Abstract
Schizophrenia is associated with abnormalities in emotional processing and social cognition. However, it remains unclear whether patients show abnormal neurophysiological responses during fast, online appraisals of the emotional meaning of social information. To examine this question, event-related potentials (ERPs) were collected while 18 schizophrenia patients and 18 demographically matched controls evaluated 2-sentence vignettes describing negative, positive, or neutral social situations. ERPs were time locked to a critical word (CW) in the second sentence that conferred emotional valence. A late positivity effect to emotional (vs neutral) CWs was seen in both groups (in controls, to negative and positive CWs; in patients, to negative CWs only). However, the controls showed a greater late positivity effect to the negative and positive (vs neutral) CWs than the schizophrenia patients at mid-posterior (negative vs neutral) and at right posterior peripheral (positive vs neutral) sites. These between-group differences arose from reduced amplitudes of the late positivity to the negative and positive CWs in the patients relative to the controls; there was no difference between the 2 groups in the amplitude of the late positivity to the neutral CWs. These findings suggest that schizophrenia is associated with a specific neural deficit during the online evaluation of emotionally valent, socially relevant information.
Keywords: schizophrenia, emotion, affect, negative symptoms, semantic, language, P300, late positivity, event-related potentials
Introduction
Abnormalities in the perception and expression of emotion have been considered an inherent part of schizophrenia since the syndrome was first described.1,2 More recently, it has been established that schizophrenia patients show marked behavioral and neural dysfunction in a variety of emotional perception, evaluation, and memory tasks.3–10 Behaviorally, patients are impaired in their ability to recognize or discriminate between different emotions expressed in faces9,11–18 and in vocal prosody.11,19–22 At a neural level, event-related potential (ERP) studies report reduced effects in patients, relative to controls, during visual and auditory emotional judgments,23–27 and neuroimaging studies reveal abnormalities within cortical and subcortical limbic regions as patients view faces with emotional expressions24,28–35 or evaluate odors36 or emotional scenes.37,38 Behavioral emotional deficits have been linked to other aspects of neuropsychological dysfunction10,39–41 and to negative symptoms.9,14,17,18,42 Importantly, they can predict social dysfunction11,18,43 and functional outcome.11,44
Although there is little doubt that schizophrenia patients show abnormalities in emotional processing, there remain several unresolved issues. First, do such impairments stem from a specific dysfunction in emotional evaluation, or are they the result of more general cognitive dysfunction? There is some evidence for the latter possibility: patients’ poor executive function can predict poor performance on emotion recognition tasks,40,45and several studies have shown that patients’ deficits in recognizing facial emotions may be explained by a more general deficit in processing face stimuli.9,14,15,40,46,47
Second, do patients’ impairments in discriminating between emotional and neutral stimuli stem from abnormalities to the emotional and/or to the neutral material? In previous studies, it has sometimes been assumed that impairments in discriminating emotional and neutral stimuli in schizophrenia arise from reduced responses to emotional stimuli, without considering the possibility of abnormal responses to neutral stimuli.9,14,17,42 However, there is evidence that, relative to controls, schizophrenia patients can show inappropriate attributions of emotional meaning (or salience) to neutral stimuli.27,48–53 These behavioral abnormalities have been particularly associated with delusions.27,48–52
At a neural level, functional magnetic resonance imaging (fMRI) studies have reported reduced differences in activity in schizophrenia patients when contrasting aversive and neutral stimuli.34,54 Again, however, because fMRI is a comparative methodology, it remains unclear whether these reduced differences arise from (a) elevated neural responses to neutral stimuli and/or (b) reduced neural responses to aversive stimuli. There is some evidence that patients show abnormally elevated amygdala activity to neutral faces.29,31 Others have reported inappropriate increases in activity to neutral, relative to emotional, stimuli within the parahippocampal gyrus in association with reality distortion symptoms,56 and within midline cortical regions in association with delusions.55 However, the evidence for reductions in neural activity to emotional stimuli in schizophrenia has been inconsistent, with reports of decreased,24,33,34,54,57 but sometimes increased,29,30,58 responses in patients relative to controls.
The present study used ERPs—a direct index of online processing in the brain with high temporal resolution—to address some of the open questions outlined above. The ERP component that has been most closely associated with emotional processing is a positivity, peaking between 300 and 900 milliseconds, which shows a larger amplitude to emotional than to neutral stimuli of multiple different types, including faces,59–61 pictures,61–65 and words.66–70 This positivity—referred to here as the late positivity—has some features in common with the P300 ERP component, classically seen to rare (oddball) stimuli.71 Importantly, however, several studies in healthy individuals have established that the late positivity evoked by emotional stimuli is not just observed when these are presented as oddballs, suggesting that it can be triggered by the inherent arousal of emotional stimuli,61–63,70 rather than simply their novelty. The increased amplitude of the late positivity to emotional, relative to neutral, stimuli is thought to reflect the increased allocation of attentional resources to such arousing stimuli61,72 and their reanalysis with respect to their surrounding context.
In schizophrenia, there have been several ERP studies documenting a reduced late positivity/P300 effect in tasks requiring the evaluation of emotional stimuli.23–26 All have used face stimuli. However, these studies have not, as yet, directly addressed the question of whether the reduced late positivity effect observed in patients derives from a selective reduction in this component to emotional stimuli and/or an abnormally increased response to neutral stimuli. In some studies, emotional stimuli were rarer than non-emotional stimuli,25,26 making it difficult to determine whether any reduction of the positivity was due to the inherent emotional properties of the stimuli or simply their novelty (it is well established that the P300 to novel oddball stimuli is reduced in schizophrenia73). Also, in all ERP studies to date, the same faces have been repeated many times over the course of an experiment23–26—sometimes more so for neutral than for emotional faces23—which may have led to differential repetition priming and habituation effects in patients relative to controls,30 confounding the interpretation of findings. One study reported a larger positivity in patients (particularly in those with paranoid symptoms) than controls to neutral faces but no differences to emotional faces,27 but the neutral and emotional faces were presented in separate blocks and using different tasks, making these findings difficult to interpret. Finally, there is some evidence that a reduced late positivity effect to emotional faces can be explained by reduced modulation of earlier components,23,24 making it difficult to determine whether schizophrenia patients can show neural abnormalities in evaluating emotional stimuli in the absence of early visual processing deficits.
In the present study, we recorded ERPs as participants evaluated the emotional meaning of 2-sentence vignettes describing real-world social situations.8 There is now robust evidence that reading mini-stories of this sort simulates real-world comprehension,74 including emotional experience,75 as readers derive mental models of the situations described.76,77 In this paradigm, the first sentence of each pair presents a neutral context and the second sentence, presented word by word, is positive, negative, or neutral in valence. The emotional meaning of the situation described is conferred by a single word in the second sentence, the critical word (CW), eg, “Sandra's old boyfriend stopped by her apartment today. This time he brought a letter/rose/gun (neutral, positive, negative word, respectively) with him.” (table 1).
Table 1.
