Two individuals undergoing orthotopic hepatic transplantation received livers from donors who were on average 10 kg smaller than themselves based on recipient ideal body weight. As a result, the donor livers in these 2 cases were 29%–59% smaller than would be expected had the donor liver and recipient been matched ideally. The liver grafts in the recipients steadily increased in size, as determined by serial computed tomography scanning, to achieve new volumes consistent with those that would have been expected in a normal individual of the recipient’s size, sex, and age. Fasting plasma levels of amino acids, glucagon, insulin, and standard liver injury tests were monitored to determine which measure best reflected the changes observed in the size of the grafts over time. No relationship between the changes observed in any of these parameters and hepatic growth was apparent. In both cases, the liver increased in volume at a rate of ~70 ml/day. These data demonstrate that a small-for-size liver transplanted into a larger recipient increases in size at a rate of ~70 ml/day until it achieves a liver volume consistent with that expected given the recipient’s size, age, and sex.
Under normal circumstances, attempts at matching the recipient of an orthotopic liver transplant (OLT) with the best available donor in terms of organ and body size are made (1,2). Occasionally, however, only a small donor is available and thus a small-for-size liver is transplanted urgently into a recipient who is considerably larger but is in serious need of a transplant. In the last 5 yr, we have had 2 cases in which a small donor liver has been transplanted into a larger recipient. In both cases, we have had the opportunity to observe the change in size of the allograft over time using serial computed tomography (CT) scans to evaluate change in graft size. In addition, in each case, we serially obtained measures of hepatic injury, plasma amino acids, and circulating levels of insulin and glucagon, the two pancreatic hormones that are thought to be trophic for the liver. Herein are reported the observations made on these unique cases.
Patients and Methods
Recipients
The two OLT recipients reported herein had been selected as candidates for OLT based on standard criteria used in Pittsburgh for establishing such candidacy. These have been reported elsewhere (1,3–5). For each recipient, the nature of the primary hepatic disease, body weight, ideal body weight, age, sex, and liver volume obtained by CT scanning before OLT are reported in Table 1.
Table 1.
Characteristics of the Two Recipients Studied
| Case No. |
Sex | Underlying hepatic disease |
Age (yr) |
Liver volume (ml) |
Body weight (kg) |
Ideal body weight (kg) |
|---|---|---|---|---|---|---|
| 1 | F | Primary biliary cirrhosis |
44 | 1768 | 47.5 | 56.3–62.7 |
| 2 | M | Postnecrotic cirrhosis |
16 | 1785 | 44.3 | 67.3–72.7 |
Donors
The two donors had a mean age of 8 yr (range 7–9 yr). Their body weights, ideal body weights, and liver volumes are shown in Table 2.
Table 2.
Characteristics of the Two Donors Studied
| Case No. |
Sex | Cause of death |
Age (yr) |
Liver volume (ml) |
Body weight (kg) |
Ideal body weight (kg) |
|---|---|---|---|---|---|---|
| 1 | M | Accident | 7 | 693 | 23.0 | 21.0–25.0 |
| 2 | F | Accident | 9 | 1193 | 30.0 | 25.0–33.3 |
Controls
Eight adult patients undergoing OLT but receiving normal-sized livers served as the controls. Each received a liver that was no greater or smaller than a liver [± 15%) that would have been expected to be in the recipient had the recipient been normal.
Liver Volume Determinations
The volume of each recipient’s original diseased organ before OLT and the recipient’s new organ after successful OLT was determined serially using a CT scanning technique that has been described and validated previously (2); liver weights and volumes determined by this method are similar on a gram per milliliter basis.
Hormone Measurements
Plasma immunoreactive insulin, glucagon, and C-peptide were determined serially after successful OLT at the time of each CT scan using radioimmunoassay kits obtained from Serono Laboratories (Boston, Mass.). The detection limits for the three kits are 5.0 μU/ml, 10 pglml, and 0.2 ng/ml, respectively. Normal values for each are as follows: insulin 2–18 μU/ml, glucagon 0–85 pglml, and C-peptide 0.3–3.0 ng/ml. All measurements were made in triplicate and the mean value was reported for each determination at each time point.
