Abstract
Background: Previous studies reported an association between weak habituation of skin conductance orienting response and psychosis proneness. The aim of this study was to investigate the relationship among neuregulin 1 (NRG1)–stimulated AKT phosphorylation (a putative marker of psychosis), orienting response habituation, delusional ideas, anxiety, and depression in nonclinical individuals. Methods: One hundred twenty individuals participated in the skin conductance measurements. Weak and strong habituators were compared on measures of NRG1-stimulated AKT phosphorylation in B lymphoblasts, delusional ideas, anxiety, and depression. The predictors of delusional ideas were determined by multiple regression analysis. Results: Weak habituators displayed higher levels of delusional ideas/anxiety and a lower ratio of phosphorylated AKT as compared with strong habituators. There were 3 significant predictors of delusional ideas: decreased habituation, NRG1-induced AKT phosphorylation, and anxiety. Age, gender, education, IQ, and depression did not predict delusional ideas. Conclusions: These results suggest that decreased habituation of arousal, NRG1-induced AKT phosphorylation, and anxiety are related to delusional ideation in the general population.
Keywords: neuregulin 1, AKT, psychosis proneness, delusional ideation, skin conductance, orienting response
Introduction
There is epidemiological evidence that psychosis-like symptoms are more common in the nonclinical population than previously believed, which may indicate an etiological continuity in psychotic symptoms.1 At the physiological level, psychosis vulnerability may be associated with the decreased adaptation of autonomic arousal. Allen et al2 provided physiological evidence for the hypothesis of psychosis continuum by examining the relationship between the adaptation of the skin conductance orienting response (autonomic arousal) and delusional ideation in nonclinical individuals. During this experiment, participants were presented with a series of auditory stimuli parallel with the measurement of the electrodermal orienting response. The orienting response was gradually reduced with stimulus repetition, which reflects the adaptation of autonomic arousal. Allen et al2 demonstrated that individuals who showed less adaptation (weak habituators) achieved higher scores on a scale of delusional ideation (especially for conviction and preoccupation), which may be a marker of psychosis proneness. This finding is similar to that found in schizophrenia-spectrum disorders. Patients with schizophrenia who experienced hallucinations and delusions3,4 and individuals with schizotypal personality disorder5,6 displayed a similarly weak adaptation of the orienting response, which supports the hypothesis of psychosis continuum. Finally, Hazlett et al7 demonstrated abnormal electrodermal activity in the prodromal phase of psychosis (for reviews of early studies on electrodermal activity and psychosis vulnerability and schizotypy, see Ohman8 and Gruzelier and Raine9).
One of the most difficult challenges is to understand the neurobiological bases of psychosis continuum and to explore its relationship with physiological parameters (eg, the orienting response), molecular-cellular features, and subjective experiences such as delusional ideation and emotional symptoms. The crucial issue is that a valid peripheral marker of psychosis is still missing. However, the data of Sei et al10 on neuregulin 1 (NRG1)–inducted cell migration and AKT phosphorylation in peripheral B lymphoblasts of patients with schizophrenia are promising. NRG1 and its receptor ErbB4 play an important role in neurodevelopment and synaptic plasticity and may be implicated in psychotic disorders.11,12 NRG1 activates the Ras-MAPK and phosphatidylinositol-3 kinase–protein kinase B (PI3K–PKB/AKT) intracellular signaling pathways through ErbB receptors.13 This pathway may be important in the regulation of cell migration and cell adhesion, which are potentially implicated in the pathophysiology of schizophrenia.10,14
Genetic studies indicated that various polymorphisms of the NRG1 gene are associated with schizophrenia and bipolar disorder, although the evidence is not conclusive.15,16 Postmortem studies have revealed altered expression of different isoforms of NRG1 in the brain of patients with schizophrenia, but these alterations are not consistently linked to functional polymorphisms.17 Some of these genetic variants have been linked to decreased brain activation during cognitive tasks and to increased risk of psychosis conversion in people who displayed subclinical psychosis-like symptoms (“at-risk” mental state).18,19 Sei et al10 demonstrated that NRG1-induced migration of B lymphoblasts of patients with schizophrenia is significantly decreased compared with control individuals. This impaired migration was related to reduced NRG1-stimulated AKT phosphorylation in the patients and was associated with polymorphisms of the NRG1 and catechol-O-methyltransferase (COMT) genes and with an epistatic interaction of these genes.10 The robust reduction of NRG1-induced AKT phosphorylation in the patients is a promising peripheral marker of psychosis, especially because AKT-related signaling pathways have been implicated in the pathophysiology of schizophrenia.20
In this study, we measured delusional ideation, emotional symptoms (depression and anxiety), habituation of autonomic arousal, and NRG1-induced AKT phosphorylation in nonclinical individuals. We hypothesized that delusional ideation is associated with weaker habituation of arousal and decreased AKT phosphorylation.
