Abstract
Objective
More than half of older adults respond only partially to first-line antidepressant pharmacotherapy. Our objective was to test the hypothesis that a depression-specific psychotherapy, Interpersonal Psychotherapy—IPT, when used adjunctively with escitalopram, would lead to a higher rate of remission and faster resolution of symptoms in partial responders than escitalopram with depression care management (DCM).
Method
We conducted a 16-week randomized clinical trial of IPT and DCM in partial responders to escitalopram, enrolling 124 outpatients aged 60 and older. The primary outcome, remission, was defined as three consecutive weekly scores of ≤7 on the Hamilton Rating Scale for Depression (17-item). We conducted Cox regression analyses of time to remission and logistic modeling for rates of remission. We tested group differences in Hamilton depression ratings over time via mixed-effects modeling.
Results
Remission rates for escitalopram with IPT and with DCM were similar in intention-to-treat (IPT versus DCM: 58 [95% CI: 46, 71] versus 45% [33,58]; p = 0.14) and completer analyses (IPT versus DCM: 58% [95% CI: 44,72] versus 43% [30, 57]; p = 0.20). Rapidity of symptom improvement did not differ in the two treatments.
Conclusion
No added advantage of IPT over DCM was shown. Depression care management is a clinically useful strategy to achieve full remission in about 50% of partial responders.
Keywords: depression, late life, escitalopram, depression care management, interpersonal psychotherapy, partial response
Introduction
Because fewer than 50% of older adults with major depressive episodes achieve remission with first-line antidepressant pharmacotherapy, the majority are left with clinically significant symptoms and functional impairment (Bruce et al. 2004; Unutzer et al. 2002). Partial response is predicted by co-existing anxiety, greater medical burden, depression severity, chronicity, prior treatment response, and cognitive impairment (Charney et al. 2003). It poses risk of chronic relapsing depression, non-adherence to other treatments for co-existing medical disorders, worsening of disability and cognitive impairment, family caregiver burden, and suicide (Charney et al. 2003). With the goal of achieving remission and faster symptomatic resolution, we designed the current study to compare two strategies for managing partial response in older adults with major depression—continuing depression care management (DCM) with pharmacotherapy at a higher dose, or continuing DCM with pharmacotherapy at a higher dose coupled with a depression-specific psychotherapy (Interpersonal Psychotherapy [IPT]) (Klerman et al. 1984). We hypothesized that treatment combining escitalopram with IPT would lead to a higher remission rate and faster symptomatic improvement in partial responders, than escitalopram with DCM. To our knowledge this is the first controlled study of partial response to antidepressant pharmacotherapy in late-life major depression.
Treatment sequencing for partial response in older adults with major depression needs to account for several age-dependent clinical factors. Many patients need longer treatment duration than is currently standard for younger adults, reflecting either hesitation to increase doses in older adults (Whyte et al. 2004) and/or age-related slower resolution of depressive symptoms (Reynolds et al. 1996). Hence, continued pharmacotherapy with the first-line agent at a higher than initial dose provides a clinically relevant control for assessing the benefit of adding psychotherapy. In addition, attention to resolving psychosocial challenges that may complicate the clinical presentation of depression in old age, precipitate suicidal behavior, prolong response time, compromise treatment adherence, or predispose to early relapse are all clinically relevant considerations that led us to further test the benefit of adding a depression-specific psychotherapy for resolving partial response.
We have reported elsewhere that partial response at six weeks (with less than 45% reduction in baseline Hamilton depression ratings) does not bode well for eventual symptom remission (Andreescu et al. 2008; Mulsant et al. 2006). Similarly, other authors have reported that by week 6, at least 60% of ultimate non-remitters can be identified by slow resolution of depressive symptoms (Sackeim et al. 2006). While a simple dose increase would be the most likely, feasible, and perhaps also the most reasonable step in general medical settings, it is not clear whether this strategy alone would be as effective in achieving remission as combined treatment with a depression-specific psychotherapy. We chose IPT because depression in later life is often associated with the core foci of IPT: losses (e.g., bereavement), social role transitions (e.g., retirement), interpersonal disputes, and social isolation (Klerman et al. 1984). Moreover, IPT encourages depressed people to accept the diagnosis of depression and to adhere closely to prescribed antidepressant medication. Therefore, the current study provides further controlled evaluation of combined IPT and pharmacotherapy with depression care management, in the specific contexts of (1) partial response to initial pharmacotherapy, (2) the desirability of treatment to full remission and of rapid symptom resolution to alleviate the anguish of depression, and (3) the need to mitigate risk for relapse and chronic illness.
