Abstract
Background: The prodromal phase of psychosis is characterized by impaired executive function and altered prefrontal activation. The extent to which the severity of these deficits at presentation predicts subsequent clinical outcomes is unclear. Methods: We employed functional magnetic resonance imaging in a cohort of subjects at clinical risk for psychosis and in healthy controls. Images were acquired at clinical presentation and again after 1 year, using a 1.5-T Signa MRI scanner while subjects were performing a verbal fluency task. SPM5 was used for the analysis of imaging data. Psychopathological assessment of the “at-risk” symptoms was performed by using the Comprehensive Assessment for the At-Risk Mental State (CAARMS) and the Positive and Negative Symptom Scale (PANSS). Results: In the at-risk mental state (ARMS) group, between presentation and follow-up, the CAARMS (perceptual disorder and thought disorder subscales) and the PANSS general scores decreased, while the Global Assessment of Functioning (GAF) score increased. Both the ARMS and control groups performed the verbal fluency task with a high degree of accuracy. The ARMS group showed greater activation in the left inferior frontal gyrus but less activation in the anterior cingulate gyrus than controls. Within the ARMS group, the longitudinal normalization of neurofunctional response in the left inferior frontal gyrus was positively correlated with the improvement in severity of hallucination-like experiences. Conclusions: The normalization of the abnormal prefrontal response during executive functioning is associated with 12-month psychopathological improvement of prodromal symptoms.
Keywords: prodromal psychosis, fMRI, executive functions, ARMS, cognition
Introduction
The first episode of psychosis is usually preceded by a prodromal phase that is characterized by subtle psychopathological signs and symptoms. Common features are attenuated psychotic symptoms (ideas of reference, magical thinking, perceptual disturbance, paranoid ideation, and odd thinking and speech) or brief limited intermittent psychotic symptoms too short in duration to meet Diagnostic and Statistical Manual of Mental Disorders criteria for psychosis.1 Subjects presenting with these symptoms show an “at-risk mental state, ARMS,” which is associated with an increased risk of developing a psychotic disorder within the following months.2 Studying the prodromal phase provides a means of determining the pathophysiological processes underlying vulnerability to psychosis and leading to the development of schizophrenia and may ultimately inform preventive interventions.3 A growing number of neuropsychological studies have taken to studying the ARMS, largely reporting cognitive impairments that are qualitatively similar although less severe to those seen in schizophrenia.4–7 Several impaired cognitive domains characterize the ARMS,5,8–10 but abnormalities in executive functions and working memory are among the most remarkable.5,9 Functional neuroimaging techniques allow us to further explore the neurophysiological correlates of the cognitive impairment during the prodromal phase. Previous reviews have indicated abnormalities in the prefrontal and temporal lobes during executive and working memory tasks in subjects at risk for psychosis.6,11 These findings were not attributable to effects of the illness or its treatment and may represent markers of increased vulnerability to psychotic disorders. However, it is still unclear what pathophysiological process underlies the transition from an ARMS to frank psychosis over time and whether this can be detected prior to illness onset.11 As the ARMS is a dynamic condition that may or may not transit to a full-blown psychotic episode, observable neurofunctional changes over time may be an important metric with regard to the later onset of psychosis. To date, only a structural longitudinal imaging study has explored transient brain abnormalities in subjects at clinical risk for psychosis. In the first longitudinal study of ARMS subjects, 21 of the 75 ARMS individuals who had a baseline magnetic resonance imaging (MRI) scan were followed up with a second MRI scan, either immediately after psychosis or after 12 months.12 At the time of the second scan, individuals who had developed psychosis showed a reduction in gray matter in the left parahippocampal, fusiform, orbitofrontal, and cerebellar cortices and the cingulate gyri. In those who had not become psychotic, longitudinal changes were restricted to the cerebellum.12 A subsequent study using data from the same subjects demonstrated an accelerated rate of gray matter retraction in prepsychotic ARMS individuals during the transition to psychosis.13 Although these studies were the first to explore the structural brain changes over time in the prodromal phases of psychosis, to the best of our knowledge, no longitudinal functional imaging data in such populations are available. Similarly, it is not clear how structural or functional alterations may be related to the “at-risk” symptoms in such population. The identification of the neurobiological correlates of prepsychotic symptoms and their assessment over time is of fundamental relevance to improve the diagnostic process and to develop preventive strategies.6,14
We employed functional magnetic resonance imaging (fMRI) in a cohort of help-seeking subjects at high risk of psychosis and in sociodemographically matched controls. The high-risk group was scanned at clinical presentation and again after 1 year while performing a verbal fluency task. On the basis of previous evidence,6 we predicted that at baseline, ARMS subjects would show altered prefrontal activation relative to controls. On the basis of previous studies indicating that prefrontal response during verbal fluency is associated with psychotic symptoms15,16 and on the basis of longitudinal dynamic changes in brain structure12,13,17 and in prodromal symptoms,18,19 we explored the relationship between neurofunctional response and psychopathology over time. We thus tested the hypothesis that over the subsequent follow-up period, baseline alterations in prefrontal function would change longitudinally in parallel with changes in the clinical status of the ARMS group.