Examples of 2-Sentence Vignettes
| First Sentence | Second Sentence (Without the Critical Word) | Neutral Critical Word | Positive Critical Word | Negative Critical Word |
| Nancy's son ended up just like his father. | He was already a ____ by age 25. | husband | millionaire | criminal |
| Stephen owned a lot of nineteenth century art. | Everyone knew that he ____ paintings of old masters. | bought | loved | forged |
| An unfamiliar man rang Lenora's doorbell one day. | He had come to _____ her. | register | congratulate | arrest |
| Every day, the local newspaper wrote about the mayor. | They always said _____ things about him. | factual | marvelous | damaging |
| Mr Jenners planned to move his family to New York. | The reason for this was _____ to the children. | obvious | reassuring | hidden |
| After Donald moved, his life went on as before. | On the west coast, his ____ only continued. | campaign | luck | misfortune |
| Cheryl's baby cried when she took him to bed. | She quieted him with a ____ that night. | pacifier | lullaby | drug |
| The letter Fred received was tightly sealed. | He knew it contained ____ when he saw it. | advertisements | money | bacteria |
This paradigm has several advantages. First, unlike faces and pictures, emotional salience of linguistic material, which must first be decoded, is typically evaluated only when its full meaning is accessed. In the present paradigm, we carefully matched the number of letters, the word frequency, and concreteness of the CWs across the neutral, positive, and negative conditions. Thus, any differential modulation of the late positivity in schizophrenia in this paradigm would most likely be driven by abnormalities in the evaluation of emotion, rather than deficits at early perceptual stages of emotional processing.
Second, we averaged ERPs separately to positive, negative, and neutral words and also avoided several confounds of the previous ERP studies discussed above: the emotional stimuli were not rarer than the neutral stimuli, and no sentence was repeated more than once. This allowed us to compare the amplitudes of ERPs evoked by emotional and neutral words separately between patient and control groups to determine the source of between-group differences in ERP effects.
Based on previous studies,66,78,79 as well as our own recent findings using this ERP paradigm in a younger group of healthy individuals,8 we predicted that controls would show a larger late positivity to emotional than to neutral words. We hypothesized that, by comparison, this late positivity effect to emotional vs neutral vignettes would be reduced in schizophrenia patients,23–28,33 particularly in those with negative symptoms, or possibly even reversed,52,55,56 particularly in patients with delusions. We aimed to determine whether such abnormalities in schizophrenia patients arose because of an abnormally reduced response in patients to emotional vignettes and/or an abnormally increased response to neutral vignettes.
Methods
Materials
The sentence-pair stimuli have been described in detail elsewhere.8 Briefly, 2-sentence descriptions of situations involving one person or more (see table 1 for examples) were generated for each of 3 experimental conditions. For each sentence pair, the first sentence (8–11 words long) was neutral and ambiguous in content, providing a non-constraining context for the second sentence. The second sentence contained 6–9 words and, before the end of the sentence, contained a word that was neutral, positively valenced, or negatively valenced (the CW). Pretests in healthy individuals who did not participate in the ERP study confirmed that the CWs and the sentence pairs as a whole were indeed positive, negative, or neutral in valence.8 The positive and negative CWs had higher mean arousal ratings than the neutral CWs, and all 3 conditions were matched in terms of concreteness, number of letters, and Kucera-Francis frequency of the CWs.8 The stimulus set was divided into 3 lists, using a Latin square design, which were counterbalanced between participants such that no individual encountered the same sentence pair more than once, but, across subjects, all vignettes were seen in all 3 conditions. Each of the 3 lists included 135 sentence pairs, with 45 sentence pairs in each condition.
Participants
Eighteen outpatients were recruited from the Erich Lindemann Mental Health Center, Boston, MA. All patients met Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria for schizophrenia, as confirmed by the Structured Clinical Interview for DSM-IV (SCID).80 Eighteen healthy volunteers were recruited by advertisement and were screened using the SCID to exclude the presence of psychiatric disorders.80 Patients’ symptomatology was assessed within 2 weeks of ERP testing using the Positive and Negative Syndrome Scale.81 Patients were receiving stable doses of antipsychotic medication: 16 were taking atypical antipsychotic medication, 1 was on typical antipsychotics, and 1 was unmedicated. One patient was also taking anticholinergic medication. There were no medication changes between clinical assessments and the ERP session. Healthy volunteers were not taking medication affecting the central nervous system.
All participants were right-handed and had normal or corrected-to-normal vision. Participants were excluded if they had a history of neurological injury, head trauma with documented cognitive sequelae, and medical disorders that can impair neurocognitive function, as well as if they met DSM-IV criteria for substance abuse within the previous 3 months or any lifetime history of substance dependence. Patients and controls matched closely in gender and race/ethnicity distributions, and there was no significant difference between the groups in age (t34 = 0.53, P = .60). The patient and control groups also showed no significant difference in premorbid IQ (t34 = 1.45, P = .16) as estimated by the North American Adult Reading Test82 or in parental socioeconomic status (t34 = 1.22, P = .23) as determined by Hollingshead Index scores.83 Demographic characteristics of all participants and clinical details for the patient group are given in table 2.
Table 2.
Demographic and Clinical Data of Healthy Controls and Patients With Schizophrenia
| Parameter | Subject Group |
|
| Controls (n = 18) | Patients (n = 18) | |
| Gender (M/F) | 12/6 | 13/5 |
| Race (C/AA) | 16/2 | 16/2 |
| Age (y) | 42.4 (7.8) | 43.7 (7.9) |
| Hollingshead Index | 3.4 (1.0) | 2.9 (1.4) |
| Premorbid verbal IQ | 111.5 (10.0) | 106.2 (11.8) |
| CPZ equivalent | — | 465.7 (436.7) |
| Duration of illness (y) | — | 16.9 (11.8) |
| PANSS negative (total) | — | 14.7 (4.2) |
| PANSS positive (total) | — | 15.3 (7.2) |
Note: SDs are shown in parenthesis. M = male, F = female, C = Caucasian, AA = African American, CPZ = chlorpromazine, PANSS = Positive and Negative Syndrome Scale.81
Written informed consent was obtained from all participants according to the guidelines of the Massachusetts General Hospital and Tufts Human Subjects Research Committees.
Stimulus Presentation and Task
Participants sat in a comfortable chair in a dimly lit room. Stimuli were presented on a computer monitor, in white font, centered on a black background, and subtended at a visual angle of about 5°. Experimental participants were randomly assigned to 1 of the 3 lists. All trials began with a white fixation cross (500 ms, interstimulus interval [ISI] = 100 ms). The first sentence was presented as a whole (3.5 s, ISI = 100 ms), and the second sentence was presented word by word (500 ms, ISI = 100 ms). A 750-millisecond blank screen interval followed the final word of the second sentence, and this was followed by a question mark cue. Participants’ task was to press 1 of 3 buttons with their right thumb (button order was counterbalanced across subjects), depending on their judgment of whether the sentence pair depicted a pleasant, unpleasant, or neutral person, place, or situation. This delayed judgment reduced any contamination of the ERPs of interest by response-sensitive components.71 Participants then proceeded to the next trial.
