enhanced proliferation and production of IFN-γ in antigen specific T cells in vitro.
Using a novel method of primary unstimulated T cell manipulation, Amaxa nucleofection of RNA, we demonstrated the sufficiency of IL-15RA up-regulation in enhancing IL-15 mediated T cell survival and proliferation. Recently, others have also applied similar electroporation based methods to manipulate T cells [35, 36]. This approach allowed rapid manipulation of negatively selected (untouched) primary T cells that maintained high viability without post-transfection addition of cytokines—all necessary conditions for naïve or unstimulated T cell survival studies. We showed that the IL-15RA gene expression level dictates whether the CD8+ T cell will respond to IL-15 in a dose-dependent manner. In the past, in order to generate CD8+ T cells with different levels of IL-15RA expression for our study, we would have to perform laborious sorting or cloning of transduced or transfected cells, which is highly difficult to perform on primary unstimulated cells. To our knowledge we are the first to use simple transfection procedures to manipulate gene expression levels in primary unstimulated T cells for subsequent in vivo function analysis.
In our experiments, we demonstrated that IL-15RA expression on CD8+ T cells predominantly presented exogenous or cell autonomous IL-15 in cis to promote T cell survival and proliferation. A very recent compelling study has demonstrated that NK-DC interaction and synapse formation was important for NK cell survival. Interestingly, this study documented that IL-15RA expression on NK cells themselves, not on the trans-presenting cell, likely contributed to synapse formation and signaling [37]. This finding is consistent with our finding regarding IL-15RA expression on T cells and its contribution to T cell survival. Other groups have clearly demonstrated that IL-15RA expressed on cell types other than T cells, including dendritic cells and macrophages, present IL-15 in trans to T cells [18-23]. We also confirmed that bone-marrow-derived dendritic cells (BMDCs) were capable of presenting IL-15 to T cells in trans. We have performed trans-presentation cell mixing experiments using IL-15RA transfected BMDCs instead of T cells using the same transfection, culture, and assay conditions as used in our T cell-T cell trans-presentation assays. Our data showed that BMDCs mixed with untransfected T cells were able to enhance T cell survival in trans. Furthermore, transfection of either T cells or BMDC with IL-15RA resulted in similar levels of survival of T cells responding in cis or in trans in response to IL-15, although in the absence of IL-15 the baseline level of survival after 4 days culture in the presence of BMDC (including IL-15RA−/− BMDC) was higher than in the absence of BMDC (unpublished observations). Based on these data, the ability to trans-present IL-15 by IL-15RA may be cell type dependent. We can only speculate as to the reason we do not see trans-presentation of IL-15 by IL-15RA bearing T cells to T cells bearing the β/γc receptor. It is becoming increasingly appreciated that IL-15RA participates in an immunological synapse between two cells [37]. A synapse between 2 smaller, unstimulated T cells that potentially lack some of the membrane components necessary for a stable synapse may not occur as efficiently as a synapse between a T cell and a larger professional APC or tumor.
Our results give insight into the potential mechanisms of injected recombinant IL-15 or vaccine derived IL-15 in boosting T cell responses. For example, our data demonstrate that the level of IL-15RA on activated and memory T cells is sufficient to drive proliferation in response to injected IL-15. This is consistent with a previous study that reported IL-15RA+/+ T cells, adoptively transferred into IL-15RA−/− hosts, preferentially proliferated in response to injected recombinant IL-15 compared to endogenous IL-15RA−/− T cells [20]. We have used IL-15RA+/+ cells to further enhance IL-15RA expression in these cells to demonstrate that responses to IL-15 including STAT5 phosphorylation, survival, and proliferation are graded responses depending on the level of IL-15RA expression, and are not an all or nothing, i.e. IL-15RA plus or minus, phenomenon. Another important distinguishing characteristic between this and our experiments is the use of IL-15RA−/−versus IL-15RA+/+ recipient mice. Our studies demonstrate that the level of IL-15RA expression on the T cell influences in vivo proliferation to injected IL-15 even in the presence of IL-15RA+/+ cells capable of trans-presentation. It is important to note that IL-15RA expression and trans-presentation of IL-15 by surrounding cells may be contributing to the observed CD8+ T cell proliferation in response to recombinant IL-15. However, in the presence of soluble IL-15, further expression of IL-15RA on the CD8+ T cell itself becomes a distinguishing factor in a T cell’s ability to proliferate. Thus our results indicate that therapeutically administered IL-15 may preferentially affect CD8+ T cells according to the level of IL-15RA on the surface of the cells. This finding may offer predictive value in assessing potential individual responses to IL-15 based vaccines or therapies.
