Abstract
Reduced mismatch negativity (MMN) in response to auditory change is a well-established finding in schizophrenia and has been shown to be correlated with impaired daily functioning, rather than with hallmark signs and symptoms of the disorder. In this study, we investigated (1) whether the relationship between reduced MMN and impaired daily functioning is mediated by cortical volume loss in temporal and frontal brain regions in schizophrenia and (2) whether this relationship varies with the type of auditory deviant generating MMN. MMN in response to duration, frequency, and intensity deviants was recorded from 18 schizophrenia subjects and 18 pairwise age- and gender-matched healthy subjects. Patients’ levels of global functioning were rated on the Social and Occupational Functioning Assessment Scale. High-resolution structural magnetic resonance scans were acquired to generate average cerebral cortex and temporal lobe models using cortical pattern matching. This technique allows accurate statistical comparison and averaging of cortical measures across subjects, despite wide variations in gyral patterns. MMN amplitude was reduced in schizophrenia patients and correlated with their impaired day-to-day function level. Only in patients, bilateral gray matter reduction in Heschl's gyrus, as well as motor and executive regions of the frontal cortex, correlated with reduced MMN amplitude in response to frequency deviants, while reduced gray matter in right Heschl's gyrus also correlated with reduced MMN to duration deviants. Our findings further support the importance of MMN reduction in schizophrenia by linking frontotemporal cerebral gray matter pathology to an automatically generated event-related potential index of daily functioning.
Keywords: magnetic resonance imaging, cortical pattern matching, mismatch negativity, event-related potential, sociooccupational functioning, auditory, temporal lobes, frontal lobes, Heschl's gyrus
Introduction
Occasional variations of acoustic stimuli in a regular sequence of sounds generate an event-related potential (ERP) of the brain known as mismatch negativity (MMN). MMN is extracted as a difference waveform by subtracting the ERP to infrequent deviant sounds from the ERP to frequent regular or standard sounds. Due to the lower probability of deviant stimuli, however, the difference waveform is also likely to include some changes in obligatory ERP components, which are reflecting physical stimulus difference and neural refractoriness in afferent mechanisms.
MMN generation is not reliant on active attention to the sound sequence and is therefore often described as preattentive, but it is reliant on a memory record of the immediate history of auditory information, a record that appears to have similar sensory resolution to auditory perception.1
A substantial body of research2 indicates that the memory system underlying MMN enables the brain to process sounds with respect to a relevant acoustic context and to automatically identify events that might be behaviorally relevant prompting an attention switch for further processing. This memory system incorporates a model of the acoustic context used to make perceptual inferences about the nature of future sound events and is considered evidence of a “primitive intelligence” in the auditory system.
MMN amplitude reduction is a well-replicated finding in schizophrenia research3 following the first report in 19914 and is considered a robust biological marker of the disorder.5 MMN abnormality is also linked to glutamate hypofunction that has been implicated in schizophrenia.6–10 Pharmacological studies demonstrate that MMN is critically dependent on the functional state of N-methyl-D-aspartate (NMDA) glutamate receptors.11–14 For instance, healthy subjects with the smallest MMN developed more severe “psychotic” reactions following administration of the glutamate antagonist, ketamine.14 NMDA receptor antagonists can also induce symptoms and cognitive impairments in healthy individuals that resemble those in schizophrenia.15 Conversely, agents that enhance NMDA receptor function increase MMN amplitude in schizophrenia.10
There is clear evidence of structural brain abnormalities in established schizophrenia,16,17 and glutamate hypofunction has also been implicated in the neuropathological processes that can lead to cortical gray matter decline.7,8,18 Furthermore, MMN reduction, as a putative measure of NMDA dysfunction, has been reported to correlate with gray matter volume reduction in Heschl's gyrus in the left hemisphere. Further MMN decline over the course of the illness has been associated with progressive gray matter loss in this region.19
This finding is largely consistent with functional brain imaging using various modalities, such as positron emission tomography (eg, Dittmann-Balcar et al20), functional magnetic resonance imaging (eg, Schall et al21), and current source density mapping (eg, Shalgi and Deouell22), indicating bilateral MMN generators in the auditory cortices with some additional contribution from frontal and prefrontal cortex. Functionally consistent with contributions to MMN generators from frontal sources are findings that reduced MMN in schizophrenia has been associated with poor proverb interpretation and verbal memory23 and poor overall functional status across psychological, social, and occupational domains.24 Importantly, these cognitive and functional measures persistently characterize schizophrenia, rather than psychotic symptoms, which tend to fluctuate.
