Abstract
Background: Cerebral morphological abnormalities in schizophrenia may be modulated by treatment, chronicity, and duration of illness. Comparing brain imaging studies of individuals with first-episode schizophrenia and neuroleptic naive (NN-FES) with that of their neuroleptic-treated counterparts (NT-FES) can help to dissect out the effect of these potential confounders. Methods: We used the anatomical likelihood estimation method to compare voxel-based morphometric studies of NN-FES (n = 162 patients) and NT-FES (n = 336 patients) studies. The analysis included a sample size weighting step based on the Liptak-Stouffer method to reflect the greater power of larger studies. Results: Patient samples were matched for age, gender, and duration of illness. An extensive network of gray matter deficits in frontal, temporal, insular, striatal, posterior cingulate, and cerebellar regions was detected in the NN-FES samples as compared with healthy controls. Major deficits were detected in the frontal, superior temporal, insular, and parahippocampal regions for the NT-FES group compared with the NN-FES group. In addition, the NT-FES group showed minor deficits in the caudate, cingulate, and inferior temporal regions compared with the NN-FES group. There were no regions with gray matter volumetric excess in the NT-FES group. Conclusion: Frontal, striato-limbic, and temporal morphological abnormalities are present in the early stage of schizophrenia and are unrelated to the effects of neuroleptic treatment, chronicity, and duration of illness. There may be dynamic effects of treatment on striato-limbic and temporal, but not frontal, regional gray matter volumes of the brain.
Keywords: meta-analysis, neuroleptic naive, subtraction analysis, brain structure, voxel-based morphometry, ALEn
Introduction
Modern research has had some success in deconstructing the neurobiology of schizophrenia, partly fuelled by the advent of magnetic resonance imaging (MRI). The enormous effort in striving for this “Holy Grail of biological psychiatry”1 can be seen from the volume of MRI-related publications in schizophrenia which now averages about 400 annually (PubMed search accessed June 15, 2009). In piecing together the complex puzzle of brain abnormality in schizophrenia, the majority of studies have focused on chronically ill populations, but more recently, attention has turned to newly diagnosed, minimally, or never-treated patients with this disorder.2,3 Strategically, recruiting such individuals helps to reduce confounders such as age of onset, illness duration, and treatment that may dilute the neuropathological findings in schizophrenia.4,5 In other words, the ideal participant in MRI research would be in his or her first episode of schizophrenia (FES).
With the plethora of structural brain findings in schizophrenia comprising reduced brain volume, lateral ventricular enlargement, frontal, temporal, limbic, and subcortical deficits,6–10 integration and characterization of all this information is formidable. Meta-analysis is a statistical tool which conveniently synthesizes these findings. Meta-analytic approaches can be divided into 2 types according to the data collected: region of interest (ROI) and voxel-based morphometry (VBM). Conventional effect-size meta-analyses of ROI studies consolidate studies based on manual tracing of targeted brain regions. These generally agree that there is significant lateral ventricular enlargement along with smaller volumes of whole brain and hippocampus in patients in their FES compared with healthy controls.11,12 On the other hand, meta-analysis of VBM studies which evaluate each voxel or “volume element” across the whole brain has revealed an extensive network of gray matter deficits in FES compared with healthy controls, including frontostriatal temporal regions and insula.13,14
However, even in studies of FES, it is not always possible to rule out brain changes partly due to drug treatment because excluding any prior exposure to antipsychotic medication is difficult in practice.11 Antipsychotic treatment is often started at the time of diagnosis, and hence, the sample size in purely neuroleptic-naive (NN)-FES studies is usually small,15 although Lui et al16 successfully recruited a particularly large NN-FES sample comprising 68 individuals. Moreover, evidence that neuroleptics can alter brain structure has accumulated17–19 and underscores the basal ganglia as the principal dopamine receptor-rich site and target of antipsychotic action.18,20,21 Indeed, first-time exposure to antipsychotic treatment in NN-FES for as short as 3-week duration has an incremental effect on caudate size22 and increases thalamus volume by 8 weeks.23 In addition, 4- to 6-week treatment with risperidone has been reported to cause gray matter increase in superior and middle temporal gyrus and decrease in left frontal lobe and rectal gyrus.24 This early effect on brain structure is consistent with substantial evidence that the maximal clinical efficacy may be at the third and fourth weeks of treatment,25 or even earlier at the first and second weeks.26
Thus, the neuroleptic effect on brain volume and psychotic symptoms in schizophrenia may be prominent as early as 1 month after drug treatment. Even with an average treatment duration as brief as 1.6 months27 or 1.7 months,28 it is still possible that neuroleptic-related brain changes would already be impossible to exclude. This makes it difficult to separate neurotoxic changes relating to the disorder per se, from neuroplastic or other changes caused by pharmacotherapy. In those studies that have successfully recruited individuals with NN-FES, some discrepancies remain: for example, thalamic deficits in FES were only found in males15 but not in other NN-FES studies.6,16,29,30 Insular deficits were detected by some29,31 but not all studies.6,16
Thus, it is possible that an admixture of NT and NN individuals might hamper the interpretation of previous meta-analyses of FES. Over time, it has become more likely that samples of patients with NN-FES be included in meta-analytic studies. Honea et al32 included one study of NN-FES15 and 2 studies with neuroleptic-treated patients (NT-FES).28,33 Ellison-Wright et al14 included 3 NN-FES studies6,15,31 and 4 NT-FES studies.27,28,33,34 However, as more studies of NN patients have been carried out in the interim, it seems timely to attempt to isolate the effect of early drug treatment from FES itself.
The anatomical likelihood estimation (ALE) approach permits automated meta-analysis of either functional or structural voxel-based neuroimaging data sets.35,36 In synthesizing data generated from VBM studies, ALE attempts to identify the regions most consistently implicated across all VBM studies of the relevant disorder. We and others have described its successful application in meta-analyses of attention deficit hyperactivity disorder (ADHD),37 depression,38 and schizophrenia.13,14,39–41 The basis of ALE is to create a Gaussian probability distribution around the coordinates reported in individual VBM studies.36 In this way, the probability that any given voxel is implicated in the target condition can be estimated. Essentially, ALE will merge coordinates from different studies that are spatially close. The resultant clusters therefore reflect brain regions most often reported or “common” to the majority of studies. A useful extension of ALE is that a subtraction analysis can be carried out. For example, previous ALE studies of schizophrenia estimated the progression of brain pathology by “subtracting” FES data from data generated in studies of chronic schizophrenia.13,14
However, ALE may have a disadvantage when studies of different sample sizes are treated equally. In allocating the same weight to coordinates from every constituent study, regardless of the study sample size, ALE is excessively “democratic.” Indeed, this issue was also recently highlighted by Ellison-Wright et al.37,42 In general, increasing sample size improves statistical power by reducing the standard error of the sample mean so ideally, larger studies deserve more weight in an analysis. In an elegant solution to this problem,37,42 the sum-rank method from genome scan meta-analysis was adapted to rank VBM data according to sample size and generate “sum-rank images” for a nonparametric permutation analysis.43 However, to date, weighted data have not been entered into “subtraction” analyses.