ERP Recording Procedure
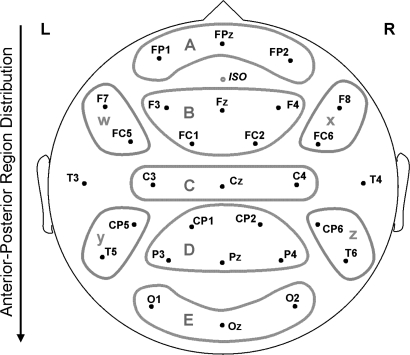
The electroencephalogram (band-pass, 0.01–40 Hz, 6-dB cutoffs; sampling rate, 200 Hz) was recorded from 29 scalp electrodes (Electro-Cap International, Inc, Eaton, OH; for full montage see figure 1), as well as below and at the outer canthus of an eye and over the right mastoid; all recordings were referenced to the left mastoid. ERPs were averaged off-line at each electrode site for each experimental condition using a 100-millisecond prestimulus baseline and lasting until 1170 milliseconds after word onset. Trials contaminated with eye artifact (exceeding 50 μV) or amplifier blockage were excluded from analyses. Artifact contamination led to a rejection rate of 12.81% (SD = 8.04%) in patients and 8.27% (SD = 5.79%) in controls.
Fig. 1.
Electrode Montage and Regions Used in Analysis. Midregions: A, anterior frontal; B, frontal; C, central; D, central posterior; E, posterior. Peripheral regions: W, left anterior; X, right anterior; Y, left posterior; Z, right posterior. This and all other figures are available in color as online supplementary material.
Behavioral Analysis
It was first determined that individual participants were able to perform the task by considering just the positive and negative items and determining whether these were accurately discriminated, using d′: z(hit rate) − z(false alarm rate). Here, the hit rate was calculated as the percentage of items in one emotional condition that were classified consistently with the preclassification (eg, percentage of negative items classified as negative), and the false alarm rate was calculated as the percentage of items in the other emotional condition that were classified inconsistently with the preclassification (eg, percentage of positive items classified as negative).
In addition, for each of the 3 preclassified sentence types (positive, negative, and neutral), the percentage of trials that each individual classified as positive, negative, and neutral during the ERP experiment—consistent responses—was entered into a 2 (group) by 3 (affect) analysis of variance (ANOVA) to determine how these classifications differed between the patient and control groups across the 3 sentence types.
ERP Analysis
Because we were primarily interested in the electrophysiological response in relation to participants’ affective classifications, ERPs were averaged according to participants’ explicit classifications of the sentences as positive, negative, and neutral (as reported in the behavioral accuracy section of the results, these classifications were usually consistent with the a priori affective classifications of these sentences based on norming studies). ERPs to these trials were quantified by calculating mean amplitudes (relative to the 100-ms baseline preceding the CW) for components of interest. Modulation of the P2 and N400 components was captured across the 100- to 250-millisecond and 300- to 500-millisecond time window, respectively. Modulation of the late positivity was examined within the 500- to 700-millisecond time window. This time window was chosen on the basis of our previous study using the same paradigm in a separate cohort of healthy individuals,8 and it captured the late positivity component in both patients and controls. Based on our a priori hypotheses and the findings of our previous study,8 we proceeded to ANOVAs that contrasted the negative and positive sentence pairs with the neutral sentence pairs. For each of these contrasts, a mixed-design ANOVA was conducted in order to examine the relative modulation of the mean amplitude of the late positivity across conditions at mid and peripheral sets of scalp regions. Each of these omnibus analyses had a within-subject factor of affect (2 levels: negative vs neutral or positive vs neutral) and a between-subject factor of group (2 levels: patients vs controls). In order to examine how the modulation of the waveforms varied across the scalp surface, the scalp was subdivided into regions along the anterior-posterior (AP) distribution of the scalp surface at both mid and peripheral sets of regions; see figure 1. Each ANOVA also included the within-subject scalp topography factors of AP region distribution and, for the peripheral regions, hemisphere. Significant interactions involving the affect and AP region distribution factors were first parsed by assessing the ERPs at each region or, for the peripheral analysis, pairs of regions. Significant interactions involving both affect and group interactions were parsed in 2 ways: first by examining the effect of affect in each participant group and second by examining the effect of group for the ERPs evoked by each type of CW (positive, negative, or neutral). The Geisser-Greenhouse correction was applied to repeated measures with more than 1 df,84 and a significance level of α = .05 was used as, in all cases, a priori hypotheses were tested.
Spearman correlations were used to examine how the late positivity effects to the negative (vs neutral) and positive (vs neutral) CWs varied in relation to clinical characteristics (shown in table 2) within the patient group. These late positivity effects were calculated by subtracting the averaged amplitudes of ERPs evoked by neutral CWs from the averaged amplitudes between 500ms and 700ms of ERPs evoked by negative and positive CWs across electrode sites within (a) the mid-posterior region and (b) the right peripheral posterior regions where, as reported below, effects differentiated between the patient and control groups. For a priori hypotheses about the relationships between delusions and negative symptoms and the late positivity effects (see “Introduction”), α = .05 was used. For other, more exploratory correlations (with total positive symptoms, conceptual disorganization and hallucinations), computed to establish specificity of any findings, the significance level was determined based on the Bonferroni correction.
Results
Behavioral Data
All individual participants were able to distinguish between the negative and positive sentence types (all individual d′ scores >1.75) (table 3).
Table 3.
Mean Percentage of Responses Classified as Positive, Negative, and Neutral During the ERP Experiment in Relation to Prior Classified Sentence Types
| Sentence Type (Prerated) | Participants’ Classifications During ERP Experiment |
Total |
||||||
| Positive |
Negative |
Neutral |
||||||
| Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | |
| Positive | 85.8 (7.3) | 77.2 (13.1) | 3.6 (7.0) | 2.9 (3.6) | 20.4 (14.6) | 27.2 (20.1) | 33.7 (0.9) | 33.7 (0.8) |
| Negative | 1.7 (1.6) | 2.5 (3.6) | 87.4 (12.4) | 86.4 (11.0) | 3.8 (3.7) | 9.3 (9.4) | 33.4 (1.0) | 33.3 (0.4) |
| Neutral | 12.5 (7.5) | 20.3 (12.6) | 9.1 (9.8) | 10.7 (10.2) | 75.9 (13.9) | 63.5 (24.3) | 32.8 (0.5) | 33.0 (0.6) |
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Note: SDs are shown in parenthesis. ERP, event-related potential.
In examining the percentages of participants’ responses that were consistent with the 3 a priori classified conditions, there was a main effect of affect (F2,68 = 11.74, P< .0005) that arose because participants were less consistent in classifying the neutral sentence pairs than either the positive (t35= 2.65, P< .02) or negative (t35= 4.46, P< .0001) sentence pairs. Additionally, negative sentence pairs were classified more consistently than positive sentence pairs (t35= 2.38, P< .05). A significant main effect of group (F1,34 = 8.04, P< .009) was due to significantly greater consistency (across all sentence types) in controls than patients (t34= 2.88, P< .01). There was no significant interaction between group and affect (F2,68= 1.30, P= .272).
Event-Related Potentials
The grand average ERPs, time locked to the presentation of the CWs in sentence pairs classified as positive, negative, and neutral, are plotted in controls in figure 2 and in patients in figure 3. In both groups, an N1-P2 complex can be seen in the first 250 milliseconds after word onset. The P2 did not show differential modulation by affect across the patient and control groups: mid-region and peripheral region omnibus ANOVAs failed to reveal significant interactions involving affect and/or group across the 100- to 250-millisecond time window, all P values >.05.