There is some indication in the literature that MMN to distinct deviant features may be differentially sensitive to auditory processing deficits in schizophrenia and may differ across the course of the illness.3 There is now some evidence consistent with a progressive decline in frequency MMN amplitude across the course of the illness (see Todd et al25 for review) suggesting that the tonotopic organization of frequency encoding may render this index particularly useful in studying progressive changes in cortical gray matter volume.
The current study investigated associations between MMN to duration, frequency, and intensity deviants and cerebral gray matter from high-resolution magnetic resonance images (MRIs) using cortical pattern matching techniques26 in schizophrenia and closely age- and gender-matched control subjects. This method permits the aggregation of MRI data by using the same anatomical reference locations across subjects and explicitly models individual differences in cortical folding patterns as well as overall brain size. Using this technique, accurate in vivo measurements of cortical gray matter can be calculated in grouped data and correlated with functional measures.27
If MMN indexes (at least in part) pathology that results in cortical volume loss, then this pathology is unlikely to be restricted to the auditory cortex. Firstly, we predicted that MMN amplitude would be correlated with cerebral gray matter reduction in many areas associated with dysfunction in schizophrenia (eg, temporal, frontal, and prefrontal brain regions). Secondly, we predict that the gray matter-MMN association will be best represented by frequency deviance as an index of illness progression25 linked to more pronounced cerebral pathology and lower sociooccupational function levels.28
Methods
The study was approved by the Hunter New England Human Research Ethics Committee. All participants gave informed written consent to participate in the study.
Participants
Participants were recruited from the community by advertisement, from the Schizophrenia Research Register,29 and from the Hunter Medical Research Institute Volunteer Register. All subjects were tested for intact hearing and examined for relevant medical and neurological conditions, including standard MRI exclusion criteria and alcohol and illicit substance use.
Eighteen subjects (13 males and 5 females, age range = 16–67 y, mean age = 32.8 y, SD = ±12.3) met diagnostic criteria for schizophrenia according to the International Classification of Diseases (Tenth Edition; World Health Organization) that was confirmed by the Diagnostic Interview for Psychosis.30 Mean duration of illness was 11.8 years (SD = ±8.8 y and ranging from 2 to 32 y). Age and duration of illness were highly correlated (r = 0.90, P < .001, 2-tailed significance).
All but one schizophrenia participant was stabilized on first- (n = 3) and/or second-generation (n = 14) antipsychotic medication and not acutely psychotic at the time of testing. The remaining participant was well but reported taking nil medication for the 3 months prior to testing.
Eighteen healthy control subjects (age range = 17–65 y, mean age = 33.0 y, SD = ±12.3) without personal or family history in first- and second-degree biological relatives were pairwise age- (<3 y) and gender-matched to the schizophrenia subjects. The groups did not differ on premorbid IQ estimates (schizophrenia group: 110.4, SD = 6.5; healthy control subjects: 111.8, SD = 8.8) as assessed by the National Adult Reading Test,31 but they did differ (t = 3.5, P < .002) in years of formal education (schizophrenia group: 11.3 y, SD = 2.1; healthy control subjects: 14.4 y, SD = 2.9). In addition, sociooccupational function levels were determined in schizophrenia subjects using the Social and Occupational Functioning Assessment Scale32 (SOFAS) that closely corresponds to the Global Assessment of Functioning Scale32 with less symptom severity confounds.33
Stimuli and MMN Recording
MMN recording procedures closely followed those described by Todd et al.25 Participants were presented with a sequence of 2400 tones (regular 450-ms stimulus-onset asynchrony) in a pseudorandom presentation order of standard tones (P = .82, 50 ms, 1000 Hz, 80-dB sound pressure level [SPL]) and 3 types of deviant tone (P = .06 each): “duration” (125 ms), “frequency” (1200 Hz), and “intensity” (90-dB SPL). Participants were instructed to watch a video and to ignore the sounds presented via headphones.
Continuous electroencephalographic (EEG) data sampled at 500 Hz were obtained from 28 scalp sites in accordance with the 10-20 system, the left and right mastoid, and a nose reference using Neuroscan software and hardware (Neuroscan, El Paso, TX). Vertical and horizontal electrooculogram was monitored by electrodes above and below the left eye and 1 cm to the side of the outer canthi of each eye, respectively.