Methods
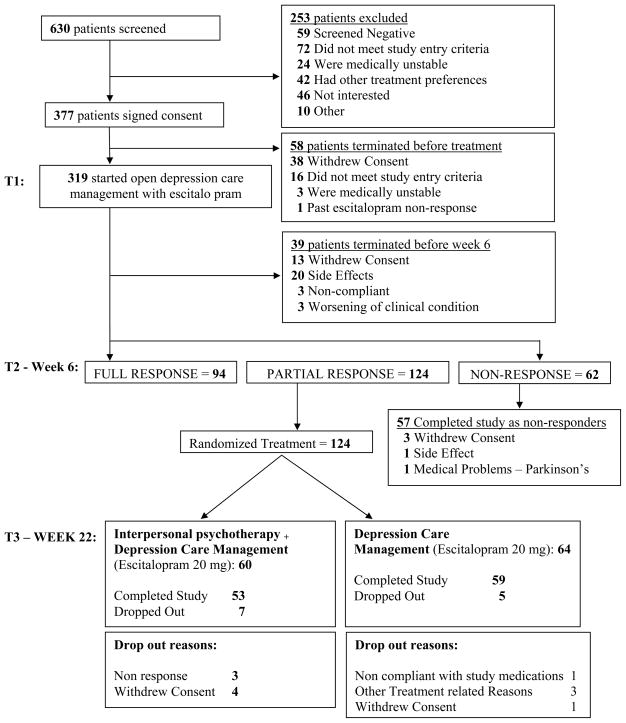
Figure 1 depicts recruitment and retention of study participants. Of 630 persons screened, 377 (59.8%) signed consent and 319 (50.6%) started open treatment with escitalopram. Of these 94 (29.5%) responded by week 6 (HRSD-17 score of ≤10) and exited the study. One hundred twenty-four (38.9%) were partial responders (defined by HRSD-17 scores of 11–14 at six weeks) and were randomly assigned to treatment with IPT (n = 60) or depression care management without IPT (n = 64). Participants in both treatment arms had their daily dose of escitalopram increased to 20mg, and those randomly assigned to IPT also continued to receive depression care management. Non-responders exited the study and were offered treatment with SNRI + adjunctive aripipiazole, as reported elsewhere (Sheffrin et al. 2009)
Figure 1.
Participant Accrual and Retention
We imposed relatively few exclusion criteria, accepting both primary care and specialty mental-health patients age 60 and older with a wide range of medical and psychosocial comorbidities (acceptable as long as patients were sufficiently stable medically as not to require inpatient treatment for co-existing medical disorders). Participants satisfied SCID/DSM-IV criteria (First et al. 1994) for current major depressive episode (non-psychotic, non-bipolar), with 17-item Hamilton Depression Rating Scale scores (Hamilton 1960) of 15 or higher. Subjects with suicidal ideation, with a history of suicide attempt, or with a current suicide plan were eligible as long as study participation was deemed to be safe and the subject was not in imminent risk of self-harm (consistent with the Institute of Medicine’s recommendation) (Institute of Medicine of the National Academies 2002). To achieve a clinically representative study group, we included subjects with mild neurocognitive impairment (Folstein Mini-Mental State Examination scores of 18 or greater; (Folstein et al. 1975)) but not with previously diagnosed dementia.
Patients received a medical evaluation before treatment, including a physical and neurological examination, electrocardiogram, and metabolic blood work (liver, renal, thyroid, and electrolytes). If a patient’s heart rate was lower than 50 beats per minute, we asked the primary care physician or cardiologist for permission to enroll the patient. If pretreatment serum sodium was 137 or lower, we repeated the sodium level after 7–10 days to monitor for hyponatremia (Fabian et al. 2004). One subject left the trial due to persistent hyponatremia, before randomization.