Materials and Methods
Subjects
ARMS Group.
Individuals meeting Personal Assessment and Crisis Evaluation Clinic (PACE) criteria for the ARMS20 (n = 15) were recruited from the OASIS team (Outreach and Support in South London).21 The diagnosis was based on assessment by 2 experienced clinicians using the Comprehensive Assessment for the ARMS (CAARMS1) and a consensus meeting with the clinical team. An individual met PACE criteria for the ARMS if he displayed one or more of the following: “attenuated” positive symptoms, frank psychotic symptoms that last less than 1 week and resolve without treatment, a recent decline in function coupled with either schizotypal personality disorder or a first-degree relative with psychosis. The subjects recruited were antipsychotic naive and were representative of the local population of people presenting with an ARMS in terms of age, gender, ethnicity, and duration and intensity of symptoms.21 They underwent a baseline scanning and a second scan after 1 year.
Controls.
Healthy volunteers (n = 15) were recruited via advertisements in the local media. All subjects lived in the same borough of London as the clinical subjects (Lambeth), were native speakers of English, and were right-handed.
Clinical Measures
Subjects were excluded if there was a history of neurological disorder or they met Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria for a substance misuse disorder. Estimated IQ was assessed by using the National Adult Reading Test.22 Severity of symptoms in the clinical groups was assessed at the time of scanning using the following instruments: the Positive and Negative Symptom Scale (PANSS)23 and the CAARMS.1 Handedness was evaluated with the Lateral Preferences Inventory,24 while daily consumption of cups of coffee/tea was assessed during the clinical assessment. Planned independent t tests were used to compare differences in sociodemographic characteristics between ARMS and controls and between the 2 scans (baseline-follow-up).
fMRI Scanning
Image Acquisition.
Images were acquired on a 1.5-T Signa (GE) system at the Maudsley Hospital, London. T2*-weighted images were acquired with a repetition time (TR) of 2 seconds, 38 × 3 mm slices, with a 0.3-mm gap in 14 axial planes. A gradient echo sequence (TR = 4000 ms, echo time [TE] = 40 ms) was used with the acquisition of each volume compressed into the first 1250 milliseconds of the TR, creating a 2750-millisecond window in which subjects could articulate a response in the absence of scanner noise.25 To facilitate anatomical localization of activation, a high-resolution inversion recovery image dataset was also acquired, with 3-mm contiguous slices and an in-plane resolution of 3 mm (TR = 1600ms, inversion time [TI] = 180 ms, TE = 80 ms).
Overt Verbal Fluency Task
Subjects were required to overtly articulate a word beginning with a visually presented letter. The stimuli, each subtending an angle of 5°, were presented visually on a black screen, viewed through a mirror. Cognitive load was modulated with 2 levels of task difficulty: “easy” and “hard” conditions that involved letters that differed with respect to the ease with which volunteers can usually generate words beginning with them. The easy condition involved the letters L, T, C, P, and S and the hard condition, O, N, E, F, and G.25 Incorrect responses were defined as words that were proper names, repetitions or grammatical variations of the previous word, and “pass” responses. Letters were presented in 28-second (s) blocks of 7 stimuli at 4-second intervals. The control condition of word repetition comprised 28-second blocks of 7 presentations of the word “rest” at 4-second intervals, which subjects were required to read aloud. Five blocks of each condition (hard/easy/repetition) were presented in random order. Verbal responses were recorded via an MRI-compatible microphone on Cool Edit 2000 (Syntrillium Software Corporation, www.syntrillium.com). To ensure that subjects heard their responses clearly, their speech was transmitted by an MRI-compatible microphone, amplified by a computer sound card, and relayed back through an acoustic MRI sound system (Ward Ray, Hampton Court, UK) and noise-insulated stereo headphones at a volume of 91 ± 2 dB.
Image Processing and Analysis.