In the current study, we propose an alternative approach to sample size weighting which for convenience we refer to as “ALEn” (anatomical likelihood estimation weighted by number of subjects). The height of each Gaussian distribution was directly modulated by the square root of the size of the study sample37,42,44 contributing at that coordinate. Any deviation from null hypothesis was identified by statistically comparing the weighted ALE map to simulated maps of random foci assigned the same weights. The advantage of this approach is that it can be readily incorporated into the ALE subtraction analysis. In the present study, we applied ALEn to evaluate brain morphological features of first-episode schizophrenia. The first meta-analysis summarizes differences in NN-FES compared with healthy controls, that is, the effect of schizophrenia on regional gray matter volumes unconfounded by neuroleptic treatment and illness chronicity. Next, we conducted a meta-analysis of VBM studies of NT-FES compared with healthy controls. Finally, we carried out a weighted “subtraction analysis” to evaluate the difference between NT and NN patients and thereby characterize the impact of drug treatment on brain structure in FES.
Methods
Data Sources
A systematic search was performed in the PubMed and MEDLINE database to identify VBM studies that compared gray matter volumetric differences in patients with FES (NN, NT) and typically developing controls. The search keywords “voxel-based,” “morphometry,” or “morphometric” and “first-episode schizophrenia” or “schizophrenia” were used in all possible combinations to include all possible relevant studies for selection. In addition to the computerized search, the most recent titles published in advance from psychiatric journals (including American Journal of Psychiatry, Archives of General Psychiatry, Schizophrenia Bulletin, Schizophrenia Research, and Biological Psychiatry) in the year of 2008 and January 2009 were screened manually to identify suitable articles for inclusion. The reference sections of all the articles identified were also searched and the reference lists of 6 key reviews and meta-analyses of VBM studies13,14,32,39,45,46 were cross-referenced.
The inclusion criteria were (1) full research articles published in the English language, (2) VBM methods used for whole-brain analysis, (3) comparison with healthy control group in at least 1 cross-sectional analysis, and (4) coordinates of gray matter differences reported in stereotactic space, or else the corresponding author was contacted by e-mail for further details. FES was defined by the authors (the longest mean duration of illness reported was 2.5 years65). NN patients were defined as without any prior antipsychotic/neuroleptic exposure (not even within a day or 2 of the scan) and no history of substance abuse before and/or at the time of MRI scan, whereas NT patients had prior antipsychotic/neuroleptic treatment before the time of MRI scan. For studies using overlapping samples, the one with the largest number of subjects34 or in which the goal of the study most matched the goal of the current meta-analysis33 was included. Meda et al29 comprised 200 patients from 4 hospitals, and only the analysis of NN-FES samples from the Western Psychiatric Institute and Clinic at the University of Pittsburgh was included. Fifteen studies met the inclusion criteria. Six of them recruited NN-FES (n = 162) while the other 9 studies recruited NT-FES (n = 336).
ALE Methodology
Statistics and Weighting.
Studies were grouped into either NN group or NT group. In each group, a master list of foci was created by tabulating the Talairach coordinates of the peak maxima of regional differences reported in all constituent studies, with one focus per row. Coordinates reported in MNI format were transformed into Talairach using the “Lancaster transform,” icbm2tal programme from GingerALE.47 Coordinates that had been transformed to Talairach space by the Brett transformation were transformed back to the original MNI48 and reconverted to Talairach using icbm2tal. For each focus, a weighting factor was calculated, based on the study sample size (N) following the Liptak-Stouffer method44:
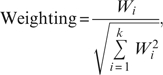 |
where 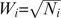 , Ni is the sample size of the current study and k is the total number of studies.
, Ni is the sample size of the current study and k is the total number of studies.
Construction of Weighted ALE Maps, Histogram Generation, and Probability Estimation.
Using downloaded software for ALE36 (http://csl.georgetown.edu/software/), the probability of locating each coordinate obtained from the master list of included studies was modeled with a 3-dimensional Gaussian distribution, with a full-width half-maximum of 8 mm.36 The height of this distribution was then modulated with the corresponding sample size weighting of each focus and a weighted ALE map was constructed. The weighted ALE map was compared with 5000 randomly generated maps to test for deviation from null hypothesis.35 These randomly generated maps had the same number of foci, and same weightings as the actual master list, but with coordinates randomly selected. Thus, the probability of voxels on the resultant ALE map having a particular intensity by chance could be tested. The probability map was thresholded by setting the false discovery rate (FDR) at P < .05. Subsequently, a cluster filter of minimal size of 150 contiguous voxels was applied.35,36 These final steps of thresholding and reporting resultant clusters were done using the most recent version of GingerALE software (http://www.brainmap.org/ale/).
Subtraction Analysis.
Subtraction analysis allows comparison of differences in brain anatomy between 2 different ALE paradigms.13,14,41,45 In this study, subtraction analysis between NN and NT was done to identify the effect of medication on FES. Because the number of foci of NN (74 foci) and NT (122 foci) was not balanced, a “normalization” procedure is needed to balance the subtraction. In nonweighted ALE, normalization can be done by equalizing the number of foci contributed from each paradigm—foci are picked randomly from the bigger paradigm to match the foci number of the smaller paradigm.14 In the current weighted ALE subtraction, normalization was achieved by balancing the total weightings from each paradigm (NN-FES and NT-FES). This had the advantage of using all the foci information from contributing studies. The sum of weights of foci in NN-FES paradigm was 26.52 units while the sum of weights from the NT-FES paradigm was 48.13 units. Therefore, the individual foci weights in the NN-FES paradigm were “normalized” by a factor of 1.82. The 2 sets of foci were entered into subtraction analysis with opposite signs assigned. The significance of the resultant weighted ALE subtraction map was tested against a simulated ALE subtraction map generated from randomly selected foci allocated the same weights as the actual foci.