Standard Laboratory Data
Total bilirubin and aspartate aminotransferase levels were determined daily on venous blood by the Clinical Chemistry Division of the Department of Pathology, Presbyterian University Hospital, using standard methods. Fasting plasma amino acid levels were determined in venous blood obtained in the morning of the day on which each CT scan study was obtained. The plasma was separated by centrifugation at 4°C and plasma proteins were removed by precipitation with 4% sulfosalicylic acid. The levels of the individual amino acids were determined using a Beckman amino acid analyzer (Beckman Spinco, Palo Alto, Calif.). Results were reported as micromoles per deciliter and the molar ratio of the sum of the aromatic amino acids, phenylalanine and tyrosine, to the sum of the branched chain amino acids, leucine, isoleucine, and valine, was determined.
Determination of Normal Liver Weight for a Given Individual Using Age- and Sex-Matched Controls
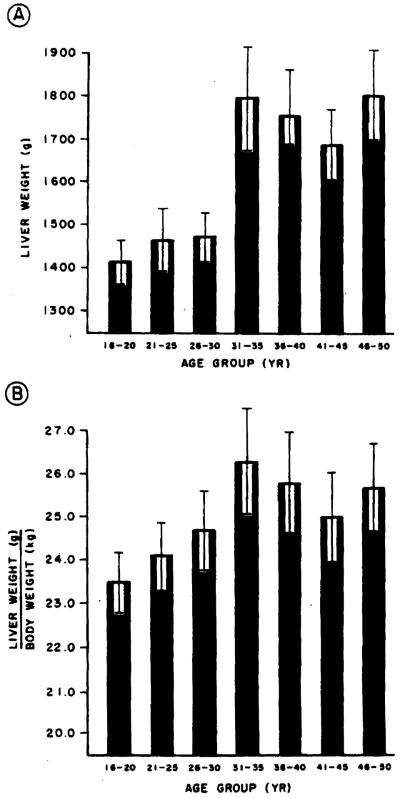
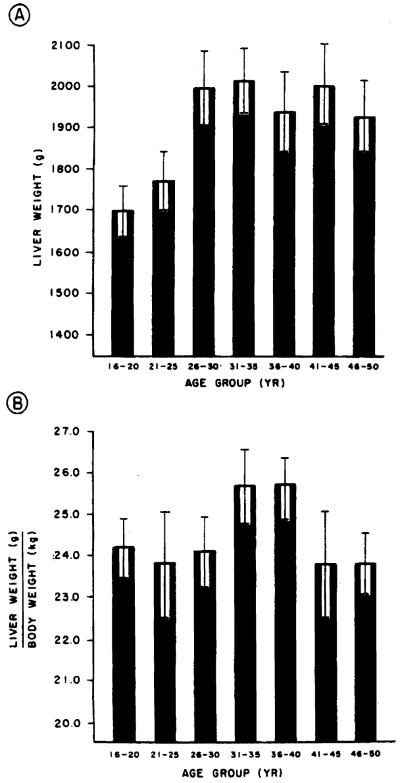
The records of the Allegheny County Coroner’s Office were used to identify individuals who had died suddenly of an accident or homicide and who had no hepatic pathology at the time of their death. The records of the individuals meeting these criteria were reviewed and verified. Forty such individuals for each age group (21–25, 26–30, 31–35, 36–40, 41–45, and 46–50 yr) were selected for each sex to serve as control data for the determination of normal hepatic weight (volume) for each age group (Figures 1 and 2).
Figure 1.
Liver weight in grams (A) and liver weight in grams corrected for body weight in kilograms (B] for women aged 16–50 yr grouped in 5-yr intervals. The bars represent mean values. The brackets represent the standard error of the mean. Note that liver size increases until age 26–30 and then achieves a plateau.
Figure 2.
Liver weight in grams (A) and liver weight in grams corrected for body weight in kilograms (B) for men aged 16–50 yr grouped in 5-yr intervals. The bars represent mean values. The brackets represent the standard error of the mean. Note that as was the case in women, a plateau in liver mass is reached after age 26 yr.
Statistical Analysis
The rate and amount of growth of the transplanted livers was calculated from serial CT volume measurements using linear regression analysis. Only successive points that showed an increase in liver volume of at least 50 ml were used for these calculations to avoid using points obtained after the transplanted liver had reached a plateau in size.
Results
The data obtained for normal liver weight for each sex at 5-yr intervals between ages 21 and 50 yr are shown in Figures 1 and 2.