Functional MRI data were analyzed with Statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK) running under the MATLAB7.1 environment. All volumes were realigned to the first volume, corrected for motion artifacts, mean adjusted by proportional scaling, normalized into standard stereotactic space (template provided by the Montreal Neurological Institute), and smoothed using a 6mm full-width-at-half-maximum Gaussian kernel. The time series were high pass filtered to eliminate low-frequency components (filter width = 128 s) and adjusted for systematic differences across trials. The onset times (in seconds) for each trial convolved with a canonical hemodynamic response function. For the verbal fluency paradigm, each task condition (easy, hard) was then contrasted against the baseline condition (repeating the word rest). To test our hypothesis that there were between-group differences, the activation for each task condition was then compared between the groups (controls and ARMS), using an analysis of variance between-subjects test. Whole-brain voxel-wise threshold was set at P < .05 family-wise-error-rate (FWE) corrected. Small volumes correction (sphere of 12-mm radius) was used for clusters observed in hypothesized regions of interest (prefrontal cortex). To explore the longitudinal changes in brain function, pairwise t tests between the 2 scans were employed. To investigate the relation between blood oxygen level–dependent (BOLD) response and functional outcomes, cluster average beta values were extracted for the region of between-group differences by using the MarsBar option available in SPM5. These were correlated with the clinical longitudinal outcomes assessed using the changes (baseline-follow-up) in score on the CAARMS and PANSS. All correlational analyses are reported at a threshold of P < .05 and were corrected for multiple comparisons using the Bonferroni test. Cook d test was used to assess of the extent to which any correlations reflected the influence of outliers.
Results
Clinical and Demographic Characteristics of the Sample
There were no significant differences between the ARMS and control groups with respect to age (control: mean = 25.18 y, SD = 5.07, ARMS: mean = 24.36 y, SD = 4.48; F = 0.026, P = .873), IQ (control: mean = 102.6, SD = 9.2; ARMS: mean = 101.7, SD = 12.3; F = 1.733, P = .204), or gender (ARMS females: n = 7; control females: n = 6; χ2 = 1.502, P = .220). All controls and ARMS subjects were right handed. The PANSS and CAARMS general scores and the Global Assessment of Functioning (GAF) scores at baseline and follow-up are shown in table 1. After 1 year, the overall psychopathological and functional status of the ARMS sample had significantly improved, despite 2 out of the 15 subjects having developed a full-blown psychosis. Thus, between presentation and follow up, the CAARMS (perceptual disorder and thought disorder subscales) and PANSS general scores decreased, while the GAF score increased (P < .05). However, the changes in the CAARMS speech disorder subscale and in the PANSS positive, negative, and general subscales were not significant (P > .05) (table 1). When the 2 subjects who made transition to psychosis were excluded, a significant longitudinal improvement was observed in some additional psychopathological domains (PANSS positive, negative, general and CAARMS speech disorders). During the follow-up period, all ARMS subjects received case management (which included psychoeducation, crisis intervention, family counseling, and assistance with education or work-related difficulties, according to need) and cognitive behavioral therapy; in addition, 7 of them gave their consent to psychopharmacological treatment with low dosages of antipsychotics (quetiapine, dosage range = 25–150mg/d, mean dosage = 100 mg/d).
Table 1.
Longitudinal Changes in the “At-Risk Symptoms”
| Baseline |
Follow-up |
|||||
| Mean | SD | Mean | SD | t | P | |
| GAF | 59.57 | 6.59 | 71.07 | 17.45 | −2.307 | .029 |
| CAARMS perceptual disorders | 3.50 | 1.34 | 2.00 | 2.00 | 2.329 | .028 |
| CAARMS thought disorders | 3.71 | 0.83 | 2.21 | 1.85 | 2.774 | .010 |
| CAARMS speech disorder | 1.86 | 1.56 | 0.93 | 1.33 | 1.695 | .102 |
| PANSS positive | 10.14 | 3.75 | 13.14 | 7.84 | −1.291 | .208 |
| PANSS negative | 10.93 | 3.97 | 11.50 | 6.77 | −0.272 | .787 |
| PANSS general | 25.57 | 9.85 | 17.93 | 5.57 | 2.526 | .018 |
| PANSS total | 46.57 | 12.09 | 42.86 | 15.29 | 0.713 | .482 |
Note: GAF, Global Assessment of Functioning; CAARMS, Comprehensive Assessment for the At-Risk Mental State; PANSS, Positive and Negative Symptom Scale.
Performance
Both groups performed the task with a high degree of accuracy. During the easy condition of the verbal fluency task, there was no significant difference in the number of incorrect responses between ARMS subjects (mean = 6.09, SD = 4.03) (mean = 82.21%) and controls (mean = 4.92, SD = 6.41) (84.39%) (P > .05). During the hard condition of the verbal fluency task, there was no significant difference in the number of incorrect responses between ARMS subjects (mean = 18.09, SD = 11.01) and controls (mean = 10.35, SD = 11.37) (P > .05). Within the ARMS, verbal fluency performance was correlated with the PANSS scores (R = 0.637, P = .014). However, the correlations were not reliable as they were significantly influenced by one outlier (as detected with the Cook d test). No interactions between group and cognitive load were observed (P > .05). At the follow-up, no significant changes in the verbal fluency performance during the easy and hard condition of the verbal fluency task were found (P > .05).
fMRI Results
Cross-sectional Results.