EEG data were analyzed off-line. Epochs beginning 100 milliseconds before each stimulus and ending 450 milliseconds after stimulus onset were extracted and the scalp sites rereferenced to the algebraic sum of the 2 mastoids. Eye blink artifacts were corrected with procedures implemented in Neuroscan, and epochs exceeding 100 μV were excluded. The averaged responses to standard and deviant tones were corrected for linear trends and low pass filtered at 30 Hz. The MMN was obtained by subtracting the standard from the deviant ERP for each deviant type. The MMN waveforms were low pass filtered at 20 Hz. Peak MMN amplitudes within a 100- to 300-millisecond poststimulus window relative to 100-millisecond prestimulus baseline were computed for the frontal-central (Fz) site.
MRI Data Collection and Analysis
MRI data were acquired from a Siemens Magnetom Vision 1.5-T MRI scanner (John Hunter Hospital, Newcastle, Australia) to obtain high-resolution scans (3D MPRAGE protocol with repetition time = 9.7 ms, echo time = 4 ms, and flip angle = 12°) with voxel volumes 1 mm3. Two modes of structural analyses were pursued using cortical pattern matching.26 The first involved the cerebral cortical gray matter and the second a region of interest approach, where the temporal lobe cortical gray matter was examined in isolation in order to examine deeper cortical structures, such as Heschl's gyrus, in more detail.
Subject's MRIs were transformed to International Consortium for Brain Mapping34 space using mritotal35 or manually using the software program Register (available at http://www.bic.mni.mcgill.ca/software/register/register.html) and a 12-parameter affine transformation. This was followed by a radio frequency bias correction.36 Masking of the cerebrum was then performed, and a model of the cerebral cortex was extracted from the resulting image.37 This model was used together with the subject's MRI to identify and trace sulcal and control lines (defining the midline hemisphere boundaries) on each hemisphere in adherence with the anatomical protocol available at http://www.loni.ucla.edu/∼esowell/edevel/new_sulcvar.html.
Using the cerebral cortex models and lines from all 36 subjects, a geometric average model was created, and each individual cortex was deformed to match it using cortical pattern matching.26 This establishes a computed correspondence mapping between the vertices comprising the subject's cerebral cortex in the average model space and the voxels of each individual subject's MRI in native space.
To avoid confounds when measuring cortical gray matter in each individual subject,38 all cortical gray matter measures were computed in the native space of each subject and then transformed to the average model space. For these calculations, in native space, each subject's MRI was corrected for intensity inhomogeneity due to radio frequency bias fields36 in areas defined by a dilated native space version of their cerebral mask. This was followed by a skull stripping of the radio frequency bias–corrected MRI using this dilated mask.
A fully automated tissue classification was then performed on each subject's scan using a partial volume classifier.39 The cerebrum masks (in native space) were used to crop the cerebrum from the tissue-classified volumes that were then supersampled reducing their voxel dimensions by one-third. In native space, the overall cerebral volume for each subject was calculated as well as the average cerebral volume for the 36 subjects.
Using the corresponding locations within native space of each vertex comprising a subject's deformed cerebral cortex model, the proportion of gray matter to gray plus white matter within a subject-specific kernel (a sphere of radius 15 mm multiplied by the ratio of the individual to average cerebral volume) was determined in native space from the subject's tissue-classified volume and allocated to the corresponding vertex in average space. The subject-specific kernel sizes were used to account for varying cerebral volumes across the subjects.
A deformable version of the “Montreal Neurological Institute (MNI) colin27” cerebral atlas27,40 was also used to label the gyral structures of the average cortex, thus enabling gyrus-wise averaging of the gray matter measure for each subject.
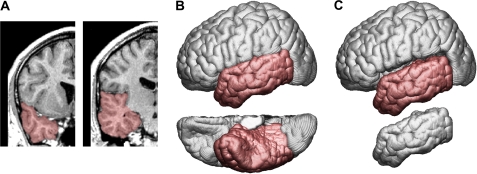
Masking and extraction of the temporal lobes as well as a subset of the sulcal lines (ie, superior temporal, inferior temporal, collateral, and occipital-temporal sulcus) were used to generate the average temporal lobe models using cortical pattern matching. The masking of the temporal lobes involved the boundaries defined by the temporal poles and the Sylvian fissure from the lateral border of the temporal lobes and medially along the cerebral peduncle (including the hippocampus, parahippocampal gyrus, and uncus). The insular and inferior insular cortices were excluded and used to define boundaries. The posterior boundary of the temporal lobe was defined by a surface line beginning medially at the junction of the parietooccipital sulcus and the calcarine fissure passing along the emerging basal temporal sulci and ending at the occipital temporal notch as well as going along the posterior boundaries of the inferior, middle, and superior temporal gyri (figure 1). All other methods followed those described for the cerebrum.