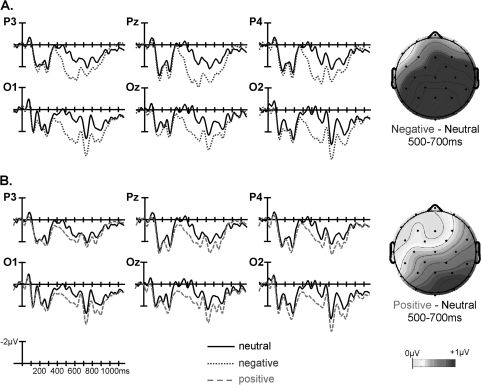
Fig. 2.
Grand Averaged Waveforms and Voltage Maps Comparing Electrophysiological Response to (A) Negative Vs Neutral and (B) Positive Vs Neutral Critical Words in the Control Group (n = 18). This figure is available in color as online supplementary material.
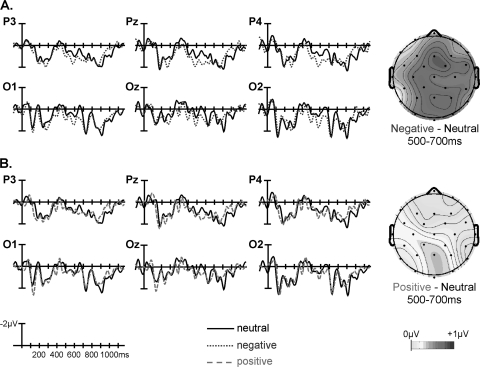
Fig. 3.
Grand Averaged Waveforms and Voltage Maps Comparing Electrophysiological Response to (A) Negative Vs Neutral and (B) Positive Vs Neutral Critical Words in the Patient Group (n = 18). This figure is available in color as online supplementary material.
The P2 was followed by a negativity (the N400). This overlapped with a positive shift in the waveform that was particularly marked to the emotional words. Because this positive shift began within the N400 time window, any differential modulation across groups in the modulation of the N400 component by affect could not be isolated in this paradigm (see Discussion): across the 300- to 500-millisecond time window, the contrast between negative and neutral CWs revealed a significant main effect of affect in the mid-region omnibus ANOVA (F1,34 = 8.533, P = .006), this was due to the overlapping larger positivity to the emotional words than the neutral words. Across the 300- to 500-millisecond time window, there were no interactions between affect and group in either the mid-region or peripheral region omnibus ANOVA for either the contrast between negative and neutral or between positive and neutral CWs, across the 300- to 500-millisecond time window. The later part of the positivity appeared to be differentially modulated between conditions and groups, and this was further examined within the 500- to 700-millisecond time window (the late positivity).
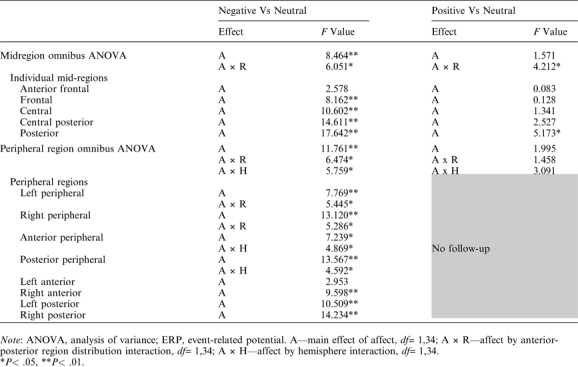
The late positivity was first contrasted between negative and neutral CWs. Both the mid-region and peripheral region omnibus ANOVAs revealed main effects of affect as well as significant interactions between affect and AP region distribution (table 4, left). These interactions were followed up with ANOVAs in each of the regions or, for the peripheral analysis, pairs of regions (table 4, left). These follow-up ANOVAs showed main effects of affect in all regions/pairs of regions except the most anterior mid-prefrontal region, reflecting significant widespread late positivity effects to negative (vs neutral) CWs across both groups. In addition, the peripheral region omnibus ANOVA revealed an interaction between affect and hemisphere: this arose because the late positivity effect was larger over right than left peripheral regions (follow-ups in each hemisphere separately revealed significant main effects of affect in both hemispheres, but effects were larger on the right).
Table 4.
Midregion and Peripheral Region ANOVAs Examining ERP Modulation Across the 500- to 700-ms Time Window, Indicating Main Effects and Interactions Involving Affect but not Group, ie, Modulation of the Late Positivity by Affect Across All Participants (Patients and Controls)
 |
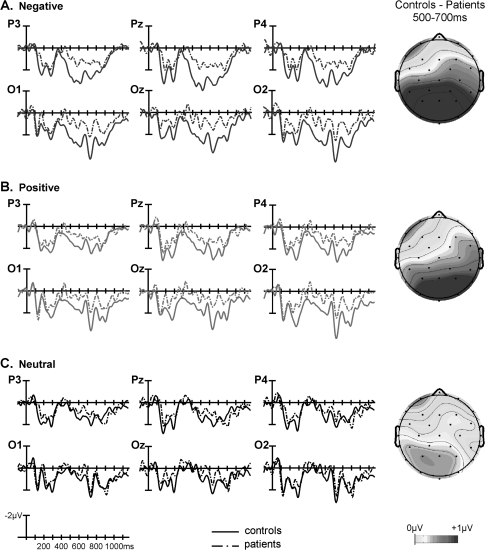
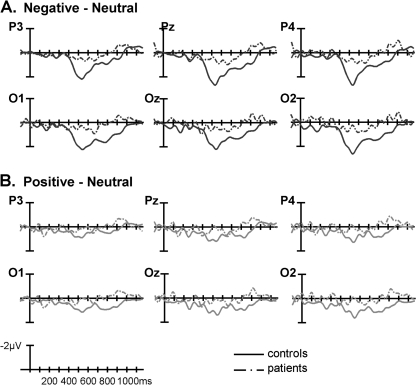
Notably, both the mid-region and peripheral region omnibus ANOVAs revealed significant interactions between affect, AP region distribution, and group (table 5, left). Follow-ups in each mid-region/pair of peripheral regions revealed interactions between affect and group that reached significance in the mid-posterior region and that approached significance in the posterior peripheral regions (table 5, left), indicating that, in these regions, the modulation of the late positivity by affect differed between the 2 groups. The significant interaction in the mid-posterior region was followed up in 2 ways. First, ERPs evoked by the negative and neutral words separately were compared between the 2 groups. This revealed a significantly larger late positivity to the negative CWs in controls than patients (main effect of group, F1,34 = 4.571, P = .040) but no such between-group difference to the neutral CWs (F1,34 = 0.553, P = .462); see figure 4A and 4C. Second, ERP effects to negative (vs neutral) CWs were examined within each group separately. This revealed significant effects in both controls (t17 = 3.606, P< .01, see figure 2A) and patients (t17= 2.158, P< .05, see figure 3A), although this effect was smaller in patients than controls (see figure 5A).
Table 5.