Results
Table 1 summarizes the studies included in the analyses. Six studies reported 74 coordinates from a total of 162 patients with NN-FES and 165 healthy controls. Nine studies reported 122 coordinates from a total of 336 patients with NT-FES and 484 healthy controls. The percentages of male in all patient and healthy control groups were higher than that of female. The mean age of the patients and healthy controls was balanced about a mean of 26 years old for NN study and 22 years old for NT study. Thus, the data were relatively unconfounded by gender or age. Importantly, the median duration of illness in NN-FES samples was not significantly different from that reported in NT-FES samples, implying that differences in illness chronicity would not likely contribute to the results.
Table 1.
Details of the NN and NT-FES Studies Included in the Meta-analysis
| FES Study | Total Number of Subject |
% of Male Subject |
Mean Age |
Mean Duration of Illness (Months) | Number of Foci of GM Deficits | |||
| FES | Control | FES | Control | FES | Control | |||
| NN | ||||||||
| Meda et al29 | 22 | 21 | 64 | 62 | 25 | 26 | — | 31 |
| Lui et al16 | 68 | 68 | 44 | 46 | 24 | 25 | 8.6 | 3 |
| Chua et al6 | 26 | 38 | 42 | 45 | 32 | 33 | 4.0 | 10 |
| Jayakumar et al31 | 18 | 18 | 50 | 50 | 25 | 26 | 10.3 | 13 |
| Salgado-Pineda et al15 | 13 | 13 | 100 | 100 | 24 | 23 | — | 15 |
| Prasad et al30a | 15 | 7 | 73 | 43 | 26 | 24 | 27.8 | 2 |
| Total/mean of NN-FES | 162 | 165 | 62 | 58 | 26 | 26 | [9.5] | 74 |
| NT | ||||||||
| Janssen et al98 | 25 | 51 | 76 | 69 | 15 | 15 | 3.8 | 2 |
| Meisenzahl et al99 | 93 | 177 | 72 | 69 | 28 | 32 | 9.1 | 48 |
| Yoshihara et al100 | 18 | 18 | 50 | 50 | 16 | 16 | 14 | 1 |
| Douaud et al101 | 25 | 25 | 72 | 68 | 16 | 16 | 14.4 | 23 |
| Kaspárek et al27 | 22 | 18 | 100 | 100 | 24 | 24 | 9.6 | 7 |
| Schaufelberger et al64 | 62 | 94 | 71 | 56 | 28 | 30 | 6.3 | 6 |
| Whitford et al34 | 41 | 47 | 63 | 70 | 20 | 19 | <3 | 14 |
| Job et al33 | 34 | 36 | 68 | 47 | 21 | 21 | — | 12 |
| Kubicki et al28 | 16 | 18 | 88 | 89 | 26 | 24 | — | 9 |
| Total/mean of NT-FES | 336 | 484 | 73 | 69 | 22 | 22 | [9.1] | 122 |
Note: FES, first-episode schizophrenia; NN, neuroleptic naive; NT, neuroleptic treated; GM, gray matter. The values within the brackets indicate the median duration of illness (months).
Only included participants who had been exposed to Herpes Simplex Virus 1.
Weighted Meta-analysis of VBM Studies in First-Episode Schizophrenia
Gray Matter Deficits in NN-FES.
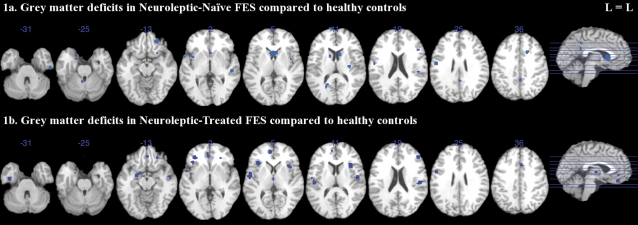
Lower gray matter volumes in NN-FES compared with controls were observed in bilateral caudate, insula, uncus, superior, and inferior temporal gyrus; left posterior cingulate, precentral and superior frontal gyrus, and culmen; and right cingulate, middle frontal, inferior frontal gyrus, claustrum, and cerebellar tonsil (see figure 1a, table 2).
Fig. 1.
Gray Matter Deficits in Neuroleptic-Naive and Neuroleptic-Treated FES Compared With Healthy Controls With ALEn. Significant clusters were thresholded with a false discovery rate P<.05 and a cluster extent of 150 voxels. The left side (L) of each axial section represents the left side of the brain. FES, first-episode schizophrenia; ALEn, anatomical likelihood estimation weighted by number of subjects.
Table 2.