Fig. 1.
Coronal Views of a Single Subject's structural Magnetic Resonance Image with Temporal Lobe Masked in Red (A). Left lateral (top) and ventral (bottom) views of the cerebrum with temporal lobe indicated in red (B). Left lateral views of the temporal lobe semidetached (top) and detached (bottom) from the cerebrum (C).
Statistical Analyses
Data distributions were assessed for compliance with the assumptions of parametric tests. MMN peak amplitude group differences were tested by 2-sample t test for each deviant type, and correlations with age were assessed with Pearson correlation coefficients. A 2-sided P value of <.05 was considered statistically significant. Associations of SOFAS with MMN measures were assessed with Spearman ρ. A 1-sided P value of <.05 was considered statistically significant by taking the directional nature of our hypothesis into account.24
Correlation maps of cerebral gray matter measures with MMN peak amplitudes for each MMN type were calculated, statistically thresholded at P < .05 (uncorrected), and permutation tested41 at P < .05 for each group and hemisphere. Associations at the gyral level were tested post hoc by Pearson correlation coefficients at P < .05 (2 sided).
Results
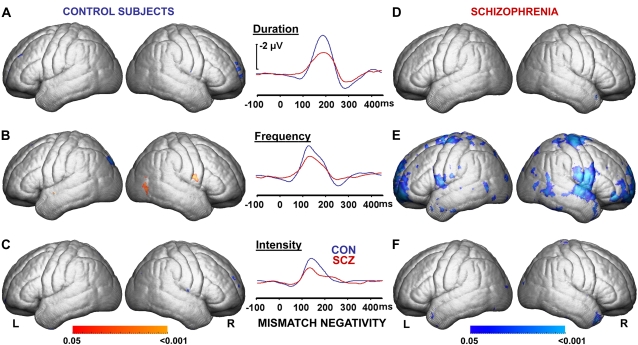
MMN peak amplitudes recorded at Fz were significantly lower in schizophrenia subjects vs healthy control subjects for frequency deviants (t = 2.2, P < .04. figure 2), with similar trends for duration (t = 2.0, P < .06) and intensity deviants (t = 1.9, P = .06). Duration MMN was significantly correlated with age (r = 0.37, P < .03) in the combined dataset but did not reach statistical significance in the individual groups (patients: r = 0.32, P = .21, and control participants: r = 0.31, P = .22). Frequency MMN was correlated with age only in patients (r = 0.66, P = .003). Sociooccupational function levels of schizophrenia subjects (SOFAS scores) correlated with frequency MMN recorded at Fz (rρ = −0.43, P < .04, figure 3C) and also tended to correlate with duration (rρ = −0.38, P = .06) but not with intensity (rρ = 0.10, P = .45) MMN peak amplitudes.
Fig. 2.
Correlation Maps of Mismatch Negativity (MMN) Recorded at frontal-central by Gray Matter Measures Coregistered on Averaged Left and Right Cerebral Hemispheres of 18 Healthy Control Subjects (CON: A, B, C) and 18 Schizophrenia Patients (SCZ: D, E, F). Cerebral correlation maps were statistically thresholded at P < .05 (uncorrected) and permutation tested for each hemisphere at P < .05. Blue map colors indicate an association of reduced gray matter measures with reduced MMN peak amplitudes, while red map colors indicate reversed relationship. (E) Permutation testing confirmed association of MMN with gray matter in schizophrenia patients for the left and right hemisphere for frequency deviants (see figure 5 for detailed analysis at gyral level).