The co-administration of IL-15 with IL-15RA is a promising approach to enhance the in vivo effects of IL-15 [38, 39]. One study suggests that IL-15RA may mainly function to prolong IL-15 signaling by retaining IL-15 intracellularly and on the cell surface [40]. Our study demonstrates that in addition to prolonging IL-15 signals, IL-15RA influences early IL-15 mediated signals including STAT5 phosphorylation. Because IL-15RA does not itself activate STAT5 phosphorylation, we reason that IL-15RA on T cells enhances STAT5 phosphorylation in cis by binding and presenting IL-15 to the β/γc complex on the same cell. We predict that IL-15RA signaling in cis on T cells is β/γc dependent for 3 reasons; 1) the β/γc complex has been shown to be indispensable for IL-15 function, 2) we observed that transfection of IL-15RA with a nearly complete deletion of the cytoplasmic domain (32 A.A.) did not affect the ability of IL-15RA to enhance survival responses to IL-15 in vitro (unpublished observations), and 3) we have performed transfection experiments using an IL-15/IL-15RA construct that contains a single point mutation of an amino acid in IL-15 (designated IL-15 (Q108D)/IL-15RA) that is critical for IL-15 interaction with the γc chain and IL-15 bioactivity in vitro [29, 41]. We observed that despite similar levels of surface expression between IL-15/IL-15RA and IL-15(Q108D)/IL-15RA after transfection, IL-15/IL-15RA transfection significantly enhanced CD8+ T cell survival to culture in the absence of added cytokines while IL-15(Q108D)/IL-15RA transfection did not significantly enhance CD8+ T cell survival compared to controls (Supporting Information Figure 6). This indicates that abolishment the interaction of IL-15 with the β/γc complex on CD8+ T cells impairs the ability of IL-15 to signal in cis. We are currently performing further studies to more fully characterize this mutant construct.
In our study we showed that while transfection of IL-15 alone did not significantly influence T cell function, transfection of IL-15 + IL-15RA allowed T cells to survive and proliferate cell autonomously. In human T cells, IL-15 has been detected in both CD4+ and CD8+ cells by RT-PCR and flow cytometry. Interestingly, in the same study IL-15 was detected on the surface of central memory T (TCM) cells which, like our T cells transfected with IL-15 and IL-15RA, proliferated cell autonomously ex-vivo. Proliferation of TCM cells which expressed detectable surface IL-15 was higher than proliferation of the naïve T cells (TN) and effector memory T cell (TEM) subsets in which surface IL-15 was undetectable [42]. Thus, it is possible that T cell derived IL-15, in the presence of T cell expressed IL-15RA, contributes to T cell function in certain situations.
Since IL-15 has multiple immune enhancing functions on CD8 T cells [1, 4, 7, 28, 43], IL-15 represents one of the few promising candidates for modification of tumor antigen specific T cells for the use of adoptive cellular therapies [24]. Our data suggests that additional expression of IL-15RA in T cells modified by IL-15, or the expression of the IL-15/IL-15RA chimeric protein on T cells may further improve the anti-tumor effect of adoptively transferred T cells. We show evidence in support of this hypothesis using human papillomavirus type 16 (HPV-16) E7 tumor antigen-specific T cells. We found that the E7-specific T cells transfected with the IL-15/IL-15RA chimeric protein demonstrated significant proliferation in the presence of E7-expressing tumors in vitro compared to the mock-transfected controls. In addition, IL-15/IL-15RA transfected cells demonstrated enhanced IFN-γ production upon tumor stimulation compared to control transfected cells. One intriguing study has suggested the potential importance of IL-15RA expression on T cells used for adoptive transfer therapy. In this study, T cells that specifically demonstrated high levels of IL-15RA expression were treated with IL-15 prior to transfer into leukemia bearing mice. Treatment of T cells in this manner reversed their tolerance and demonstrated increased therapeutic benefit [44]. We have preliminary evidence that transduction of E7 antigen specific T cells with IL-15RA provides prolonged and high levels of IL-15RA expression in transduced T cells (unpublished observations). Because we have seen that IL-15RA transfected T cells preferentially respond to injected IL-15 in vivo, we imagine that we will be able to boost at will the response of such transduced adoptively transferred cells, without affecting bystander cells, via injection of recombinant IL-15.