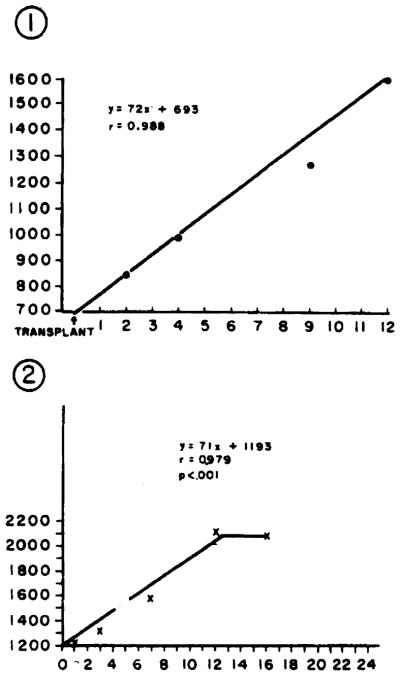
The small-for-size intact donor organs transplanted into the larger recipients grew rapidly over a 2–3-wk period within their new hosts. The rate of hepatic growth in each recipient varied only slightly and was linear at an approximate rate of 70 ml/day until a new hepatic mass was achieved at 10–20 days posttransplantation (Figure 3).
Figure 3.
Growth of an intact small-for-size normal liver transplanted into a larger recipient. The changes observed in graft size for the 2 cases reported are shown in the component parts of the figure. The ordinate for each component graph is milliliters of liver determined by CT scanning. The number in the left upper corner of each figure reflects the patient number referred to in the text.
There was no correlation between laboratory measurements of liver injury (bilirubin, transaminases) obtained before or immediately after transplantation and either the absolute change in liver mass or the rate of change in liver mass (data not shown).
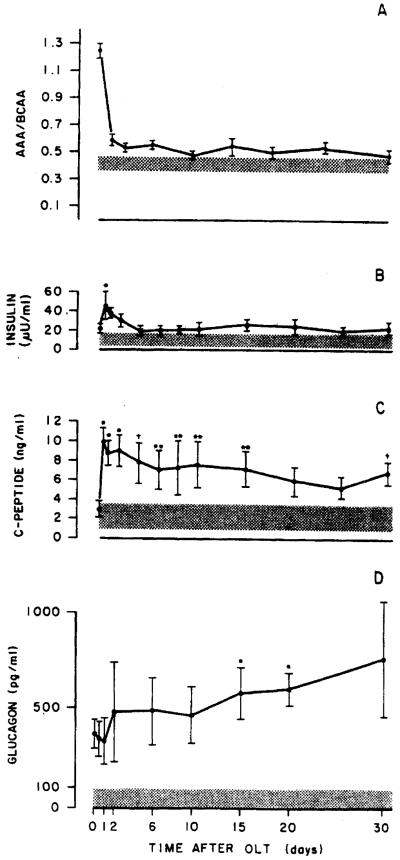
The molar ratio of aromatic amino acids to branched chain amino acids in the serum of the recipients studied before OLT was increased; the ratio declined initially after liver transplantation, but returned to an elevated level several days posttransplantation (Figures 4 and 5). The changes observed in this measure did not correlate with either the absolute change or rate of change in liver mass noted in the recipients. The plasma levels of insulin, C-peptide, and glucagon observed at various times before and after OLT in the 2 patients studied are shown in Figures 4 and 5. Note that in each case, glucagon levels were initially elevated and remained elevated posttransplantation, whereas insulin levels declined toward normal levels with time after transplantation; note also that the time-course of these hormonal changes varied considerably. Similar studies performed in 8 OLT patients not receiving an organ mismatched for size (i.e., within ±15% of ideal size) are shown in Figure 6.
Figure 4.
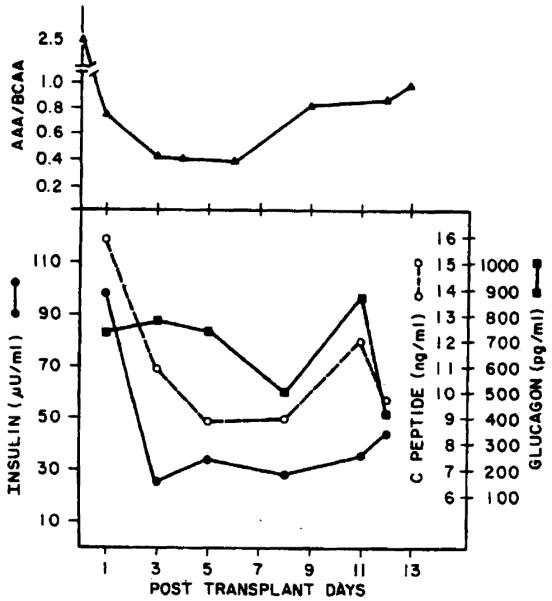
Schematic figure showing the sequential changes in the ratio of aromatic amino acids to branched chain amino acids, insulin. C-peptide, and glucagon levels in case 1.