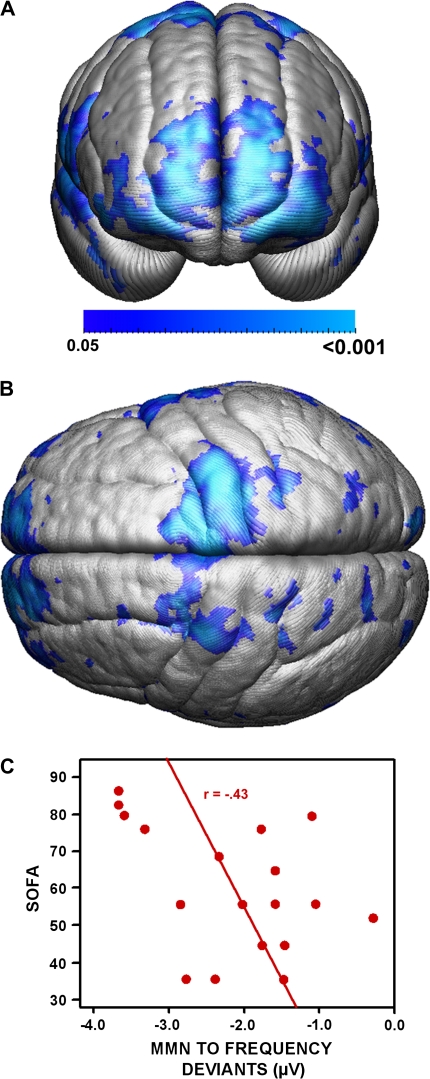
Fig. 3.
(A) Anterior View and (B) Superior View of Correlation Maps Between Frequency Mismatch Negativity (MMN) Recorded at frontal-central and Measures of Frontal Gray Matter. r Values are thresholded at P < .05 (uncorrected) and confirmed to be significant by permutation testing for both hemispheres at P < .05. (C) Low levels of sociooccupational functioning ratings (Social and Occupational Functioning Assessment Scale [SOFAS]) are correlated (P < .05, 1 tailed) with lower MMN amplitudes to frequency deviants in schizophrenia subjects.
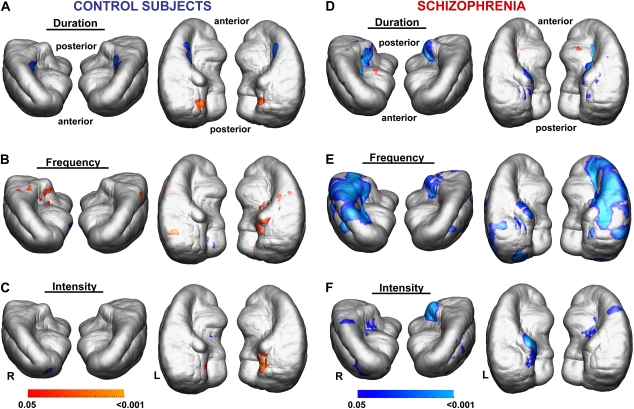
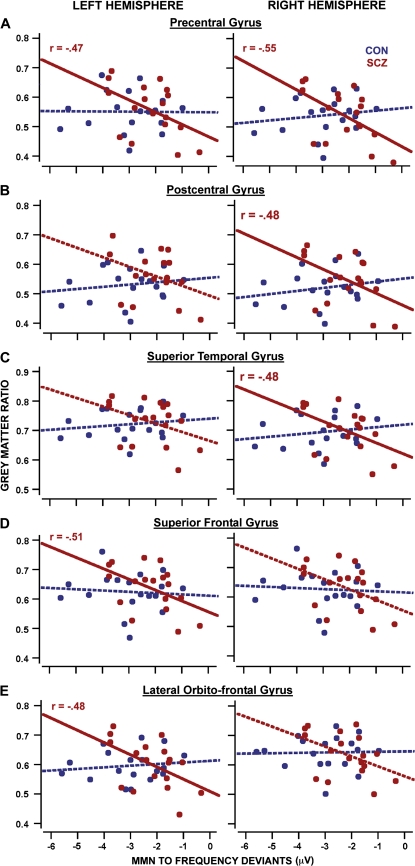
Correlations of gray matter measures with reduced frequency MMN peak amplitudes recorded at Fz were confirmed in schizophrenia subjects by permutation tests for the left and right cerebrum (figure 2E), for left and right temporal lobes (including Heschl's gyrus bilaterally, figure 4E), as well as for duration MMN for right Heschl's gyrus (figure 4D). Such an association was not confirmed for healthy subjects with any MMN type (figures 2A—C, figures 4A–C). Post hoc correlation analyses in schizophrenia patients confirmed (P < .05) an association of reduced gray matter measures with reduced frequency MMN peak amplitudes recorded at Fz for left and right precentral gyri, right postcentral and right superior temporal gyri, left superior frontal gyri, and left lateral frontoorbital gyri as determined by a deformable version of the MNI colin27 cerebral atlas (figure 5A–E).