In summary, our data suggest that IL-15RA can present IL-15 on T cells in cis to promote STAT5 phosphorylation, survival, and proliferation of primary unstimulated CD8+ T cells. Over-expression of IL-15RA alone in primary unstimulated CD8+ T cells confers enhanced IL-15 mediated proliferation in vivo. Furthermore, expression of IL-15 linked to IL-15RA in CD8+ T cells promotes cell autonomous survival and in vivo proliferation. We have provided evidence that this strategy may potentially be used for the modification of T cells for adoptive T cell immunotherapy.
Materials and Methods
Reagents
All antibodies were used according to manufacturer recommendations. The antibodies to CD8A, Thy1.1, CD3ε, CD28, CD44, IFN-γ, phospho-STAT5, SA-PE, and annexin V and 7-AAD were purchased from BD Biosciences, San Jose, CA. IL-15RA antibody was purchased from R & D Systems, Minneapolis, MN. Amaxa™ murine T cell transfection kits were purchased from Amaxa ™ Biosystems, Gaithersburg, MD. CFSE was purchased from Molecular Probes, Carlsbad, CA. mMessage mMachine T7 ultra In vitro transcription kits were purchased from Ambion, Inc., Austin, TX. Gel Extraction, RNeasy, and Endofree Plasmid Maxi purification kits were purchased from Qiagen, Inc., Valencia, CA. CD4+ and CD8+ negative purification kits were purchased from Miltenyi Biotec Inc., Auburn, CA. PORF-IL-15 was purchased from Invivogen, San Diego, CA. IL-2 and rhIL-15 was purchased from eBioscience, San Diego, CA. Dexamethasone was purchased from Sigma-Aldrich, St. Louis, MO. Sterile filtered FACS buffer was made with .2% BSA in DPBS, pH 7.4.
Mice
THY1.1, CD45.1, IL-15RA KO, and B6129SF2/J mice were purchased from The Jackson Laboratory, Bar Harbor, ME. The C57BL/6 mice were acquired from the National Cancer Institute, Bethesda, MD, USA. All animals were maintained under specific pathogen-free conditions, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Plasmids
GFP and Luciferase-ires-Thy1.1 cDNA have been previously described [45] and were sub-cloned into PCDNA3.1 using restriction enzyme cloning. IL-15RA was cloned from murine splenocyte cDNA into PCDNA3.1 as previously described [46]. PORF IL-15 was subcloned into PCDNA3.1. The signal peptide of IL-15 was replaced with IL-2 signal peptide using the long primer Bamh1 – 5′ ACC GGA TCC GCC GCC ACC ATG TAC AGC ATG CAG CTC GCA TCC TGT GTC ACA TTG ACA CTT GTG CTC CTT GTC AAC AGC AAC TGG ATA GAT GTA AGA. IL-15 was linked to IL-15RA using a strategy similar to one previously described linking human IL-15 to human IL-15 [31]. Recombinant PCR primers IL-15RA F 5′ CGG AGG TGG TGG TTC CGG TGG TGG TGG TAG TGG AGG TGG TAG TCT TCA GGG CAC CAC GTG TC and IL-15 R 5′ CAC CGG AAC CAC CAC CTC CGG AAC CGC CTC CAC CGG AAC ACC GCC GGA GGA CGT GTT GAT G were used, and the product was cloned into PCDNA3.1 using Bamh1/Ecor1 restriction sites. The cytoplasmic domain of IL-15RA was deleted using primer Ecor1 5′ ACA GAA TTC TTA CCT TGA TTT GAT GTA CCA GGC. Large-scale plasmid preps were purified using Qiagen Endo Free Plasmid Maxi kits.
mRNA production
In vitro transcription from restriction enzyme digested plasmid, subsequent DNAse digestion, and poly A tailing was performed using Ambion mMessage Machine T7 ultra in-vitro transcription kit (Austin, TX) following the manufacturer’s protocol. Each reaction was purified using Qiagen RNeasy RNA purification kit and RNA was eluted with dH20 to a final concentration of approximately 1 μg/mL. Eluted RNA was kept on ice during all subsequent procedures. RNA was quantified using a wavelength of 260 nm on a spectrophotometer and checked for quality of poly A tailed RNA by agarose gel electrophoresis. Resultant capped, poly A tailed mRNA was immediately frozen at −80°C for later use.