The largest source of recruitment was self-referral (n=47, 37.9%). The second largest source of participants was from mental health specialtists (n=46, 37.1%) followed by primary care (n=31, 25%). We used IRB-approved advertisements in the print and on-air media, as well as research registries from the University of Pittsburgh Medical Center. While the study group was not a randomly selected community sample, its gender and racial attributes were similar to those of older adults in Pittsburgh and Allegheny County, and its clinical attributes (Table 1) resembled those of subjects who participated in a previous primary-care study (Bruce et al. 2004). Subjects randomly assigned to IPT were by chance 2.3 years younger on average than those in DCM. (Hence, our analyses comparing remission rates were adjusted for age.) Although at pre-intervention baseline IPT participants scored higher on measures of anxiety, the two groups had equivalent scores by week 6, the point of randomization to IPT or DCM. Both groups showed decreases of about 30% in depression ratings at week 6, consistent with partial response and, hence, need for further treatment.
Table 1.
Demographic and Clinical Measures (mean ± SD)
| Measure | IPT N=60 | Depression Care Management N=64 | X2 or t | df | p |
|---|---|---|---|---|---|
| Recruitment Source (%) | 1.49 | 2 | 0.48 | ||
| primary care | 16 (27%) | 15 (23%) | |||
| self-referral | 25 (42%) | 22 (34%) | |||
| mental health + social service | 19 (32%) | 27 (42%) | |||
| Demographic Measures | |||||
| Age | 71.1 (7.1) | 73.4 (7.7) | 1.69 | 122 | 0.094 |
| %Women | 73% | 64% | 1.24 | 1 | 0.27 |
| %White | 95% | 89% | 1.47 | 1 | 0.23 |
| Education in years | 13.6 (2.8) | 14.1 (2.8) | 0.88 | 122 | 0.38 |
| Marital | 2.54 | 3 | 0.47 | ||
| %Married/cohabit | 48% | 51% | |||
| %Never Married | 7% | 5% | |||
| %Separated/Divorce | 20% | 11% | |||
| %Widowed | 25% | 33% | |||
| Pre-intervention Clinical Measures | |||||
| Cumulative Illness Rating Scale – total | 10.1 (4.1) | 10.4 (3.7) | 0.37 | 122 | 0.71 |
| Mattis Dementia Rating Scale - total | 133.6 (7.6) | 134.2 (6.8) | 0.47 | 115 | 0.64 |
| %Recurrent MDD | 50% | 52% | 0.30 | 1 | 0.86 |
| Duration of Illness (years) | 16.2 (18.9) | 15.1 (16.7) | 0.34 | 122 | 0.73 |
| Duration of current episode in weeks+ | 129 (209) | 133 (153) | 0.58 | 122 | 0.57 |
| Hamilton Depression Score | 19.0 (3.5) | 18.0 (2.5) | 1.90 | 122 | 0.06 |
| Hamilton Anxiety Score | 18.8 (5.0) | 16.8 (3.8) | 2.52 | 121 | 0.01 |
| Medical Outcomes Survey - Physical | 42.0 (10.8) | 42.4 (11.3) | 0.22 | 122 | 0.82 |
| Medical Outcomes Survey - Mental | 33.1 (11.4) | 33.7 (8.7) | 0.33 | 122 | 0.74 |
| Randomization at 6 weeks | |||||
| Hamilton Depression Score | 12.5 (1.0) | 12.5 (1.1) | 0.17 | 122 | 0.86 |
| Hamilton Anxiety Score | 12.4 (3.1) | 13.2 (3.6) | 1.36 | 120 | 0.18 |
Natural log transformation prior to statistical comparison
All participants provided informed consent and signed IRB-approved consent forms. A Data Safety Monitoring Board convened twice yearly to oversee the conduct of the study.