Main Effect of Task (Independent of Group)
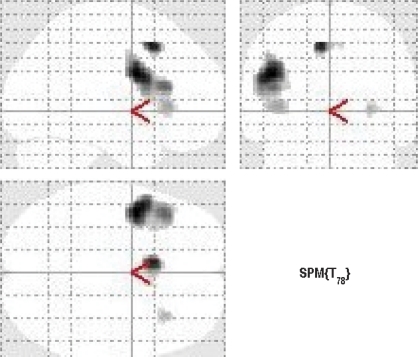
At baseline, verbal fluency (ARMS + controls), relative to word repetition, was associated with activation in a network of areas that included the inferior frontal gyri, the right superior frontal gyrus, and the left middle frontal gyrus (P < .05 FWE) (figure 1, table 2).
Fig. 1.
Activation During the Verbal Fluency Task, Independent of Group (P < .05, FWE Corrected). Left of the figure is left on the brain.
Table 2.
Blood Oxygen Level–Dependent Response During Verbal Fluency
| Brain Region | Side | BA | MNI |
Number of Voxel | Z Scores | P, FWE | ||
| x | y | z | ||||||
| Main effect for the task | ||||||||
| Inferior frontal gyrus | L | 9 | −44 | 4 | 28 | 1283 | 7.04 | .000 |
| Middle frontal gyrus | L | 46 | −42 | 26 | 18 | 6.69 | .000 | |
| Inferior frontal gyrus | L | 47 | −38 | 28 | 5 | 5.20 | .002 | |
| Superior frontal gyrus | R | 8 | 6 | −16 | 50 | 141 | 6.65 | .000 |
| Superior frontal gyrus | R | 6 | 8 | 14 | 50 | 4.71 | .018 | |
| Inferior frontal gyrus | R | 47 | 32 | 26 | 2 | 49 | 5.02 | .000 |
Note: BA, Brodmann area; MNI, Montreal Neurological Institute; L, left; R, right.
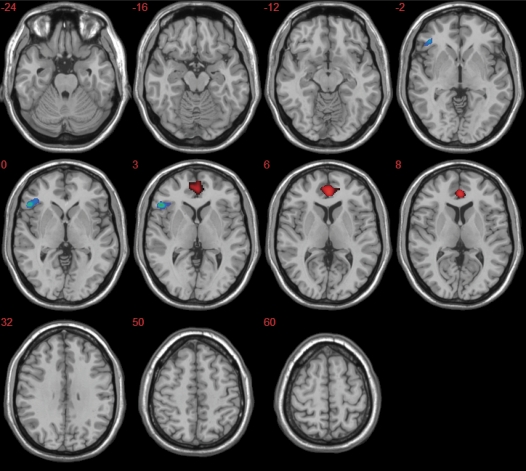
Fig. 2.
Group Differences in Activation During Verbal Fluency (Red Clusters: Controls > ARMS, Blue Clusters: ARMS > Controls) (P < .05, FWE Corrected). Left of the figure is left on the brain.
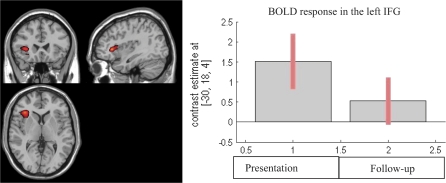
Fig. 3.
Longitudinal Reduction in Left Inferior Frontal Activation in the ARMS Group (Baseline–Follow-up) (P < .05, FWE Corrected). Left of the figure is left on the brain.
Main Effect of Cognitive Load (Independent of Group)
At baseline, the main effect for cognitive load (ARMS + controls) was nonsignificant (P > .05): there was no difference between activation during the more demanding (hard) condition of verbal fluency than during the less demanding easy condition. No interactions between cognitive loads and groups were detected (P > .05).
Group Differences in Activation
When performing the verbal fluency task, the healthy volunteers showed greater activation than the ARMS subjects in the anterior cingulate gyrus bilaterally (x = −2, y = 40, z = 6; x = 18, y = 38, z = −8) (P < .05 FWE) (figure 2). Conversely, ARMS subjects showed greater activation than controls in the left inferior frontal gyrus (x = −52, y = 26, z = 0) (P < .05 FWE) (figure 2).