Significant Gray Matter Deficits in First-Episode Schizophrenia Compared With Healthy Controls With ALEn
| Side | Brain Region | Coordinates |
ALE Extrema Value | Cluster Size | ||
| x | y | z | (mm3) | |||
| Gray matter deficits in neuroleptic-naive group | ||||||
| Frontal lobe | ||||||
| L | Precentral gyrus | −50 | −10 | 24 | 0.0027 | 440 |
| L | Superior frontal gyrus | −8 | 66 | 10 | 0.0021 | 320 |
| R | Middle frontal gyrus | 22 | 38 | −14 | 0.0036 | 760 |
| R | Inferior frontal gyrus extending to middle frontal gyrus | 46 | 12 | 16 | 0.0017 | 288 |
| 46 | 20 | 18 | 0.0017 | |||
| Temporal lobe | ||||||
| R | Superior temporal gyrus | 46 | −30 | 16 | 0.0050 | 864 |
| 50 | −26 | 0 | 0.0051 | 816 | ||
| L | −56 | 4 | −4 | 0.0024 | 256 | |
| R | Inferior temporal gyrus | 52 | −20 | −32 | 0.0026 | 384 |
| L | −52 | −22 | −34 | 0.0028 | 320 | |
| Limbic lobe | ||||||
| L | Uncus | −18 | 0 | −22 | 0.0024 | 264 |
| R | Uncus extending to inferior frontal gyrus | 28 | 8 | −20 | 0.0024 | 360 |
| 22 | 14 | −14 | 0.0014 | |||
| R | Cingulate gyrus | 18 | 16 | 34 | 0.0050 | 864 |
| 2 | −48 | 26 | 0.0023 | 304 | ||
| 2 | −46 | 38 | 0.0024 | 248 | ||
| L | Posterior cingulate | −20 | −62 | 14 | 0.0018 | 304 |
| −18 | −58 | 12 | 0.0017 | |||
| Subcortical region | ||||||
| R | Caudate | 10 | 10 | 12 | 0.0019 | 1936 |
| L | 0 | 12 | 4 | 0.0057 | ||
| R | Insula | 40 | 0 | 4 | 0.0023 | 288 |
| L | −36 | 4 | 0 | 0.0026 | 504 | |
| R | Claustrum | 32 | −16 | 14 | 0.0028 | 448 |
| Cerebellum | ||||||
| L | Culmen | −4 | −50 | −22 | 0.0024 | 296 |
| R | Cerebellar tonsil | 28 | −42 | −34 | 0.0023 | 280 |
| Gray matter deficits in neuroleptic-treated group | ||||||
| Frontal lobe | ||||||
| R | Precentral gyrus | 48 | −10 | 12 | 0.0082 | 520 |
| R | Medial frontal gyrus | 2 | 36 | −16 | 0.0078 | 480 |
| L | Medial frontal gyrus extending to anterior cingulate | −6 | 48 | 8 | 0.0057 | 928 |
| −2 | 36 | −2 | 0.0044 | |||
| R | Middle frontal gyrus | 44 | 36 | 18 | 0.0059 | 304 |
| R | Inferior frontal gyrus | 24 | 34 | −8 | 0.0115 | 832 |
| L | −32 | 34 | −4 | 0.0080 | 528 | |
| Temporal lobe | ||||||
| R | Superior temporal gyrus | 52 | −8 | −8 | 0.0085 | 560 |
| L | −52 | −8 | 4 | 0.0089 | 1088 | |
| Limbic lobe | ||||||
| L | Parahippocampal gyrus (amygdala) | −20 | −6 | −14 | 0.0076 | 568 |
| L | Uncus | −38 | −14 | −30 | 0.0077 | 448 |
| R | Cingulate gyrus | 4 | 16 | 38 | 0.0046 | 352 |
| 4 | 12 | 42 | 0.0045 | |||
| Subcortical region | ||||||
| R | Thalamus | 2 | −14 | 4 | 0.0048 | 352 |
| R | Insula | 34 | 16 | 12 | 0.0108 | 856 |
| 50 | −22 | 18 | 0.0056 | 456 | ||
| L | −32 | 20 | 6 | 0.0083 | 808 | |
| −48 | −22 | 14 | 0.0066 | 504 | ||
Note: ALEn, anatomical likelihood estimation weighted by number of subjects; L, Left; R, Right.
Gray Matter Deficits in NT-FES.
Gray matter volume in NT-FES was lower than controls in bilateral insula, medial frontal, inferior frontal, and superior temporal gyrus; left parahippocampal gyrus (amygdala), uncus, and anterior cingulate; and right thalamus, cingulate, precentral, and middle frontal gyrus (see figure 1b, table 2).
Subtraction Analysis NT-FES Minus NN-FES.
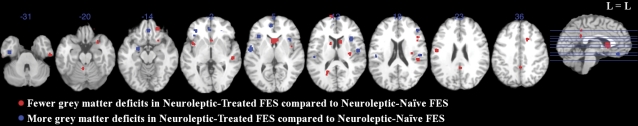
Gray matter volume deficits were less extensive in NT-FES than NN-FES in bilateral caudate and inferior temporal gyrus; left posterior cingulate, precentral, superior frontal gyrus, and culmen; right cingulate, middle frontal, and superior temporal gyrus and claustrum. Conversely, gray matter volume deficits were more extensive in NT-FES than NN-FES in bilateral insula, medial frontal, and inferior frontal gyrus; left parahippocampal gyrus (amygdala) and superior temporal gyrus; and right precentral gyrus (see figure 2, table 3).
Fig. 2.
Gray Matter Deficits in Neuroleptic-Treated FES Compared With Neuroleptic-Naive FES by Subtraction Analysis with ALEn. Significant clusters were thresholded with a false discovery rate P < .05 and a cluster extent of 150 voxels. The left side (L) of each axial section represents the left side of the brain. FES, first-episode schizophrenia; ALEn, anatomical likelihood estimation weighted by number of subjects.
Table 3.
Significant Gray Matter Deficits in NT Compared With NN First-Episode Schizophrenia by Subtraction Analysis With ALEn
| Side | Brain Region | Coordinates |
ALE Extrema Value | Cluster Size | ||
| x | y | z | (mm3) | |||
| Gray matter deficits: NT < NN group | ||||||
| Frontal lobe | ||||||
| L | Precentral gyrus | −50 | −10 | 24 | 0.0133 | 400 |
| L | Superior frontal gyrus | −8 | 66 | 10 | 0.0105 | 320 |
| R | Middle frontal gyrus | 22 | 38 | −14 | 0.0172 | 568 |
| R | Middle frontal gyrus extending to inferior frontal gyrus | 46 | 20 | 18 | 0.0085 | 256 |
| 46 | 12 | 16 | 0.0087 | |||
| R | Inferior frontal gyrus extending to uncus | 22 | 14 | −14 | 0.0076 | 296 |
| 28 | 8 | −20 | 0.0103 | |||
| Temporal lobe | ||||||
| R | Superior temporal gyrus | 50 | −26 | 0 | 0.0143 | 352 |
| 46 | −30 | 16 | 0.0130 | 248 | ||
| R | Inferior temporal gyrus | 52 | −20 | −32 | 0.0130 | 384 |
| L | −52 | −22 | −34 | 0.0142 | 312 | |
| Limbic lobe | ||||||
| R | Cingulate gyrus | 18 | 16 | 34 | 0.0142 | 360 |
| 2 | −46 | 38 | 0.0103 | 176 | ||
| 2 | −48 | 26 | 0.0097 | 160 | ||
| L | Posterior cingulate | −18 | −58 | 12 | 0.0087 | 296 |
| −20 | −62 | 14 | 0.0090 | |||
| Subcortical region | ||||||
| R | Caudate | 10 | 10 | 12 | 0.0106 | 1992 |
| L | 0 | 12 | 4 | 0.0276 | ||
| L | −12 | 6 | 12 | 0.0095 | 264 | |
| R | Insula | 40 | 0 | 4 | 0.0097 | 160 |
| L | −38 | 6 | 0 | 0.0114 | 408 | |
| R | Claustrum | 32 | −16 | 14 | 0.0140 | 440 |
| Cerebellum | ||||||
| L | Culmen | −4 | −50 | −22 | 0.0102 | 200 |
| Gray matter deficits: NT > NN group | ||||||
| Frontal lobe | ||||||
| R | Precentral gyrus | 48 | −10 | 12 | 0.0143 | 432 |
| L | Medial frontal gyrus extending to anterior cingulate | −6 | 48 | 8 | 0.0098 | 632 |
| −2 | 36 | −2 | 0.0077 | |||
| R | Medial frontal gyrus | 2 | 36 | −16 | 0.0135 | 384 |
| R | Middle frontal gyrus | 44 | 36 | 18 | 0.0112 | 280 |
| R | Inferior frontal gyrus | 24 | 34 | −6 | 0.0185 | 512 |
| L | −32 | 34 | −4 | 0.0151 | 488 | |
| Temporal lobe | ||||||
| R | Superior temporal gyrus | 52 | −8 | −8 | 0.0154 | 472 |
| L | −52 | −8 | 4 | 0.0156 | 792 | |
| Limbic lobe | ||||||
| L | Parahippocampal gyrus (amygdala) | −20 | −6 | −14 | 0.0132 | 408 |
| L | Uncus | −38 | −14 | −30 | 0.0134 | 376 |
| Subcortical region | ||||||
| R | Insula | 34 | 16 | 12 | 0.0187 | 776 |
| 40 | 12 | 4 | 0.0075 | |||
| 50 | −20 | 18 | 0.0098 | 280 | ||
| L | −34 | 22 | 4 | 0.0145 | 664 | |
| −48 | −22 | 14 | 0.0111 | 296 | ||
Note: ALEn, anatomical likelihood estimation weighted by number of subjects; NN, neuroleptic naive; NT, neuroleptic treated; L, Left; R, Right.