ANOVAs at Mid and Peripheral Regions Examining ERP Modulation Across the 500- to 700-ms Time Window, Indicating Main Effects and Interactions Involving Both Affect and Group, ie, Differential Modulation of the Late Positivity by Affect Across the 2 Groups
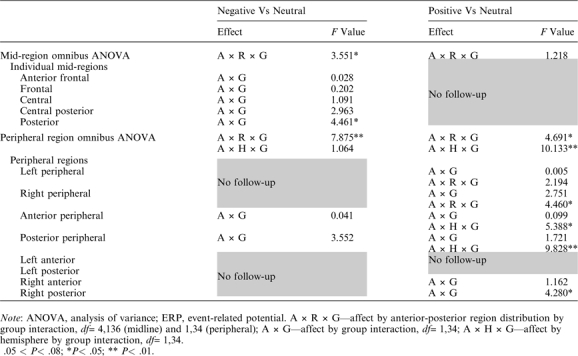 |
Fig. 4.
Grand Averaged Waveforms and Voltage Maps Comparing Patients’ and Controls’ Electrophysiological Response to (A) Negative, (B) Positive, and (C) Neutral Critical Words. This figure is available in color as online supplementary material.
Fig. 5.
Grand Averaged Difference Waveforms Comparing Patients’ and Controls’ Electrophysiological Response to (A) Negative Minus Neutral and (B) Positive Minus Neutral Critical Words. This figure is available in color as online supplementary material.
The late positivity was next contrasted between positive and neutral CWs. Here, the mid-region omnibus ANOVA revealed interactions between affect and AP region distribution; no such interaction was seen in the peripheral region ANOVA (table 4, right). Follow-ups in each of the mid-regions revealed a main effect of affect only in the mid-posterior region, reflecting a larger late positivity to positive than to neutral CWs across both patient and control groups.
There were no interactions involving group and affect in the mid-region omnibus ANOVA, but, in the peripheral region ANOVA, significant 3-way interactions were observed between group, affect, and hemisphere, as well as between group, affect, and AP region distribution (table 5, right). The group by affect by hemisphere interaction was parsed by conducting analyses at each right and left peripheral region separately, and this revealed a group by affect by AP region distribution interaction in right peripheral but not left peripheral regions. Further follow-up in right peripheral regions indicated that the modulation of the late positivity differed between the 2 groups at the right posterior peripheral region (group by affect interaction: F1,34 = 4.280, P = .046) but not at the right anterior peripheral region (group by affect interaction: F1,34 = 1.162, P = .289). Once again, this interaction in the right posterior peripheral region was followed up in 2 ways. First, direct comparisons between the 2 groups of ERPs evoked by the positive and neutral CWs separately revealed a significantly larger late positivity to the positive CWs in controls than patients (main effect of group: F1,34 = 5.755, P< .03, see figure 4B) but no such effect to the neutral words (main effect of group: F1,34= 0.006, P> .90). Second, when ERP effects to positive (vs neutral) CWs were examined in each group separately, controls showed a larger late positivity to positive (vs neutral) CWs (t17 = 2.936, P = .009, see figure 2B), but patients showed no late positivity effects to positive (vs neutral) CWs (t17 = −0.152, P = .881, see figure 3B and figure 5B).
In sum, in the mid-posterior region, patients, relative to controls, showed a significantly smaller late positivity effects to negative (vs. neutral) CWs, and in the right peripheral posterior region, they showed a significantly smaller late positivity effect to positive (vs neutral) CWs. In both cases, these reduced effects arose because the late positivity evoked by the emotional CWs was smaller in patients than controls, but there was no between-group difference in the late positivity evoked by the neutral CWs.
Finally, ERPs to negative and positive CWs were contrasted directly in these same 2 posterior regions. Significant main effects of affect reflected a larger late positivity to negative than positive CWs across the 2 groups (mid-posterior region: F1,34 = 4.481, P = .042; right peripheral posterior region: F1,34 = 4.866, P = .034). There were no interactions between affect and group, P values >.3, ie, modulation to negative relative to positive CWs did not differ significantly across the 2 groups. Consistent with the results described above, a main effect of group approached or reached significance in these posterior regions (mid-posterior region: F1,34 = 6.690, P = .014; right posterior peripheral region: F1,34 = 3.792, P = .060), reflecting a larger late positivity to emotional (both positive and negative) CWs in controls than patients.
Correlations Between ERPs and Clinical Measures
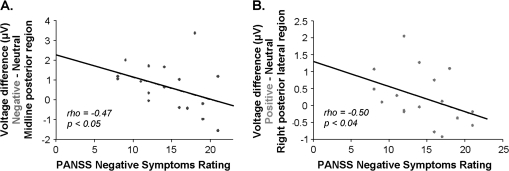
Severity of negative symptoms was negatively correlated with the late positivity effect to the positive (vs neutral) CWs in the right peripheral posterior region (r = −.50, P = .035) and to the negative (vs neutral) CWs in the mid-posterior region (r = −.47, P = .047), ie, the more the negative symptoms, the smaller the late positivity effect to both negative and positive (vs neutral) CWs (figure 6A and 6B). There was no correlation between severity of delusions and the late positivity effect in either region (P values > .1). Total positive symptoms, hallucinations, and thought disorder also did not correlate with the late positivity effect to negative or positive (vs neutral) CWs in either of these regions (P values > .09). There were also no correlations between dose of antipsychotic medication (in chlorpromazine equivalents) and the late positivity effects to either negative (vs neutral) or positive (vs neutral) CWs in either of these regions (P values > .12).
Fig. 6.
Correlations Between the Late Positivity Effects to Negative (Vs Neutral) Critical Words (A) and Positive (Vs Neutral) Critical Words (B) and Negative Symptoms in the Patient Group.
Discussion
This study used ERPs to examine the neurocognitive processes engaged as schizophrenia patients and healthy controls judged the valence of 2-sentence descriptions of affectively positive, negative, and neutral social vignettes. Controls showed a late positivity effect to both negative and positive (vs neutral) words within these vignettes. The effect to CWs in the negative (vs neutral) sentences was observed at widespread regions across the scalp but was maximal at mid-posterior and right posterior regions. The effect to positive (vs neutral) sentences was generally smaller and more localized to posterior regions. Both these observations replicate results of our previous study in younger healthy individuals using the same paradigm.8 We interpret the late positivity effect to emotional (vs neutral) stimuli as reflecting the increased allocation of attentional resources to reevaluate emotional events, with respect to their preceding neutral context, as a result of their intrinsic motivational significance.61,72
Importantly, as predicted, there were significant differences in both these effects (ie, to CWs in both the negative and positive, vs neutral, sentence pairs) between patients and controls. Of note, these group differences were not widespread and were localized to posterior regions. Within the mid-posterior region, the late positivity effect to CWs in negative (vs neutral) sentences was larger in controls than patients, and within a right posterior peripheral region, controls showed a late positivity effect to CWs in positive (vs neutral) sentences but patients showed no such effect. Although it is possible that these differences in distribution reflect distinct impairments in the underlying neural generators that discriminate positive and negative stimuli from neutral stimuli in schizophrenia, given the poor resolution of ERPs, we do not draw any firm conclusions about the anatomical sources of these effects.
In both cases, group differences in the modulation of the late positivity arose because the amplitude of the late positivity to CWs in emotional (both positive and negative) sentences was smaller in patients than controls, but there was no between-group difference in the amplitude of the late positivity to CWs in the neutral sentences. Because ERPs were averaged according to each participant's end-of-sentence classifications of emotional valence (positive, negative, or neutral), the between-group neural differences were not simply a by-product of patients being unable to perform the task: even when stimuli were behaviorally classified as positive, negative, or neutral, patients still showed reduced immediate neural responses to the critical emotional words.