Methods
Participants
One hundred twenty volunteers, aged between 18 and 45 years, with negative history for psychiatric and neurological disorders participated in the study. They were recruited via internet community networks or were members of the hospital staff and their acquaintances. All participants were screened for psychiatric disorders using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) Axis I disorders (SCID-CV).21 Individuals with psychiatric disorders, including psychoactive substance misuse, were excluded from the study. General intellectual functions were assessed by the revised version of the Wechsler Adult Intelligence Scale (WAIS-R).22 All participants gave written informed consent, and the study was approved by the local ethics board.
Assessment of Delusional Ideation and Emotional Symptoms
Delusional ideas were measured using the Peters et al. Delusion Inventory (PDI),23 which is a self-report instrument consisting of modified items from the Present State Examination.24 The PDI assesses delusional conviction, preoccupation, and distress. The original scale consists of 40 items, which are divided into 8 categories: (1) delusions of control; (2) misinterpretations, misidentification, and delusions of reference; (3) delusions of persecution; (4) expansive delusions; (5) delusions concerning various types of influence and primary delusions; (6) other delusions (delusions related to body image and smell); (7) simple delusions based on guilt, depersonalization, hypochondriasis; (8) thought reading, insertion, echo, broadcast. First, the participant was asked a yes-no question (“Do you ever feel as if people seem to drop hints about you or say things with a double meaning?” or “Do you ever feel as if electrical devices such as computers can influence the way you think?”). Participants were asked to fill the conviction, preoccupation, and distress scales only for the statements that they endorsed (“yes” response). In the case of a “no” response, they were asked to proceed to the next question without filling in the scales, and a score of 0 was given. Each dimension was represented by a 5-point Likert scale (from “Not at all distressing” [point 1] to “Very distressing” [point 5] for distress; from “Hardly ever think about it” [point 1] to “Think about it all the time” [point 5] for preoccupation; and from “Don't believe it's true” [point 1] to “Believe it is absolutely true” [point 5] for conviction). In the present study, the 21-item version of the PDI was used25. The Cronbach α was .84, indicating a good internal consistency. The dependent measure was the total PDI score, which was the sum of the conviction, preoccupation, and distress subscales.
Anxiety and depression were measured by the Beck Anxiety Inventory (BAI)26 and by the Beck Depression Inventory (BDI)27, respectively.
Orienting Response
To measure the orienting response, the classic method of Venables and Christie28 was implemented in a modified version. An in-house made instrument was used that was linked to a HP workstation. Silver/silver chloride electrodes were placed on the index and middle fingers of the dominant hand of the participants. The duration of baseline recording without any stimulus was 5 minutes. After the baseline recording, the stimulus presentation began. Stimuli were 10 consecutive tones presented binaurally through headphones (80 dB, 800 Hz). The interstimulus interval varied between 40 and 80 seconds. The skin conductance response was recorded during the baseline period and during the orienting response to the tones. The orienting response was measured in a latency window of 0–5 seconds after stimulus offset. The amplitude threshold was 0.05 μS. Nonrespondents were excluded from the experiment (12 volunteers not included in the sample description).