Treatment
Participants received depression care management (described below) and open treatment with escitalopram 10 mg daily for six weeks, provided by the same clinician who subsequently provided randomized treatment. After six weeks, those meeting criteria for response (Hamilton depression scores of 10 or less) left the study. Patients with scores in the partial responder range (11–14) had their daily dose of escitalopram increased to 20 mg and were randomly assigned to 1) IPT or 2) DCM (16 weekly sessions). (Note: initially our protocol specified a 10-week randomization phase, with only 10 IPT sessions; we modified the protocol early in the study after consulting with the DSMB and NIMH program staff, in order to conduct a more rigorous evaluation of augmentation with IPT. Eighteen of 124 subjects participated in the randomized 10-week phase (9 receiving IPT, 9 in depression care management), while 106 participated in the 16-week phase. Over all, the mean numbers (SD) of IPT sessions was 11.8 (3.6); and of DCM sessions, 12.6 (2.9).) The final mean dose of escitalopram in IPT was 17.7 (4.5) mg/day; and in depression care management, 17.0 (4.6) mg/day. (Some participants were unable to tolerate 20mg and had their doses adjusted to 10–15mg/day.) We assessed pharmacotherapy adherence using patient self-report. In both treatment arms 60% of participants reported missing less than on dose weekly.
Depression Care Management
Depression care management (provided during both the initial 6-week phase of the study and during randomized treatment in both study arms) was supportive and educational, with an emphasis on encouraging treatment adherence and managing risk for suicide, and involved discussions with family members and caregivers to elicit their support of the treatment plan. DCM consisted of nine components: 1) education about depression in later life, 2) education about the medications used to treat depression in later life, 3) education about good sleep practices, 4) review of symptoms, 5) review of side effects, 6) management of side effects, 7) education about suicide and assessment of suicidality, 8) availability of a 24-hour on-call service, and 9) encouragement to stay the course long enough to benefit. DCM visits typically lasted 45–50 minutes, while IPT study visits lasted 60–75 minutes and included both manualized IPT as well as DCM.
Treatment Fidelity
Both DCM and IPT were conducted by the same study clinicians (masters-prepared psychiatric nurses and social workers, as well as PhD-level psychologists) trained in both procedures. These study clinicians had participated in previous studies of maintenance IPT in late-life depression (Reynolds et al. 1999; Reynolds et al. 2006). To document fidelity with randomized assignment, trained raters evaluated a seven-minute audio-taped segment beginning five minutes after the start of each session using the 27-item Therapy Rating Scale (Wagner et al. 1992). They rated for the presence of specific IPT and DCM components. Rating scale scores confirmed a high degree of fidelity, such that participants randomly assigned to IPT were shown to receive IPT, while those assigned to DCM received supportive and educational clinical management but not IPT. Study clinicians participated in weekly group supervision with three of the coauthors (MDM, JMC, and EF) to ensure continuing adherence with IPT and DCM manual-based procedures. (Copies of both manuals are available upon request.) Participants were not seen routinely by study psychiatrists, except on a prn basis (e.g., to assess suicidal risk). The principal investigator (CFR) supervised all aspects of study conduct and patient management via weekly conferences with study personnel.
Randomization
Randomization was under the control of the biostatistician coauthor (SM) and research pharmacist. Other investigators did not have access to the randomization schedule. Randomization was site-specific, using a single, permuted-block randomization stratified on site (e.g., primary care practice, mental health specialty clinic) to ensure that equal numbers of subjects entered into each treatment arm at each site (Table 1).
Outcomes
Outcomes assessment was conducted by independent raters blind to treatment. Our primary categorical outcome measure was remission of major depression, defined by three consecutive weekly Hamilton scores of 7 or lower. (“Response” was indicated by three final scores of 8–10.) Because Hamilton data were collected on everyone throughout randomized treatment, we were also able to document trajectory and speed of symptom change continuously via weekly scores on the Hamilton.
Statistical Analysis
We used all available data from the 124 participants in intent-to-treat analyses. Study analysts (PRH, MAD, and SM) conducted Cox regression analyses of time to, and logistic modeling of rates of, remission. Group differences in Hamilton depression ratings over time were tested via mixed-effects modeling. All analyses adjusted for age. Since we tested a small number of a priori hypotheses, we used an alpha of 0.05 for each. We powered the study to detect a 36% vs 62% difference in remission rates (for DCM versus IPT, respectively). Our aim was to randomize 80 patients to each condition, yielding a power to detect this difference of .89 (two-tailed alpha of .05).
Results (see Table 2)
Table 2.