Fig. 4.
Correlation Maps Showing Associations Between Mismatch Negativity (MMN) Recorded at frontal-central and Local Gray Matter Measures, Coregistered on Averaged Left and Right Temporal Lobes for Each MMN Type (Anterior and Superior Views). Maps are thresholded at P < .05 (uncorrected). Permutation testing confirmed the association of reduced gray matter with reduced MMN peak amplitudes in schizophrenia for (D) duration MMN in the right Heschl's gyrus and (E) bilaterally for frequency MMN, at P < .05.
Fig. 5.
Post Hoc Correlation Analyses of Reduced Gray Matter Measures—Averaged Over Specific Gyri Defined on the Deformed “Montreal Neurological Institute colin27” Brain Template—With Reduced Frequency Mismatch Negativity (MMN) Peak Amplitudes Recorded at frontal-central in Control Subjects (Blue) and Schizophrenia Patients (Red). Solid regression lines indicate significant correlation (P < .05, uncorrected).
Discussion
Our findings confirm an association of MMN reduction recorded at Fz in schizophrenia with MRI-derived measures of cortical gray matter estimates in areas subserving auditory processing (eg, Heschl's gyrus; compare Salisbury et al19). The most robust correlation was found for MMN measures derived from frequency deviants suggesting that frequency MMN is more linearly associated with pathological changes in schizophrenia than duration or intensity MMN. We have previously raised the question of whether deviance in different sound features could provide complimentary evidence of the pathology underlying MMN reduction in schizophrenia. The observation of Salisbury et al19 suggests that at least some aspect of this pathology is progressive and that the frequency MMN amplitude change is accompanied by a change in the gray matter volume of auditory processing areas. Our present findings are certainly consistent with this observation and with our previous findings showing a progressive decline of MMN in the course of illness predominantly in response to frequency but not to duration and intensity deviants.25
When different forms of deviant are used, there are many factors that complicate a comparison between the resultant MMNs elicited (see Todd et al25 for discussion). However, we hypothesize that it is likely that the multiple tonotopic representations of frequency information in auditory cortex could explain why gray matter volume is more linearly associated with frequency MMN in the present study. These multiple representations might afford frequency MMN some degree of resilience to initial disruption of auditory processing, but as the disruption spreads engulfing other tonotopic representations, frequency MMN declines, rendering it particularly sensitive to a linear progression of the pathology.25 That frequency-derived MMN in particular is closely associated with bilateral gray matter reduction in Heschl's gyrus in schizophrenia suggests a neuropathology that affects the integrity of the tonotopic organization of frequency representation and sound processing in primary auditory cortex with progression of illness. These findings are consistent with observations that perceptual resolution of frequency differences (frequency discrimination) is impaired in schizophrenia42 and becoming more severe with chronicity of illness.43
While our findings suggest that frequency MMN is more closely associated with illness chronicity than MMN to duration and intensity deviants, it is possible that our sample size did not provide sufficient power to detect potentially weaker associations of gray matter deficits, for instance, with intensity-elicited MMN. It is also possible that the reduction in MMN to intensity (and potentially duration) change is not as well suited to linear association with progressive pathology. There is evidence that these features are represented in a distributed fashion across the auditory cortex,44 anterior cingulate, and presupplementary motor area45 and as such, could be less resistant to early signs of the pathology or simply less linearly affected.
As age and duration of illness are highly correlated, and gray matter volume declines with age over the human life span,46 reduction of MMN amplitude and its association with reduced gray matter could be confounded by normal ageing. Our findings, however, clearly indicate that pairwise age- and gender-matched healthy control subjects do not show an association of MMN with gray matter. Furthermore, frequency MMN is correlated with age only in patients and not in healthy participants. Hence, the association of frequency-elicited MMN with gray matter measures appears to be specific to progressive neuropathology in schizophrenia and not driven by genuine age-related variability across the 2 measures when accepting age as a proxy measure of duration of illness.
Medication is another important factor when interpreting our findings. Both human as well as animal research47,48 suggest reduction of gray matter with haloperidol exposure and to exposure to second-generation antipsychotics, though to a lesser extent when compared with first-generation antipsychotics.17,49,50
However, there is no consistent evidence that antipsychotic medication affects MMN generation (eg, Catts et al51; Schall et al52), although a relatively intact MMN to frequency deviants predicts a favorable response to clozapine treatment in chronic schizophrenia patients who fail to respond to other antipsychotics.53 Nonetheless, more systematic pharmacological research, including animal models, is required to establish whether MMN generation is unaffected by exposure to antipsychotic medication and whether medication effects on gray matter are linked to illness-related changes of cortical gray matter.