To define individuals with weak and strong adaptation, the habituation index was calculated for each participant. To obtain the index, the orienting response amplitude of the third trial was subtracted from the amplitude of the first trial, given that habituation is the most pronounced during the first 3 trials.6 Positive values of the index indicate normal (strong) habituation, whereas 0 or negative values indicate weak habituation.2 Participants with positive values were included in the strong habituation group, and participants with a negative or 0 value were included in the weak habituation group. The habituation index was rounded to 2 decimal places. For example, a habituation index of 0.03 would have been rounded to 0 and included in the low habituation group2.
NRG1-Induced AKT Phosphorylation
We adopted the protocol of Sei et al.10 B lymphocytes in the mononuclear cell preparation were transformed by infection with Epstein-Barr virus and were grown and maintained as described by Pressman and Rotter29 and Sei et al.10 B lymphoblasts were stimulated with NRG1a (296-HR, 65 amino acid residue recombinant protein from the epidermal growth factor domain of NRG1a [177–241]; R&D system Inc, Minneapolis, MN; 200 ng/ml) for 30 minutes. Protein isolated from the cells was analyzed by Western blotting. To quantify the level of phosphorylation, the immunoblots were stained with antibodies specific to phosphorylated AKT (pAKT)1 at Ser473 (60 kDa) and were then reprobed with total AKT (both antibodies were purchased from Upstate, Charlottesville, VA; 1 μg/ml). For the immunoblot analysis, cells were disrupted in Bioforce homogenizers (buffer: 250 mM Tris–HCl, 100 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% deoxycholic acid, 1 mM p-nitrophenylguanidinobenzoate, and protease inhibitor cocktail I and II [Sigma, Saint Louis, MO]). The homogenized material was incubated for 20 minutes at 41 °C, and then was centrifugated (14000 g for 15 min) to obtain supernatants for further analysis. Following the collection of supernatants, the protein samples were separated (sodium dodecyl sulfate-polyacrylamide gel electrophoresis on Tris-glycine gel [Invitrogen, Carlsband, CA]), transferred to polyvinylidene difluoride membrane (Millipore Corp, Bedford, MA), and then probed with the primary antibodies. The primary antibodies were detected using horseradish peroxidase–conjugated anti-rabbit IgG antibodies (Promega, Madison, WI), which was followed by enhanced chemiluminescence detection (Amersham Biosciences, Buckinghamshire, UK). The protein bands within the linear range of the standard curve were imaged. The relative optical density was measured using National Institutes of Health Image software. The dependent measure was the ratio of pAKT and total AKT at baseline (without NRG1 stimulation) and after NRG1 stimulation (pAKT/AKT). The phosphorylated form/total ratio was calculated after a quantitative densitometric analysis of each band within a linear range.10
Data Analysis
The STATISTICA 7.0 software was used for data analysis (StatSoft Inc, Tulsa, OK). All data were checked for the normality of distribution using Kolmogorov-Smirnov tests. In the first analysis, delusional ideation (PDI), emotional symptoms (BAI, BDI), IQ (WAIS-R), and demographic parameters were compared with a series of independent 2-tailed t tests in individuals with strong and weak orienting response habituation. These analyses were corrected for multiple comparisons using the Bonferroni method (the α value divided by the number of comparisons). In the second part of the analysis, we investigated pAKT/AKT using an analysis of variance (ANOVA), in which group (strong vs weak habituators) was the between-subject factor and stimulation condition (baseline vs NRG1 stimulation) was the within-subject factor. Scheffé’s tests were used for post hoc comparisons. In the third part of analysis, we investigated the factors that predicted the level of delusional ideation. In this forward stepwise linear regression analysis, the dependent variable was the PDI score, and the independent (predictor) variables were age, education, gender, IQ, BAI, BDI, and pAKT/AKT (baseline and NRG1 stimulated). The level of significance was set at α < .05.