Outcomes in Intent-to-Treat and Completer Samples*
| Interpersonal Psychotherapy N=60 | Depression Care Management N=64 | HR/OR** [95% CI] | X2 Df=1 | p | |||
|---|---|---|---|---|---|---|---|
| Raw rates [95% CI] | Age adjusted rates* | Raw rates [95% CI] | Age adjusted rates* | ||||
| Intent to Treat Responded1 during trial | 49 (82%) [72,91] | 83% | 49 (77%) [66,87] | 79% | 1.15 [0.77,1.72] | 0.47 | 0.50 |
| Intent to Treat Remitted2 during trial | 35 (58%) [46,71] | 61% | 29 (45%) [33,58] | 48% | 1.46 [0.88,2.41] | 2.13 | 0.14 |
| Completers3 Analysis Remission at 16 weeks | N=48 28 (58%) [44,72] |
58% | N=53 23 (43%) [30,57] |
43% | 1.69 [0.76,3.77] | 1.63 | 0.20 |
Three consecutive HRSD scores of 10 or less
Three consecutive HRSD scores of 7 or less
Average of last 3 HRSD scores: Remission of 7 or less, response of 10 or less, partial of 11–14 and nonresponse at 15 and above
Age adjusted rates using prognostic calculation for ITT sample and logistic predicted values for completer analyses.
We report hazard ratios (HR) for intent-to-treat Cox regression and odds ratio (OR) for logistic modeling in completer analysis.
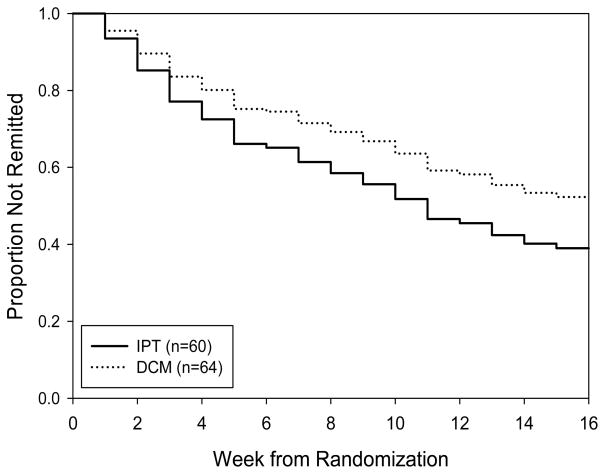
Both groups showed decreases in Hamilton depression ratings of about 30% after six weeks of depression care management with escitalopram 10mg daily. After randomization, dropout rate was 9.7% (11.7% in IPT and 7.8% in DCM). Intent-to-treat response rates were 82% [95% CI: 72,91] with IPT and 77% [66,87] with DCM, with similar median times to response (5 and 6 weeks, respectively). Both intent-to-treat (ITT) and completer analyses found similar remission rates for IPT and DCM: 58% [46,71] versus 45% [33,58], respectively, in the intent-to-treat (p = 0.14); and 58% [44,72] versus 43% [30,57], respectively, in the completer analysis (p = 0.20). (Note that p-values were based upon age-adjusted analyses, which yielded similar estimates of remission rates to those obtained from observed data.) Median time to remission was 11 weeks in IPT, while fewer than 50% of DCM-randomized patients achieved remission. (See Figure 2.)
Figure 2.
Remission in IPT and DCM
Remission rates did not differ for Interpersonal Psychotherapy (IPT) and Depression Care Management (DCM): 58% [46,71] versus 45% [33,58] (p=.14). Time to remission also did not differ (LR chi square = 1.47, p=.23).
We examined the speed of symptom improvement in age-adjusted Hamilton depression scores measured weekly during randomized treatment. The two groups did not differ in speed of symptom decrease (i.e., group by time interactive effect from mixed effects analysis, F = 2.59; df = 1, 108; p = .11).
Discussion
To our knowledge this is the first controlled evaluation of an adjunctive depression-specific psychotherapy for partial response to pharmacotherapy in late-life depression. We detected similar response and remission rates for IPT and DCM, suggesting that IPT had no added advantage over depression care management. Overall, completing a course of antidepressant phamacotherapy with either DCM or IPT resulted in cumulative response rates of 79–83% and remission rates of 45–58% in the intent-to-treat sample of partial responders to initial pharmacotherapy. These data suggest that the challenge of partial response in older adults with major depression may be amenable to straightforward depression care management strategies, with or without IPT.