Some recent anatomical evidence from postmortem data is consistent with impaired auditory information processing in schizophrenia.54 The authors stereologically quantified the density of synaptophysin-immunoreactive axon terminals in the gray matter of Brodmann areas 41 and 42. Feedback auditory pathways in layer 1 of area 41 and layer 3 of area 42 were intact in schizophrenia, while reduced terminal densities were found in feed-forward pathways of layer 3 in area 41. This may affect the spread of activation within primary auditory cortex in response to acoustic stimuli and, in turn, may affect the performance of neural circuits involved with sound discrimination and MMN generation.
Importantly, the authors found no evidence that long-term exposure to antipsychotic medication reduces synaptophysin-immunoreactive axon terminals in nonhuman primates.54 If confirmed by more data, this finding would be consistent with a lack of effect of antipsychotics on MMN generation in schizophrenia. However, further animal research is required to better understand the neurocircuitry of auditory mismatch processing and how individual pathways contribute to auditory mismatch generation.
Our data revealed that associations between frequency-elicited MMN and reduced gray matter measures in schizophrenia were not limited to auditory areas; they also extended into other cortical areas involved in motor organization and executive function. The topographic distribution of MMN/gray matter associations in schizophrenia is consistent with current source density studies (eg, Shalgi and Deouell22) and functional brain imaging data (e.g., Dittmann-Balcar et al20; Schall et al21) implicating temporal and frontal cortical areas in the generation of MMN. Such an association was not detected in healthy control subjects or for duration and intensity-elicited MMN in schizophrenia. In this respect, associations of MMN in response to frequency deviants with frontal gray matter reduction indicate a more widespread brain pathology—beyond auditory cortex—that is linked to MMN reduction with longer duration of illness.
We also confirmed earlier reports of a correlation of smaller MMN amplitudes with poor sociooccupational functioning in our schizophrenia sample,24 although in our sample, this correlation was once again most robust for frequency MMN. However, one explanation of this relationship that emerges from our data is that the relationship between decreased MMN and impaired functional skills occurs, not because these deficits are causally linked but because they are both related to frontal lobe pathology.
In summary, the current study shows that MMN is a useful tool to investigate important features of the disorder, such as progression of illness, clinical outcomes, and potentially treatment response, as well as the importance of the choice of the physical characteristics of the deviant stimuli when conducting MMN research in schizophrenia. MMN also holds the potential to provide more profound insights into the underlying pathophysiology by identifying core neurophysiological deficits of the disorder, how they develop, and how they give rise to the clinical features of the condition.
Funding
National Health and Medical Research Council of Australia (209828, 252480).
Acknowledgments
We are grateful to C. Atkinson, N. Bilton, G. Cooper, R. Fulham, K. Gjermundsen, S. Hudson, R. Inkpen, K. Khoo, C. Loughland, N. Matthews, G. O'Connor, P. Thiruthaneeswaran, F. Tradefelt, and P. Wynne for their valuable assistance in this project. “BrainSuite2” was supported by National Institute of Biomedical Imaging and Bioengineering grant R01 EB002010 and National Center for Research Resources grant RR013642. Infrastructure support was received from the Hunter Medical Research Institute, the Schizophrenia Research Institute, and New South Wales Health.