Figure 5.
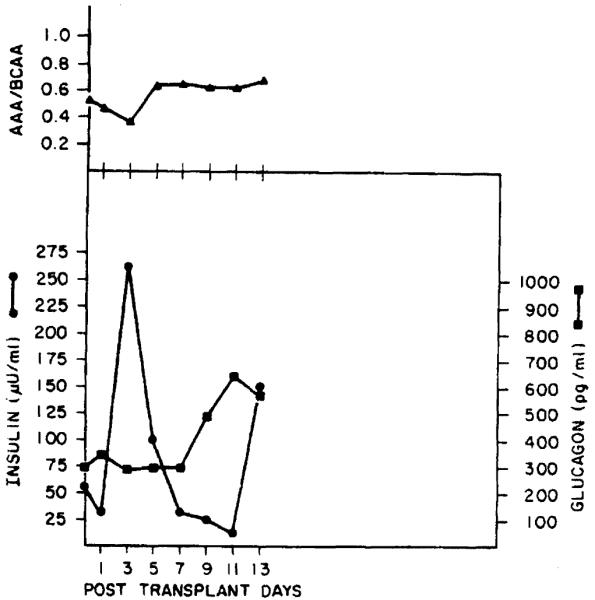
Schematic figure showing the sequential changes in the ratio of aromatic amino acids to branched chain amino acids, insulin, and glucagon levels in case 2.
Figure 6.
Schematic figure showing the sequential changes in the ratio of AAA to BCAA, insulin, C-peptide, and glucagon in 8 patients undergoing OLT but not having a mismatch in terms of liver size. The shaded orea is the range for normal individuals; the hrockets represent the SEM. The asterisk in panel B represents the single point at which it was statistically different (p < 0.051 from that observed at time zero. In panel C, a single asterisk indicates p < 0.005, a double asterlsk indicates p < 0.05, and a cross indicates p < 0.01 from that obtained at time zero. In panel D the asterlsk identifies the two times at which the glucagon level was significantly different from the level obtained at time zero. In panel A, all points obtained after time zero are significantly different from the data obtained at time zero.
Discussion
The data presented clearly demonstrate that a small-for-size intact liver transplanted into a larger host increases in size rapidly over 2–3 wk to achieve a size consistent with that of a normal-sized liver in a normal individual of the same size, age, and sex as the recipient. Several mechanisms may be at least partially responsible for this observation. In each case, the small donor liver was likely subjected to a relatively high volume of portal venous blood flow after transplantation into a larger recipient. This increase in hepatic blood flow relative to liver size could be hypothesized to have produced hepatocellular edema; the fact that the transplanted liver maintained its new volume during the 1–2 wk of observation after the plateau in volume was reached suggests, however, that the apparent growth of the allograft was not due simply to edema. It is also possible that increases in the hepatic content of glycogen, fat, or inflammatory cells (due to mild rejection) may have contributed to the increase in liver mass observed after transplantation in these two recipients. This possibility may account, at least in part, for the increased liver weighthody weight ratio observed in these recipients after successful transplantation, Again, the fact that the liver volume stabilized at the larger volume achieved 2–3 wk after OLT makes this possibility less likely but does not rule it out. Moreover, comparable evidence for stabilization of newly increased liver volume in animals after transplantation of a small-for-size graft into a larger recipient has been reported elsewhere (6). These observations also make this hypothesis less likely. A remaining possibility is that hepatic regeneration occurred and accounted for the change in liver volume in the small-for-size allograft recipient.
The changes observed in serum bilirubin, aminotransferase levels, and the ratio of aromatic amino acids to branched chain amino acids after OLT did not correlate with the observed changes in hepatic mass of the allograft. In addition, as the hepatic mass increased and normal liver function returned after OLT, the preexistent abnormalities in the systemic plasma levels of insulin and C-peptide associated with cirrhosis and liver failure corrected at least partially. In contrast, glucagon levels continued to be increased and remained so as the small-for-size donor organ increased in size in the larger recipient. It must be noted, however, that similar changes were observed in the plasma of the 8 patients who did not receive mismatched livers; thus it is unlikely that the changes observed in the levels of these biochemical markers were directly responsible for the hepatic growth observed.