An important question is whether the selectively reduced late positivity to emotional words in posterior regions in patients, relative to controls, can be explained as an instance of a reduced P300—an ERP that is well known to be attenuated in simple oddball paradigms in schizophrenia.73 Work in healthy volunteers suggests that the emotional late positivity and P300 are related and part of the same overall family of components: They may both, in part, reflect attentional reallocation processes and a reanalysis of the context surrounding the critical stimulus. Nonetheless, their triggers are different, and there is some evidence that they reflect at least partially distinct underlying neurocognitive processes: whereas the oddball P300 is triggered by attention to novelty within a given stimulus set, the emotional late positivity is evoked by the intrinsic emotional salience or arousing quality of a given stimulus, which is not necessarily novel within a given stimulus set,8,65,72,85 and does not necessarily require volitional attention.8,72,86,87 fMRI studies also reveal both common and distinct neuroanatomical circuitries engaged in response to novelty and emotion, with amygdala involvement in processing emotion88,89 that persists under conditions when attention is minimized or absent.88–93
In the present study, the late positivity was elicited by the emotional meaning of the words rather than by the novelty of the emotional sentences across the entire experiment: the emotional sentences were, in fact, less rare (less novel) than the neutral sentences (two-thirds of the stimuli were emotional, while one-third were neutral). Nonetheless, it may still be that a general impairment in attentional reallocation and context updating processes can explain both the reduced P300 seen in response to oddball stimuli in schizophrenia, as well as the reduced emotional late positivity seen here, especially given that participants were asked to pay explicit attention to the emotional valence of the stimuli. In young healthy individuals, we have shown that the late positivity to CWs in these same emotional (vs neutral) sentences was still present but reduced when the task was simply to read the sentences for comprehension.8 A follow-up study in schizophrenia using this more passive comprehension task will enable us to determine whether patients still show a reduced late positivity effect to emotional (vs neutral) stimuli when they are not required to pay explicit attention to emotional valence. (Reductions in late positivities in schizophrenia have also been documented during other aspects of higher order language processing94–96 and real-world comprehension.97 Here again, while late positivities may have some functional commonalities with the P300, they usually show characteristic patterns of modulation within their individual domains of function and are likely to arise from at least some distinct underlying neuroanatomical sources.)
In the present study, the positivity evoked by the emotional words was studied within the 500- to 700-millisecond time window. However, examination of the waveforms suggest that the positive shift to the emotional words began well before 500 milliseconds and overlapped with the N400—an ERP component that is thought to reflect the ease or difficulty of mapping the meaning of individual words into their preceding context,98 with respect to semantic memory structure99 and real-world knowledge.100 This component overlap meant that we were unable to examine modulation of the N400 to emotional stimuli. In our study, using these same stimuli, in young healthy individuals,8 we observed a small N400 effect to both positive and negative emotional (vs neutral) CWs under more passive reading conditions when, as discussed above, the late positivity was relatively reduced (see also Van Berkum et al101). It will be important to determine whether, in schizophrenia patients, this “emotional” N400 effect is abnormally modulated to these stimuli under these conditions.
Another question for future studies is whether between-group differences in the modulation of the late positivity to emotional words within sentences were driven, in part, by neural deficits in perceptual processing. There is some evidence that early perceptual processing deficits can contribute to abnormalities in processing nonlinguistic emotional stimuli, such as faces, in schizophrenia.102–105 In the present study, it seems unlikely that the reduced late positivity in patients was driven directly by bottom-up early perceptual processing deficits. This is because, by their very nature, linguistic stimuli must first be decoded to derive conceptual meaning.106 Nonetheless, given that, on some accounts, emotional meaning is represented or embodied at perceptual and motor levels, rather than at more abstract levels,75,107 it may be that reduced activation of such perceptual representations may have contributed to the reduced late positivity. We also cannot exclude the possibility that patients showed abnormalities at presemantic lexical (or sublexical) stages of processing the emotional words (see Gaillard et al108 for evidence that the emotional nature of words can be registered at early processing stages in healthy adults): although, in the present study, there was no evidence that patients showed reduced modulation of the P2 component, the activation of pre-semantic lexical representations is thought to decay rapidly, and it is therefore unlikely that any abnormalities would be detected under the experimental conditions used in the present study.109 Future studies examining the processing of emotional words under masked priming conditions will be able to test this possibility.
The demonstration of a reduced difference in neural activity to emotionally salient vs neutral stimuli in schizophrenia builds upon some fMRI findings that have reported reduced differences in hemodynamic activity (within the medial temporal lobe) in contrasting an emotional faces discrimination task with an age discrimination task33 and in comparing emotional faces with neutral faces.34,54 Indeed, some studies have shown reversed patterns of modulation in patients, relative to controls, in paralimbic regions: one study reported increased activity within the right parahippocampal gyrus in response to increasingly fearful facial expressions,56 and we recently found increases in activity within medial prefrontal and posterior cingulate cortices in response to the same emotional vs neutral sentence pairs as those used in the present study.55 Because fMRI is a comparative methodology, it is unclear whether these abnormal hemodynamic modulations stemmed primarily from decreases in activity to emotional stimuli and/or increases in activity to neutral stimuli. The present findings suggest that they may have, at least in part, stemmed from immediate decreases in neural activity to the emotional material.
Nonetheless, this does raise the question of why the present ERP study failed to show an increased response to neutral stimuli in patients relative to controls—the pattern seen in our recent fMRI study using these stimuli.55 The reason for this discrepancy is unclear, but we suggest that it may be partially explained by a difference in the time course of evaluating the meaning of neutral vs emotional stimuli. As discussed above, evaluating the meaning of emotional stimuli occurs very fast (within the order of hundreds of milliseconds) and begins immediately after encountering the stimulus that confers emotional salience. This makes evolutionary sense: upon encountering an emotionally salient stimulus, it is necessary to immediately evaluate it, in relation to its context, for survival.110–112 Because ERPs measure neural response to individual events, they provide a very sensitive measure of such fast, time-locked evaluation processes and are therefore likely to detect any abnormal reduction in patients’ immediate neural response to emotional stimuli. In contrast, abnormal responses to neutral stimuli in schizophrenia may be associated with neural responses that take longer to build up and that are less closely time locked to the onset of a critical event. fMRI, which indexes hemodynamic activity spread over many words and during decision making, may have been better suited to capturing such increased activity to neutral stimuli in patients. (In the present study, participants were asked to make their decisions after a 750-ms time interval in order to reduce any contamination of the ERP waveform measured at the CW by later response-sensitive ERPs,71 limiting any interpretation of reaction time measures at this late point). Interestingly, a previous ERP study that presented neutral and emotional faces in blocks, rather than randomizing them among each other, reported a larger positivity to neutral faces in patients (particularly those with paranoid symptoms) than controls: this blocked presentation may have allowed time for any increased neural response to neutral stimuli in patients to accumulate.27
A second possibility for why, unlike in our recent fMRI study, increased activity to neutral stimuli was not observed in patients is that aberrant neural responses to neutral material may be dependent on the level of active psychosis, in particular delusions.27,48–52,56 Consistent with this possibility, patients in the present study showed less evidence of delusions than those included in our fMRI study using the same stimuli.55
Finally, within the patient group, the magnitude of the late positivity effect at posterior regions to CWs in both the negative and positive (vs neutral) sentence pairs showed an inverse correlation with the severity of negative symptoms. This observation is consistent with several previous behavioral findings reporting that more severe negative symptoms are associated with poorer performance on emotion labeling/matching tasks.9,14,18,23,42 Although this finding should be considered preliminary, if replicated in a larger patient group, it would suggest that the social impairment and reduced affective engagement that is characteristic of negative symptomatology might, in part, stem from deficits in the neural response that occur immediately following an emotionally significant stimulus.