Discussion
Our ALEn study builds on recent adaptations of the ALE method to allow weighting by sample size.37,42 The key findings were that an extensive network of gray matter deficits in the frontal, temporal, insular, striatal, posterior cingulate, and cerebellar regions characterizes NN patients with schizophrenia compared with healthy controls. To our knowledge, this is the first ALE evaluation of brain morphology in schizophrenia free of confounds of both neuroleptic treatment and illness duration. We also conducted a meta-analysis of NT-FES studies. The results from a subtraction analysis of NT and NN-FES suggested that the gray matter volume deficit in a number of regions in patients with schizophrenia is minimized by neuroleptic treatment. This “reversal” was most evident in the striatum.
Our findings partly agree with those reported in a previous meta-analysis of FES using ALE.14 We did not identify gray matter deficits in thalamus in NN-FES but found deficits in bilateral inferior and superior temporal gyrus. One explanation for this may be that NN and NT patients were previously included together.14 Publication of more FES studies in the intervening period meant that we were able to group NN and NT-FES studies separately. In addition, we carried out a weighted analysis. Thus, our analysis suggests that gray matter abnormalities in thalamus are not especially prominent in the early stage of illness prior to treatment.
We found that, unlike NN patients, patients who had been treated with neuroleptic medication no longer had caudate volume deficits. Lower volumes in caudate nuclei have been consistently reported in FES studies, especially those with NN patients. Consistent with this, our ALEn analysis generated the largest cluster of significantly lower gray matter volume in NN-FES in the caudate. In all, 4 out of 6 of the NN-FES studies included in this meta-analysis contributed to this result.6,15,29,31 This finding is also in accord with a number of ROI studies comparing NN-FES to controls showing that volume reduction in the basal ganglia is relatively specific to caudate nucleus rather than putamen,54–56 though not all reports on NN-FES agree.57–59 In contrast, NT-FES was associated with lower gray matter volume in the thalamus. This conflicts with a previous ROI study, suggesting thalamic enlargement related to use of atypical antipsychotic medication.21 However, the present thalamus result did not survive subtraction analysis, suggesting that neuroleptic-related changes in thalamic volume are not especially prominent in VBM studies.
Caudate pathology and dysfunction in NN-FES may be clinically relevant. For example, abnormal spontaneous movements in NN patients with schizophrenia can be explained by basal ganglia dysfunction,60 and chronically ill, NN patients may suffer tardive dyskinesia.61 Moreover, volume reduction in the caudate nuclei in NN-FES appears to be significantly related to the severity of positive symptomatology and longer duration of untreated psychosis.62 These data support the observation from Kestler et al63 that “the initiation of neuroleptic treatment interrupts a neuropathological process that involves an age-related change in dopamine receptors.” While there is good consensus that dopamine disregulation in striatal systems is strongly associated with schizophrenia,64 possibly causing loss of initiative and drive commonly seen in schizophrenia,65 more recently other neurochemical abnormalities in schizophrenia have been explored. In particular, a progressive reduction of serotonergic receptor density has been reported in the caudate of individuals at risk of schizophrenia who later converted to FES but not in nonconverters.66 Together, the evidence suggests that caudate pathology is intrinsic to the pathology of schizophrenia,67 and MRI measures early in the disease process have been suggested to constitute a possible biomarker.6,22,23
Our subtraction analysis by ALEn revealed significantly larger caudate volumes in NT-FES compared with NN-FES, suggesting that the bilateral caudate deficits observed in NN-FES may “reverse” in NT-FES. Also, the meta-analysis of NT-FES studies alone did not show any significant gray matter deficits in bilateral caudate nucleus (figure 1b). Although caudate volumes have been reported to expand following typical drug treatment for 10 months18 and 4 years,19 the only VBM study reporting gray matter excess in NT-FES localized it to the frontal and occipital lobes but not in the caudate nucleus.34 This may be consistent with a “normalizing” rather than “hypertrophic” influence of antipsychotic treatment on caudate volume.22 However, our study aimed to capture changes over short periods of treatment, and longer duration of medication has been reported to cause basal ganglia enlargement.18,19 It has also been suggested that typical and atypical antipsychotics may have differential effects on the basal ganglia structure.17,68 On average, more patients in the studies included were treated with atypical (approximately 76%) than typical antipsychotics. Even so, no gray matter excesses in caudate were found in the one study which had more subjects treated with typical (69%) than atypical antipsychotics (31%).53 Thus, although the exact mechanism of volume change in basal ganglia is still unknown,69 typical and atypical drugs are all dopamine antagonists which may contribute to neuroplastic change in the acute phase of treatment.