Of the two pancreatic hormones measured, the glucagon levels more accurately reflected a putative hepatic regeneration stimulus, as demonstrated by concomitantly increasing glucagon levels and liver volume. These data suggest the possibility that the factors that regulate glucagon secretion may regulate the process of hepatic regeneration under appropriate circumstances or be a signal for hepatic regeneration initiated by some other immediate cause. It is also important to note that, although the insulin levels declined after successful OLT, insulin extraction from the portal venous blood was not determined and hepatic insulin extraction may have been increased and have produced the net reduction in the plasma levels of the hormone measured, independent of the secretion rate of the hormone accessed by measurement of plasma C-peptide levels.
Considerable experimental data exist for the concept that both insulin and glucagon are hepatotrophic factors (7–25). Each has been shown to enhance the nuclear labeling of hepatocytes with tritrium-labeled thymidine both in vitro and in vivo after partial hepatectomy. Moreover, it has been reported that after partial hepatectomy, plasma levels of glucagon increase while the plasma levels of insulin fall, and that the turnover rates of both insulin and glucagon decrease almost twofold (13,14,20,21). Coincidentally, hepatic insulin receptor content has been shown to increase twofold, whereas hepatic glucagon receptor content declines by 50% under these conditions (8,14,25). In addition, numerous studies performed in both experimental animals and humans after portacaval shunting procedures have provided considerable evidence that insulin acts as a potent hepatotrophic substance (8–10,12–21,23–27). Specifically, the liver atrophies rapidly after portal vein diversion despite a threefold to fourfold increase in the systemic level of insulin (15,16,21,22,28). The unique hepatotrophic effect of insulin has been documented even more clearly in “double liver” experiments in which one side of the liver has been perfused via the portal vein with its greater insulin levels while the other side receives systemic venous blood with its lower insulin content (9). Even the more recent observations that multiple factors other than insulin. particularly a host of insulinlike peptides, act as hepatotrophic factors do not negate the central role of insulin as a hepatotrophic factor (23,24,29,30). This concept has been demonstrated best by the studies in which hepatic regeneration occurs in the absence of all spianchnic viscera if exogenous insulin is added to the portal venous blood (23,24).
Taken together, the present data and that of many others suggest that there may be an ideal hepatic mass for adults of each age and sex and that when such a hepatic mass is achieved, the putative signals for hepatic regeneration are either turned off or counterregulatory signals are initiated to inhibit the regenerative process. The specific nature of these signals, however, has not been elucidated and remains to be determined.
Acknowledgments
This work was supported in part by grants NIAAA AA04425 and NIAMDD R01 AM32556 and a grant from the Gastroenterology Medical Foundation of Southwestern Pennsylvania.
Abbreviations used in this paper
- CT
computed tomography
- OLT
orthotopic liver transplant
References
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–36. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Gastroenterology. 1985;88:1812–917. doi: 10.1016/0016-5085(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 3.Van Thiel DH, Schade RR, Hakala TR, Starzl TE, Denny D. Liver procurement for orthotopic transplantation: an analysis of the Pittsburgh experience. Hepatology. 1984;4:66S–71S. doi: 10.1002/hep.1840040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Thiel DH, Schade RR, Gavaler JS, Shaw BW, Jr, Iwatsuki S, Starzl TE. Medical aspects of liver transplantation. Hepatology. 1984;4:79S–83S. doi: 10.1002/hep.1840040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Thiel DH. Liver transplantation. Pediatr Ann. 1985;14:474–40. doi: 10.3928/0090-4481-19850701-05. [DOI] [PubMed] [Google Scholar]
- 6.Kam I, Lynch S, Svanas G, et al. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology. 1987;7:362–6. doi: 10.1002/hep.1840070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann FC. The portal circulation and restoration of the liver after partial removal. Surgery. 1940;8:225–38. [Google Scholar]