That IPT and DCM did not differ in remission rates could reflect several possibilities. First, we used depression care management in both arms of the trial, and DCM is a psychosocial intervention in its own right. Consistent with this view, results of the recent CREATE trial (Cardiac Randomized Evaluation of Antidepression and Psychotherapy Efficacy) also confirmed the antidepressant effects of citalopram with clinical management, but no added benefit of IPT over clinical management, in patients with coronary artery disease (Lesperance et al. 2007). A second possibility is that the lack of difference in remission rates could reflect the fact that the same clinicians worked in both study arms. This seems unlikely because independent, blind ratings confirmed fidelity to randomized treatment assignment. A third possibility is that our study was underpowered to detect observed differences in intent-to-treat remission rates (13%). However, the clinical significance and utility of a 13% difference could be marginal in relation to the additional effort and cost of IPT. Number needed to treat (NNT) in the intent-to-treat sample was 7.7, consistent with a modest clinical effect. Nonetheless, while not large, the 13% difference is still greater than the overall difference between drug and placebo reported in a recent meta-analysis of RCT’s (with 2,337 participants) in late-life depression: 32.6% versus 26.5%, respectively (Nelson et al. 2008). This again suggests the need for a larger sample to detect modest clinical differences. Of note, the remission rates reported in the current study appear to be larger (45–58%) in partial responders than those estimated in the meta-analysis (26.5–32.6%) of all patients (not just partial responders).
The generalizability of the current findings is bolstered by the enrollment of older adults from primary care, use of relatively few exclusion criteria, treatment by mental health specialists (nurses, social workers, psychologists) working in primary care settings, and use of a widely prescribed antidepressant (escitalopram) with good efficacy and tolerability (Cipriani et al. 2009). The practicability of depression care management and delivery of IPT has been shown by our earlier study (PROSPECT [Prevention of Suicide in Primary Care Elderly: Collaborative Trial]) (Bruce et al. 2004). We do not know if similar results would have been found, had treatment been delivered by general medical clinicians rather than mental health specialists. However, results were similarly positive in the IMPACT study (Improving Mood-Promoting Access to Collaborative Treatment for Late-Life Depression), where general medical clinicians carried out guideline-based depression care, including problem solving therapy, with backup from psychiatrists as needed (Unutzer et al. 2002).
Participants demonstrated partial response (Hamilton depression scores averaging 12.5; see Table 1 and Figure 1) by the time of entry into randomized treatment at 6 weeks. Improvement during the first 4–6 weeks of treatment is a good prognostic indicator for full response by 12 weeks in older depressed adults, while minimal improvement (typically less than 40% reduction in depression ratings, as was the case with our study participants) does not support the likelihood of full response, absent a change in treatment strategy (Andreescu et al. 2008; Mulsant et al. 2006; Sackeim et al. 2006). These data provided the empirical rationale for the choice of six weeks as the randomization point for partial responders.
It is clinically important to emphasize that “staying the course” with a higher dose of pharmacotherapy helps improve rate of remission following partial responses after six weeks of initial pharmacotherapy. However, providing depression care management in conjunction with pharmacotherapy goes beyond providing extended pharmacotherapy alone. Depression care management is an active intervention that provides subjects with support, psychoeducation, and behavioral interventions (e.g., sleep hygiene), as well as emphasizing adherence with pharmacotherapy, all elements that may be key to optimizing treatment outcomes among older depressed patients. Clinicians should not assume that they can expect the same frequency of remission just by prescribing a 30-day supply of antidepressant medication with refills to depressed older patients.
We note an alternative possibility that a less intensive intervention strategy that increases medication dose and then focuses additional resources on those who are not having a satisfactory response by 12 weeks could have similar outcomes, but greater feasibility and cost-effectiveness. In fact, the study design does not allow an influence about which specific intervention led to further improvement. Was it simply a dose increases from 10mg to 20mg? Is contact this intensive necessary for improvement? These are important issues for further investigation.