Results
Demographic Parameters, Inventory Scores, and Skin Conductance Habituation
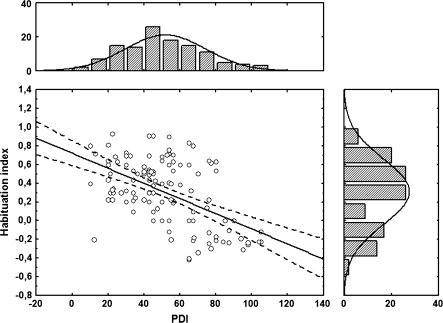
The demographic data and results from the inventories are depicted in table 1. The distribution of the habituation index and the PDI scores are shown in figure 1. The mean habituation index in the strong habituator group was 0.48 μS (SD = 0.22, trial 1: 0.60 μS, trial 3: 0.12 μS), whereas in the weak habituator group this value was −0.15 μS (SD = 0.13, trial 1: 0.42 μS, trial 3: 0.57 μS). In the whole sample, the mean total PDI score was 51.6 (SD = 22.3), which is similar to the published norms.25 Strong and weak habituators did not differ in age, gender, education, IQ, and BDI scores. However, weak habituators displayed higher scores on BAI and PDI compared with strong habituators, suggesting more pronounced anxiety and paranoid ideation in weak habituators (table 1).
Table 1.
Demographical Parameters of Weak and Strong Habituators
| Weak Habituators (n = 33) | Strong Habituators (n = 87) | |
| Gender (male/female) | 13/20 | 45/42 |
| Age (y) | 30.4 (7.4) | 29.3 (6.5) |
| Education (y) | 11.4 (3.6) | 11.9 (3.2) |
| IQ | 109.9 (15.1) | 110.8 (11.0) |
| BDI | 7.2 (3.1) | 6.4 (3.3) |
| BAI* | 10.0 (5.2) | 6.7 (3.7) |
| PDI** | 74.3 (19.0) | 43.6 (16.2) |
Note: Data are mean (SD) with the exception of gender distribution. Means were compared with 2-tailed t tests; gender distributions were compared with a χ2 test. IQ was measured by the revised version of the Wechsler Adult Intelligence Scale. BDI, Beck Depression Inventory, BAI, Beck Anxiety Inventory, PDI, Peters et al. Delusion Inventory.
*t118 = 3.88, P < .001; **t118 = 8.83, P < .0001.
Fig. 1.
The central correlation graph shows the relationship between the Peters et al. Delusion Inventory (PDI) scores and the habituation index. The upper panel of the figure shows the distribution (number of observed cases) of the PDI scores, which are depicted on the horizontal axis of the central correlation graph. The right-side panel shows the distribution of the habituation index, which is depicted on the vertical axis of the central correlation graph.
pAKT/AKT Ratio in Strong and Weak Habituators
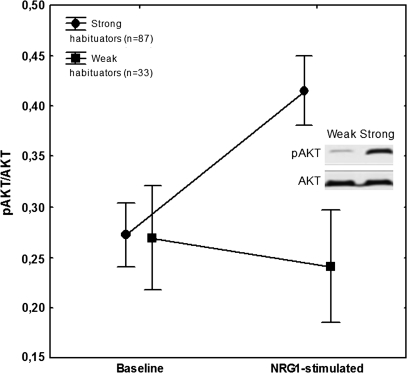
The ANOVA conducted on the pAKT/AKT revealed significant main effects of group (strong vs weak habituators) (F1,118 = 9.93, P < .01) and condition (baseline vs NRG1 stimulated) (F1,118 = 14.94, P < .001). The interaction between group and condition was significant (F1,118 = 32.96, P < .0001). Scheffé’s test indicated that the 2 groups did not differ in the baseline condition (P = .9). In contrast, in the NRG1-stimulated condition, the weak habituators had significantly lower pAKT/AKT ratio compared with that of the strong habituators (P < .0001). In strong habituators, NRG1 significantly increased the pAKT/AKT ratio (P < .0001), whereas in weak habituators this effect was absent (P = .7) (figure 2).
Fig. 2.
Mean phosphorylated AKT (pAKT)/AKT ratios at baseline and after neuregulin 1 (NRG1) stimulation in weak and strong habituators. Error bars indicate 95% confidence intervals. The panel illustrates results from the immunoblot analysis showing AKT and pAKT bands from the weak and strong habituator groups after stimulation.