Longitudinal Data.
Changes in Activation Over Time
The ARMS group showed greater activation in the left inferior frontal gyrus (x = −32, y = 26, z = 2) at baseline than follow-up (P < .05 FWE) (figure 3). Conversely, there were no brain regions that showed more activation at follow-up as compared with baseline. When data from the ARMS group at follow-up were compared with data from the control group at baseline, no statistical significant differences in brain activation were observed (P > .05).
Psychopathological Correlations
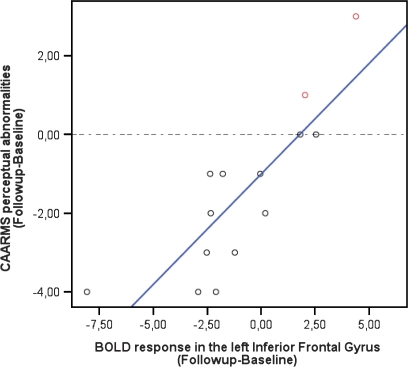
Within the ARMS group, the longitudinal change in the BOLD response in the left inferior frontal gyrus was positively correlated with the change in the severity of perceptual disorders (as assessed using the CAARMS; n = 15; r = 0.833; F = 27, 290; P < .001; adjusted R2 = 0.669) (figure 4). This correlation survived the elimination of potential outliers using Cook d test (n = 12, r = 0.783, P = .03). Post hoc analyses revealed that 2 subjects did not show any clinical improvement over time as they made transition to psychosis (figure 4, top right). For these 2 subjects, the change (follow-up-baseline) in activation of the left inferior frontal gyrus was above the 90th percentiles. There were no significant correlations between the longitudinal change in left inferior frontal activation and changes between presentation and follow scores on the GAF, PANSS (negative, positive, general, total), or other aspects of the CAARMS (speech disorders, disorder of thought content) (P > .05).
Fig. 4.
Correlation Between Longitudinal Changes in Left Inferior Frontal Activation and Symptom Severity Within the ARMS Sample (P < .05). The greater the reduction in activation, the greater the improvement in perceptual symptoms, such as hallucinations. The dotted line separates subjects who showed a clinical improvement (black circles) from those who became psychotic (in red).
Effects of Medication
At baseline, no significant differences in brain activation during the verbal fluency task were detected between ARMS subjects treated with antipsychotics and drug-naive ARMS subjects (P > .05). Similarly, no cluster survived correction for multiple comparisons in the longitudinal analysis comparing treated and untreated ARMS subjects (P > .05).
Discussion
The present study used fMRI in a longitudinal design to explore the dynamic changes of prefrontal activity subserving executive functioning during the prodromal phases of psychosis. Subjects with an ARMS were scanned while they performed a verbal fluency task when they first presented to clinical services and after an interval of 1 year. The study uncovered a direct relationship between the neurofunctional response in the prefrontal cortex of ARMS subjects and their clinical improvement over time.
Task performance did not differ between ARMS and matched controls, and no interaction between group and cognitive load was detected at any time points. As the present study was tailored to detect differences at a neurophysiological level, it may have been underpowered to detect differences at behavioral level. In fact, neuropsychological studies in larger samples indicated that the ARMS is associated with cognitive impairments that are qualitatively similar to, but less severe than those seen in schizophrenia, with consistent evidence of deficits on tasks that engage executive functions.9,26 The impairment in verbal fluency performance is particularly marked, with performance about 1 SD below the normative level.7
For these reasons, verbal fluency paradigms have been widely used as a probe to test cognitive functions subserved by the prefrontal cortex during the early phases of psychosis.6 Verbal fluency tasks can assess the intrinsic generation of a verbal response, suppression of inappropriate responses, and the holding of information about previous responses online.27 The verbal fluency task used in the scanner was paced to facilitate image acquisition and minimize performance differences between subjects, which can confound the interpretation of differential activation in functional imaging studies.28 This was reflected in the high response accuracy, which was near ceiling in both groups, and probably accounted for the absence of the behavioral differences between the ARMS and control groups that we previously observed when we used an unpaced verbal fluency paradigm as is typically employed in neuropsychological studies.29 In line with these considerations, speed of information processing is considered a core cognitive deficit in schizophrenia and might be mediating a broader diversity of executive functions.30
Baseline scans confirmed that the verbal fluency task selectively engages a prefrontal network that includes a number of ventrolateral prefrontal areas, in line with previous findings (for a meta-analysis see Costafreda et al31). Furthermore, this network was found to be highly left lateralized, as previously observed.31 In particular, the inferior frontal gyrus on the left side of the brain has been linked to word production, selection, and retrieval and is robustly activated by verbal fluency paradigms.31 Although ARMS subjects and controls shared similar accuracy rates, their neurofunctional response during the task was significantly different. At baseline, ARMS subjects showed a reduced brain response in the anterior cingulate and an increased response in the inferior frontal gyrus as compared with the healthy controls. A number of studies