Our meta-analysis collated VBM data from a large number of mostly male patients and suggested that the bilateral insular volumes were lower in NN patients with FES relative to healthy controls. Several ROI studies have also reported that insular volume deficits already exist at early stages in schizophrenia, but the results have been inconsistent. One reason for this may be gender effects. In an ROI study of males with NN-FES, significant reduction in gray matter volume was found in left insular cortex.70 In other ROI studies with predominantly male participants, bilateral insular reduction was observed.29,71 However, when equal numbers of male and female NN participants with FES were included, significantly smaller volumes were measured in the right insula in female patients only72 making the gender effect difficult to interpret.
Complicating this are observations that symptom severity is related to insula volume measurements. A recent MRI study showed that psychotic patients had smaller anterior insular cortices at the early phase of disease and continued to lose insular gray matter as the illness progressed, and this correlated with severity of positive and negative symptoms.73 This negative correlation between insular volume and psychotic symptoms has been replicated in previous ROI studies.70,72 Subtraction analysis revealed more extensive bilateral insular deficits in NT-FES compared with NN-FES, suggesting that the deficits observed in NN-FES were not “reversing” in NT-FES. Conversely, the gray matter deficits in the bilateral insula persisted or even became more extensive in the medicated group. Potentially, the insula is very vulnerable in schizophrenia, and some evidence supporting this comes from readily detectable gray matter deficits in people at high risk of schizophrenia.13,74
Consistent with Ellison-Wright et al14 we found bilateral gray matter deficits in uncus/amygdala region common to NN-FES studies. The uncus is anatomically closely related to the amygdala,75 and they are sometimes examined together as one ROI. However, the amygdala findings in schizophrenia have been contradictory.76 This may be partly due to the challenges of data confounders. For example, bilateral amygdala deficits in a group of mixed sex NN patients with FES77 has been reported, but Gur et al78 have noted a gender effect on the size of amygdala in patients with schizophrenia such that women with FES have larger size and men with FES have smaller size compared with healthy controls.
In terms of drug effect on the amygdala, our subtraction analysis revealed significantly smaller left uncus/amygdala volume in FES after medication. In other words, only the right uncus/amygdala deficit was “reversed” by medication but not the left side. This is also consistent with Ellison-Wright's previous finding of progressive reduction in left uncus/amygdala with illness chronicity and medication.14 In general, left amygdala pathology seems more pronounced than right in schizophrenia77,79–81 and in young offspring of schizophrenia patients82 left amygdala volume negatively correlated with memory impairment in schizophrenia,83and hypoactivation in the left amygdala and bilateral hippocampus of patients compared with healthy controls has been observed during an emotional valence task.84 These are remiscent of Reynolds's seminal findings of raised dopamine levels in the amygdala, left greater than right, in postmortem brains in schizophrenia.85 Conversely, only a slight increase in dopamine in the caudate nuclei was found, and thus, he concluded that amygdala dopamine excess was more likely to be related to the illness rather than to neuroleptic treatment in this chronically treated population.85
There were considerable gray matter deficits found in the frontal lobe in NN-FES compared with control groups. These ubiquitous frontal abnormalities in the early stage of schizophrenia should therefore reflect disease-related pathology unconfounded by medication. A recent ROI study of NN-FES showed differential volume and thickness deficits in various regions of the prefrontal cortex (PFC). Damage to this region is thought to result in impaired social functioning and working memory, core dysfunctions in schizophrenia.86 Many functional studies have replicated a finding of reduced activation in NN-FES compared with controls in the PFC during working memory tasks.87–90 The significant reduction of gray matter volume in PFC in schizophrenia may contribute an anatomical basis to such underactivations in patients with NN-FES and may help to explain their impaired ability to encode information into working memory, even at an early stage of the illness.86,91
Frontal volumetric abnormalities were also observed in the treated group and might be related to the eventual development of negative symptoms, also unresponsive to drug treatment and characteristically associated with smaller frontal volume.92,93 Indeed, rather than a “reversal” of volume deficits following drug treatment, subtraction analysis revealed significantly more extensive gray matter volume deficits in bilateral medial frontal and inferior frontal gyrus in NT-FES compared with NN-FES. Similarly, longitudinal functional studies before and after 6 weeks of atypical treatment in NN-FES have found that pretreatment cognitive deficits are exacerbated by antipsychotics.86,91 The authors suggested that this adverse cognitive effect of antipsychotics might be related to changes in prefrontal dopaminergic systems particularly the D1 receptor system. Even 4 weeks of atypical treatment did not improve hypoactivation in dorso-lateral prefrontal cortex in FES.94 However, the literature is not entirely consistent on the direction of changes following treatment and associated symptomatic improvement. Others have found that drug-induced decreases in frontal and hippocampal activity are linked with fewer delusions and hallucinations95 while clinical improvement is associated with gray matter reduction in childhood-onset schizophrenia.96 Therefore, it seems that the impact of antipsychotic treatment on anatomical or functional abnormalities in the frontal lobe in schizophrenia needs further evaluation.
Study Limitations
First, in our study as in all meta-analyses, a major limitation is the “file drawer” problem. That is, studies which find no significant differences are less likely to be reported. In ALE, even if studies finding no differences between groups are published, only those listing foci can be incorporated into the analysis. For example, Prasad et al30 studied subjects with and without viral markers and found no significant gray matter differences only among the patients with FES and controls who do not have the viral markers; however, this kind of result cannot be represented by ALE. Thus, published studies and meta-analyses on schizophrenia may overpromote the assumption that there must be gray matter abnormalities in schizophrenia. Related to this, it is possible that there was some selection bias in the original studies. For example, never-treated FES patients may show more negative symptoms or less aggression than treated patients; this can potentially influence within-scanner cooperation and may lead to selection bias as a study limitation.
A second issue is that meta-analyses should ideally take into consideration the significance level of the contributing results. However, there is no common reporting system for VBM studies. Some studies report the T value for individual peak maxima and some do not; some report P values corrected for multiple comparison and some report uncorrected P values. While sample size can reflect the power of a result, difficulties arise with unbalanced designs, where the total number of patients and controls in the original study is large but number in one group far outweighs the other.
Third, VBM methodology changes over time. Voxelwise gray matter differences may be quantified in terms of intensity or modulated to yield volume measures but attention has only lately come to focus on the extent these shifts impact upon the results reported.42 Many other factors, including smoothing kernel, small volume correction statistics and thresholding of the spatial extent of clusters can influence how coordinates are generated and reported in original VBM studies,32,35,45,97 and this has lead to a call for more “rigorous standards of data reporting” which we would echo here.35,45
Forth, approximately 20% of the NT-FES subjects were under 18 years old, who may undergo an early-onset pathology and have faster gray matter loss compared with those who were above 18.98 Finally, we are aware of the recent debate on the relative efficacy of first- and second-generation antipsychotics,99,100 but this meta-analysis is not able to address their relative contribution to brain morphology in this meta-analysis because the number of studies did not permit separation into these categories.