Predictors of Delusional Ideation
The forward stepwise multiple regression analysis revealed that the habituation index was the best predictor of PDI scores (F1,118 = 49.57, P < .0001, R2 change = 0.30) (Figure 1). The second was NRG1-stimulated pAKT/AKT ratio (F1,118 = 24.68, P < .0001, R2 change = 0.12), and the third was BAI scores (F1,118 = 12.68, P < .001, R2 change = 0.06). Age, education, gender, IQ, BDI scores, and baseline pAKT/AKT ratio did not predict PDI scores (F < 2, P > .1).
Discussion
In this study, we investigated the relationship between NRG1-induced AKT phosphorylation and markers of psychosis proneness in nonclinical individuals. As expected from previous studies, participants with weak habituation of arousal displayed more paranoid ideation than strong habituators,2 and the habituation index accounted for 30% of variance of the PDI. The key finding of the study was that the pAKT/AKT ratio after NRG1 induction was also lower in weak habituators than in strong habituators and accounted for 12% of variance of the PDI. These predictors could be promising, but the underlying mechanism leading to psychosis proneness and even psychosis needs further investigation.
The finding that the pAKT/AKT ratio is lower in psychosis-prone individuals is consistent with the data of Emamian et al20 and Sei et al10 who described similar phenomena in patients with schizophrenia. However, some differences should be mentioned. Emamian et al20 demonstrated decreased AKT protein levels in the peripheral lymphocytes and brains of patients with schizophrenia, a significant association between schizophrenia and an AKT haplotype associated with lower AKT protein levels and a greater sensitivity to the sensorimotor gating-disruptive effect of amphetamine related to AKT deficiency. The decreased AKT protein level and its association with genetic variants were not replicated in a Japanese population of schizophrenia patients.30 Ide et al30 also found unaltered pAKT/AKT ratio in the lymphocytes of schizophrenia patients. The paradigm of Sei et al10 went further and investigated the effect of NRG1 stimulation on AKT phosphorylation, which may be a more specific marker. In the baseline (nonstimulated) condition of Sei et al,10 the pAKT/AKT ratio difference between patients with schizophrenia and controls was small, whereas in the NRG1-stimulated condition, the pAKT/AKT ratio was approximately 4 times higher in controls than in patients with schizophrenia. We observed a highly similar pattern in nonclinical individuals with higher delusional ideations and weaker habituation: These persons differed from less delusional strong habituators only in the NRG1-stimulated condition but not in the baseline condition (Figure 1).
It is interesting that NRG1-induced AKT phosphorylation is also influenced by the genetic polymorphism of the COMT gene (Val108/158Met)10,31. Specifically, NRG1-induced AKT phosphorylation is significantly impaired in Val carriers compared with Met carriers in both healthy subjects and patients with schizophrenia, which may indicate that poorer NRG1-induced cell adhesion and migration in Val carriers are due at least in part to decreased AKT activation10,31. At the level of symptoms, this polymorphism may be associated with anxiety-spectrum phenotypes.32
The causal relationship between NRG1, AKT, and orienting response habituation remains elusive. In this study, a relatively indirect system was applied to measure AKT phosphorylation induced by exogenous NRG1 in B lymphoblasts. Even though both NRG1 and AKT seem to be implicated in the pathophysiology of schizophrenia, the underlying signaling cascades from NRG1 to AKT are complex. At the behavioral level, evidence suggests that that NRG1 is important in sensory inhibition and adaptation when repeated stimuli evoke smaller responses. Hong et al33 found that a missense mutation of the NRG1 gene is associated with impaired prepulse inhibition in healthy individuals and in patients with schizophrenia. This is consistent with rodent models in which NRG1 heterozygous animals showed significantly impaired prepulse inhibition.34 Recently, Chen et al35 demonstrated that the type III isoform of NRG1 is required to maintain normal sensory gating, which is a marker of the integrity of corticostriatal circuits. In the original study of Emamian et al,20 the deficit of AKT was also related to sensorimotor gating disturbances. The PI3K/AKT system is important in dopaminergic and glutamatergic neurotransmission,36,37 and AKT is linked to dopamine-related prefrontal functions and structural alterations similar to those found in schizophrenia.38,39
Previous studies suggested that there is a relationship between anxiety and psychosis-like experiences,40,41 with a special reference to persecutory delusions.42,43 However, the results are not consistent. For example, Allen et al2 did not find significant differences in BAI scores between weak and strong habituators with high and low psychosis proneness, respectively. These discrepancies may be due to differences in the characteristics of the individuals included in separate studies or may be the consequence of low statistical power. In our study, weak habituators showed higher anxiety than strong habituators, and anxiety was an independent predictor of delusional ideation in addition to the habituation index and NRG1-stimulated pAKT/AKT.