reported reduced activation of the anterior cingulate gyrus in first-episode psychosis during verbal fluency,32 executive control task,33 and manipulation phase of working memory.34 The anterior cingulate cortex has been implicated in schizophrenia by several lines of evidence: postmortem neuropathology,35 structural MRI,36 positron emission tomography studies,37 increased glutamatergic metabolites on 1H magnetic resonance spectroscopy (MRS),38 and deficits in membrane phospholipids on 31P MRS39 in this region. The anterior cingulate cortex has extensive anatomical connections to the prefrontal cortex, motor areas, and thalamus and a presumed role in initiation of action, selective attention, selection, and monitoring of conflicting responses and error detection.40,41 As the verbal fluency task entails all these cognitive processes, the observed abnormalities in the anterior cingulate may represent the neurophysiological basis of the reported executive dysfunctions in the ARMS group. There was also a significant group difference in ventrolateral prefrontal cortex, but in this region ARMS subject showed greater activation that controls. Previous studies in first episode32,42,43 or high-risk samples27 found both reduced and increased activation in this area during similar verbal fluency tasks relative to a baseline scan. Discrepant findings in the literature might be simply due to differences in experimental design, technical differences of fMRI data acquisition, demographic characteristics, generalized cognitive impairment, and functional heterogeneity in subjects at risk for schizophrenia or to other cofounders such as different exposure to medication, head motion, and lack of diagnostic reassessment during the follow-up.6 Nevertheless, taken together these studies suggest that prefrontal functional alteration is evident prior to or early in the onset of the first episode of illness and even in subjects who are at high risk but will never become psychotic. In particular, the increased activation in the left inferior frontal gyrus may serve to compensate for the anterior cingulate neural dysfunction and maintain the performance on the verbal fluency test within the normative range, even if the overall network architecture is inefficient.44 Such an interpretation is in line with the hypothesis that the recruitment of ventrolateral compensatory networks among schizophrenics represents a compensatory response to a reduced activity of the dorsolateral prefrontal cortex.44 Moreover, the finding of functional abnormalities in ventrolateral and anterior cingulate cortex in high-risk subjects is consistent with data from structural MRI studies of the early phases of psychosis, which generally report reduced gray matter volume in these regions.12 As the ARMS group had a high risk of developing a psychotic disorder but were not psychotic at the time of the baseline scanning, these dysfunctional abnormalities underlying executive functioning can be seen as a correlate of their increased vulnerability to psychosis.6
ARMS subjects were followed for 1 year at which point they underwent a second clinical assessment and fMRI scan. Overall, the ARMS group showed a significant improvement in functional and clinical outcome measures (GAF, PANSS general scores, and CAARMS perceptual and thought disorders). When the neurophysiological response of the ARMS group at this time point was compared with that observed at the baseline, we found relatively decreased activation in the left inferior frontal gyrus. It is possible that the normalization of the abnormal neural response in the ventrolateral prefrontal cortex is associated with the clinical improvement of the overall sample. In line with this assumption, we found no difference in brain activation between ARMS at follow-up and controls at baseline, suggesting a normalization of prefrontal functioning over time. As the 12-month period makes unlikely any practice effect, these initial results are suggestive of dynamic neurofunctional changes that would be consistent with clinical changes manifest in these patients. To test the relationship between dynamic brain changes and symptomatic and functional outcomes (PANSS and CAARMS), we extracted the parameter estimates from left inferior frontal gyrus cluster and correlated them with these clinical measures. The changes in the activation of inferior frontal gyrus were positively correlated with the changes in the perceptual disorder subscale of the CAARMS. As the neural response decreased, ARMS subjects showed lower perceptual abnormalities and vice versa. The correlation between the normalization of inferior frontal activation in the ARMS group and the improvement in perceptual symptoms is interesting, as increased inferior frontal activity has previously been associated with the presence of auditory hallucinations.45,46 Changes in prefrontal activity are evident when psychotic symptoms are induced in healthy subjects by ketamine,47–49 while auditory verbal hallucinations seem to activate predominantly the right inferior frontal cortex.50 Furthermore, ARMS who later developed schizophrenia had a smaller gray matter volume in a region including the inferior frontal gyrus.51 These differences were located in similar parts of the right inferior frontal cortex to those identified by Pantelis et al.12 The involvement of this region in mediating perceptual symptoms may thus have contributed to its response being most altered when subjects were most symptomatic.