- 8.Starzl TE, Terblanche J. Hepatotrophic substances. In: Popper H, Schaffner F, editors. Progress in liver diseases. Volume 7. Grune & Stratton; New York: 1982. pp. 135–51. [PubMed] [Google Scholar]
- 9.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:139–99. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Putman CW, Porter KA, et al. Portal diversion for the treatment of glycogen storage disease in humans. Ann Surg. 1973;178:525–39. doi: 10.1097/00000658-197310000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Chase HP, Putman CW, Porter KA. Portacaval shunt in hyperlipoproteinaemla. Lancet. 1973;ii:940–4. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- 12.Bucher NLR, Swaffield MN. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Scl USA. 1975;72:1157–60. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley CGD, Kuku S, Rubenstein AH, Boyer JL. Serum hormone levels following partial hepatectomy in the rat. Biochem Biophys Res Commun. 1975;67:653–61. doi: 10.1016/0006-291x(75)90862-1. [DOI] [PubMed] [Google Scholar]
- 14.Leffert H, Alexander NM, Faloona G, Rubalcava B, Unger R. Specific endocrine and hormonal receptor changes associated with liver regeneration in adult rats. Proc Natl Acad Sci USA. 1975;72:4033–6. doi: 10.1073/pnas.72.10.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Porter KA, Putman CW. Intraportal insulin protects from the liver injury of portacaval shunt in dogs. Lancet. 1975;ii:1241–2. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Lee IY, Porter KA, Putman CW, Starzl TE, Porter KA, Kashiwagi N, Lee IY, Russell Wjj, Putman CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. The effect of diabetes mellitus on portal biood hepatotrophic factors in dogs. Surg Gynecol Obstet. Surg Gynecol Obstet. 1975;1975;140140:381–96. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Porter KA, Kashiwagi N, Putman CW. Portal hepatotrophic factors, diabetes mellitus and acute liver atrophy, hypertrophy and regeneration. Surg Gynecol Obstet. 1975;141:843–58. [PMC free article] [PubMed] [Google Scholar]
- 19.Bucher NLR, Weir GC. Insulin, glucagon, liver regeneration, and DNA synthesis. Metabolism. 1976;25:1423–5. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- 20.Richman RA, Claus TH, Pilkis SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci USA. 1976;73:3589–93. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Porter KA, Watanabe K, Putman CW. The effects of insulin glucagon and insulin/glucagon infusions upon Liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;i:821–5. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 22.Putman CW, Porter KA, Starzl TE. Hepatic encephalopathy and light and electron microscopic changes of the baboon liver after portal diversion. Ann Surg. 1976;184:155–61. doi: 10.1097/00000658-197608000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;147:193–207. [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Francavilla A, Porter KA, Benichou J, Jones AF. The effect of splanchnic viscera removal upon canine liver regeneration. Surg Gynecol Obstet. 1978;147:193–207. [PMC free article] [PubMed] [Google Scholar]
- 25.Leffert HL, Koch KS, Rubalcava B, Sell S, Moran T, Boorstein R. Hepatocyte growth control: in vitro approach to problems of liver regeneration and function. Natl Cancer lnst Monogr. 1978;48:87–101. [PubMed] [Google Scholar]
- 26.Strecker W, Goldberg M, Feeny DA, Ruhenstroth-Bauer G. The influence of extended glucagon infusion on liver cell regeneration after partial hepatectomy in the rat. Acta HepatoGastroenterol. 1979;26:439–41. [PubMed] [Google Scholar]
- 27.Wagle SR, Ingerbretsen WR, Jr, Sampson L. Studies on the effects of insulin on glycogen synthesis and ultrastructure in isolated rat liver hepatocytes. Biochem Biophys Res Commun. 1973;53:937–43. doi: 10.1016/0006-291x(73)90182-4. [DOI] [PubMed] [Google Scholar]
- 28.Rubin E, Gevirtz NR, Gohan P, Tomita F, Jacobson IH. Liver cell damage produced by portacaval shunt. Proc Soc Exp Biol Med. 1965;118:235–7. doi: 10.3181/00379727-118-29807. [DOI] [PubMed] [Google Scholar]
- 29.Leffert HL, Koch KS. Ionic events at the membrane initiate rat Liver regeneration. Ann NY Acad Sci. 1980;339:201–15. doi: 10.1111/j.1749-6632.1980.tb15979.x. [DOI] [PubMed] [Google Scholar]
- 30.Koch KS, Leffert HL. Growth control of differentiated adult rat hepatocytes in primary culture. Ann NY Acad Sci. 1980;341:111–27. doi: 10.1111/j.1749-6632.1980.tb29520.x. [DOI] [PubMed] [Google Scholar]