Conclusions
In summary, we used a combined approach to characterize psychosis proneness in nonclinical individuals. This included the measurement of the peripheral modulation of intracellular signal transduction associated with psychotic disorders (NRG1-induced AKT phosphorylation), a physiological marker (habituation of autonomic arousal), and self-report rating scales of subjective experiences (overvalued ideas and emotional distress). The results revealed a characteristic pattern of relationships among these measures, which raises the possibility that such a combined approach may be useful for future studies aimed at investigating psychosis proneness and psychosis risk factors in the general population.
Funding
Hungarian Research Fund (OTKA NL72488); Marie Curie Fellowship (EUTwinSS 0359271 to I.S.).
Acknowledgments
We thank the VitaTrade Kft and John Bowen for technical assistance. The authors declare no conflict of interest.
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 2.Allen P, Freeman D, McGuire P. Slow habituation of arousal associated with psychosis proneness. Psychol Med. 2007;37:577–582. doi: 10.1017/S0033291706009615. [DOI] [PubMed] [Google Scholar]
- 3.Cooklin R, Sturgeon D, Leff J. The relationship between auditory hallucinations and spontaneous fluctuations of skin conductance in schizophrenia. Br J Psychiatry. 1983;142:47–52. doi: 10.1192/bjp.142.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Dawson ME, Nuechterlein KH, Schell AM, Gitlin M, Ventura J. Autonomic abnormalities in schizophrenia. State or trait indicators? Arch Gen Psychiatry. 1994;51:813–824. doi: 10.1001/archpsyc.1994.03950100061006. [DOI] [PubMed] [Google Scholar]
- 5.Raine A, Lencz T, Benishay D. Schizotypal Personality and Skin Conductance Orienting. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- 6.Raine A, Benishay D, Lencz T, Scarpa A. Abnormal orienting in schizotypal personality disorder. Schizophr Bull. 1997;23:75–82. doi: 10.1093/schbul/23.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Hazlett H, Dawson ME, Schell AM, Nuechterlein KH. Electrodermal activity as a prodromal sign in schizophrenia. Biol Psychiatry. 1997;41:111–113. doi: 10.1016/s0006-3223(96)00351-4. [DOI] [PubMed] [Google Scholar]
- 8.Ohman A. Electrodermal activity and vulnerability to schizophrenia: a review. Biol Psychol. 1981;12:87–145. doi: 10.1016/0301-0511(81)90008-9. [DOI] [PubMed] [Google Scholar]
- 9.Gruzelier J, Raine A. Bilateral electrodermal activity and cerebral mechanisms in syndromes of schizophrenia and the schizotypal personality. Int J Psychophysiol. 1994;16:1–16. doi: 10.1016/0167-8760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 10.Sei Y, Ren-Patterson R, Li Z, et al. Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol Psychiatry. 2007;12:946–957. doi: 10.1038/sj.mp.4001994. [DOI] [PubMed] [Google Scholar]
- 11.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Kanakry CG, Li Z, Nakai Y, Sei Y, Weinberger DR. Neuregulin-1 regulates cell adhesion via an ErbB2/phosphoinositide-3 kinase/Akt-dependent pathway: potential implications for schizophrenia and cancer. PLoS ONE. 2007;2:e1369. doi: 10.1371/journal.pone.0001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munafò MR, Thiselton DL, Clark TG, Flint J. Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:539–546. doi: 10.1038/sj.mp.4001817. [DOI] [PubMed] [Google Scholar]
- 16.Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33:905–911. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt A, Parlapani E, Gruber O, Wobrock T, Falkai P. Impact of neuregulin-1 on the pathophysiology of schizophrenia in human post-mortem studies. Eur Arch Psychiatry Clin Neurosci. 2008;258(suppl 5):35–39. doi: 10.1007/s00406-008-5019-x. [DOI] [PubMed] [Google Scholar]