Primary T cell transfection
Preparation of mouse splenocytes generally followed the Amaxa protocol for splenocyte preparation. Briefly, mice were sacrificed and spleens removed and immediately placed in PBS with 5% FBS, ground using the back of a 10 ml syringe and passed through a 70 μm nylon mesh filter. Cells were spun at for 10-15 min. at 950 rpm in a Beckman-Coulter GH 3.8 rotor and resuspended in PBS with 5% FBS buffer for magnetic purification. Cells were purified using magnetic negative selection for CD8+ T cells following manufacturer protocols. T Cells were spun as above at R.T. for 12-15 min. RNA was prepared by adding no more than 15μl of RNA per transfection to a separate 1.5 ml eppendorf tubes for each transfection. Amaxa transfection reagents were prepared according to manufacturer directions. RNA was kept in Eppendorf tubes at room temperature (temperature is critical) until transfection. Pelleted cells were resuspended in nucleofection solution at 1-3×106 cells/100 μl/transfection. 100 μl of cells in nucleofection solution was added to the RNA in the eppendorf tube and rapidly mixed by pipetting. Immediately the cells were transferred to a cuvette and transfected using program x-01 (mouse T cell). Immediately after transfection, cells were removed from the cuvette according to manufacturers directions and each transfection was added to 1 mL supplied media/well in a 48 well plate. When duplicates or triplicates were done, the transfections were alternated between groups.
IL-15 Stimulation and dexamethasone treatment
At 2-3 hrs post transfection 100μl (1×105 cells) of transfected cells were removed and plated into a 96 well U-shaped plate or added to 5 ml media in a 6 well plate and where indicated, recombinant mIL-15 was added to the wells at doses indicated. In some cases transfected versus control transfected were mixed at time of re-plating. In some cases dexamethasone or vehicle was added to wells to a final concentration of 10−8 M at time of IL-15 stimulation. Cells were incubated at 37 °C in 5% CO2 incubator for 4 days. In case of in vivo injection, rIL-15 was suspended in PBS at 2 μg or 10 μg/100μl and 100μl was injected into mice i.p.
Cell staining, viability, and transfection efficiency
Cells were stained according to directions and antibody concentration given by supplier. Analysis was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry Systems, Mountain View, CA).
T cell Stimulation
For CD3 stimulation, 2 × 106 purified CD8+ T cells were added to 1 mL of media in 24 well plates that were pre-coated for 90 minutes with 5 μg/mL anti-CD3ε antibody. 1 μg/mL anti-CD28 and 100 U/mL IL-2 were added at time of stimulation. Cells were stimulated for 2 days before flow cytometry analysis. The E7 specific CD8+ T cell line was maintained as previously described [47]. Irradiated TC-1 cells were prepared as previously described [48]. For E7 specific T cell stimulation, E7 T cells from day 5 after the previous stimulation were labeled with CFSE. Cells were transfected as above. Following transfection, cells were mixed with irradiated TC-1 cells at a ratio of 5:1 and cultured in 96 well plates for 3 days prior to analysis. High numbers of ex-vivo CD8+ T cells from E7 vaccinated mice were generated from C57BL/6 mice via a gene gun prime vaccinia boost regimen as has previously been described [49]. Briefly, mice were vaccinated intradermally via gene gun with DNA vaccine, Sig/E7/LAMP-1. Mice were boosted one week later with intraperitoneal injection of SigE7/LAMP-1-expressing vaccinia. 7 days after the last vaccination, CD8+ T cells were isolated from the spleens of vaccinated mice. Following transfection with RNA, E7 specific T cells were mixed at a ratio of 5:1 with TC-1 tumor cells transduced with either empty vector or IL-15/IL-15RA. Cultures were centrifuged briefly and incubated for 4 hours in the presence of golgi plug. Following incubation, cells were stained for intracellular IFN-γ as has been previously described [49].
CFSE labeling and adoptive transfers
Splenocytes were labeled with CFSE before magnetic purification as previously described. [50] Labeled or unlabeled cells were transfected as described above. 3-4 hours after transfection, media was gently removed from the top of the cells. 1 mL of HBSS was added to each well and cells were transferred to 15 mL tubes containing 10 mL of HBSS. Cells were centrifuged at 950 rpm for 15 minutes at 4 °C. Cells were brought to a concentration of 1×106 cells/100μl in HBSS and 100μl/mouse was injected intravenously into tail veins of recipient mice. In the case where a reference population was used, cells were mixed 1:1 or 1.5:1, depending on the experiment, with the reference population, and brought to a final concentration of 1×106 cells/100μl before i.v. injection.