Although the clinical status of the ARMS group overall improved, 2 subjects developed psychosis. When these subjects were removed from the analysis, the clinical improvement of the ARMS sample was even more marked, and this was evident in additional improvements in specific outcome measures (PANSS positive, negative, and total and CAARMS speech disorders). Interestingly, in the subjects who became psychotic, the normalization of prefrontal hyperactivation that was a feature of the rest of the group was not evident (figure 4), suggesting that the onset of psychosis may be associated with the persistence of the original perturbation in prefrontal function. This is consistent with the above theories of inefficient executive functioning in psychosis and with structural MRI findings of reduced gray matter volume in PFC in the ARMS.51 Specifically, ARMS subjects who later developed a psychotic episode had gray matter abnormalities in the inferior frontal gyrus, as compared with the ones who did not transit to psychosis.51 This brain region may thus play a crucial role in the development of psychosis transition. Unluckily, our small sample size prevented any longitudinal comparison of ARMS subjects who made transition vs the ones who remained in an “at-risk state.” Future works await replication with larger cohorts. A limitation of the current study was the lack of follow-up scans in the control group to control for nonspecific time or effects. However, fMRI activation during sentence completion and52 word generation tasks53 appears to be consistent over time in controls and genetic high-risk subjects who are clinically stable. In line with these observations, the previous longitudinal imaging studies in subjects at clinical risk for psychosis did not repeat the control scan at follow-up, assuming influent brain changes in such group.12,13
Other limitations of the present study include exposure to low dosages of quetiapine for about half of the ARMS subjects. Although the contrast between treated and untreated ARMS subjects did not uncover significant effects of medication on brain activity, this may be simply due to small sample sizes. Available evidence indicates that antipsychotics can affect the neurofunctional response during cognitive functioning in first-episode psychosis.42,54 As a consequence, the attenuation of prefrontal activity over time in the high-risk group may have been confounded by antipsychotic-induced changes rather than reflecting true pathophysiological changes.
Taken together, our findings provide evidence that active neurofunctional changes occur in patients at risk for psychosis and that brain changes concur with symptomatic improvement. As during the follow-up, our subjects received the standard active interventions offered by the prodromal service for people at risk for psychosis,55 whether the longitudinal neurofunctional changes in prodromal psychosis are caused by the disease itself or are a consequence of the active treatment still remains unanswered. On the other hand, our results emphasize the importance of early interventions in the treatment of schizophrenia, suggesting that the observed neurophysiological abnormalities are something that could perhaps be modulated by active interventions before the psychosis onset. As ventrolateral prefrontal cortex has been reported to be sensitive to antipsychotic treatment in first-episode psychosis,42 the question of the functional significance of dynamic ventrolateral changes in the prodromal phases of psychosis may have some potential clinical implications.56,57 Although longitudinal randomized controlled fMRI trials in the prodromal population are extremely complex, they will clarify the respective contribution of disease progression and clinical interventions in the prodromal phases preceding the illness onset.
Conclusion
The prodromal phase of psychosis is associated with abnormalities in the ventrolateral prefrontal cortex during executive functioning. The normalization of the neural response was correlated with psychopathological improvement at 1 year.