- 18.Hall J, Whalley HC, Job DE, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 19.Kéri S, Kiss I, Kelemen O. Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high-risk for psychosis. Mol Psychiatry. 2009;14:118–119. doi: 10.1038/mp.2008.1. [DOI] [PubMed] [Google Scholar]
- 20.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 23.Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory) Schizophr Bull. 1999;25:553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- 24.Wing JK, Cooper JE, Sartorius A. Management and Classification of Psychiatric Symptoms. Cambridge: Cambridge University Press; 1974. [Google Scholar]
- 25.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Cons Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Venables PH, Christi MJ. Electordermal activity. In: Martin I, Venables PH, editors. Techniques in Psychophysiology. Chichester, UK: John Wiley & Sons; 1980. pp. 2–67. [Google Scholar]
- 29.Pressman S, Rotter JI. Epstein–Barr virus transformation of cryopreserved lymphocytes: prolonged experience with technique. Am J Hum Genet. 1991;49:467. [PMC free article] [PubMed] [Google Scholar]
- 30.Ide M, Ohnishi T, Murayama M, et al. Failure to support a genetic contribution of AKT1 polymorphisms and altered AKT signaling in schizophrenia. J Neurochem. 2006;99:277–287. doi: 10.1111/j.1471-4159.2006.04033.x. [DOI] [PubMed] [Google Scholar]
- 31.Sei Y, Li Z, Song J, et al. Washington, DC: 2008. Effects of catechol-o-methyltransferase val/met polymorphism on NRG1-ErbB signaling: Akt1-mediated mechanism. Poster presented at: 38th Annual Meeting of the Society for Neuroscience (760.7/EE25); November 2008. [Google Scholar]
- 32.Hettema JM, An SS, Bukszar J, et al. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry. 2008;64:302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol Psychiatry. 2008;63:17–23. doi: 10.1016/j.biopsych.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YJ, Johnson MA, Lieberman MD, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaulieu JM, Sotnikova TD, Marion S, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Howlett E, Lin CC, Lavery W, Stern M. A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000277. e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai WS, Xu B, Westphal KG, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci USA. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan HY, Nicodemus KK, Chen Q, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen P, Freeman D, McGuire P, et al. The prediction of hallucinatory predisposition in non-clinical individuals: examining the contribution of emotion and reasoning. Br J Clin Psychol. 2005;44:127–132. doi: 10.1348/014466504X20044. [DOI] [PubMed] [Google Scholar]
- 41.Freeman D, Dunn G, Garety PA, et al. The psychology of persecutory ideation I: a questionnaire survey. J Nerv Ment Dis. 2005;193:302–308. doi: 10.1097/01.nmd.0000161687.12931.67. [DOI] [PubMed] [Google Scholar]
- 42.Freeman D, Garety PA, Kuipers E. Persecutory delusions: developing the understanding of belief maintenance and emotional distress. Psychol Med. 2001;31:1293–1306. doi: 10.1017/s003329170100455x. [DOI] [PubMed] [Google Scholar]
- 43.Freeman D, Gittins M, Pugh K, Antley A, Slater M, Dunn G. What makes one person paranoid and another person anxious? The differential prediction of social anxiety and persecutory ideation in an experimental situation. Psychol Med. 2008;38:1121–1132. doi: 10.1017/S0033291708003589. [DOI] [PMC free article] [PubMed] [Google Scholar]