The present study included patients with chronic schizophrenia who were all taking psychotropic medication. It seems unlikely, however, that medication alone can explain the pattern of findings reported here: in the posterior regions that discriminated maximally between patients and controls, and where effects were generally largest, there was no correlation within the patient group between the size of the late positivity effect to emotional (vs neutral) CWs and either medication dosage or chronicity of illness within the schizophrenia group. It will, however, be important to replicate these findings in patients in the earliest stages of illness and without medication.
To conclude, the present study demonstrates a selective reduction in the immediate neural response to emotional vignettes describing real-world social situations, in schizophrenia patients, relative to healthy controls, during emotional evaluation. No such reduction was observed to vignettes describing neutral situations. These findings suggest that schizophrenia patients fail to evaluate the emotional significance of socially relevant emotionally salient stimuli and allocate neural resources to such stimuli, within several hundred milliseconds after their first encounter.
Supplementary Material
Supplementary figures 1–6 are available in color at http://schizophreniabulletin.oxfordjournals.org and http://kuperberglab.nmr.mgh.harvard.edu/publications/papers/Kuperberg_SczBull_2009_suppl_figures.pdf.
Funding
National Institute of Mental Health (RO1 MH02034 to G.R.K., K23 MH076054 to D.J.H.); National Alliance for Research in Schizophrenia and Depression with the Sidney J. Baer Trust (to G.R.K., D.J.H.); Judge Baker Center Clinical Research Training Program (to D.J.H.); GlaxoSmithKline Severe Mental Illness Award (to D.J.H.); Jerome Lyle Rappaport Charitable Foundation (to D.J.H.).
Acknowledgments
We thank Phillip Holcomb, Scott Rauch, Spencer Lynn, and Tali Ditman for their assistance. We are also grateful to Christine Portal for collecting the clinical ratings and Kaila Norman for subject recruitment.
References
- 1.DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th Rev ed. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 2.Kraepelin E. Dementia Praecox and Paraphrenia. New York, NY: Krieger; 1971. [Google Scholar]
- 3.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hall J, Harris JM, McKirdy JW, Johnstone EC, Lawrie SM. Emotional memory in schizophrenia. Neuropsychologia. 2007;45:1152–1159. doi: 10.1016/j.neuropsychologia.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Herbener ES, Rosen C, Khine T, Swseeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol. 2007;116:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 7.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 8.Holt DJ, Lynn S, Kuperberg GR. Neurophysiological correlates of comprehending emotional meaning in context. J Cogn Neurosci. doi: 10.1162/jocn.2008.21151. In press 2009; http://www.nmr.mgh.harvard.edu/kuperberglab/publications/papers/HOLTetal_Neurophyscorr_080508.pdf. Accessed March 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry. 2000;48:127–136. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- 10.Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 11.Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 12.Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia: a review. Schizophr Bull. 1998;24:399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- 13.Morrison RL, Bellack AS, Mueser KT. Deficits in facial-affect recognition and schizophrenia. SchizophrBull. 1988;14:67–83. doi: 10.1093/schbul/14.1.67. [DOI] [PubMed] [Google Scholar]
- 14.Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, Katschnig H. Facial recognition deficits and cognition in schizophrenia. Schizophr Res. 2004;68:27–35. doi: 10.1016/S0920-9964(03)00131-2. [DOI] [PubMed] [Google Scholar]
- 15.Salem JE, Kring AM, Kerr SL. More evidence for generalized poor performance in facial emotion perception in schizophrenia. J Abnorm Psychol. 105:480–483. doi: 10.1037//0021-843x.105.3.480. [DOI] [PubMed] [Google Scholar]
- 16.Schneider F, Gur RC, Koch K, et al. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 163:442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- 17.Suslow T, Droste T, Roestel C, Arolt V. Automatic processing of facial emotion in schizophrenia with and without affective negative symptoms. Cogn Neuropsychiatry. 2005;10:35–56. doi: 10.1080/13546800344000318. [DOI] [PubMed] [Google Scholar]
- 18.van't Wout M, Aleman A, Kessels RP, Cahn W, de Haan EH, Kahn RS. Exploring the nature of facial affect processing deficits in schizophrenia. Psychiatry Res. 2007;150:227–235. doi: 10.1016/j.psychres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Bozikas VP, Kosmidis MH, Anezoulaki D, Giannakou M, Andreou C, Karavatos A. Impaired perception of affective prosody in schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18:81–85. doi: 10.1176/jnp.18.1.81. [DOI] [PubMed] [Google Scholar]
- 20.Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Leitman DI, Hoptman MJ, Foxe JJ, et al. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry. 2007;164:474–482. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- 22.Shea TL, Sergejew AA, Burnham D, et al. Emotional prosodic processing in auditory hallucinations. Schizophr Res. 2007;90:214–220. doi: 10.1016/j.schres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- 25.An SK, Lee SJ, Lee CH, et al. Reduced P3 amplitudes by negative facial emotional photographs in schizophrenia. Schizophr Res. 2003;63:10. doi: 10.1016/s0920-9964(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 26.Ueno T, Morita K, Shoji Y, Yamamoto M, Yamamoto H, Maeda H. Recognition of facial expression and visual P300 in schizophrenic patients: differences between paranoid type patients and non-paranoid patients. Psychiatry Clin Neurosci. 2004;58:585–592. doi: 10.1111/j.1440-1819.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann MJ, Reif A, Jabs BE, Jacob C, Fallgatter AJ. Facial affect decoding in schizophrenic disorders: a study using event-related potentials. Psychiatry Res. 2006;141:247–252. doi: 10.1016/j.psychres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 29.Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Hall J, Whalley HC, McKirdy JW, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Schneider F, Weiss U, Kessler C, et al. Differential amygdala activation in schizophrenia during sadness. Schizophr Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- 33.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 34.Williams LM, Das P, Harris AW, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 35.Kosaka H, Omori M, Murata T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 36.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H, Koeda M, Oda K, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. NeuroImage. 22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Kee KS, Kern RS, Green MF. Perception of emotion and neurocognitive functioning in schizophrenia: what's the link? Psychiatry Res. 1998;81:57–65. doi: 10.1016/s0165-1781(98)00083-3. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker JF, Deakin JF, Tomenson B. Face processing in schizophrenia: defining the deficit. Psychol Med. 2001;31:499–507. doi: 10.1017/s0033291701003701. [DOI] [PubMed] [Google Scholar]
- 41.Silver H, Shlomo N. Perception of facial emotions in chronic schizophrenia does not correlate with negative symptoms but correlates with cognitive and motor dysfunction. Schizophr Res. 2001;52:265–273. doi: 10.1016/s0920-9964(00)00093-1. [DOI] [PubMed] [Google Scholar]
- 42.Martin F, Baudouin JY, Tiberghien G, Franck N. Processing emotional expression and facial identity in schizophrenia. Psychiatry Res. 2005;134:43–53. doi: 10.1016/j.psychres.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Mueser K, Doonan R, Penn D, et al. Emotion recognition and social competence in chronic schizophrenia. J Abnorm Psychol. 1996;105:271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- 44.Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- 45.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Johnston PJ, Devir H, Karayanidis F. Facial emotion processing in schizophrenia: no evidence for a deficit specific to negative emotions in a differential deficit design. Psychiatry Res. 2006;143:51–61. doi: 10.1016/j.psychres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- 48.Corlett PR, Honey GD, Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol. 2007;21:238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- 49.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 50.Maher B. Anomalous experience in everyday life: its significance for psychopathology. Monist. 1999;82:23. [Google Scholar]