In conclusion, we performed an ALE meta-analysis of first-episode schizophrenia, weighted for sample size differences between constituent studies, and found marked frontotemporal including insular gray matter volume deficits in the NT condition compared with the NN one. There were only minor deficits in gray matter in the striato-limbic-temporal regions in the NT condition. Taken together, these findings suggest that prior to neuroleptic treatment, there is lower striatal volume, which may possibly “reverse” following treatment. In addition, our data suggest that frontal regions are less prone to dynamic volume change than striatal, limbic, and temporal regions in the early phase of treatment. This concurs with the existing literature in which frontal gray matter volume is relatively lower in first-episode patients in the early weeks of treatment23 with frontal involvement being particularly marked within the first year of psychosis.101 Following the acute phase of treatment for positive symptoms, there may be unmasking of negative symptoms which are conventionally associated with reduced frontal lobe volume and less amenable to treatment.92,93 Taken together, antipsychotic drugs may unmask or even induce negative symptoms which are in turn related to cerebral gray matter loss especially in the first year. These findings indicate that the early phase of treatment deserves fuller exploration to determine how the balance of symptom control and cerebral gray matter integrity can be optimized in the first episode of schizophrenia.
Funding
The University of Hong Kong Research Postgraduate studentship to M.L.; The University of Hong Kong Funding to S.C.
Acknowledgments
We thank Dr KMR Prasad who provided additional information about his study. We also thank Dr Ian Ellison-Wright for his helpful comments on study weighting options. All authors declare no conflict of interest.
References
- 1.Harrison PJ, Roberts GW. The Neuropathology of Schizophrenia: Progress and Interpretation. Oxford, NY: Oxford University Press; 2000. [Google Scholar]
- 2.Arango C, Moreno C, Martinez S, et al. Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 2008;34:341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dazzan P, Murray RM. Schizophrenia is (not simply) a neurodevelopmental disorder. Epidemiol Psichiatr Soc. 1999;8:235–241. doi: 10.1017/s1121189x00008137. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 6.Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Chua SE, McKenna PJ. Schizophrenia—a brain disease? A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry. 1995;166:563–582. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Gur RE, Turetsky BI, Bilker WB, Gur RC. Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry. 1999;56:905–911. doi: 10.1001/archpsyc.56.10.905. [DOI] [PubMed] [Google Scholar]
- 9.McCarley RW, Wible CG, Frumin M, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 11.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 12.Vita A, Peri LD, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode and chronic schizophrenia: An activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. doi: 10.1093/schbul/sbp073. July 24, 2009; doi:10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado-Pineda P, Baeza I, Pérez-Gómez M, et al. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–375. doi: 10.1016/s1053-8119(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 16.Lui S, Deng W, Huang X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- 17.Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 18.Chakos MH, Lieberman JA, Bilder RM, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 19.Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 20.Gur RE, Kohler C, Turetsky BI, et al. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 22.Chua SE, Deng Y, Chen EYH, et al. Early striatal hypertrophy in first-episode psychosis within 3 weeks of initiating antipsychotic drug treatment. Psychol Med. 2009;39:793–800. doi: 10.1017/S0033291708004212. [DOI] [PubMed] [Google Scholar]
- 23.Deng MY, McAlonan GM, Cheung C, et al. A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naive, newly diagnosed schizophrenia. Psychopharmacology. doi: 10.1007/s00213-009-1619-z. July 30, 2009; doi:10.1007/s00213-009-1619-z. [DOI] [PubMed] [Google Scholar]
- 24.Girgis RR, Diwadkar VA, Nutche JJ, Sweeney JA, Keshavan MS, Hardan AY. Risperidone in first-episode psychosis: a longitudinal, exploratory voxel-based morphometric study. Schizophr Res. 2006;82:89–94. doi: 10.1016/j.schres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone EC, Crow TJ, Frith CD, Carney MW, Price JS. Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet. 1978;1:848–851. doi: 10.1016/s0140-6736(78)90193-9. [DOI] [PubMed] [Google Scholar]
- 26.Leucht S, Busch R, Hamann J, Kissling W, Kane JM. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57:1543–1549. doi: 10.1016/j.biopsych.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Kaspárek T, Prikryl R, Mikl M, Schwarz D, Cesková E, Krupa P. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:151–157. doi: 10.1016/j.pnpbp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meda SA, Giuliani NR, Calhoun VD, et al. A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 2008;101:95–105. doi: 10.1016/j.schres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad KMR, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12:105–113. doi: 10.1038/sj.mp.4001915. 101. [DOI] [PubMed] [Google Scholar]
- 31.Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–591. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 33.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–889. [PubMed] [Google Scholar]
- 34.Whitford TJ, Grieve SM, Farrow TFD, et al. Progressive grey matter atrophy over the first 2-3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32:511–519. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 37.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Chan RCK, McAlonan GM, Gong Q-Y. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. doi: 10.1093/schbul/sbn190. May 20, 2009; doi:10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Levinson DF, Levinson MD, Segurado R, Lewis CM. Genome scan meta-analysis of schizophrenia and bipolar disorder, part I: methods and power analysis. Am J Hum Genet. 2003;73:17–33. doi: 10.1086/376548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folks J. Handbook of statistics v.4: nonparametric methods. In: Krishnaiah PR, Sen PK, editors. Combination of Independent Tests. Amsterdam, The Netherlands: Elsevier Science Pub. Co.; 1984. [Google Scholar]
- 45.Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Torrey EF. Studies of individuals with schizophrenia never treated with antipsychotic medications: a review. Schizophr Res. 2002;58:101–115. doi: 10.1016/s0920-9964(02)00381-x. [DOI] [PubMed] [Google Scholar]
- 47.Laird A. User's Manual for BrainMap GingerALE 1.1. San Antonio, TX: Research Imaging Center, UTHSC; 2007. [Google Scholar]
- 48.Brett M. MRC Cognition and Brain Sciences Unit in Cambridge: Cambridge Imagers; 1999. The MNI brain and the Talairach atlas. http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html. [Google Scholar]
- 49.Janssen J, Reig S, Parellada M, et al. Regional gray matter volume deficits in adolescents with first-episode psychosis. J Am Acad Child Adolesc Psychiatry. 2008;47:1311–1320. doi: 10.1097/CHI.0b013e318184ff48. [DOI] [PubMed] [Google Scholar]