References
- 1.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD, Cornblatt B, McGorry P. The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull. 2007;33:661–664. doi: 10.1093/schbul/sbm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Valmaggia L, McGuire P. Can antidepressants prevent psychosis? Lancet. 2007;370:1746–1748. doi: 10.1016/S0140-6736(07)61732-2. [DOI] [PubMed] [Google Scholar]
- 4.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res. 2007;93:266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusar-Poli P, Perez J, Broome MR, et al. Neurofunctional correlates of liability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Simon AE, Cattapan-Ludewig K, Zmilacher S, et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33:761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Nieman D, Becker H, van de Fliert R, et al. Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophr Res. 2007;95:54–60. doi: 10.1016/j.schres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Wood SJ, Pantelis C, Velakoulis D, Yucel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Allen P, McGuire P. Neuroimaging studies of the early stages of psychosis: a critical review. Eur Psychiatry. 2008;23:237–244. doi: 10.1016/j.eurpsy.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Fu CH, Abel KM, Allin MP, et al. Effects of ketamine on prefrontal and striatal regions in an overt verbal fluency task: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:92–102. doi: 10.1007/s00213-005-0154-9. [DOI] [PubMed] [Google Scholar]
- 16.Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry. 2005;162:485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- 17.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins KA, Keefe RS, Christensen BK, et al. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophr Res. 2008;105:1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis—a step towards indicated prevention of schizophrenia. Br J Psychiatry. 1998;172:14–20. [PubMed] [Google Scholar]
- 21.Broome M, Woolley J, Tabraham P, et al. What causes the onset of psychosis? Schizophr Res. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Nelson HE, Willison JR. National Adult Reading Test (NART): Test Manual. 2nd ed. Windsor, UK: NFER-Nelson. 1991 [Google Scholar]
- 23.Kay SR. Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q. 1990;61:163–178. doi: 10.1007/BF01064966. [DOI] [PubMed] [Google Scholar]
- 24.Coren S. Measurement of handedness via self-report: the relationship between brief and extended inventories. Percept Mot Skills. 1993;76(3 pt 1):1035–1042. doi: 10.2466/pms.1993.76.3.1035. [DOI] [PubMed] [Google Scholar]
- 25.Fu CH, Morgan K, Suckling J, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. NeuroImage. 2002;17:871–879. [PubMed] [Google Scholar]
- 26.Pukrop R, Schultze-Lutter F, Ruhrmann S, et al. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol. 2006;28:1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- 27.Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- 28.Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howes O, Montgomery A, Asselin M, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Sanchez JM, Crespo-Facorro B, Gonzalez-Blanch C, Perez-Iglesias R, Vazquez-Barquero JL. Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. Br J Psychiatry Suppl. 2007;51:s107–s110. doi: 10.1192/bjp.191.51.s107. [DOI] [PubMed] [Google Scholar]
- 31.Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boksman K, Theberge J, Williamson P, et al. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Snitz BE, Macdonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 34.Tan HY, Choo WC, Fones CS. Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- 35.Benes FM. Model generation and testing to probe neural circuitry in the cingulate cortex of postmortem schizophrenic brain. Schizophr Bull. 1998;24:219–230. doi: 10.1093/oxfordjournals.schbul.a033322. [DOI] [PubMed] [Google Scholar]
- 36.Yucel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 37.Kim JJ, Mohamed S, Andreasen NC, et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:542–548. doi: 10.1176/appi.ajp.157.4.542. [DOI] [PubMed] [Google Scholar]
- 38.Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 39.Jensen JE, Al-Semaan YM, Williamson PC, et al. Region-specific changes in phospholipid metabolism in chronic, medicated schizophrenia: (31)P-MRS study at 4.0 Tesla. Br J Psychiatry. 2002;180:39–44. doi: 10.1192/bjp.180.1.39. [DOI] [PubMed] [Google Scholar]
- 40.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(pt 10):2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 41.Spence SA, Liddle PF, Stefan MD, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- 42.Jones HM, Brammer MJ, O'Toole M, et al. Cortical effects of quetiapine in first-episode schizophrenia: a preliminary functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:938–942. doi: 10.1016/j.biopsych.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Schaufelberger M, Senhorini MC, Barreiros MA, et al. Frontal and anterior cingulate activation during overt verbal fluency in patients with first episode psychosis. Rev Bras Psiquiatr. 2005;27:228–232. doi: 10.1590/s1516-44462005000300013. [DOI] [PubMed] [Google Scholar]
- 44.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(suppl 1):i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 45.McGuire P, Shah G, Murray R. Increased blood flow in Broca's area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- 46.Shergill S, Brammer M, Williams S, Murray R, McGuire P. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 47.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 48.Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- 49.Vollenweider FX, Leenders KL, Scharfetter C, et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) Eur Neuropsychopharmacol. 1997;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- 50.Sommer I, Ramsey NF, Mandl R, Van Oel C, Kahn R. Language activation in monozygotic twins discordant for schizophrenia. Br J Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- 51.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Whalley HC, Gountouna VE, Hall J, et al. fMRI changes over time and reproducibility in unmedicated subjects at high genetic risk of schizophrenia. Psychol Med. 2008:1–11. doi: 10.1017/S0033291708004923. [DOI] [PubMed] [Google Scholar]
- 53.Brannen JH, Badie B, Moritz CH, Quigley M, Meyerand ME, Haughton VM. Reliability of functional MR imaging with word-generation tasks for mapping Broca's area. AJNR Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 54.Fusar-Poli P, Broome MR, Matthiasson P, Williams SC, Brammer M, McGuire PK. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur Neuropsychopharmacol. 2007;17:492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Broome MR, Woolley JB, Johns LC, et al. Outreach and support in south London (OASIS): implementation of a clinical service for prodromal psychosis and the at risk mental state. Eur Psychiatry. 2005;20:372–378. doi: 10.1016/j.eurpsy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 57.Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45–60. doi: 10.1016/j.schres.2005.05.009. [DOI] [PubMed] [Google Scholar]