- 51.Phillips ML, Senior C, David AS. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med. 2000;30:157–167. doi: 10.1017/s0033291799001397. [DOI] [PubMed] [Google Scholar]
- 52.Holt DJ, Titone D, Long LS, et al. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006;83:247–256. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- 53.Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 54.Phillips ML, Williams L, Senior C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 55.Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Dysfunction of a Cortical Midline Network during Emotional Appraisals in Schizophrenia. Schizophrenia Bulletin. doi: 10.1093/schbul/sbp067. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surguladze S, Russell T, Kucharska-Pietura K, et al. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006;60:423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 58.Russell TA, Reynaud E, Kucharska-Pietura K, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45:107–123. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 59.Krolak-Salmon P, Fischer C, Vighetto A, Mauguiere F. Processing of facial emotional expression: spatio-temporal data as assessed by scalp event-related potentials. Eur J Neurosci. 2001;13:987–994. doi: 10.1046/j.0953-816x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- 60.Vanderploeg RD, Brown WS, Marsh JT. Judgments of emotion in words and faces: ERP correlates. Int J Psychophysiol. 1987;5:193–205. doi: 10.1016/0167-8760(87)90006-7. [DOI] [PubMed] [Google Scholar]
- 61.Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 62.Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int J Psychophysiol. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- 63.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- 64.Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- 65.Johnston VS, Miller DR, Burleson MH. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23:684–693. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 66.Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Res. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 67.Naumann E, Bartussek D, Diedrich O, Laufer ME. Assessing cognitive and affective information processing functions of the brain by means of the late positive complex of the event-related potential. J Psychophysiol. 1992;6:285–298. [Google Scholar]
- 68.Naumann E, Maier S, Diedrich O, Becker G, Bartussek D. Structural, semantic and emotion-focussed processing of neutral and negative nouns: event-related potential correlates. J Psychophysiol. 1997;11:158–172. [Google Scholar]
- 69.Kiehl KA, Hare RD, McDonald JJ, Brink J. Semantic and affective processing in psychopaths: an event-related potential (ERP) study. Psychophysiology. 1999;36:765–774. [PubMed] [Google Scholar]
- 70.Herbert C, Kissler J, Junghofer M, Peyk P, Rockstroh B. Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology. 2006;43:197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 71.Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:355–372. [Google Scholar]
- 72.Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. J Neurosci. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 74.Barsalou LW, Kyle Simmons W, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends Cogn Sci. 2003;7:84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 75.Glenberg A, Havas D, Becker R, Rinck M. Grounding language in bodily states: the case for emotion. In: Pecher D, Zwaan RA, editors. The Grounding of Cognition: The Role of Perception and Action in Memory, Language and Thinking. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 76.Zwaan RA, Radvansky GA. Situation models in language comprehension and memory. Psychol Bull. 1998;123:162–185. doi: 10.1037/0033-2909.123.2.162. [DOI] [PubMed] [Google Scholar]
- 77.Johnson-Laird PN. Mental Models. Cambridge, Mass: Harvard University Press; 1983. [Google Scholar]
- 78.Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. Int J Psychophysiol. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- 79.Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD. Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 81.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 82.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989:129–136. [Google Scholar]
- 83.Hollingshead AB. Two Factor Index of Social Position. New Haven, Conn: Yale University Press; 1965. [Google Scholar]
- 84.Greenhouse S, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 85.Kissler J, Assadollahi R, Herbert C. Emotional and semantic networks in visual word processing: insights from ERP studies. Prog Brain Res. 2006;156:147–183. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- 86.Williamson S, Harpur TJ, Hare RD. Abnormal processing of affective words by psychopaths. Psychophysiology. 1991;28:260–273. doi: 10.1111/j.1469-8986.1991.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 87.Schupp HT, Ohman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- 88.Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. NeuroImage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- 89.Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Res. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 92.Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40:2156–2166. doi: 10.1016/s0028-3932(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 93.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruchsow M, Trippel N, Groen G, Spitzer M, Kiefer M. Semantic and syntactic processes during sentence comprehension in patients with schizophrenia: evidence from event-related potentials. Schizophr Res. 2003;64:147–156. doi: 10.1016/s0920-9964(02)00482-6. [DOI] [PubMed] [Google Scholar]
- 95.Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: electrophysiological evidence for abnormal interactions between semantic and syntactic processing. J Abnorm Psychol. 2006;115:243–256. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- 96.Kuperberg GR, Kreher DA, Ditman T. What can event-related potentials tell us about language, and perhaps even thought, in schizophrenia? Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2009.09.005. Special Issue. In press; http://www.nmr.mgh.harvard.edu/kuperberglab/publications/papers/ERP&language&scz_spissue_IJP_Kuperberg_inpress.pdf. Accessed March 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sitnikova T, Goff D, Kuperberg GR. Abnormalities in conceptual processing dissociate disorganization and negative symptomatology during real-world behavior in schizophrenia. J Abnorm Psychol. In press. [Google Scholar]
- 98.Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- 99.Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- 100.Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- 101.Van Berkum JJA, Holleman B, Nieuwland M, Otten M, Murrel J. Right or wrong? The brain's fast response to morally objectionable statements. Psychol Sci. doi: 10.1111/j.1467-9280.2009.02411.x. In press; http://www.mpi.nl/world/persons/private/josber/vanberkum-nsrevision-final.pdf. Accessed march 10, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Yeap S, Kelly SP, Sehatpour P, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 103.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 104.Butler PD, Schechter I, Zemon V, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 105.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jackendoff R. Foundations of Language. Brain, Meaning, Grammar, Evolution. Oxford, NY: Oxford University Press; 2002. [DOI] [PubMed] [Google Scholar]
- 107.Yaxley RH, Zwaan RA. Simulating visibility during language comprehension. Cognition. 2007;105:229–236. doi: 10.1016/j.cognition.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 108.Gaillard R, Del Cul A, Naccache L, Vinckier F, Cohen L, Dehaene S. Nonconscious semantic processing of emotional words modulates conscious access. Proc Natl Acad Sci U S A. 2006:7524–7529. doi: 10.1073/pnas.0600584103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grainger J, Holcomb PJ. Watching the word go by: on the time-course of component processes in visual word recognition. Lang Linguist Compass. 2009;3:128–156. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- 111.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 112.LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon and Schuster; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.