- 50.Meisenzahl EM, Koutsouleris N, Bottlender R, et al. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res. 2008;104:44–60. doi: 10.1016/j.schres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Yoshihara Y, Sugihara G, Matsumoto H, et al. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann Gen Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 53.Schaufelberger MS, Duran FLS, Lappin JM, et al. Grey matter abnormalities in Brazilians with first-episode psychosis. Br J Psychiatry Suppl. 2007;51:s117–s122. doi: 10.1192/bjp.191.51.s117. [DOI] [PubMed] [Google Scholar]
- 54.Glenthoj A, Glenthoj BY, Mackeprang T, et al. Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res. 2007;154:199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Keshavan MS, Haas GL, Kahn CE, et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 56.Shihabuddin L, Buchsbaum MS, Hazlett EA, et al. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry. 1998;55:235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- 57.Cahn W, Hulshoff Pol HE, Bongers M, et al. Brain morphology in antipsychotic-naïve schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl. 2002;43:s66–s72. doi: 10.1192/bjp.181.43.s66. [DOI] [PubMed] [Google Scholar]
- 58.Gunduz H, Wu H, Ashtari M, et al. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol Psychiatry. 2002;51:801–808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- 59.Lang DJ, Kopala LC, Vandorpe RA, et al. An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am J Psychiatry. 2001;158:625–631. doi: 10.1176/appi.ajp.158.4.625. [DOI] [PubMed] [Google Scholar]
- 60.Busatto GF, Kerwin RW. Schizophrenia, psychosis, the basal ganglia. Psychiatr Clin North Am. 1997;20:897–910. doi: 10.1016/s0193-953x(05)70351-8. [DOI] [PubMed] [Google Scholar]
- 61.McCreadie RG, Thara R, Padmavati R, Srinivasan TN, Jaipurkar SD. Structural brain differences between never-treated patients with schizophrenia, with and without dyskinesia, and normal control subjects: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:332–336. doi: 10.1001/archpsyc.59.4.332. [DOI] [PubMed] [Google Scholar]
- 62.Crespo-Facorro B, Roiz-Santiáñez R, Pelayo-Terán JM, et al. Reduced thalamic volume in first-episode non-affective psychosis: correlations with clinical variables, symptomatology and cognitive functioning. Neuroimage. 2007;35:1613–1623. doi: 10.1016/j.neuroimage.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 63.Kestler LP, Walker E, Vega EM. Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behav Pharmacol. 2001;12:355–371. doi: 10.1097/00008877-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry. 1993;150:1343–1348. doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- 65.Graybiel AM. The basal ganglia and cognitive pattern generators. Schizophr Bull. 1997;23:459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 66.Hurlemann R, Matusch A, Kuhn K-U, et al. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl) 2008;195:579–590. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- 67.Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S. Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry. 1999;46:712–720. doi: 10.1016/s0006-3223(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 68.Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 69.McClure RK, Phillips I, Jazayerli R, Barnett A, Coppola R, Weinberger DR. Regional change in brain morphometry in schizophrenia associated with antipsychotic treatment. Psychiatry Res. 2006;148:121–132. doi: 10.1016/j.pscychresns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 71.Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- 72.Duggal HS, Muddasani S, Keshavan MS. Insular volumes in first-episode schizophrenia: gender effect. Schizophr Res. 2005;73:113–120. doi: 10.1016/j.schres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 74.Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 76.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joyal CC, Laakso MP, Tiihonen J, et al. The amygdala and schizophrenia: a volumetric magnetic resonance imaging study in first-episode, neuroleptic-naive patients. Biol Psychiatry. 2003;54:1302–1304. doi: 10.1016/s0006-3223(03)00597-3. [DOI] [PubMed] [Google Scholar]
- 78.Gur RE, Turetsky BI, Cowell PE, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- 79.Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 80.Sumich A, Chitnis XA, Fannon DG, et al. Temporal lobe abnormalities in first-episode psychosis. Am J Psychiatry. 2002;159:1232–1235. doi: 10.1176/appi.ajp.159.7.1232. [DOI] [PubMed] [Google Scholar]
- 81.Hulshoff Pol HE, Schnack HG, Mandl RC, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- 82.Keshavan MS, Dick E, Mankowski I, et al. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- 83.Killgore WDS, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cogn Behav Neurol. 2009;22:28–37. doi: 10.1097/WNN.0b013e318192cc67. [DOI] [PubMed] [Google Scholar]
- 84.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 85.Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529. doi: 10.1038/305527a0. [DOI] [PubMed] [Google Scholar]
- 86.Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- 87.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 88.Boksman K, Théberge J, Williamson P, et al. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 89.Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- 90.Harrison BJ, Yücel M, Shaw M, et al. Dysfunction of dorsolateral prefrontal cortex in antipsychotic-naïve schizophreniform psychosis. Psychiatry Res. 2006;148:23–31. doi: 10.1016/j.pscychresns.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Reilly JL, Harris MSH, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry. 2007;62:818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 92.Chua SE, Wright IC, Poline JB, et al. Grey matter correlates of syndromes in schizophrenia. A semi-automated analysis of structural magnetic resonance images. Br J Psychiatry. 1997;170:406–410. doi: 10.1192/bjp.170.5.406. [DOI] [PubMed] [Google Scholar]
- 93.Crow TJ, Cross AJ, Johnstone EC, Owen F, Owens DG, Waddington JL. Abnormal involuntary movements in schizophrenia: are they related to the disease process or its treatment? Are they associated with changes in dopamine receptors? J Clin Psychopharmacol. 1982;2:336–340. [PubMed] [Google Scholar]
- 94.Snitz BE, MacDonald A, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 95.Liddle PF, Lane CJ, Ngan ET. Immediate effects of risperidone on cortico-striato-thalamic loops and the hippocampus. Br J Psychiatry. 2000;177:402–407. doi: 10.1192/bjp.177.5.402. [DOI] [PubMed] [Google Scholar]
- 96.Sporn AL, Greenstein DK, Gogtay N, et al. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry. 2003;160:2181–2189. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- 97.Fox PT, Laird AR, Lancaster JL. Coordinate-based voxel-wise meta-analysis: dividends of spatial normalization. Report of a virtual workshop. Hum Brain Mapp. 2005;25:1–5. doi: 10.1002/hbm.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60:553–564. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]
- 100.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 101.Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]