References
- 1.Näätänen R. Mismatch negativity: clinical research and possible applications. Int J Psychophysiol. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 2.Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. Primitive intelligence in the auditory cortex. Trends Neurosci. 2001;24:283–288. doi: 10.1016/s0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- 3.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 5.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42:177–194. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson ML, Carlsson A, Nilsson M. Schizophrenia: from dopamine to glutamate and back. Curr Med Chem. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- 7.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 9.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 11.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 13.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 14.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 15.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 16.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in Schizophrenia with different treatments. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn152. doi:10.1093/cercor/bhn152. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dittmann-Balcar A, Jüptner M, Jentzen W, Schall U. Dorsolateral prefrontal cortex activation during automatic auditory duration-mismatch processing in humans: a positron emission tomography study. Neurosci Lett. 2001;308:119–122. doi: 10.1016/s0304-3940(01)01995-4. [DOI] [PubMed] [Google Scholar]
- 21.Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. Neuroimage. 2003;20:729–736. doi: 10.1016/S1053-8119(03)00398-7. [DOI] [PubMed] [Google Scholar]
- 22.Shalgi S, Deouell LY. Direct evidence for differential roles of temporal and frontal components of auditory change detection. Neuropsychologia. 2007;45:1878–1888. doi: 10.1016/j.neuropsychologia.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kuta M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007;13:653–663. doi: 10.1017/S1355617707070816. [DOI] [PubMed] [Google Scholar]
- 24.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 25.Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Thompson PM, Hayashi KM, Sowell ER, et al. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 27.Rasser PE, Johnston P, Lagopoulos J, et al. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. Neuroimage. 2005;26:941–951. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Pantelis C, Velakoulis D, Wood SJ, et al. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19:371–379. doi: 10.1080/09540260701512079. [DOI] [PubMed] [Google Scholar]
- 29.Loughland CM, Carr V, Lewin T. The NISAD schizophrenia research register: why do we need a database of schizophrenia volunteers? Aust N Z J Psychiatry. 2001;35:660–667. doi: 10.1080/0004867010060516. [DOI] [PubMed] [Google Scholar]
- 30.Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36:69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- 31.Nelson HE, Willison JR. The Revised National Adult Reading Test—Test Manual. Windsor, England: NFER-Nelson; 1991. [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 33.Lehoux C, Everett J, Laplante L, et al. Fine motor dexterity is correlated to social functioning in schizophrenia. Schizophr Res. 2003;62:269–273. doi: 10.1016/s0920-9964(02)00327-4. [DOI] [PubMed] [Google Scholar]
- 34.Mazziotta JC, Toga AW, Evans AC, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 36.Sled JG, Zijdenbos AP, Evans AC. A non-parametric method for automatic correction of intensity non-uniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald D, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance imaging. In: Robb RA, editor. Visualization in Biomedical Computing. Rochester, MN: Proceedings of SPIE; 1994;2359:160–169. [Google Scholar]
- 38.Allen JS, Bruss J, Mehta S, Grabowski T, Brown CK, Damasio H, et al. Effects of spatial transformation on regional brain volume estimates. Neuroimage. 2008;42:535–547. doi: 10.1016/j.neuroimage.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 40.Rasser PE, Johnston P, Ward P, Thompson PM. A deformable brodmann area atlas. In: Leahy R, editor. IEEE International Symposium on Biomedical Imaging: Nano to Macro, 2004. Arlington, VA: IEEE; 2004;1:400–403. [Google Scholar]
- 41.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 44.Bueti D, van Dongen EV, Walsh V. The role of superior temporal cortex in auditory timing. PLoS ONE. 2008;3:e2481. doi: 10.1371/journal.pone.0002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastor MA, Macaluso E, Day BL, Frackowiak RS. The neural basis of temporal auditory discrimination. Neuroimage. 2006;30:512–520. doi: 10.1016/j.neuroimage.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 46.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human lifespan. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 47.Reinke A, Martins MR, Lima MS, Moreira JC, Dal-Pizzol F, Quevedo J. Haloperidol and clozapine, but not olanzapine, induces oxidative stress in rat brain. Neurosci Lett. 2004;372:157–160. doi: 10.1016/j.neulet.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Wright AM, Bempong J, Kirby ML, Barlow RL, Bloomquist JR. Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res. 1998;788:215–222. doi: 10.1016/s0006-8993(97)01551-5. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 50.Molina V. Structural effects of atypical antipsychotics: implications for the meaning of cortical volume deficit in schizophrenia. Eur J Psychiatry. 2005;19:231–242. [Google Scholar]
- 51.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 52.Schall U, Catts SV, Chaturvedi S, et al. The effect of clozapine therapy on frontal lobe dysfunction in schizophrenia: neuropsychology and event-related potential measures. Int J Neuropsychopharmacol. 1998;1:19–29. doi: 10.1017/S146114579800100X. [DOI] [PubMed] [Google Scholar]
- 53.Schall U, Catts SC, Karayanidis F, Ward PB. Auditory event-related potential indices of fronto-temporal information processing in schizophrenia syndromes: valid outcome prediction of clozapine therapy in a three-year follow-up. Int J Neuropsychopharmacol. 1999;2:83–93. doi: 10.1017/S1461145799001418. [DOI] [PubMed] [Google Scholar]
- 54.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]