Statistical analysis
Statistics were performed in most cases using a one-sided student T test. In some cases where indicated, a two-sided student T test was used. All p values shown are unadjusted p values. Statistical significance was determined at α= 0.05 after bonferroni adjustment for the number of statistical comparisons made.
Supplementary Material
Acknowledgements
This work was supported by the National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
References
- 1.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seder RA. High-dose IL-2 and IL-15 enhance the in vitro priming of naive CD4+ T cells for IFN-gamma but have differential effects on priming for IL-4. J Immunol. 1996;156:2413–2422. [PubMed] [Google Scholar]
- 3.Ye W, Young JD, Liu CC. Interleukin-15 induces the expression of mRNAs of cytolytic mediators and augments cytotoxic activities in primary murine lymphocytes. Cell Immunol. 1996;174:54–62. doi: 10.1006/cimm.1996.0293. [DOI] [PubMed] [Google Scholar]
- 4.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 5.Demirci G, Li XC. IL-2 and IL-15 exhibit opposing effects on Fas mediated apoptosis. Cell Mol Immunol. 2004;1:123–128. [PubMed] [Google Scholar]
- 6.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 7.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambricki E, Shigeoka A, Kishimoto H, Sprent J, Burakoff S, Carpenter C, Milford E, McKay D. Signaling T-cell survival and death by IL-2 and IL-15. Am J Transplant. 2005;5:2623–2631. doi: 10.1111/j.1600-6143.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 12.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland NG, Gilbert DJ, Jenkins NA, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 16.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. Embo J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 18.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 22.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluns KS, Stoklasek T, Lefrancois L. The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both? Int J Biochem Cell Biol. 2005;37:1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 26.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 27.Di Sabatino A, Ciccocioppo R, Cupelli F, Cinque B, Millimaggi D, Clarkson MM, Paulli M, Cifone MG, Corazza GR. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–477. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S. Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007;282:37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 30.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 32.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 33.Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, Reiling N, Bulfone-Paus S. Soluble Interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- 34.Rowley J, Monie A, Hung C-F, Wu T-C. Inhibition of tumor growth by NK1.1+ cells and CD8+ T cells activated by IL-15 through receptor beta/common gamma signaling in trans. Journal of Immunology. 2008 doi: 10.4049/jimmunol.181.12.8237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaft N, Dorrie J, Muller I, Beck V, Baumann S, Schunder T, Kampgen E, Schuler G. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol Immunother. 2006;55:1132–1141. doi: 10.1007/s00262-005-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brilot F, Strowig T, Roberts SM, Arrey F, Munz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest. 2007;117:3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettit DK, Bonnert TP, Eisenman J, Srinivasan S, Paxton R, Beers C, Lynch D, Miller B, Yost J, Grabstein KH, Gombotz WR. Structure-function studies of interleukin 15 using site-specific mutagenesis, polyethylene glycol conjugation, and homology modeling. J Biol Chem. 1997;272:2312–2318. doi: 10.1074/jbc.272.4.2312. [DOI] [PubMed] [Google Scholar]
- 42.Miranda-Carus ME, Benito-Miguel M, Llamas MA, Balsa A, Martin-Mola E. Human T cells constitutively express IL-15 that promotes ex vivo T cell homeostatic proliferation through autocrine/juxtacrine loops. J Immunol. 2005;175:3656–3662. doi: 10.4049/jimmunol.175.6.3656. [DOI] [PubMed] [Google Scholar]
- 43.Dooms H, Van Belle T, Desmedt M, Rottiers P, Grooten J. Interleukin-15 redirects the outcome of a tolerizing T-cell stimulus from apoptosis to anergy. Blood. 2000;96:1006–1012. [PubMed] [Google Scholar]
- 44.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 45.Kim D, Hung CF, Wu TC. Monitoring the trafficking of adoptively transferred antigen-specific CD8-positive T cells in vivo, using noninvasive luminescence imaging. Hum Gene Ther. 2007;18:575–588. doi: 10.1089/hum.2007.038. [DOI] [PubMed] [Google Scholar]
- 46.Bulfone-Pau SS, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. Faseb J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 47.Ji H, Wang TL, Chen CH, Pai SI, Hung CF, Lin KY, Kurman RJ, Pardoll DM, Wu TC. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 48.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 49.Chen CH, Wang TL, Hung CF, Pardoll DM, Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–2022. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 50.Parish CR, W. HS. Unit 4.9 Use of the Intracellular Fluorescent Dye CFSE to monitor Lymphocyte Migration and Proliferation. In: Coligan JE, K. AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons, Inc.; 2002. a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.