Many older depressed adults express a preference for psychotherapy over pharmacotherapy (Hanson and Scogin 2008). IPT is well standardized for use in older adults (Bruce et al. 2004), and briefer courses of IPT have been shown to be effective for depression in non-geriatric adults (Swartz et al. 2008), a fact that may enhance its acceptance and feasibility in primary care settings. Further research should address whether a briefer course of IPT delivered by general medical clinicians working in primary care is effective; and to determine which sociodemographic (e.g., gender, race) and clinical factors (e.g., anxiety, co-existing medical burden, or cognitive impairment) may modify its effect on depression remission. Such data will help to identify which patients are likely to benefit from IPT. Complete remission of late-life depression, especially in previously partial responders, may also benefit the health and well-being of family care-givers who struggle to cope with patients’ depressive symptoms (Martire et al. 2008). Finally, the use of IPT may lead to a more sustained remission and better social role functioning, as previously shown for older adults with recurrent major depression (Lenze et al. 2002; Reynolds et al. 1999). The current study provides support for the use of depression care management: it appears to be as effective or almost as effective as well-delivered IPT in late-life depression and substantially better than previously reported rates of response and remission in usual care (Bruce et al. 2004; Unutzer et al. 2002) and in a meta-analysis of placebo-controlled RCT’s (Nelson et al. 2008).
Key Points.
Partial response to first-line antidepressant pharmacotherapy is common in old-age depression.
Partial response can be successfully managed by continuing pharmacotherapy with either adjunctive depression care management (DCM) or interpersonal psychotherapy (IPT).
Either adjunctive DCM or IPT yields similar remission rates in partial responders of around 50%.
This study failed to show added advantage of IPT over DCM, but patient preference needs to be taken into account.
Acknowledgments
Funding/Support: The principal source of support is the National Institute of Mental Health (MH37869, MH52247, MH071944, and MH065547). The University of Pittsburgh Medical Center (UPMC) endowment in geriatric psychiatry and the John A. Hartford Foundation provided support for Drs. Reynolds, Martire, Karp, Dombrovski, and Andreescu. Forest Laboratories provided supplies of escitalopram at no cost to the study.
Footnotes
Trial Registration: NCT00177294
Author Contributions: As principal investigator of the study, Dr. Reynolds had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Reynolds, Dew, and Frank
Acquisition of data: Reynolds, Martire, Miller, Cyranowski, Lenze, Whyte, Mulsant, Pollock, Karp, Gildengers, Szanto, Dombrovski, Andreescu, Butters, Morse, Stack, and Frank
Analysis and interpretation of data: Reynolds, Dew, Martire, Miller, Cyranowski, Lenze, Whyte, Mulsant, Pollock, Karp, Gildengers, Szanto, Dombrovski, Andreescu, Butters, Morse, Houck, Bensasi, Mazumdar, and Frank
Drafting of the manuscript: Reynolds, Dew, Martire, Houck, Mazumdar
Critical revisions of the manuscript for important intellectual content: Reynolds, Dew, Martire, Miller, Cyranowski, Lenze, Whyte, Mulsant, Karp, Gildengers, Szanto, Dombrovski, Andreescu, Morse, Houck, Mazumdar, and Frank
Statistical expertise: Dew, Houck, and Mazumdar
Obtained funding: Reynolds, Dew, Mazumdar, Frank
Administrative, technical, or material support: Reynolds, Dew, Miller, Lenze, Karp, Bensasi, and Stack
Supervision: Reynolds, Dew, Miller, Cyranowski, Frank, Mazumdar, and Bensasi
Reference List
- Andreescu C, Mulsant BH, Houck PR, Whyte EM, Mazumdar S, Dombrovski AY, Pollock BG, Reynolds CF. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry. 2008;165(7):855–862. doi: 10.1176/appi.ajp.2008.07081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ML, Ten Have TR, Reynolds CF, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: A randomized controlled trial. JAMA. 2004;291(9):1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- Charney DS, Reynolds CF, Lewis L, Lebowitz BD, Sunderland T, Alexopoulos GS, Blazer DG, Katz IR, Meyers BS, Arean PA, Borson S, Brown C, Bruce ML, Callahan CM, Charlson ME, Conwell Y, Cuthbert BN, Devanand DP, Gibson MJ, Gottlieb GL, Krishnan KR, Laden SK, Lyketsos CG, Mulsant BH, Niederehe G, Olin JT, Oslin DW, Pearson J, Persky T, Pollock BG, Raetzman S, Reynolds M, Salzman C, Schulz R, Schwenk TL, Scolnick E, Unutzer J, Weissman MM, Young RC. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Fabian TJ, Amico JA, Kroboth PD, Mulsant BH, Corey SE, Begley AE, Bensasi SG, Weber E, Dew MA, Reynolds CF, Pollock BG. Paroxetine-induced hyponatremia in older adults: A 12-week prospective study. Arch Intern Med. 2004;164(3):327–332. doi: 10.1001/archinte.164.3.327. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), Version 2.0. New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Folstein MF, Folstein SW, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AE, Scogin F. Older adults’ acceptance of psychological, pharmacological, and combination treatments for geriatric depression. Journal of Gerontology B Psychological Sciences and Social Sciences. 2008;63(4):245–248. doi: 10.1093/geronb/63.4.p245. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. Reducing Suicide: A National Imparative. National Academic Press; Washington, D.C: 2002. [Google Scholar]
- Klerman GL, Weissman MM, Rounsaville BJ, Chevron E. Interpersonal Psychotherapy of Depression. Academic Press, Basic Books Inc; New York: 1984. [Google Scholar]
- Lenze EJ, Dew MA, Mazumdar S, Begley AE, Cleon C, Miller MD, Imber SD, Frank E, Kupfer DJ, Reynolds CF. Combined pharmacotherapy and psychotherapy in maintenance treatment for late-life depression: Effects on social adjustment. Am J Psychiatry. 2002;159(3):466–468. doi: 10.1176/appi.ajp.159.3.466. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297(4):367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- Martire LM, Schulz R, Reynolds CF, Morse JQ, Butters MA, Hinrichsen GA. Impact of close family members on older adults’ early response to depression treatment. Psychol Aging. 2008;23(2):447–452. doi: 10.1037/0882-7974.23.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsant BH, Houck PR, Gildengers AG, Andreescu C, Dew MA, Pollock BG, Miller MD, Stack JA, Mazumdar S, Reynolds CF. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113–120. doi: 10.1097/01.jcp.0000204471.07214.94. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008;16(7):558–567. doi: 10.1097/JGP.0b013e3181693288. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, Houck PR, Mazumdar S, Butters M, Stack JA, Schlernitzauer MA, Whyte E, Gildengers A, Karp J, Lenze E, Szanto K, Bensasi S, Kupfer DJ. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, Houck PR. Treatment outcome in recurrent major depression: A post hoc comparison of elderly (“young old”) and midlife patients. Am J Psychiatry. 1996;153(10):1288–1292. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Frank E, Perel JM, Imber SD, Cornes C, Miller MD, Mazumdar S, Houck PR, Dew MA, Stack JA, Pollock BG, Kupfer DJ. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: A randomized controlled trial in patients older than 59 years. JAMA. 1999;281(1):39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Roose SP, Lavori PW. Determining the duration of antidepressant treatment: Application of signal detection methodology and the need for duration adaptive designs (DAD) Biol Psychiatry. 2006;59(6):483–492. doi: 10.1016/j.biopsych.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Sheffrin M, Driscoll HC, Lenze EJ, Mulsant BH, Pollock BG, Miller MD, Butters MA, Dew MA, Reynolds CF. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: Getting to remission. J Clin Psychiatry. 2009;70(2):208–213. doi: 10.4088/jcp.07m03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HA, Frank E, Zuckoff A, Cyranowski JM, Houck PR, Cheng Y, Fleming MA, Grote NK, Brent DA, Shear MK. Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. Am J Psychiatry. 2008;165(9):1155–1162. doi: 10.1176/appi.ajp.2008.07081339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Frank E, Steiner SC. Discriminating maintenance treatments for recurrent depression: development and implementation of a rating scale. J Psychother Pract Res. 1992;22:281–291. [PMC free article] [PubMed] [Google Scholar]
- Whyte EM, Dew MA, Gildengers A, Lenze EJ, Bharucha A, Mulsant BH, Reynolds CF. Time course of response to antidepressants in late-life major depression: Therapeutic implications. Drugs Aging. 2004;21(8):531–554. doi: 10.2165/00002512-200421080-00004. [DOI] [PubMed] [Google Scholar]